Abstract
Laryngeal squamous cell carcinoma (LSCC) is a common aggressive head and neck cancer with high mortality and incidence. MicroRNAs (miRNAs) are short, non-coding and endogenous RNAs that posttranscriptionally inhibit gene expression. In this study, we showed that miR-300 expression was downregulated in LSCC tissues compared with adjacent no-tumor tissues. MiR-300 overexpression inhibited Hep-2 cell proliferation, as well as the expression of ki-67 and PCNA. Moreover, overexpression of miR-300 repressed the cell invasion in Hep-2 cells. We identified c-ros oncogene 1 receptor tyrosine kinase (ROS1) as a direct target gene of miR-300 in Hep-2 cell. Furthermore, ROS1 expression was upregulated in LSCC tissues compared with adjacent no-tumor tissues. Interesting, there were an inverse correlation between ROS1 and miR-300 expression in the LSCC tissues. Overexpression of ROS1 increased the Hep-2 cells proliferation and invasion. Overexpression of ROS1 abrogated miR-300 induced cell growth and invasion inhibition. Therefore, our data suggested that miR-300 acted as a tumor suppressive gene in LSCC.
Keywords: Laryngeal squamous cell carcinoma, microRNAs, miRNAs, miR-300, ROS1
Introduction
Laryngeal squamous cell carcinoma (LSCC) is a common aggressive head and neck cancer with high mortality and incidence [1-6]. The worldwide incidence of LSCC was about 2.4% every year [4,7]. Recent treatments such as radiation therapy, chemotherapy and surgical have some effect on the patients of early stage, but are littler effective on the patients of advanced cases [6,8-10]. The 5-year (OS) overall survival of LSCC cases is poor [11-13]. Therefore, it is important to find new biomarkers to improve diagnosis and therapy of LSCC patients.
MicroRNAs (miRNAs) are short (18-22 nucleotides), non-coding endogenous RNAs that repress gene expression through binding to 3’-UTR (3’ untranslated regions) of target mRNAs [14-20]. Aberrant expression of miRNAs has been found in a number of cancers such as bladder cancer, gastric cancer, ovarian cancer, and gallbladder and hepatocellular carcinoma [21-25]. They act as important regulators in various cell biology such as cell development, cell proliferation, apoptosis, metabolize, invasion and migration [26-29]. They are also considered as a tumor suppressors or oncogenes in tumor development [30-32].
In this study, we demonstrated that miR-300 expression was downregulated in LSCC tissues and overexpression of miR-300 suppressed the cell proliferation and invasion by targeting c-ros oncogene 1 receptor tyrosine kinase (ROS1) in LSCC cell line Hep-2.
Materials and methods
Samples cell lines and cell transfected
Human LSCC specimens (n = 30) and adjacent non-tumor samples (n = 30) were received from our department with written informed consent from each patient. All experiments were approved by the Ethics Committee of Liaocheng People’s Hospital and EENT Hospital. LSCC cell line, Hep-2, was purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China). Cells were cultured in RPMI 1640 supply with 10% FBS (fetal bovine serum) at 37°C. miR-300 mimic oligonucleotide and scramble oligonucleotide was bought from GenePharma (Shanghai, China) and was transfected to cells through Lipofectamine 2000 Reagent (Invitrogen) according to manuscript’s information.
Real-time PCR
RNA was extracted from cells and tissues by using Trizol (Invitrogen, CA) following to the manufacturer’s explanation. MiR-300 and ROS1 expression was quantified using qRT-PCR analysis. The specific primers were used as follows: MiR-300: 5’-TATACAAGGGCAGACTCTCTCT-3’; 5’-GTGCAGGTTCCGAGGT-3’; U6: 5’-CTCGCTTCGGCAGCACATATACT-3’, 5’-ACGCTTCACGAATTTGCGTGTC-3’; GAPDH: 5’-AATGGGCAGCCGTTAGGAAA-3’, 5’-TGAAGGGGTCATTGATGGCA-3’; ROS1 5’-ATGGGCTCCTGTATTGGTTG-3’ and 5’-CATCAGTGCATTCTGGGAAA-3’ was used as for internal control for miR-300 and GAPDH was performed to as control for ROS1.
Cell proliferation and invasion
For cell proliferation analysis, cells were cultured in 96-well plates. CCK-8 analysis (Dojindo, Japan) was performed to detect the cell proliferation and the absorbance was readied at 450 nM. For cell invasion analysis, transwell assays were done. Cells were cultured in Matrigel matrix coated membrane (BD Biosciences) and FBS was put into the lower membrane. After 24 hours, the noninvading cells were removed and cells on the lower membrane was stained with 0.1% crystal violet and calculated.
