Abstract
The KRAS is an important and frequently mutated gene during colorectal carcinogenesis. The expression of miR-143 is often down-regulated and it might play an important role by targeting KRAS in colorectal cancer (CRC). The purpose of this study was to investigate the antitumor effects of miR-143 with an intermediate oncolytic adenovirus (Ad) in CRC. We constructed the recombinant virus Ad-ZD55-miR-143 and verified its expression by qPCR and western blot assays. Oncolytic potency of Ad-ZD55-miR-143 was determined by cytopathic effect assays using human SW480 CRC cells and L-02 normal liver cells. MTT and cell apoptosis assays were applied to explore the biological functions of Ad-ZD55-miR-143 within SW480 cells. Dual-luciferase reporter assays were performed to validate whether KRAS was regulated by miR-143. The expression level of KRAS was measured by qPCR and western blot assays. Results showed that infection of SW480 cells with Ad-ZD55-miR-143 induced high level expression of miR-143. Furthermore, Ad-ZD55-miR-143 significantly suppressed the viability of SW480 cells in a dose-dependent pattern, but did not influence L-02 cells. Ad-ZD55-miR-143 also inhibited cell growth and induced cell apoptosis in SW480 cells. Dual-luciferase assays indicated that KRAS was a direct target of miR-143, as subsequently demonstrated by qPCR and western blot analysis showing that infection of SW480 cells with Ad-ZD55-miR-143 resulted in the down-regulation of KRAS at both mRNA and protein levels. Taken together, the recombinant virus Ad-ZD55-miR-143 exhibited specific antitumor effects by targeting KRAS, and might be a promising agent for the treatment of CRC.
Keywords: Colorectal cancer, oncolytic adenovirus, microRNA-143, KRAS
Introduction
Colorectal cancer (CRC) is one of the most common malignancies, which has become the fifth leading cause of cancer deaths in China [1]. Specific mutations in selected oncogenes and suppressor genes are involved in initiation and progression of CRC, which become a main obstacle for efforts to survival improvement. The Kirsten rat sarcoma viral oncogene homolog (KRAS) is an important and frequently mutated gene in colorectal carcinogenesis. Mutation of KRAS gene is reported as a frequency of 30-40% in CRC [2]. The prognosis of metastatic CRC with KRAS mutations was much poorer because KRAS mutations were resistant to anti-EGFR monoclonal antibodies [3-5]. Thus, it is of importance to find new therapeutics against CRC with KRAS gene mutations.
MicroRNAs (miRNAs) are endogenous noncoding regulatory RNAs that inhibit gene expression at the post-transcriptional level through binding to the 3’-untranslated region (UTR) of target mRNAs [6]. Deregulation of miRNAs is considered to be associated with human malignancies suggesting a causal role of miRNAs in cancer [7,8]. Previous studies [9,10] indicated that miR-143 was frequently down-regulated and acted as a tumor suppressor in CRC. Recent computer sequence analysis exhibited that the 3’-UTR of KRAS mRNA might represent a target of miR-143, which made us to speculate that miR-143 might play an important role in development of CRC by targeting KRAS.
Gene via virus vectors have been constructed for tumor treatment [11]. This strategy combined the advantages of both gene therapy and viro-therapy by using virus vectors to harbor anti-cancer genes. However, the virus vectors used earlier were incapable of replication thus limiting its therapeutic effect. Hence, a key question is how to design vectors that improve tumor cell specificity and anti-cancer gene expression levels in gene therapy systems. A strategy based on endogenous miRNAs has been explored to control viral replication [12]. Callegari E et al [13] introduced four copies of miR-199 target sites within the 3’-UTR of E1A gene in adenovirus (Ad), essentially for viral replication in hepatocellular cancer cell lines. Other constructed Ads also could replicate specifically in tumor cells therefore infect and kill more tumor cells while avoiding damage to normal cells [14,15].
In this study, we constructed a conditionally replicative virus, Ad-ZD55-miR-143, where the E1B55kDa encoding gene was deleted (ZD55) to restrict viral replication only in tumor cells and acted as a carrier of anti-cancer gene miR-143. The present study was aimed to determine whether Ad-ZD55-miR-143 was able to selectively target CRC cells, inhibit cell growth and induce cell apoptosis. Furthermore, we also explored whether KRAS gene was regulated by Ad-ZD55-miR-143 in CRC cells.
