Abstract
IL-22-producing helper T cells (Th22 cells) have been reported to be involved in lgA nephropathy. However, the mechanisms underlying the differentiation and immune regulation of Th22 cells in lgA nephropathy remain unknown. To elucidate the mechanisms by which Th22 cells differentiate and are recruited into the kidney in lgA nephropathy, the distribution of Th22 cells in both the kidney and blood was determined. Additionally, the impacts of proinflammatory cytokines and antigen presentation in the kidney on Th22 cell differentiation were explored. Specifically, the chemoattractant activities of chemokines produced by the kidney for Th22 cells were investigated. Th22 cells were significantly higher both in the kidney and in the blood in lgA nephropathy mice. IL-1β, IL-6, IL-21 and/or TNF-a promoted Th22 cells differentiation from CD4+ T cells. It was observed that kidneys undergoing lgA nephropathy expressed CCL20, CCL22 and CCL27, and kidney supernatants were chemotactic for Th22 cells. This activity was partially blocked by anti-CCL20, anti-CCL22, and anti-CCL27 antibodies, which also potentially improved renal lesions simultaneously. The overrepresentation of Th22 cells in lgAN may be attributable to the actions of kidney chemokines and cytokines. Our data suggest a collaborative loop between the kidney and Th22 cells in lgA nephropathy.
Keywords: Th22 cells, lgA nephropathy, CCL20, CCL22, CCL27
Introduction
lgA nephropathy (lgAN) is a disease that is caused by the presence of glomerular immune complexes, and humoral and cell-mediated immune responses may be present throughout a portion of or all subsequent pathophysiological processes. IL-22 is a member of the IL-10 cytokine family, and studies have shown that IL-22 expression is dysregulated in certain human diseases, including mucosal-associated infections and inflammatory disorders of the intestine, skin, and joints [1,2]. Studies examining new subsets have confirmed the important role of T cells in the instruction of tissue cells and have demonstrated the important role of feedback regulation for polarization toward distinct T cell subsets. The CD3/CD4 proportion is a strong prognostic marker in lgAN [3]. Th17 and Th22 cells are emerging Th cell subsets that link the immune response to tissue inflammation; these cells are driven by their respective prototype cytokines, IL-17 and IL-22. Both cytokines play roles in immune defense against extracellular bacteria: IL-17 augments inflammation, and an increased number of Th22 cells has been correlated with Th17 cells in the peripheral blood of patients with IgAN [4,5]. Poly lgG dysfunction is present in lgAN, and mucosal infections expand this dysfunction [6]. It was recently shown that chemokines, a group of small proteins, serve as key regulators of directional T cell trafficking under inflammatory conditions achieved by the differential expression of corresponding chemokine receptors on the surface of leukocyte subsets. Th22 polarized cells preferentially express CCR4, CCR6, and CCR10; the highly specific ligands for these receptors are CCL22, CCL20, and CCL27, respectively. It can be argued that these chemokines play critical roles in both B and T cell development. The expression of these receptors may change depending on the activation status of the T cell [7], For example, CCR8 is only strongly expressed in inactivated Th2 cells [8].
In a previous study, we showed that the Th17 cell numbers were significantly increased in lgAN compared with normal conditions, and the overrepresentation of Th17 cells in lgAN might be attributable to Th17 cell differentiation and expansion stimulated by kidney proinflammatory cytokines, as well as the recruitment of Th17 cells from the peripheral blood induced by kidney chemokines CCL20 [9]. Recently, it has been reported that, such as Th17 cells, Th22 cells may also contribute to the immune response in human malignancies. Th22 cells were present in higher percentages in IgAN patients with proteinuria than in IgAN patients without proteinuria and were shown to be involved in the immune responses observed in IgAN [10,11]. In the present study, we investigated the distribution of IL-22-producing CD4+ T cells in IgAN and explored the possible mechanism for the differentiation and recruitment of Th22 cells in lgAN.
Materials and methods
Animals and lgAN mode
Experiments were conducted according to the Guiding Principles in the Care and Use of Laboratory Animals. The protocol was approved by the animal experimental ethics committee of Hunan Province (Permit Number: 20150003). All efforts were made to minimize animal suffering. BABL/c female mice (19±2 g) were obtained from Experimental Animal Center of Central South University (Changsha, Hunan, China) at 6 weeks of age. All animals were raised under ideal temperature, humidity and specific pathogen-free conditions and had free access to standard mouse chow and drinking water. The IgAN model was induced by BSA (Roche, USA) in acidified water with CCl4 and castor oil combined with LPS (Sigma, USA) (50 µg) at different time points over two months after adaptive feeding for a week [9].
IgAN mice were received no treatment. The HS-IgAN group was subjected to intranasal infection with live alpha-hemolytic streptococcus (a-HS) during the 10th week. a-HS was isolated from human tonsils and inoculated intranasally at a dose of 2*108 CFUs in 10 ml of PBS/mouse (5 ml/nostril). Anti-CCL intervention groups were sensitized by the intraperitoneal injection of anti-CCL antibodies alone or in combination (Abcam, USA) (100 µg/mouse). Controls received equal amounts of distilled water. All mice were terminated at the 11th week after the administration of HS and/or CCL20 antibody [9,12-15].