Luciferase assay
To build a luciferase reporter vector, cDNA contained the miR-300 binding sites was amplified and cloned into the pGL3 luciferase vector. Cell was con-transfected with pGL3-ROS1 or mut pGL3-ROS1 vectors combine with miR-300 mimic or control by using Lipofectamine 2000 Reagent (Invitrogen) according to manuscript’s information. The luciferase data was detected using the dual-luciferase reporter kit (Promega, USA) following to manuscript’s information.
Western blot analysis
Total proteins were extracted from cell or tissues and then separated used 10% SDS-PAGE and transferred to a membrane (Bio-Rad, USA). After blocked with 5% non-fat milk for 1 hour and membrane was incubated with primary antibodies (ROS1, ki-67, PCNA, GAPDH, Sigma, USA). Enhanced chemiluminescence (ECL, USA) was performed to determine the protein concentration.
Statistical analysis
Data was shown as mean ± SD (standard deviation). Difference between groups was measured using SPSS V19.0 software. Student’s t test was performed to compare the significance differences of two groups and ANOVA (one-way analysis of variance) was used measure the difference between more than two groups. P<0.05 was considered to be significant.
Result
MiR-300 was downregulated in laryngeal squamous cell carcinoma
We firstly detected the miR-300 expression in the 30 pair’s laryngeal squamous cell carcinoma (LSCC) tissues. qRT-PCR assay demonstrated that miR-300 expression was downregulated in LSCC tissues compared to adjacent no-tumor tissues (Figure 1A). Moreover, miR-300 was downregulated in 26 cases (26/30, 87%) compared with the normal adjacent tissues (Figure 1B).
Figure 1.
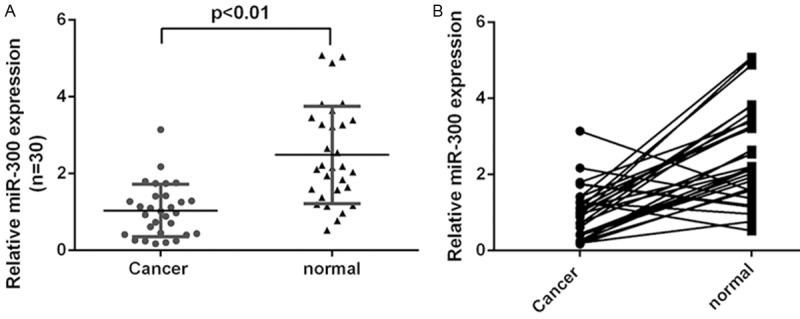
MiR-300 was downregulated in LSCC. A. The expression of miR-300 was measured by qRT-PCR. B. miR-300 was downregulated in 26 cases (26/30, 87%) compared with the normal adjacent tissues.
MiR-300 inhibited the LSCC cells proliferation and invasion
MiR-300 was upregulated after treated with miR-300 mimics in LSCC cell line Hep-2 (Figure 2A). CCK-8 analysis showed that miR-300 overexpression inhibited Hep-2 cell proliferation (Figure 2B). We also confirmed that ectopic expression of miR-300 inhibited the mRNA expression of ki-67 (Figure 2C). In line with this, western blot data proved that miR-300 overexpression suppressed the ki-67 protein expression (Figure 2D). Overexpression of miR-300 repressed the mRNA expression of PCNA (Figure 2E). Western blot data also demonstrated that miR-300 overexpression suppressed the PCNA protein expression (Figure 2F). Moreover, invasion analysis demonstrated that overexpression of miR-300 inhibited the Hep-2 cell invasion (Figure 2G).
Figure 2.