Materials and methods
Cell lines and transfection
The human colorectal cell line SW480 (positive for KRAS gene) and human normal liver cell line L-02 were purchased from Chinese Academy of Sciences (Shanghai, China). The HEK293A and HEK293T cell lines were obtained from American Type Culture Collection (ATCC, Manassas, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (both from Gibco, USA), penicillin (100 U/ml) and streptomycin (100 μg/ml) (Enpromise, China). Cells were incubated in humidified chambers supplemented with 5% CO2 at 37°C.
Plasmids and recombinant Ad reconstruction
The 3’-UTR of KRAS was amplified by polymerase chain reaction (PCR). Fragments were then sub-cloned into the XhoI site in the 3’-UTR of the psiCHECK-2 reporter vector. The reporter plasmid was allocated as psiCHECK-2/KRAS 3’-UTR.
The pShuttle-ZD55 plasmid had been constructed in our laboratory [16]. The pri-miR-143 was amplified by PCR, followed by clone into pcDNA3.1 plasmid in order to construct pcDNA-miR-143. Next, pcDNA-miR-143 was digested with the Bgl II restriction enzyme to obtain an expression cassette and miR-143 coding sequences. This cassette was sub-cloned into pShuttle-ZD55 to generate pShuttle-ZD55-miR-143. All plasmids were testified by restrictive enzyme digestion, PCR, and DNA sequencing. The Ad-ZD55 and Ad-ZD55-miR-143 were produced in HEK293A cells by homologous combinations of pShuttle-ZD55 or pShuttle-ZD55-miR-143 to the Ad packaging plasmid pAdeasy using the pAdeasy system [17], respectively. Recombinant viruses were purified by cesium chloride gradient ultracentrifugation. Virus titers were measured by using a standard plaque assay on HEK293A cells. The wild-type Ad was protected in our laboratory.
Quantitative PCR (qPCR) assay
Cells were infectioned with Ad-ZD55 or Ad-ZD55-miR-143 at a multiplicity of infection (MOI) concentration of 5 pfu/cell. After 48 h, miRNAs were harvested according to the instructions of the miRcute miRNA isolation kit (Tiangen, Beijing, China). The miR-143 primer, U6 primer and EzOmics SYBR qPCR kit were purchased from Biomics Biotechnology Inc (Jiangsu, China). The U6 primer was used as an internal control and the sequences were as follows: 5’-TGCGGGTGCTCGCTTCGCAGC-3’ (sense) and 5’-CCAGTGCAGGGTCCGAGGT-3’ (antisense), 5’-GTCCTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACAAAATATGGAAC-3’ (stem-loop primer). One Step PCR parameters for miRNA quantification were as follows: 37°C for 60 min for reverse transcription, 95°C for 10 min, and 40 cycles for 20 sec at 95°C, 62°C for 30 sec and 72°C for 30 sec. The relative expression was calculated with the 2-ΔΔCt method [18].
Total RNA was isolated using TRIzol (Invitrogen, USA) and cDNA was generated by reverse transcription using the Prime Script RT-PCR kit according to the manufacturer’s instructions (Takara). Real-time PCR (RT-PCR) was performed on a 7900HT fast RT-PCR instrument using SYBR-Green and the following primers: for KRAS, 5’-AGACAGGAGTGGAGGATGCTTTT-3’ (sense), and 5’-TTCACACAGCCAGGAGTCTTTTC-3’ (antisense); for GAPDH: 5’-AAGGTCGGAGTCAACGGATT-3’ (sense), and 5’-CTGGAAGATGGTGATGGGATT-3’ (antisense). The PCR parameters for relative quantification were as follows: 5 min at 94°C, followed by 30 cycles of 30 sec at 94°C, 45 sec at 57°C and 45 sec at 72°C.
Western blot analysis assay
Cells were harvested and re-suspended in a lysis buffer. After centrifugation at 12,000 g for 15 min at 4°C, the supernatant containing cytoplasmic fractions was used to tested for E1A, E1B55kDa, and KRAS proteins. Protein samples were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Beyotime, China). Immune complexes were formed by incubation of membranes with primary antibody (E1A and E1B55kDa from Santa Cruz and KRAS from Abcam Epitomics, USA) overnight at 4°C. Blots were then washed and incubated for 1 h with secondary antibodies. After washing with PBST, immunoreactive protein bands were detected using the Odyssey scanning system (LI-COR, Lincoln, NE, USA).