Sample collection and processing
Samples of approximately 1 milliliter of whole blood were collected from each mouse in heparin-treated tubes, then centrifuged at 500 g for 10 min. After absorbing the plasma and diluting with PBS at the ratio of 1:1, mononuclear cells were isolated by Ficoll gradient centrifugation (GE, USA) within 1 hour to evaluate T cell subsets.
Isolation of kidney lymphocytes
In brief, kidneys were finely minced and digested for 30 min at 37°C with 0.5 mg/ml collagenase IV (Sigma, USA) in DMEM medium (Roche) supplemented with 10% heat-inactivated FCS (Invitrogen). Cell suspensions were sequentially filtered through 200-um nylon mesh and washed twice with PBS. Single-cell suspensions were separated using Ficoll PAQUR PLUS (GE, USA). The leukocyte-enriched cell suspension was aspirated above the Ficoll layer. The single-cell suspension of lymphocytes was then resuspended in RPMI 1640 with 10% FCS (Invitrogen). Viability of the cells was assessed by trypan blue staining before flow cytometry.
Flow cytometry
The expression of markers on T cells from kidney and blood was determined by flow cytometry after surface or intracellular staining with anti-mouse-specific Abs conjugated with APC-Cy7, FITC, APC, PE, PE-cy7, BV-421, and APC. These mouse Abs included anti-CD3, anti-CD4, anti-IFN-γ, anti-IL-17, anti-IL-22, anti-CCR4, anti-CCR6, and anti-CCR10 Abs, which were purchased from BD Biosciences (Franklin Lakes, NJ) or R&D Systems (Minneapolis, MN). Intracellular staining for IL-22, IL-17, or IFN-γ was performed with anti-IL-22, anti-IL-17 or anti-IFN-γ mAbs on T cells stimulated with Leukocyte Activation Cocktail (2 μl/ml, BD) at 37°C in 5% CO2 for 5 hours. To identify PMCs, appropriate species-matched Abs served as isotope controls. Flow cytometry was performed on a FACS Canto II system (BD Biosciences) and analyzed using BD FCS in the FlowJo software.
Renal histopathology [16]
Renal tissues were fixed in 4% paraformaldehyde and serially cut after paraffin embedding. Tissue sections (1.5 mm in thickness) were stained with hematoxylin and eosin (HE) and periodic acid-Schiff (PAS) reagents. The stained kidney and lung sections were examined and analyzed by a renal pathologist under a light microscope. The percentage of abnormal glomeruli was evaluated by examining abnormalities in at least 50 glomeruli per mouse. Light microscopy and immunohistochemistry were performed utilizing routine procedures. Paraffin-embedded sections (2 μm) were stained with CCL20, CCL22, CCL27 mAbs.
Differentiation of Th22 cells
Purified naive CD4+ T cells (5*105) were cultured in 1 ml of complete medium containing mouse IL-2 (30 U/ml) in 6-well plates and stimulated with plate-bound anti-CD3 (1 μg/ml) and soluble anti-CD28 mAbs (1 μg/ml) for 7 d. The exogenous cytokines were IL-1β (30 ng/ml), IL-6 (200 ng/ml), IL-21 (30 ng/ml), TNF-a (30 ng/ml), and combined recombinant cytokines that were purchased from R&D Systems. Cells were harvested and intracellularly stained for IL-22 and were then analyzed by flow cytometry as described above.
Naive CD4+ T cells were isolated by MACS based on negative selection using the Naive CD4+ T cell isolation kit for mouse (GE, USA) according to the manufacturer’s instructions. The purity of naive CD4+ T cells was > 97%, as measured by flow cytometry. To isolate PMCs, whole blood cell pellets were resuspended in RPMI-1640 (Gibco, CA) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco) and 20 ng/ml epidermal growth factor (R&D Systems). The cells were seeded into 6-well-plates at a density of 2 × 105 cells/well and placed in an incubator at 37°C in 5% CO2 [17].
Chemokine-mediated chemotaxis of Th22 cells in vitro
For chemotaxis assays, 5-μm-pore polycarbonate filters in 24-well Transwell chambers (Corning Costar, Corning, NY) were used. Transwell membranes were coated with fibronectin (5 μg/ml; Chemicon International, Schwalbach, Germany) for 30 min at 37°C. Purified CD4+ T cells from blood (2*105) resuspended in RPMI 1640 medium with 0.5% FBS in a final volume of 100 μl were added into the top chamber. lgAN kidney tissue homogenates in a volume of 600 μl were placed in the bottom chamber, and the chambers were incubated for 3 h at 37°C in a 5% CO2 atmosphere. Finally, the total number of cells that migrated into the bottom chamber were harvested and intracellularly stained for IL-22 and then analyzed by flow cytometry as described above. The chemotaxis index was calculated by dividing the migrated Th22 cell numbers in response to lgAN by the migrated Th22 cell numbers in response to medium alone. To investigate whether CCL20, CCL22, or CCL27 contributed to Th22 cell migration, blocking experiments were performed by mixing the homogenates with 100 ng/ml anti-CCL20 (R&D Systems), anti-CCL22 (R&D Systems), anti-CCL27 (Abcam), a combination of these three mAbs, or an irrelevant mouse IgG isotope control (R&D Systems) [17,18].