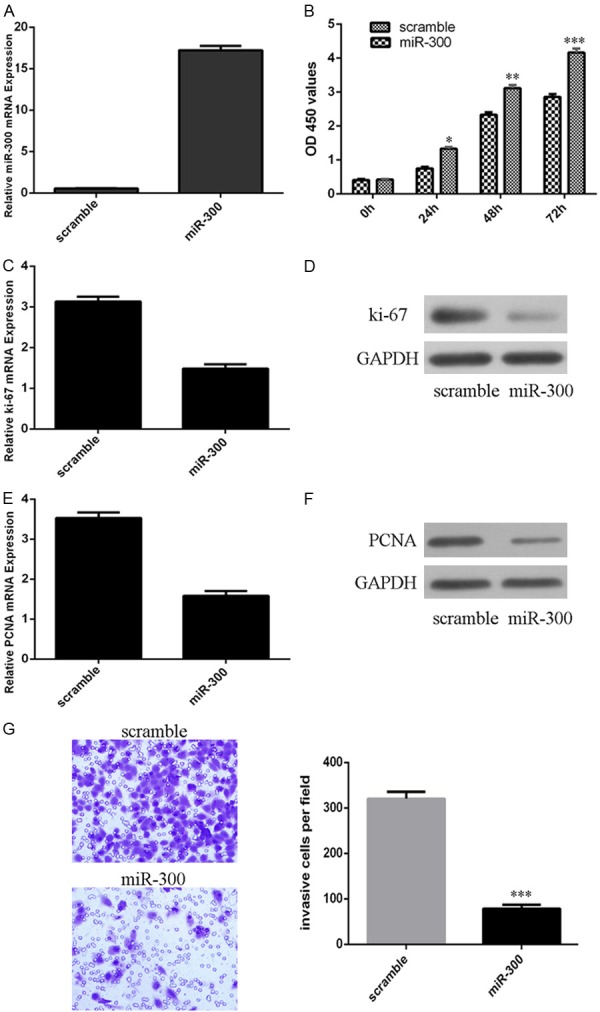
MiR-300 inhibited the LSCC cells proliferation and invasion. A. The expression of miR-300 was detected after treated by miR-300 mimic using qRT-PCR. B. CCK-8 analysis was performed to measure the cell proliferation. C. The mRNA expression of ki-67 was measured by qRT-PCR. D. The protein expression of ki-67 was detected by western blot. E. The mRNA expression of PCNA was measured by qRT-PCR. F. The protein expression of PCNA was detected by western blot. G. Overexpression of miR-300 inhibited the Hep-2 cell invasion. *p<0.05, **p<0.01 and ***p<0.001.
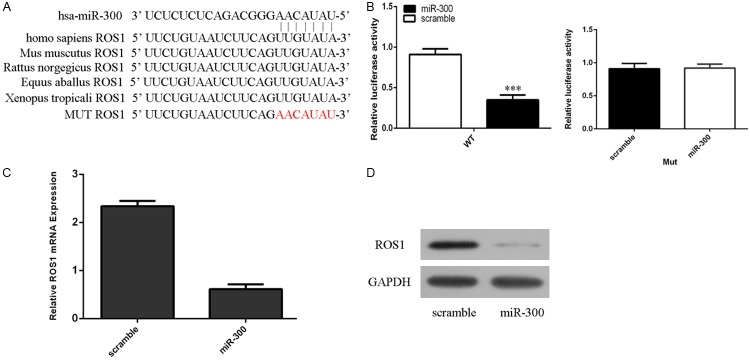
Regulation of ROS1 expression by miR-300 in LSCC
The TargetScan data showed that there were complementary seed region in the 3’-UTR of human ROS1 complementary to miR-300 (Figure 3A). Dual Luciferase Reporter analysis showed that miR-300 overexpression decreased the luciferase activity of WT 3’-UTR of ROS1 construct, however; overexpression of miR-300 did not change the luciferase activity of MUT 3’-UTR of ROS1 construct (Figure 3B). Overexpression of miR-300 inhibited the mRNA expression of ROS1 in the Hep-2 cells (Figure 3C). Moreover, miR-300 overexpression suppressed the ROS1 protein expression in the Hep-2 cells (Figure 3D).
Figure 3.
Regulation of ROS1 expression by miR-300 in LSCC. A. The Targetscan data showed that there were complementary seed region in the 3’-UTR of human ROS1 complementary to miR-300. B. miR-300 overexpression decreased the luciferase activity of WT 3’-UTR of ROS1 construct, however; overexpression of miR-300 did not change the luciferase activity of MUT 3’-UTR of ROS1 construct. C. The mRNA expression of ROS1 was measured by qRT-PCR. D. The protein expression of ROS1 was detected by western blot. ***p<0.001.
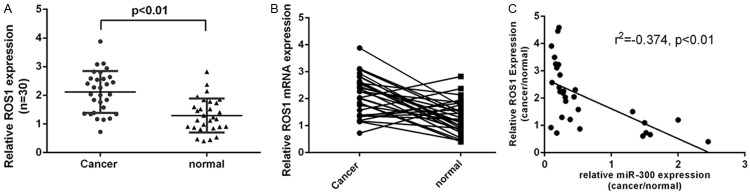
ROS1 expression was upregulated in LSCC
ROS1 expression was upregulated in LSCC tissues compared with adjacent no-tumor tissues (Figure 4A). Moreover, ROS1 was upregulated in 22 cases (22/30, 73%) compared with the normal adjacent tissues (Figure 4B). Interesting, there was an inverse correlation between ROS1 and miR-300 expression in the LSCC tissues (Figure 4C).