Cytopathic effect assay
For determination of virus-mediated cell killing ability, 5×104 cells (SW480 and L-02) were seeded in 24-well plates and infected by Ad-ZD55 or Ad-ZD55-miR-143 at various MOIs. The medium was discarded after 8 h and fresh medium containing 10% serum was added. Five days after infection, the medium was removed, and the cells were exposed to 2% crystal violet in 20% methanol for 20 min. The plates were then washed with water to remove excess dye and documented as photographs.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cell proliferation was measured by MTT assays. Cells were seeded in 96-well plates at a density of 3,000 cell/well. Then, cells were incubated with various MOIs of Ad-ZD55-miR-143 and Ad-ZD55 in serum-free DMEM at 37°C for 8 h. After incubation, serum-free DMEM containing the viruses was replaced with normal growth medium. The optical density (OD) of each well was measured with a microplate spectrophotometer at 490 nm.
Apoptosis assay
The Annexin V-FITC/PI kit was obtained from Beyotime Institute of Biotechnology (Jiangsu, China). After 20 h of co-incubation, cells were washed three times with PBS and disassociated with trypsin. After adding Annexin V-FITC reagent, the cells were incubated in the dark for 15 min at room temperature (RT). Subsequently, propidium iodide (PI) was added and the cells were incubated in dark for 5 min at RT. All samples were processed by flow cytometry (FACSCanto™ II, BD Biosciences, USA).
Dual-luciferase assay
psiCHECK-2/KRAS 3’-UTR reporter plasmids (100 ng) were co-transfected with miR-143 mimics (Gene Pharma, Shanghai, China) or negative control (NC) (100 nmol/L) into HEK293T cells, at 80% confluence, using lipofectamine 2000 (Invitrogen, USA), according to the manufacturer’s instructions. 36 h after transfection, the firefly luciferase (FL) and the renilla luciferase (RL) activity were measured by the dual-luciferase reporter assay kit (Promega, USA). The psiCHECK-2 vector that provided constitutive expression of FL was co-transfected as an internal control.
Statistical analysis
Data from at least three separate experiments are presented as mean ± standard error of the mean (SEM). Independent sample t-test was used to draw a comparison between groups. Differences were considered statistically significant only when a two-tailed P-value was less than 0.05.
Results
Verification of recombinant virus Ad-ZD55-miR-143
As depicted in Figure 1A, the 2-ΔΔCt value of miR-143 was significantly increased in Ad-ZD55-miR-143 group compared with that of wild Ad and Ad-ZD55 groups (P<0.05). These results indicated that miR-143 was successfully cloned into Ad-ZD55.
Figure 1.
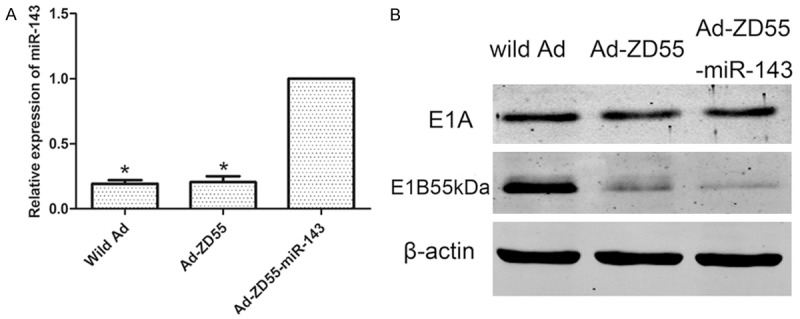
Confirming recombinant virus Ad-ZD55-miR-143. A: The expression level of miR-143 was significantly higher in Ad-ZD55-miR-143 group, comparing with wild Ad and Ad-ZD55 groups. Graph represented the 2-ΔΔCt values ± SEM. *P<0.05. B: Ad-ZD55-miR-143 was confirmed as an E1B55kDa deleted virus and expressed E1A protein by western blot. SEM, standard error of the mean.
As shown by western blot assays (Figure 1B). Ad-ZD55 and Ad-ZD55-miR-143 expressed E1A protein, but failed to express E1B55kDa protein. In contrast, wild Ad expressed both proteins. These results suggested that Ad-ZD55-miR-143 was not contaminated by wild-type Ad and Ad-ZD55-miR-143 was successfully established.