Statistical analysis
Data are expressed as the mean ± SEM (unless otherwise indicated in the figure legends). Data comparisons between different groups were performed using the Mann-Whitney U-test or the Kruskal-Wallis one-way analysis of variance for ranking. The correlations between variables were determined by calculating Spearman rank correlation coefficients. Analysis was completed with SPSS version 19.0 statistical software (Chicago, IL, USA), and p values of less than 0.05 were considered to indicate statistical significance.
Results
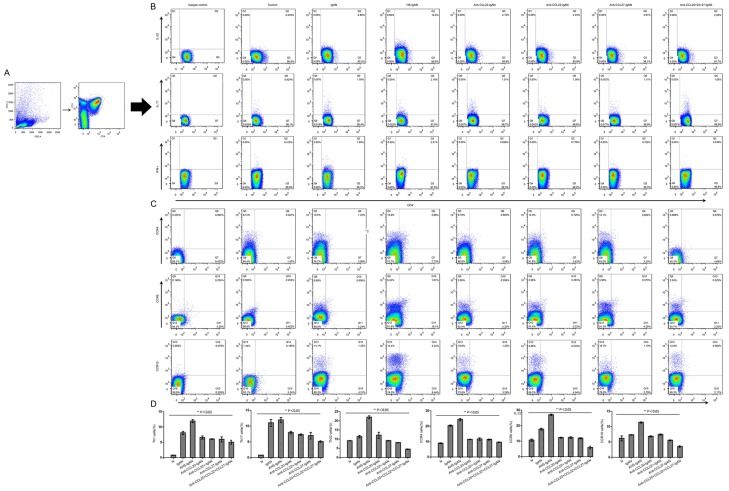
Increased proportions of Th22, Th17, Th1, and Th22 cells the and cell surface receptors CCR4, CCR6, and CCR10 in lgAN
It has been noted that Th22 cell numbers are always linked with Th17 cells and Th1 cells [10,20,21]. We first performed flow cytometry on mononuclear cells obtained from kidney and blood with gating on CD3+ and CD4+ T cells (Figure 1A). IFN-r+, IL-17+ and IL-22+ CD4+ T cells were observed in both kidney and blood (Figure 1B). Percentages of Th22 cells and the cell surface receptors CCR4, CCR6, and CCR10 demonstrated higher values both in blood (4.85±0.41%, 1.58±0.18%, 3.62±0.15%, and 1.26±0.08%, respectively) and kidney (19.35±0.63%, 20.31±0.48%, 17.65±0.63%, and 7.30±1.12%, respectively) in lgAN mice, exhibiting a significant increase compared with the percentages in the blood and kidney in the corresponding control group (0.38±0.04%, 0.41±0.02%, 0.29±0.04%, 0.36±0.09%; 9.10±0.11%, 8.9±0.13%, 10.60±0.74%, 6.16±0.81%, respectively; n=3; P<0.05). Similarly, significant increases in both Th17 and Th1 cells were observed in lgAN (1.71±0.22% and 1.71±0.12%, respectively) compared with blood (0.88±0.02% and 0.46±0.02%, respectively; n=3; both P<0.01). We observed that Th22 cells were positively correlated with numbers of Th17 and Th1 cells (r1=0.746, r2=0.627, respectively; both P<0.05). Infection with HS aggravated and treatment with CCL antibodies decreased the numbers of Th cells and CCR receptors.
Figure 1.
Percentages of Th22, Th17, and Th1 cells and Th22 chemokine receptors expressed in both blood and kidney. A. Th22, Th17, and Th1 cells within CD4+ T cells were identified based on their expression of CD3+ and CD4+. B. Representative flow chart of Th22, Th17, and Th1 cells in the blood. C. Representative flow chart of CCR4+, CCR6+, and CCR10+ within IL-22+ T cells in the blood. D. Percentages of Th22 cells and their cell surface receptors CCR4, CCR6, and CCR10 presented increased values in IgAN mice and even higher values in HS-IgAN but were significantly downregulated in the CCL intervention group and the combination group (n=3 per group). Horizontal bars indicate the mean ± SE. The percentages of Th cells and CCRs were determined by flow cytometry, and appropriate species-matched Abs served as isotype controls.
We further noted that HS aggravated Th22 cell numbers, while CCL20, CCL22, and CCL27 antibodies or a combination of these CCL antibodies reduced the increased percentage of Th22 cells following HS treatment, as expected. Percentages of Th22 cells were significantly higher in HS-lgAN (10.36±0.15%) compared with the percentages in the corresponding CCL20-lgAN, CCL22-lgAN, and CCL27-lgAN groups (4.60±0.22%, 4.30±0.03%, 3.76±0.12%, respectively; P<0.05). We also found that Th22 cell percentages were significantly lower with the combination of all CCL antibodies (2.29±0.02%) (Figure 1A, 1B, 1D).
To characterize these Th22 cells in more detail, we analyzed the expression of the chemokine receptors and found that most Th22 cells expressed high levels of CCR4, CCR6 and CCR10, although these receptors were expressed by a larger population of Th22 cells in the HS-lgAN group and a smaller population in the CCL-lgAN group; data are shown in Figure 1C, 1D.