Figure 4.
ROS1 expression was upregulated in LSCC. A. The expression of ROS1 was measured by qRT-PCR. B. ROS1 was upregulated in 22 cases (22/30, 73%) compared with the normal adjacent tissues. C. There was an inverse correlation between ROS1 and miR-300 expression in the LSCC tissues.
Overexpression of ROS1 abrogated miR-300 induced cell growth and invasion inhibition
The mRNA expression of ROS1 was upregulated after treated pcDNA-ROS1 in the Hep-2 cells (Figure 5A). Moreover, pcDNA-ROS1 also can induce the protein expression of ROS1 in the Hep-2 cells (Figure 5B). Overexpression of ROS1 increased the Hep-2 cells proliferation (Figure 5C). ROS1 overexpression promoted the Hep-2 cells invasion (Figure 5D). When miR-300 mimics and pcDNA-ROS1 was co-transfected into Hep-2 cells, ROS1 overexpression enhanced the ROS1-induced cell proliferation (Figure 5E) and invasion (Figure 5F) in Hep-2 cells.
Figure 5.
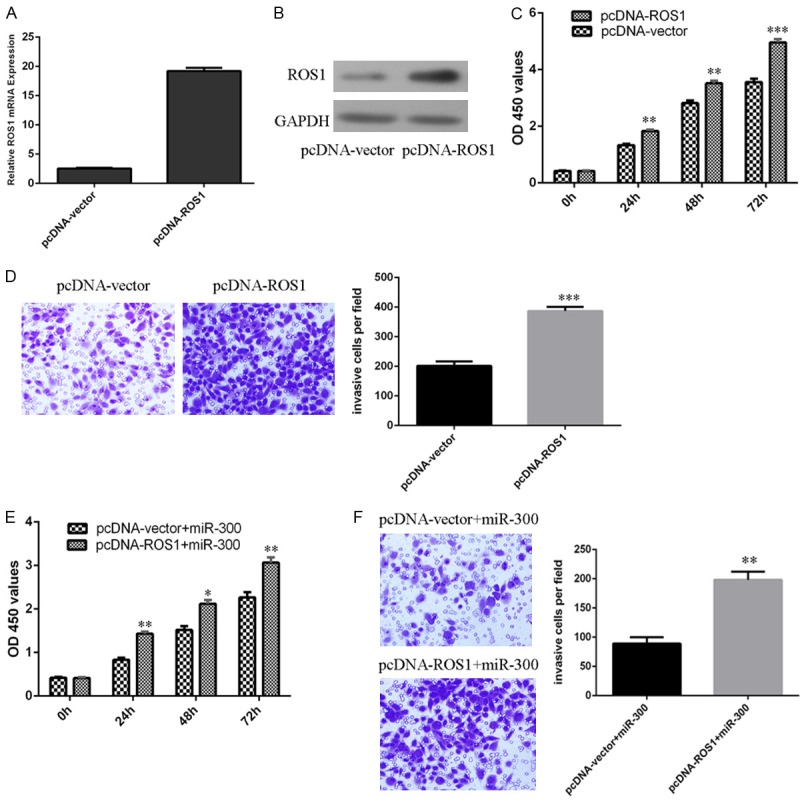
Overexpression of ROS1 abrogated miR-300 induced cell growth and invasion inhibition. A. The mRNA expression of ROS1 was measured by qRT-PCR. B. The protein expression of ROS1 was detected by western blot. C. Overexpression of ROS1 increased the Hep-2 cells proliferation. D. Overexpression of ROS1 increased the Hep-2 cells invasion. E. Cell proliferation was measured by CCK-8 analysis. F. The cell invasion was measured by transwell assays.
Discussion
In our study, we showed that miR-300 expression was downregulated in laryngeal squamous cell carcinoma (LSCC) tissues compared with adjacent no-tumor tissues. MiR-300 overexpression inhibited Hep-2 cell proliferation and inhibited the expression of ki-67 and proliferating cell nuclear antigen (PCNA). Moreover, overexpression of miR-300 repressed the Hep-2 cell invasion. We identified c-ros oncogene 1 receptor tyrosine kinase (ROS1) as a direct target gene of miR-300 in Hep-2 cell. Furthermore, ROS1 expression was upregulated in LSCC tissues compared with adjacent no-tumor tissues. Interesting, there were an inverse correlation between ROS1 and miR-300 expression in LSCC tissues. Overexpression of ROS1 increased the Hep-2 cells proliferation and invasion. Overexpression of ROS1 abrogated miR-300 induced cell growth and invasion inhibition in LSCC. Therefore, our data suggested that miR-300 acted as a tumor suppressive gene in LSCC.