Recombinant virus Ad-ZD55-miR-143 promoted cell killing ability in SW480 cells
In order to assay biological activity of the virus Ad-ZD55-miR-143, SW480 and L-02 cells were infected with Ad-ZD55 and Ad-ZD55-miR-143 virus at the dose of 0.01, 0.5, 1 and 10 MOIs, respectively. Cells with DMEM acted as mock groups. Cell killing effect was then assessed by crystal violet staining. As shown in Figure 2A, Ad-ZD55-miR-143 and Ad-ZD55 groups demonstrated a dose-dependent cell killing effect in all SW480 cells. While the killing effect of Ad-ZD55-miR-143 was higher than that of Ad-ZD55 from the dose of 0.5 MOI. However, Ad-ZD55 and Ad-ZD55-miR-143 caused limited cell death in L-02 cells (Figure 2B). The cytopathic effects indicated that Ad-ZD55-miR-143 increased the capacity of viral replication and oncolysis specifically targeting the cancer cells.
Figure 2.
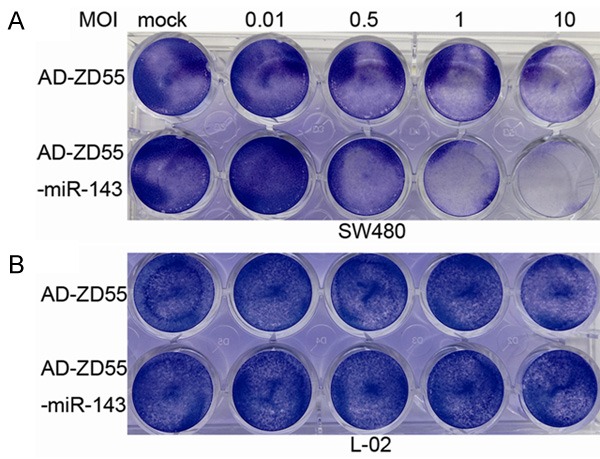
Cytopathic effects of Ad-ZD55-miR-143. SW480 and L-02 cells were infected with Ad-ZD55 and Ad-ZD55-miR-143 at 0.01, 0.5, 1 and 10 MOIs. Five days later, the cells were stained with 2% crystal violet and photographed. A: SW480 cells were sensitive to Ad-ZD55-miR-143. B: The viability of L-02 cells remained almost unchanged. MOI, multiplicity of infection.
Recombinant virus Ad-ZD55-miR-143 inhibited cell proliferation ability in SW480 cells
MTT assays were used to measure the proliferation of cells infected with recombinant virus. Cells with no infection acted as control groups. As depicted in Figure 3A, the viability of cells infected with Ad-ZD55 or Ad-ZD55-miR-143 at various MOIs was measured and compared with control groups. The cell proliferation ability of Ad-ZD55 and Ad-ZD55-miR-143 groups was obviously in a dose-dependent manner. According to the results, we chose to follow experiments at the dose of 5 MOI. Ad-ZD55 and Ad-ZD55-miR-143 inhibited growth of SW480 cells in a time-dependent manner. In addition, Ad-ZD55-miR-143 significantly inhibited the growth of SW480 cells compared with Ad-ZD55 and the control groups (Figure 3B). These results suggested that recombinant virus Ad-ZD55-miR-143 suppressed the proliferation of SW480 cells.
Figure 3.
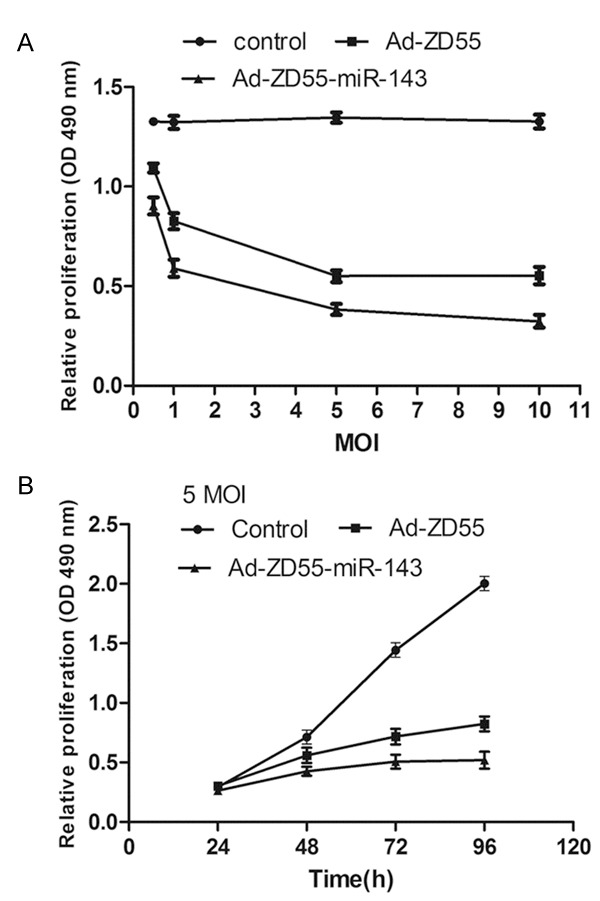
Proliferation inhibition of Ad-ZD55-miR-143 using MTT assays. Viability of Ad-ZD55-miR-143 groups was consistently and significantly lower than Ad-ZD55 and control groups in a time- and dose-dependent manner. Graph represented OD 490 nm ± SEM, *P<0.05. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; OD, optical density; SEM, standard error of the mean.