Differentiation of Th22 cells
As some proinflammatory cytokines, such as IL-1β, IL-6, IL-21 and TNF-a, have been reported to be elevated in lgAN [9,10,18,22], we evaluated the contribution of these cytokines to the differentiation of Th22 cells. IL-2-containing medium provided a baseline for comparison. IL-1β, IL-6, IL-21 and TNF-a could each promote the differentiation of Th22 cells, with the strongest effects observed for IL-6 (Figure 2). Data are shown as representative flow cytometry column diagrams from one of five independent experiments, revealing enhanced Th22 cell differentiation stimulated by IL-1β, IL-6, IL-21 and TNF-a.
Figure 2.
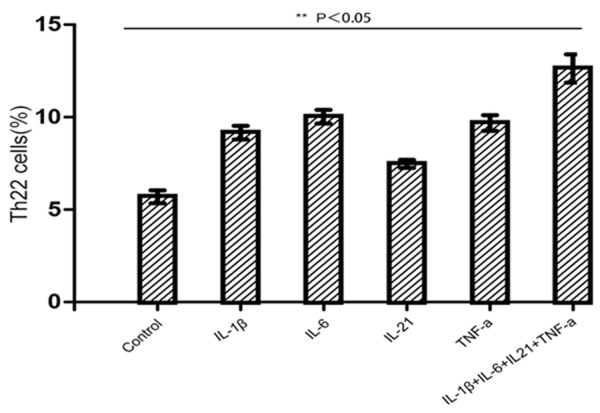
Differentiation of Th22 cells from native CD4+ T cells stimulated by various cytokines. Purified naïve CD4+ T cells isolated from the blood of lgAN mice were stimulated with plate-bound anti-CD3 and soluble anti-CD28 monoclonal antibodies (mAbs) in the presence of the designated cytokines, either alone or in combination. Seven days after activation, the cells were simulated with Leukocyte Activation Cocktail for 5 hours and analyzed for IL-22 expression after intracellular staining. The results are reported as the means and SEM from five independent experiments. Comparisons were determined by performing Kruskal-Wallis one-way analysis of variance for ranking. *P<0.05 compared with the medium control.
Recruitment of Th22 cells into the kidney was induced by chemokines
In addition to local differentiation, recruitment from peripheral blood may also contribute to the increased number of Th22 cells observed in lgAN. It is well known that lymphocyte migration is tightly regulated by chemokine/CCR interactions; we therefore sought to elucidate the effects of chemokine/CCR interactions on Th22 cell recruitment. In previous studies, we have demonstrated that the expression of CCL20, CCL22, and CCL27 In lgAN is much higher than normal in lgAN. Taken together with our observations that Th22 cells express high levels of CCR6, CCR4, and CCR10 (Figure 1C), which are ligands for CCL20, CCL22, and CCL27, respectively, we hypothesized that Th22 cells could migrate into the kidney in response to these chemokines. Anti-CCL20, anti-CCL22, or anti-CCL27 mAbs significantly blocked Th22 cell chemotaxis (Figure 3). Therefore, Th22 cells could be recruited into the kidney space via the CCL20-CCR6, CCL22-CCR4, and/or CCL27-CCR10 axes.
Figure 3.
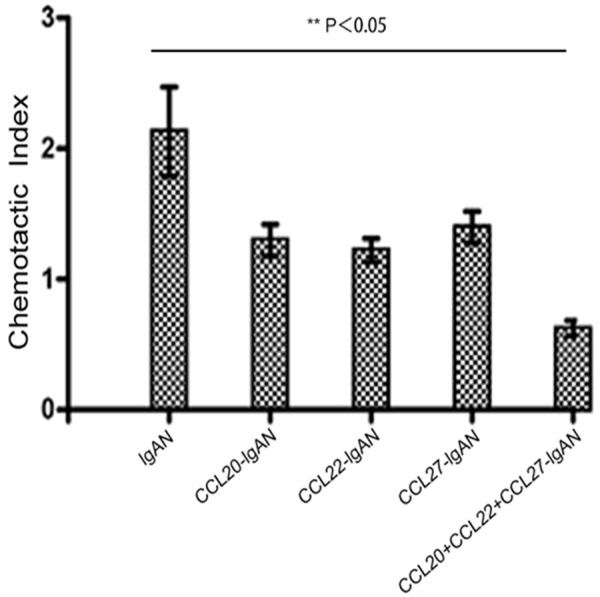
Chemokines in the kidney were chemotactic for IL-22-producing Th22 cells. CCL20, CCL22, and CCL27 were chemotactic for Th22 cells in vitro. Kidney tissue homogenates were used to stimulate the chemotaxis of Th22 cells in the absence or presence of anti-CCL20, anti-CCL22, and/or anti-CCL27 mAbs or a normal control. The chemotaxis index was calculated by dividing Th22 cell numbers that migrated in response to kidney homogenates by Th22 cell numbers that migrated in response to medium alone. Comparisons were determined by performing Kruskal-Wallis one-way analysis of variance for ranking. *P<0.05 compared with control.