Previous studies demonstrated that miR-300 played important roles in the tumor development. For example, Yu et al. showed that the expression of miR-300 was decreased in the head and neck squamous cell carcinoma (HNSCC) cells and breast cancer cell [33]. miR-300 overexpression blocked TGF-beta-induced epithelial-to-mesenchymal transition (EMT) and reversed the EMT phenotype in MDA-MB-231 and HN-12 cells. Xue et al. demonstrated that miR-300 expression was increased in osteosarcoma cells and tissues and miR-300 overexpression increased cell invasion, proliferation and EMT through regulating bromodomain-containing protein 7 (BRD7) expression [34]. Xu et al. showed that the expression of miR-300 was increased in breast cancer cell lines and tissues [35]. MiR-300 overexpression enhanced cell cycle progression and proliferation by inhibiting p53 expression. However, the role of miR-300 was still uncovered in LSCC. In this study, we demonstrated that miR-300 expression was downregulated in LSCC tissues compared with adjacent no-tumor tissues. MiR-300 was downregulated in 26 cases (26/30, 87%) compared with the normal adjacent tissues. Moreover, miR-300 overexpression inhibited Hep-2 cell proliferation and invasion and inhibited the expression of ki-67 and PCNA.
In our study, we identified ROS1 as a direct target gene of miR-300 Hep-2 cell. ROS1 is an oncogene that could activate various pathways, such as the SHP-1 and SHP-2, ERK1/2, phosphatidylinositol 3-kinase (PI3K), IRS-1 (isiulin receptor substrate 1), STAT3, VAV3 and protein kinase B signaling pathways [36-39]. ROS1 expression was detected in central nervous system, kidney, liver, stomach and colon [38,40-42]. ROS1 gene rearrangement was also found in the glioblastoma multiforme, NSCLC (nonsmall cell lung cancer), colon cancer and gastric cancer [43-47]. Recently, Zhang et al. showed that miR-33a inhibited cell metastasis and proliferation through targeting ROS1 in breast cancer [48]. In our study, we identified ROS1 as a direct target gene of miR-300 in Hep-2 cell. Furthermore, ROS1 expression was upregulated in LSCC tissues compared with adjacent no-tumor tissues. Interesting, there were an inverse correlation between ROS1 and miR-300 expression in the LSCC tissues. Overexpression of ROS1 increased the Hep-2 cells proliferation and invasion. Overexpression of ROS1 abrogated miR-300 induced cell growth and invasion inhibition.
In conclusion, we showed that miR-300 expression was downregulated in LSCC tissues and overexpression of miR-300 suppressed the LSCC cell line Hep-2 cell proliferation and invasion by targeting ROS1 expression. These data suggested that miR-300 acted as a tumor suppressive gene in LSCC.
References
- 1.Yungang W, Xiaoyu L, Pang T, Wenming L, Pan X. miR-370 targeted FoxM1 functions as a tumor suppressor in laryngeal squamous cell carcinoma (LSCC) Biomed Pharmacother. 2014;68:149–154. doi: 10.1016/j.biopha.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Tian L, Zhang J, Ge J, Xiao H, Lu J, Fu S, Liu M, Sun Y. MicroRNA-205 suppresses proliferation and promotes apoptosis in laryngeal squamous cell carcinoma. Med Oncol. 2014;31:785. doi: 10.1007/s12032-013-0785-3. [DOI] [PubMed] [Google Scholar]
- 3.Wu H, Liu T, Wang R, Tian S, Liu M, Li X, Tang H. MicroRNA-16 targets zyxin and promotes cell motility in human laryngeal carcinoma cell line HEp-2. IUBMB Life. 2011;63:101–108. doi: 10.1002/iub.417. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Wang HL, Peng X, Zhou HF, Wang X. miR-1297 mediates PTEN expression and contributes to cell progression in LSCC. Biochem Biophys Res Commun. 2012;427:254–260. doi: 10.1016/j.bbrc.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y, Fu W, Chen H, Shang C, Zhong M. miR-24 functions as a tumor suppressor in Hep2 laryngeal carcinoma cells partly through down-regulation of the S100A8 protein. Oncol Rep. 2012;27:1097–1103. doi: 10.3892/or.2011.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X, Wu Y, Liu Y, Deng H, Shen Z, Xiao B, Guo J. miR-21, miR-106b and miR-375 as novel potential biomarkers for laryngeal squamous cell carcinoma. Curr Pharm Biotechnol. 2014;15:503–508. doi: 10.2174/1389201015666140519110616. [DOI] [PubMed] [Google Scholar]