Recombinant virus Ad-ZD55-miR-143 induced cell apoptosis in SW480 cells
To investigate whether recombinant viruses could induce apoptosis of SW480 cells, SW480 cells were infected with 5 MOI of Ad-ZD55 and Ad-ZD55-miR-143 for 36 h before analysis. Apoptotic cell death rate was evaluated using flow cytometric analysis of Annexin V-FITC/PI staining. As shown in Figure 4, the percentage of the early and late apoptotic cells was higher in the Ad-ZD55-miR-143 groups compared with the Ad-ZD55 and control groups (P<0.05). These results demonstrated that Ad-ZD55-miR-143 could induce cell apoptosis in SW480 cells.
Figure 4.
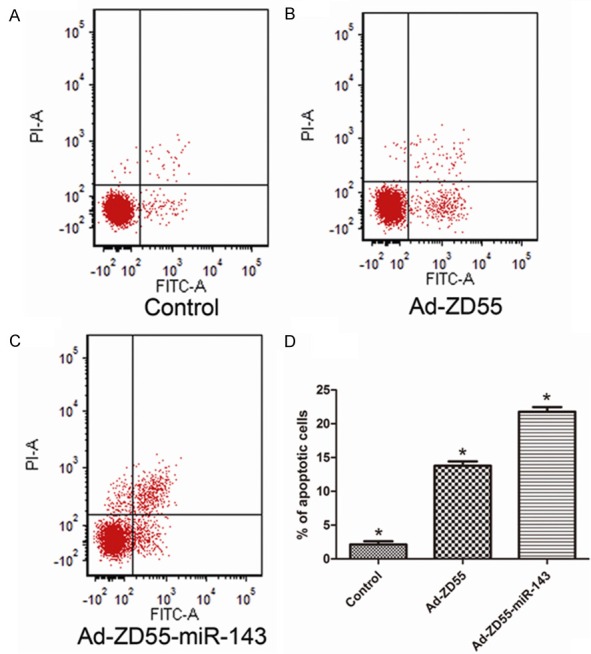
Ad-ZD55-miR-143 induced cell apoptosis in SW480 cells. (A-C) Ad-ZD55-miR-143 significantly induced early and late apoptosis of SW480 cells (C). In contrast, Cells of Ad-ZD55 (B) and control (A) groups exhibited low levels of apoptosis. (D) The percentage of apoptotic cells. Data represent means ± SEM, *P<0.05. SEM, standard error of the mean value.
KRAS was a direct target of miR-143
We found two potential binding sites for miR-143 which were located 1720-1727 bp and 3772-3779 bp downstream from the 5’ end of the KRAS 3’-UTR (Figure 5A). Next, we constructed a psiCHECK-2/KRAS 3’-UTR vector, which contained the renilla luciferase (RL) gene and the 3’-UTR region of KRAS. This construction was transfected into 293T cells together with either miR-143 or NC mimics, and luciferase activity was analyzed. The ratio of FL/RL was calculated, and the results showed that the miR-143 group had approximately 4-fold higher activity than that of the NC group (P<0.05) (Figure 5B). These results suggested that miR-143 directly interacted with the KRAS 3’-UTR in the psiCHECK-2 reporter plasmid and led to the degradation of RL mRNA.
Figure 5.