Renal pathomorphological changes
As a first step, we analyzed HE staining of the lung to ensure that HS infection was successful. The results showed that after streptococcal infection, lung infiltration of inflammatory cells increased significantly. Next, we aimed to characterize the relative distribution of CCL20, CCL22 and CCL27 in the kidneys of each group. We performed immunohistochemistry on kidney sections for each group and showed abundant renal infiltration of CCL20, CCL22 and CCL27, predominantly in the interstitial and periglomerular areas. Additionally, the frequency of CCL20, CCL22 and CCL27 in HS-lgAN mice was markedly increased compared with non-nephritic controls. In comparison with lgAN mice, the frequency of CCL20, CCL22 and CCL27 in the CCL Ab intervention group appeared to be notably reduced. Semiquantitative scoring of CCL20, CCL22 and CCL27 revealed significantly increased deposition in lgAN and HS-lgAN. Using image analysis software selection, as visualized by the yellow areas in the images indicating immunohistochemical reactants, we measured the average optical densities of these areas (CCL20, CCL22 and CCL27: 4402±0.5, 3645±0.1, and 1559±0.2, respectively. The control group was 97.2±0.3, 382±0.1, and 105.7±0.3, respectively. P<0.05) (Figure 4).
Figure 4.
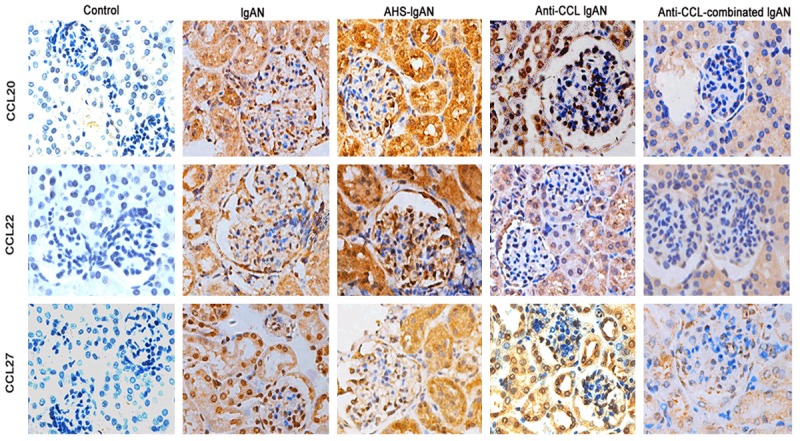
CCL20, CCL22, CCL27 expression in the kidney. Representative images of immunohistochemistry staining (brown) of anti-CCL20, anti-CCL22, and anti-CCL27 in renal tissue from the control group, IgAN mice and the anti-CCL intervention groups. CCL20, CCL22, and CCL27 are predominantly expressed in the renal tubular epithelial cells. Compared with control mice, the expression of CCL20, CCL22, and CCL27 was much higher in IgAN mice, whereas the expression was upregulated with HS treatment but was more significantly downregulated in the anti-CCL-groups and combination intervention group.
To evaluate whether the expression of CCL antibodies has functional relevance for their recruitment to the inflamed kidney, HE staining, periodic acid-Schiff (PAS) staining, immunofluorescence and electron microscopy were performed for each group. HE-stained kidney sections revealed basic histological changes. IgAN mice demonstrated pronounced proliferation of the mesangium compared with control mice; proliferation was exacerbated in HS-IgAN mice but reduced in CCL-treated mice and was most obvious in the combined CCL Ab intervention group. Similar results were obtained when PAS-stained sections of kidneys from these mice were examined.
Immunofluorescence staining was performed to visualize IgA deposition in the glomerular mesangial area and the capillary wall in the IgAN group. The fluorescence intensity was stronger in the HS-IgAN group but weaker in the CCL-treated groups (Figure 5), which is consistent with the electron microscopy results. Moreover, there was mild mesangial proliferation and significant segments or focal fusion of podocytes in the lgAN group, as well as mesangial matrix proliferation and endothelial cell hypertrophy. Podocyte fusion lesions were more serious in the HS-lgAN group, whereas the CCL antibody intervention groups demonstrated no mesangial proliferation, IgA deposition and electron dense deposition, similar to the control group. All of these results suggest that HS aggravates and CCL antibodies may improve renal damage in IgAN mice.
Figure 5.
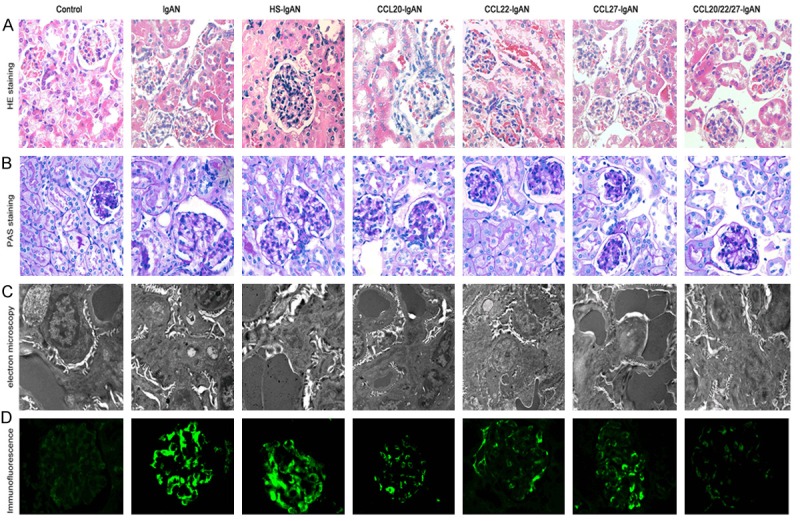
A. Representative photographs of kidney sections stained with HE and PAS and visualized by immunofluorescence and electron microscopy. A, B. IgAN mice demonstrated pronounced proliferation of the mesangium compared with the control group. Proliferation was exacerbated in the HS-IgAN group but reduced in the CCL-treated mice; this effect was more obvious in the combined Ab intervention group. C. Electron microscopy indicated that podocyte fusion lesions were more serious in the HS-IgAN group than in the IgAN group but improved in the anti-CCL intervention groups. D. Immunofluorescence staining for IgA deposition in the glomerular mesangial area and the capillary wall in IgAN mice. The fluorescence intensity was stronger in the HS-IgAN group but weaker in the CCL-treated groups.