- 7.Saito K, Inagaki K, Kamimoto T, Ito Y, Sugita T, Nakajo S, Hirasawa A, Iwamaru A, Ishikura T, Hanaoka H, Okubo K, Onozaki T, Zama T. MicroRNA-196a is a putative diagnostic biomarker and therapeutic target for laryngeal cancer. PLoS One. 2013;8:e71480. doi: 10.1371/journal.pone.0071480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu A, Huang JJ, Xu WH, Jin XJ, Li JP, Tang YJ, Huang XF, Cui HJ, Sun GB. miR-21 and miR-375 microRNAs as candidate diagnostic biomarkers in squamous cell carcinoma of the larynx: association with patient survival. Am J Transl Res. 2014;6:604–613. [PMC free article] [PubMed] [Google Scholar]
- 9.Wu TY, Zhang TH, Qu LM, Feng JP, Tian LL, Zhang BH, Li DD, Sun YN, Liu M. MiR-19a is correlated with prognosis and apoptosis of laryngeal squamous cell carcinoma by regulating TIMP-2 expression. Int J Clin Exp Pathol. 2014;7:56–63. [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Lei DP, Jin T, Zhao XN, Li G, Pan XL. Altered expression of miR-21 and PTEN in human laryngeal and hypopharyngeal squamous cell carcinomas. Asian Pac J Cancer Prev. 2011;12:2653–2657. [PubMed] [Google Scholar]
- 11.Li W, Ma H, Sun J. MicroRNA34a/c function as tumor suppressors in Hep2 laryngeal carcinoma cells and may reduce GALNT7 expression. Mol Med Rep. 2014;9:1293–1298. doi: 10.3892/mmr.2014.1929. [DOI] [PubMed] [Google Scholar]
- 12.Nohata N, Hanazawa T, Kinoshita T, Inamine A, Kikkawa N, Itesako T, Yoshino H, Enokida H, Nakagawa M, Okamoto Y, Seki N. Tumour-suppressive microRNA-874 contributes to cell proliferation through targeting of histone deacetylase 1 in head and neck squamous cell carcinoma. Br J Cancer. 2013;108:1648–1658. doi: 10.1038/bjc.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minor J, Wang X, Zhang F, Song J, Jimeno A, Wang XJ, Lu X, Gross N, Kulesz-Martin M, Wang D, Lu SL. Methylation of microRNA-9 is a specific and sensitive biomarker for oral and oropharyngeal squamous cell carcinomas. Oral Oncol. 2012;48:73–78. doi: 10.1016/j.oraloncology.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Yu X, Shen J, Law PT, Chan MT, Wu WK. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget. 2015;6:13914–13924. doi: 10.18632/oncotarget.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Yu X, Shen J, Chan MT, Wu WK. MicroRNA in intervertebral disc degeneration. Cell Prolif. 2015;48:278–283. doi: 10.1111/cpr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Yu X, Shen J, Wu WK, Chan MT. MicroRNA expression and its clinical implications in Ewing’s sarcoma. Cell Prolif. 2015;48:1–6. doi: 10.1111/cpr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu X, Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis (review) Int J Mol Med. 2014;34:923–933. doi: 10.3892/ijmm.2014.1853. [DOI] [PubMed] [Google Scholar]
- 18.Luo X, Dong Z, Chen Y, Yang L, Lai D. Enrichment of ovarian cancer stem-like cells is associated with epithelial to mesenchymal transition through an miRNA-activated AKT pathway. Cell Prolif. 2013;46:436–446. doi: 10.1111/cpr.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Yu M, Liu C, Zhu H, He X, Peng S, Hua J. miR-34c works downstream of p53 leading to dairy goat male germline stem-cell (mGSCs) apoptosis. Cell Prolif. 2013;46:223–231. doi: 10.1111/cpr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, You T, Jing J. MiR-125b inhibits cell biological progression of Ewing’s sarcoma by suppressing the PI3K/Akt signalling pathway. Cell Prolif. 2014;47:152–160. doi: 10.1111/cpr.