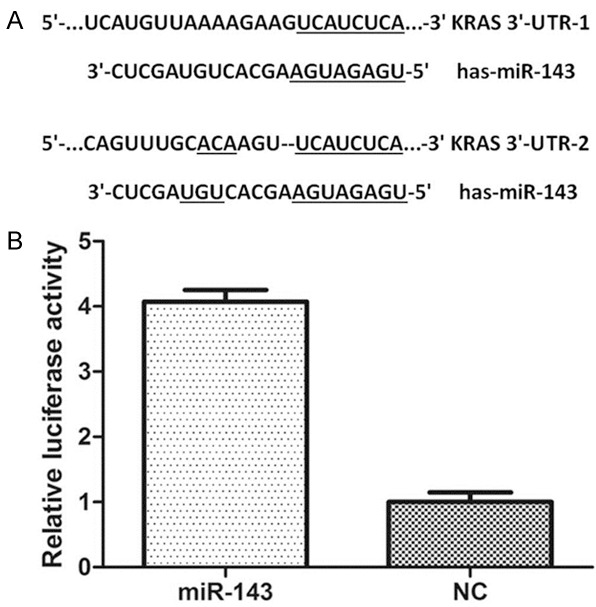
miR-143 targeted KRAS. A: The miR-143 binding site in KRAS 3’-UTR. B: The relative luciferase activity (FL/RL) was measured in HEK293T cells (*P<0.05). 3’-UTR, 3’-untranslated region; NC, negative control.
Recombinant virus Ad-ZD55-miR-143 regulated KRAS expression in SW480 cells
SW480 cells were infected with 5 MOI of Ad-ZD55 and Ad-ZD55-miR-143. After 48 h, qPCR and western blot analysis were performed to determine KRAS expression in SW480 cells. We demonstrated that Ad-ZD55-miR-143 groups significantly decreased KRAS expression at both mRNA and protein levels (Figure 6).
Figure 6.
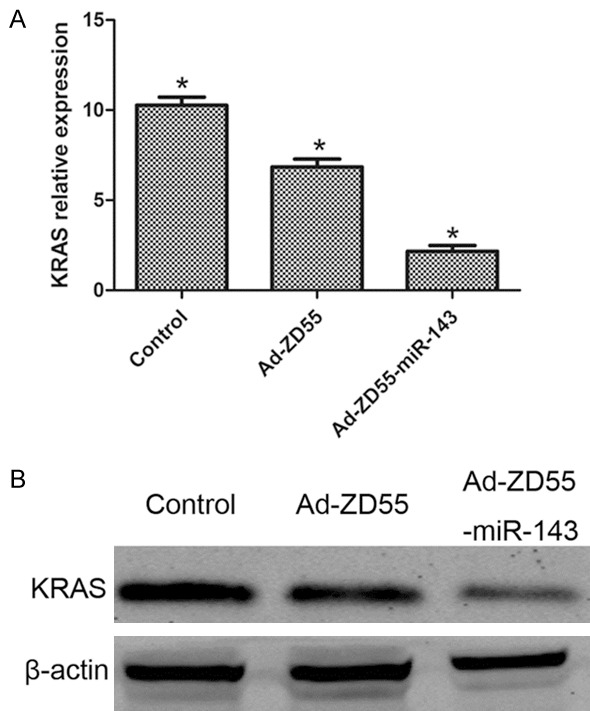
Ad-ZD55-miR-143 inhibited KRAS expression in SW480 cells. (A) qPCR and (B) western blot analysis of KRAS mRNA and protein levels in control, Ad-ZD55 and Ad-ZD55-miR-143 groups. *P<0.05.
Discussion
It has been reported that the KRAS mutation is involved in transition of adenoma to carcinoma in CRC [19,20]. Since KRAS is an important component of the EGFR pathway, tumors with KRAS mutation could be resistant to anti-EGFR therapy [21]. A study identified that KRAS was a direct miR-543 and downstream target and miR-543 over-expression inhibited CRC cell proliferation and metastasis by targeting KRAS [22]. Among the miR-143 target genes predicted by Targetscan, KRAS gene particularly hint a role in CRC progression.
The oncolytic Ad has several biological properties including ease of production, oncolytic ability and a large packaging capacity. Consequently, it is becoming one of the most customizable vectors in clinical and preclinical studies for human cancer therapy. Deleting viral genes encoding proteins necessary for normal cells but not for tumor cells is a strategy to induce a tumor-specific viral replicative lysis [15]. The early representative of Ad was Onyx-015, which was engineered without expression of the E1B55kDa viral protein [23,24]. However, Some clinical trials showed that the antitumor effect of single application of Onyx-015 was not ideal for tumor therapy [25,26]. Oncolytic viruses carrying the anti-cancer gene could solve this problem. The strategy took advantage of the therapeutic gene and the oncolytic virus itself to improve cancer therapy.