To more accurately quantify pathological changes in the kidney, numbers of abnormal glomeruli (AG) were evaluated by PAS staining to complement the morphological results. The frequency of AG was significantly increased in IgAN mice (23.06%±0.10) and in HS-IgAN mice (31.20%±0.21). However, the CCL-IgAN mice demonstrated significantly decreased ratios compared with IgAN mice and the control group (3.06%±0.02, both P<0.05) (data not shown).
Discussion
Several lines of evidence indicate that T cell-mediated tissue damage plays an important role in the immunopathogenesis of renal inflammatory diseases. Chemokines play a critical role in glomerulonephritis, preventing the renal infiltration of harmful T cells; thus, maintaining the intact migration of protective T cells appears to be a potential approach for the treatment of human glomerulonephritis. Such selective control of T cell migration under inflammatory conditions could potentially be achieved by targeting T helper cell lineage-specific chemokine receptors. IL-22 regulates tissue homeostasis and inflammation. Although Th22, Th17 and Th1 cells have been found to be increased in lgAN, the underlying mechanisms have been poorly investigator and there has been no further pathologic analysis. Here, we have explored of role of Th22 cells and their surface receptors CCR4, CCR6, and CCR10 in lgAN mice. Our data have indicated that the numbers of Th22, Th17, and Th1 cells present in lgAN mice were much higher than in their control counterparts. HS infection may worsen the situation, while targeted CCL Abs reduce the relative cellular proportions and improve renal disease. Additionally, Th22 cells express high levels of CCR4, CCR6, and CCR10 on their surface.
Furthermore, we validated in vitro experiments over the same period of time and discussed the possible mechanism underlying the observed cellular dynamics. We found that IL-1β, IL-6, IL-21 and TNF-a could significantly promote the differentiation of Th22 cells from naive CD4+ T cells, and the combination of these chemokines promoted Th22 cell differentiation to an even higher extent. The concentrations of cytokines used in our in vitro experiments were determined in preliminary experiments. An in vitro migration assay confirmed that kidneys could induce the migration of Th22 cells and that anti-CCL20, anti-CCL22 or anti-CCL27 mAbs individually or in combination significantly inhibited the ability of the kidney to stimulate Th22 cell chemotaxis. Therefore, we may speculate that the CCL20-CCR6, CCL22-CCR4, and/or CCL27-CCR10 axes facilitate chemoattraction for Th22 cell recruitment into kidneys affected by lgAN. The increased ratios of Th22 cells in lgAN kidneys might also be attributable to Th22 cell recruitment from the blood. Immunohistochemistry experiments to investigate CCL20, CCL22, and CCL27 expression in the kidney further support this conclusion.
Although not extensively studied to date, it is clear that CD4+-driven immune responses play a leading role in nephritis [22]. Chemokines are a group of small molecules that regulate the cell trafficking of various types of leukocytes. It has now become evident that chemokines play fundamental roles in the development, homeostasis, and function of the immune system. Chemokine nomenclature is based on the current chemokine receptor nomenclature, which uses CC, CXC, XC, or CX3C followed by R (for receptor) and then a number, The chemokine receptors also exhibit strong specificity for dendritic cell subsets. Immature dendritic cells have been shown to express CCR6 and respond to CCL20, similar to CCR4 responding to CCL22 and CCR10 responding to CCL27. The expression of these receptors may change depending on the activation status of the T cell [23,24]. It is conceivable that IL-22 may play a role during tumor genesis because IL-22 stimulates signaling pathways that are involved in the regulation of cell growth, cell proliferation, and cell cycle control [25,26]. Recently, some studies reported the expression of high concentrations of IL-22 in primary tumor tissue and the sera of patients with non-small cell lung cancer and suggested that the overexpression of IL-22 was potentially correlated with the occurrence and progression of lung cancer [27]. Furthermore, circulating Th17, Th22 and Th1 cells are increased in psoriasis [28], CCR1 inhibition ameliorates the progression of lupus nephritis in NZB/W mice [29], and CCR6 may regulate renal outcome in human mesangioproliferative glomerulonephritis [30]. These innate immune responses activate circulating monocytes and resident glomerular cells to release inflammatory mediators and initiate adaptive, antigen-specific immune responses that collectively damage glomerular structures. It is well known that IL-22 binds to a heterodimeric receptor complex composed of IL-22 receptor 1 and IL-10 receptor 2 at the cell surface, resulting in the activation of the Janus kinase-STAT pathway and leading to the phosphorylation of STAT3 and STAT1/STAT5 [31]. IL-22 has also been shown to activate ERK, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase [32]. IL-22 can reduce tumor growth by inhibiting signaling pathways such as STAT3 phosphorylation, resulting in decreased levels of ERK 1/2 and AKT phosphorylation [33]. To our knowledge, the current study is the first to investigate the possible mechanisms underlying the differentiation and recruitment of Th22 cells into lgAN and the potential roles of Th22 cells in the development of lgAN. Our study emphasizes the potential consequences of pharmacologic interventions that harness the chemokine system to treat renal autoimmune disease; overall, chemokine blockade may control the inflammatory response.