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itesako T, Seki N, Yoshino H, Chiyomaru T, Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa M, Enokida H. The microRNA expression signature of bladder cancer by deep sequencing: the functional significance of the miR-195/497 cluster. PLoS One. 2014;9:e84311. doi: 10.1371/journal.pone.0084311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Jin J, Liu Y, Huang Z, Deng Y, You T, Zhou T, Si J, Zhuo W. Snail-regulated MiR-375 inhibits migration and invasion of gastric cancer cells by targeting JAK2. PLoS One. 2014;9:e99516. doi: 10.1371/journal.pone.0099516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei Y, Yang Q, Liu J, Wei JJ, Shao C, Liu Z, Kong B. miR-145 inhibits tumor growth and metastasis by targeting metadherin in high-grade serous ovarian carcinoma. Oncotarget. 2014;5:10816–10829. doi: 10.18632/oncotarget.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang Y, Liu C, Yang J, Liu G, Feng F, Tang J, Hu L, Li L, Jiang F, Chen C, Wang R, Yang Y, Jiang X, Wu M, Chen L, Wang H. MiR-20a triggers metastasis of gallbladder carcinoma. J Hepatol. 2013;59:518–527. doi: 10.1016/j.jhep.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Hung TM, Ho CM, Liu YC, Lee JL, Liao YR, Wu YM, Ho MC, Chen CH, Lai HS, Lee PH. Up-regulation of microRNA-190b plays a role for decreased IGF-1 that induces insulin resistance in human hepatocellular carcinoma. PLoS One. 2014;9:e89446. doi: 10.1371/journal.pone.0089446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, Liu C, Song W, Wang F, Zhang J, Shen J, Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, Li Z, Shen J, Wu WK, Liang J, Weng X, Qiu G. MicroRNA-10b Promotes Nucleus Pulposus Cell Proliferation through RhoC-Akt Pathway by Targeting HOXD10 in Intervetebral Disc Degeneration. PLoS One. 2013;8:e83080. doi: 10.1371/journal.pone.0083080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Liu G, Cao P, Chen H, Yuan W, Wang J, Tang X. MiR-27a regulates apoptosis in nucleus pulposus cells by targeting PI3K. PLoS One. 2013;8:e75251. doi: 10.1371/journal.pone.0075251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song QC, Shi ZB, Zhang YT, Ji L, Wang KZ, Duan DP, Dang XQ. Downregulation of microRNA-26a is associated with metastatic potential and the poor prognosis of osteosarcoma patients. Oncol Rep. 2014;31:1263–1270. doi: 10.3892/or.2014.2989. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Zhang SY, Gao YM, Liu YF, Liu YB, Zhao ZG, Yang K. MicroRNAs as oncogenes or tumour suppressors in oesophageal cancer: potential biomarkers and therapeutic targets. Cell Prolif. 2014;47:277–286. doi: 10.1111/cpr.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Yu X, Shen J, Jiang Y. MicroRNA dysregulation in uveal melanoma: a new player enters the game. Oncotarget. 2015;6:4562–4568. doi: 10.18632/oncotarget.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang W, He J, Huang C, Chen L, Tao D, Wu X, Wang M, Luo G, Xiao X, Zeng F, Jiang G. miR-106b-5p targets tumor suppressor gene SETD2 to inactive its function in clear cell renal cell carcinoma. Oncotarget. 2015;6:4066–4079. doi: 10.18632/oncotarget.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J, Xie F, Bao X, Chen W, Xu Q. miR-300 inhibits epithelial to mesenchymal transition and metastasis by targeting Twist in human epithelial cancer. Mol Cancer. 2014;13:121. doi: 10.1186/1476-4598-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue Z, Zhao J, Niu L, An G, Guo Y, Ni L. Up-Regulation of MiR-300 Promotes Proliferation and Invasion of Osteosarcoma by Targeting BRD7. PLoS One. 2015;10:e0127682. doi: 10.1371/journal.pone.0127682. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Xu XH, Li DW, Feng H, Chen HM, Song YQ. MiR-300 regulate the malignancy of breast cancer by targeting p53. Int J Clin Exp Med. 2015;8:6957–6966. [PMC free article] [PubMed] [Google Scholar]