In this study, the recombinant virus Ad-ZD55-miR-143 was constructed by inserting the miR-143 into the Ad-ZD55 vector, which is an E1B55kDa gene deleted vector we previously constructed. We analyzed the expression level of miR-143 in wild Ad, Ad-ZD55 and Ad-ZD55-miR-143 by qPCR. The results of qPCR assays indicated that miR-143 was successfully cloned into Ad-ZD55-miR-143. The results of western blot assays indicated Ad-ZD55-miR-143 expressed E1A protein, and failed to express E1B55kDa protein. Ad-ZD55-miR-143 was successfully established. E1A plays a key role in adenoviral replication. E1A mediated host cell apoptosis is antagonized by adenoviral E1B55kDa. If E1B55kDa is deleted, the Ad will survive in cancer cells, but not in normal cells. In crystal violet staining assays, we found that Ad-ZD55 and Ad-ZD55-miR-143 caused limited cell death in L-02 cells, which indicated that recombinant viruses have tumor-specific ability. The killing effect of Ad-ZD55-miR-143 was higher than that of Ad-ZD55 in vitro from 0.5 MOI. We considered miR-143 strengthened killing ability. To explore the potential impact of Ad-ZD55-miR-143 on proliferation of SW480 cells, cell viability was also measured by MTT assays. Compared with the Ad-ZD55 and control groups, Ad-ZD55-miR-143 significantly repressed the growth of SW480 cells. Suppression of cell growth followed a time- and dosage-dependent manner. Then, SW480 cells were infected with 5 MOI of Ad-ZD55-miR-143 and Ad-ZD55 to conduct apoptosis assays. The percentage of the early and late apoptotic cells was highest in the Ad-ZD55-miR-143 group compared with the Ad-ZD55 and control groups.
Interaction between miR-143 and KRAS mRNA has not been reported so far. To test whether KRAS was a real target of miR-143, we constructed psiCHECK-2/KRAS 3’-UTR plasmid which contained 3’-UTR of KRAS. Through dual-luciferase assays, we predicated KRAS as a direct target of miR-143. Additionally, we found that the mRNA and protein levels of KRAS were significantly lower in Ad-ZD55-miR-143 groups than those in control and Ad-ZD55 groups in SW480 cells, these findings supported the consideration that KRAS was a downstream target of miR-143.
In summary, our findings suggested that the recombinant virus Ad-ZD55-miR-143 could specifically replicate in SW480 cells, without killing normal L-02 cells. Under infection of Ad-ZD55-miR-143, SW480 cell growth was repressed and apoptosis was induced. Moreover, the dual-luciferase, qPCR and western blot assays illustrated KRAS was a downstream target of miR-143. The recombinant virus Ad-ZD55-miR-143 could offer a new promising approach against colorectal cancer with KRAS gene mutation.
Acknowledgements
This study was made possible with financial supports from the Shanghai Science Committee Foundation, for the project 134119b0600, Project of Shanghai Municipal Health Bureau (no. 20134194), Jiaxing Science Committee Foundation of Zhejiang province (no. 2015AY23071) and the Technology Plan Project of Medicine and Health of Zhejiang Province (no. 2016KYB295).