There is a unified theory to describe how microbes cause mucosal inflammation in asthma. The respiratory mucosa is a key microbial interface where epithelial and dendritic cells interact with a range of functionally distinct lymphocytes. Lymphoid cells then control a range of pathways, both innate and specific, that organize the host mucosal immune response. This theory provides a rationale for new, specific treatments that can be assessed in clinical trials. Based on these new ideas, specific host biomarkers might allow personalized treatment to become a reality for lgAN patients as well.
It should be mentioned that our study possesses certain defects, such as the small volume of blood obtained from mice, resulting in lower numbers of extracted lymphocytes and sorted CD4+ T lymphocytes; as a result, no differences were observed with the application of combinations of interventional antibodies or cytokines. Due to the high costs associated with antibodies, the experiment was only repeated 3 times. Currently, related studies employing the kidney cells of human lgAN patients are underway.
In conclusion, our data showed that, similar to Th17 and Th1 cells, Th22 cell numbers in the kidney were significantly increased compared with blood, and the overrepresentation of Th22 cells in the kidney may be attributable to local increases in proinflammatory cytokines and chemokines. It will be necessary to evaluate potential therapeutic approaches by carrying out detailed studies in experimental models of glomerulonephritis, which would provide a basis to develop novel immune-boosting strategies as lgAN therapies.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 81173401, 81470933).
Disclosure of conflict of interest
None.
References
- 1.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 2.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faria B, Henriques C, Matos AC, Daha MR, Pestana M, Seelen M. Combined C4d and CD3 immunostaining predicts immunoglobulin (Ig)A nephropathy progression. Clin Exp Immunol. 2015;179:354–361. doi: 10.1111/cei.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng ZG, Tian J, Cui XQ, Xian WH, Sun HB, Li EG, Geng LN, Zhang LW, Zhao P. Increased number of Th22 cells and correlation with Th17 cells in peripheral blood of patients with IgA nephropathy. Hum Immunol. 2013;74:1586–1591. doi: 10.1016/j.humimm.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 6.Jones CL, Powell HR, Kincaid-Smith P, Roberton DM. Polymeric IgA and immune complex concentrations in IgA-related renal disease. Kidney Int. 1990;38:323–331. doi: 10.1038/ki.1990.204. [DOI] [PubMed] [Google Scholar]
- 7.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 8.Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D’Ambrosio D, O’Garra A, Robinson D, Rocchi M, Santoni A, Zlotnik A, Napolitano M. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]
- 9.Meng T, Li XZ, Ao X, Zhong Y, Tang R, Peng WS, Yang JH, Zou MX, Zhou QL. Hemolytic Streptococcus may exacerbate kidney damage in IgA nephropathy through CCL20 response to the effect of Th17 cells. PLoS One. 2014;9:e108723. doi: 10.1371/journal.pone.0108723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 11.Miyagaki T, Sugaya M, Suga H, Kamata M, Ohmatsu H, Fujita H, Asano Y, Tada Y, Kadono T, Sato S. IL-22, but not IL-17, dominant environment in cutaneous T-cell lymphoma. Clin Cancer Res. 2011;17:7529–7538. doi: 10.1158/1078-0432.CCR-11-1192. [DOI] [PubMed] [Google Scholar]
- 12.Fahy OL, Townley SL, Coates NJ, Clark-Lewis I, McColl SR. Control of Salmonella dissemination in vivo by macrophage inflammatory protein (MIP)-3alpha/CCL20. Lab Invest. 2004;84:1501–1511. doi: 10.1038/labinvest.3700176. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Dileepan T, Briscoe S, Hyland KA, Kang J, Khoruts A, Cleary PP. Induction of TGF-beta1 and TGF-beta1-dependent predominant Th17 differentiation by group A streptococcal infection. Proc Natl Acad Sci U S A. 2010;107:5937–5942. doi: 10.1073/pnas.0904831107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Lin SX, Agha-Majzoub R, Overbergh L, Mathieu C, Chan LS. CCL27 is a critical factor for the development of atopic dermatitis in the keratin-14 IL-4 transgenic mouse model. Int Immunol. 2006;18:1233–1242. doi: 10.1093/intimm/dxl054. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter KJ, Hogaboam CM. Immunosuppressive effects of CCL17 on pulmonary antifungal responses during pulmonary invasive aspergillosis. Infect Immun. 2005;73:7198–7207. doi: 10.1128/IAI.73.11.7198-7207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner JE, Paust HJ, Steinmetz OM, Peters A, Riedel JH, Erhardt A, Wegscheid C, Velden J, Fehr S, Mittrücker HW, Tiegs G, Stahl RA, Panzer U. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol. 2010;21:974–985. doi: 10.1681/ASN.2009070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye ZJ, Zhou Q, Yuan ML, Du RH, Yang WB, Xiong XZ, Huang B, Shi HZ. Differentiation and recruitment of IL-22-producing helper T cells stimulated by pleural mesothelial cells in tuberculous pleurisy. Am J Respir Crit Care Med. 2012;185:660–669. doi: 10.1164/rccm.201107-1198OC. [DOI] [PubMed] [Google Scholar]