- 36.Keilhack H, Muller M, Bohmer SA, Frank C, Weidner KM, Birchmeier W, Ligensa T, Berndt A, Kosmehl H, Gunther B, Muller T, Birchmeier C, Bohmer FD. Negative regulation of Ros receptor tyrosine kinase signaling. An epithelial function of the SH2 domain protein tyrosine phosphatase SHP-1. J Cell Biol. 2001;152:325–334. doi: 10.1083/jcb.152.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HJ, Seol HS, Kim JY, Chun SM, Suh YA, Park YS, Kim SW, Choi CM, Park SI, Kim DK, Kim YH, Jang SJ. ROS1 receptor tyrosine kinase, a druggable target, is frequently overexpressed in non-small cell lung carcinomas via genetic and epigenetic mechanisms. Ann Surg Oncol. 2013;20:200–208. doi: 10.1245/s10434-012-2553-6. [DOI] [PubMed] [Google Scholar]
- 38.Zeng L, Sachdev P, Yan L, Chan JL, Trenkle T, McClelland M, Welsh J, Wang LH. Vav3 mediates receptor protein tyrosine kinase signaling, regulates GTPase activity, modulates cell morphology, and induces cell transformation. Mol Cell Biol. 2000;20:9212–9224. doi: 10.1128/mcb.20.24.9212-9224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cilloni D, Carturan S, Bracco E, Campia V, Rosso V, Torti D, Calabrese C, Gaidano V, Niparuck P, Favole A, Signorino E, Iacobucci I, Morano A, De Luca L, Musto P, Frassoni F, Saglio G. Aberrant activation of ROS1 represents a new molecular defect in chronic myelomonocytic leukemia. Leuk Res. 2013;37:520–530. doi: 10.1016/j.leukres.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Infarinato NR, Park JH, Krytska K, Ryles HT, Sano R, Szigety KM, Li Y, Zou HY, Lee NV, Smeal T, Lemmon MA, Mosse YP. The ALK/ROS1 inhibitor PF-06463922 overcomes primary resistance to crizotinib in ALK-driven neuroblastoma. Cancer Discov. 2016;6:96–107. doi: 10.1158/2159-8290.CD-15-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu TL, Deng X, Huang F, Tucker M, Crosby K, Rimkunas V, Wang Y, Deng G, Zhu L, Tan Z, Hu Y, Wu C, Nardone J, MacNeill J, Ren J, Reeves C, Innocenti G, Norris B, Yuan J, Yu J, Haack H, Shen B, Peng C, Li H, Zhou X, Liu X, Rush J, Comb MJ. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One. 2011;6:e15640. doi: 10.1371/journal.pone.0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Sanea MM, Abdelazem AZ, Park BS, Yoo KH, Sim T, Kwon YJ, Lee SH. ROS1 Kinase Inhibitors for Molecular-Targeted Therapies. Curr Med Chem. 2016;23:142–160. doi: 10.2174/0929867322666151006093623. [DOI] [PubMed] [Google Scholar]
- 43.Lim SM, Choi J, Chang JH, Sohn J, Jacobson K, Policht F, Schulz J, Cho BC, Kim SH. Lack of ROS1 Gene Rearrangement in Glioblastoma Multiforme. PLoS One. 2015;10:e0137678. doi: 10.1371/journal.pone.0137678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu S, Liang Y, Lin YB, Wang F, Huang MY, Zhang ZC, Wang J, Cen WJ, Shao JY. The Frequency and Clinical Implication of ROS1 and RET Rearrangements in Resected Stage IIIA-N2 Non-Small Cell Lung Cancer Patients. PLoS One. 2015;10:e0124354. doi: 10.1371/journal.pone.0124354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheffler M, Schultheis A, Teixido C, Michels S, Morales-Espinosa D, Viteri S, Hartmann W, Merkelbach-Bruse S, Fischer R, Schildhaus HU, Fassunke J, Sebastian M, Serke M, Kaminsky B, Randerath W, Gerigk U, Ko YD, Kruger S, Schnell R, Rothe A, Kropf-Sanchen C, Heukamp L, Rosell R, Buttner R, Wolf J. ROS1 rearrangements in lung adenocarcinoma: prognostic impact, therapeutic options and genetic variability. Oncotarget. 2015;6:10577–10585. doi: 10.18632/oncotarget.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackinnon AC Jr, Luevano A, de Araujo LC, Rao N, Le M, Suster S. Cribriform adenocarcinoma of the lung: clinicopathologic, immunohistochemical, and molecular analysis of 15 cases of a distinctive morphologic subtype of lung adenocarcinoma. Mod Pathol. 2014;27:1063–1072. doi: 10.1038/modpathol.2013.227. [DOI] [PubMed] [Google Scholar]
- 47.Lee J, Lee SE, Kang SY, Do IG, Lee S, Ha SY, Cho J, Kang WK, Jang J, Ou SH, Kim KM. Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer. 2013;119:1627–1635. doi: 10.1002/cncr.27967. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C, Zhang Y, Ding W, Lin Y, Huang Z, Luo Q. MiR-33a suppresses breast cancer cell proliferation and metastasis by targeting ADAM9 and ROS1. Protein Cell. 2015;6:881–889. doi: 10.1007/s13238-015-0223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]