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worthley DL, Leggett BA. Colorectal cancer: molecular features and clinical opportunities. Clin Biochem Rev. 2010;31:31–38. [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters M, Douillard JY, Van Cutsem E, Siena S, Zhang K, Williams R, Wiezorek J. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J. Clin. Oncol. 2013;31:759–765. doi: 10.1200/JCO.2012.45.1492. [DOI] [PubMed] [Google Scholar]
- 4.De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y, Van Cutsem E, Tejpar S. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 6.Liu WH, Ren LN, Wang X, Wang T, Zhang N, Gao Y, Luo H, Navarro-Alvarez N, Tang LJ. Combination of exosomes and circulating microRNAs may serve as a promising tumor marker complementary to alpha-fetoprotein for early-stage hepatocellular carcinoma diagnosis in rats. J Cancer Res Clin Oncol. 2015;141:1767–1778. doi: 10.1007/s00432-015-1943-0. [DOI] [PubMed] [Google Scholar]
- 7.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 8.Herranz H, Cohen SM. MicroRNAs and gene regulatory networks: managing the impact of noise in biological systems. Genes Dev. 2010;24:1339–1344. doi: 10.1101/gad.1937010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo H, Chen Y, Hu X, Qian G, Ge S, Zhang J. The regulation of Toll-like receptor 2 by miR-143 suppresses the invasion and migration of a subset of human colorectal carcinoma cells. Mol Cancer. 2013;12:77. doi: 10.1186/1476-4598-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Shi K, Wang Y, Song M, Zhou W, Tu H, Lin Z. Clinical value of integrated-signature miRNAs in colorectal cancer: miRNA expression profiling analysis and experimental validation. Oncotarget. 2015;6:37544–37556. doi: 10.18632/oncotarget.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012-an update. J Gene Med. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 12.Ylosmaki E, Hakkarainen T, Hemminki A, Visakorpi T, Andino R, Saksela K. Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J Virol. 2008;82:11009–11015. doi: 10.1128/JVI.01608-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callegari E, Elamin BK, D’Abundo L, Falzoni S, Donvito G, Moshiri F, Milazzo M, Altavilla G, Giacomelli L, Fornari F, Hemminki A, Di Virgilio F, Gramantieri L, Negrini M, Sabbioni S. Anti-tumor activity of a miR-199-dependent oncolytic adenovirus. PLoS One. 2013;8:e73964. doi: 10.1371/journal.pone.0073964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alemany R. Designing adenoviral vectors for tumor-specific targeting. Methods Mol Biol. 2009;542:57–74. doi: 10.1007/978-1-59745-561-9_2. [DOI] [PubMed] [Google Scholar]
- 16.Xu B, Zheng WY, Jin DY, Wang DS, Liu XY, Qin XY. Treatment of pancreatic cancer using an oncolytic virus harboring the lipocalin-2 gene. Cancer. 2012;118:5217–5226. doi: 10.1002/cncr.27535. [DOI] [PubMed] [Google Scholar]
- 17.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N, Beranek M, Jandik P, Benamouzig R, Jullian E, Laurent-Puig P, Olschwang S, Muller O, Hoffmann I, Rabes HM, Zietz C, Troungos C, Valavanis C, Yuen ST, Ho JW, Croke CT, O’Donoghue DP, Giaretti W, Rapallo A, Russo A, Bazan V, Tanaka M, Omura K, Azuma T, Ohkusa T, Fujimori T, Ono Y, Pauly M, Faber C, Glaesener R, de Goeij AF, Arends JW, Andersen SN, Lovig T, Breivik J, Gaudernack G, Clausen OP, De Angelis PD, Meling GI, Rognum TO, Smith R, Goh HS, Font A, Rosell R, Sun XF, Zhang H, Benhattar J, Losi L, Lee JQ, Wang ST, Clarke PA, Bell S, Quirke P, Bubb VJ, Piris J, Cruickshank NR, Morton D, Fox JC, Al-Mulla F, Lees N, Hall CN, Snary D, Wilkinson K, Dillon D, Costa J, Pricolo VE, Finkelstein SD, Thebo JS, Senagore AJ, Halter SA, Wadler S, Malik S, Krtolica K, Urosevic N. Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer. 2001;85:692–696. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atsumi J, Hanami T, Enokida Y, Ogawa H, Delobel D, Mitani Y, Kimura Y, Soma T, Tagami M, Takase Y, Ichihara T, Takeyoshi I, Usui K, Hayashizaki Y, Shimizu K. Eprobe-mediated screening system for somatic mutations in the KRAS locus. Oncol Rep. 2015;33:2719–2727. doi: 10.3892/or.2015.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 22.Fan C, Lin Y, Mao Y, Huang Z, Liu AY, Ma H, Yu D, Maitikabili A, Xiao H, Zhang C, Liu F, Luo Q, Ouyang G. MicroRNA-543 suppresses colorectal cancer growth and metastasis by targeting KRAS, MTA1 and HMGA2. Oncotarget. 2016;7:21825–39. doi: 10.18632/oncotarget.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heise CC, Williams AM, Xue S, Propst M, Kirn DH. Intravenous administration of ONYX-015, a selectively replicating adenovirus, induces antitumoral efficacy. Cancer Res. 1999;59:2623–2628. [PubMed] [Google Scholar]
- 24.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 25.Crompton AM, Kirn DH. From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development. Curr Cancer Drug Targets. 2007;7:133–139. doi: 10.2174/156800907780058862. [DOI] [PubMed] [Google Scholar]
- 26.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, Randlev B, Gillenwater AM, Bruso P, Kaye SB, Hong WK, Kirn DH. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]