- 18.Okada N, Gao JQ, Sasaki A, Niwa M, Okada Y, Nakayama T, Yoshie O, Mizuguchi H, Hayakawa T, Fujita T, Yamamoto A, Tsutsumi Y, Mayumi T, Nakagawa S. Anti-tumor activity of chemokine is affected by both kinds of tumors and the activation state of the host’s immune system: implications for chemokine-based cancer immunotherapy. Biochem Biophys Res Commun. 2004;317:68–76. doi: 10.1016/j.bbrc.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Akdis M, Palomares O, van de Veen W, van Splunter M, Akdis CA. TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J Allergy Clin Immunol. 2012;129:1438–1449. z1450–z1451. doi: 10.1016/j.jaci.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Paust HJ, Turner JE, Riedel JH, Disteldorf E, Peters A, Schmidt T, Krebs C, Velden J, Mittrücker HW, Steinmetz OM, Stahl RA, Panzer U. Chemokines play a critical role in the cross-regulation of Th1 and Th17 immune responses in murine crescentic glomerulonephritis. Kidney Int. 2012;82:72–83. doi: 10.1038/ki.2012.101. [DOI] [PubMed] [Google Scholar]
- 21.Yeste A, Mascanfroni ID, Nadeau M, Burns EJ, Tukpah AM, Santiago A, Wu C, Patel B, Kumar D, Quintana FJ. IL-21 induces IL-22 production in CD4+ T cells. Nat Commun. 2014;5:3753. doi: 10.1038/ncomms4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tipping PG, Holdsworth SR. T cells in crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1253–1263. doi: 10.1681/ASN.2005091013. [DOI] [PubMed] [Google Scholar]
- 23.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 24.Kaminuma O, Ohtomo T, Mori A, Nagakubo D, Hieshima K, Ohmachi Y, Noda Y, Katayama K, Suzuki K, Motoi Y, Kitamura N, Saeki M, Nishimura T, Yoshie O, Hiroi T. Selective down-regulation of Th2 cell-mediated airway inflammation in mice by pharmacological intervention of CCR4. Clin Exp Allergy. 2012;42:315–325. doi: 10.1111/j.1365-2222.2011.03847.x. [DOI] [PubMed] [Google Scholar]
- 25.Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6:322–327. doi: 10.1016/S1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- 26.Bernardi RJ, Trump DL, Yu WD, McGuire TF, Hershberger PA, Johnson CS. Combination of 1alpha, 25-dihydroxyvitamin D(3) with dexamethasone enhances cell cycle arrest and apoptosis: role of nuclear receptor cross-talk and Erk/Akt signaling. Clin Cancer Res. 2001;7:4164–4173. [PubMed] [Google Scholar]
- 27.Zhang W, Chen Y, Wei H, Zheng C, Sun R, Zhang J, Tian Z. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14:6432–6439. doi: 10.1158/1078-0432.CCR-07-4401. [DOI] [PubMed] [Google Scholar]
- 28.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bignon A, Gaudin F, Hemon P, Tharinger H, Mayol K, Walzer T, Loetscher P, Peuchmaur M, Berrebi D, Balabanian K. CCR1 inhibition ameliorates the progression of lupus nephritis in NZB/W mice. J Immunol. 2014;192:886–896. doi: 10.4049/jimmunol.1300123. [DOI] [PubMed] [Google Scholar]
- 30.Villa L, Boor P, Konieczny A, Kunter U, van Roeyen CR, Denecke B, Gan L, Neusser MA, Cohen CD ERCB Consortium. Eitner F, Scholl T, Ostendorf T, Floege J. Late angiotensin II receptor blockade in progressive rat mesangioproliferative glomerulonephritis: new insights into mechanisms. J Pathol. 2013;229:672–684. doi: 10.1002/path.4151. [DOI] [PubMed] [Google Scholar]
- 31.Wu PW, Li J, Kodangattil SR, Luxenberg DP, Bennett F, Martino M, Collins M, Dunussi-Joannopoulos K, Gill DS, Wolfman NM, Fouser LA. IL-22R, IL-10R2, and IL-22BP binding sites are topologically juxtaposed on adjacent and overlapping surfaces of IL-22. J Mol Biol. 2008;382:1168–1183. doi: 10.1016/j.jmb.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 32.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 33.Weber GF, Gaertner FC, Erl W, Janssen KP, Blechert B, Holzmann B, Weighardt H, Essler M. IL-22-mediated tumor growth reduction correlates with inhibition of ERK1/2 and AKT phosphorylation and induction of cell cycle arrest in the G2-M phase. J Immunol. 2006;177:8266–8272. doi: 10.4049/jimmunol.177.11.8266. [DOI] [PubMed] [Google Scholar]