Abstract
Reduced placental growth factor (PLGF) during pregnancy is known to be a reason for developing preeclampsia (PE) and gestational diabetes mellitus (GDM), but the underlying mechanisms remain unclear. Recently, it has been shown that reduced PLGF may induce GDM through suppressing beta-cell mass growth in a PI3k/Akt signalling-dependent manner. Here, we dissected the interaction between beta-cells and islet endothelial cells in this model. We analysed proliferation of beta-cells and islet endothelial cells at different time points of gestation in mice. We cultured mouse islet endothelial cells (MS1), with or without PLGF. We cultured primary mouse beta-cells in conditioned media from PLGF-treated MS1. We cultured MS1 cells in conditioned media from proliferating beta-cells that were activated with conditioned media from PLGF-treated MS1 cells. We analysed cell proliferation by BrdU incorporation. We analysed cell growth by a MTT assay. We found that during mouse gestation, the increases in cell proliferation occurred earlier in beta-cells than in islet endothelial cells. In vitro, PLGF itself failed to induce proliferation of MS1 cells. However, conditioned media from the PLGF-treated MS1 cells induced beta-cell proliferation, resulting in increases in beta-cell number. Moreover, proliferation of MS1 cells significantly increased when MS1 cells were cultured in conditioned media from proliferating beta-cells activated with conditioned media from PLGF-treated MS1 cells. Thus, our data suggest that gestational PLGF may stimulate islet endothelial cells to release growth factors to promote beta-cell proliferation, and proliferating beta-cells in turn release endothelial cell growth factor to increase proliferation of endothelial cells. PE-associated reduction in PLGF impairs these processes to result in islet growth impairment, and subsequently the onset of GDM.
Keywords: Preeclampsia, placental growth factor (PLGF), beta-cell proliferation, MS1, gestational diabetes mellitus (GDM)
Introduction
A successful pregnancy needs significantly augmented systemic metabolism to meet the requirements for nutrition and support for the embryo growth. Failure of meeting these requirements leads to development of a number of gestation-associated diseases, including preeclampsia (PE) and gestational diabetes mellitus (GDM) [1-5]. Interestingly, PE and GDM share many symptoms and pathogenesis processes, which may cause multi-organ dysfunction and may increase risk of the occurrence of cardiovascular disease [1-5]. Moreover, PE and GDM also share many risk factors such as obesity, elevated blood pressure, dyslipidaemia, insulin resistance and hyperglycemia [1-5]. However, the relationship between development of PE and GDM in terms of mechanic bases is much lacking.
Placental growth factor (PLGF) is a member of the vascular endothelial growth factor (VEGF) family, and previous studies have demonstrated a pivotal role of PLGF in gestational period [6,7]. Interestingly, reduced PLGF levels have been associated with the onset of PE, which is characterized with inferior placental vascularization [6,7]. PLGF has a unique receptor, VEGF receptor 1 (VEGFR1) or Flt-1, through which PLGF conducts its effects. In the islets where beta cells situate, VEGFR1 is exclusively expressed in the islet endothelial cells [8-12]. Therefore, beta-cells do not directly responded to PLGF, and their responses to PLGF have to be mediated through PLGF-targeted islet endothelial cells. Indeed, interaction between beta-cells and islet endothelial cells has been well studied, and compelling data have been shown to demonstrate a close relationship between beta-cells and islet endothelial cells during development [12-17] and tissue homeostasis [8-12].
Recently, it has been shown that impairment in gestational beta-cell mass growth may result from PE-associated reduction in PLGF, and this impairment in gestational beta-cell mass growth may progress to GDM [18]. Moreover, the PLGF-induced beta-cell proliferation during gestation has been found to be mediated by islet endothelial cells, and involves activation of PI3k/Akt signalling pathway in beta-cells [19]. However, the exact molecular mechanisms remain unclear.
Here, we studied the mechanisms underlying PLGF-regulated beta-cell proliferation during gestation, paying special attention to the crosstalk between beta-cells and islet endothelial cells. During mouse gestation, we found that the increases in cell proliferation occurred earlier in beta-cells than in islet endothelial cells. In vitro, PLGF itself failed to induce proliferation of MS1 cells. However, conditioned media from the PLGF-treated MS1 cells induced beta-cell proliferation, resulting in increases in beta-cell number. Moreover, proliferation of MS1 cells significantly increased when MS1 cells were cultured in conditioned media from proliferating beta-cells activated with conditioned media from PLGF-treated MS1 cells. Together, these data suggest that gestational PLGF may stimulate islet endothelial cells to release growth factors to promote beta-cell proliferation, and proliferating beta-cells in turn release endothelial cell growth factor to increase proliferation of endothelial cells.
Materials and methods
Animals
MIP-GFP mice were purchased from Jackson Labs (Bar Harbor, ME, USA). This strain has been described before [20]. All mouse experiments were approved by and performed according to the guidelines of the IACUC of Ruijin Hospital. Only 12-week-old female MIP-GFP mice were used for analysing proliferating beta-cells and islet endothelial cells at different time points during gestation, and for isolation of beta-cells for in vitro studies. The mice were kept in specific pathogen free (SPF) conditions. For quantification of proliferating cells, BrdU (100 mg/kg body weight, Sigma-Aldrich, St. Louis, MO, USA) was injected 2 hours before sacrifice of the mice at different time points during gestation.
Isolation of mouse beta-cells
The MIP-GFP mouse pancreas was first perfused with 0.125 mg/ml LiberaseTL (Roche, Nutley, NJ, USA) from the common bile duct, as has been described in published protocol [21,22], then was incubated in 0.125 mg/ml LiberaseTL in a 37°C shaker at 200 rpm for 45 minutes. Histopaque 1077 and Histopaque 1119 (Sigma-Aldrich) were mixed at a ratio of 9:33 (vol:vol) to generate Histopaque 1110. Combined centrifugation in Histopaque 1100 and hand-pickings were then performed to purify islets. Purified islets were further dissociated into signal cells and beta-cells were purified by flow cytometry based on GFP, as has been described in published protocol [23].
Cell culture
MS1 was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA), and is a pancreatic islet endothelial cell line by transducing primary islet endothelial cells with a temperature sensitive SV40 large T antigen construct [24]. MS1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA). The beta-cells were cultured in the same culture media as MS1. Purified beta-cells were cultured alone, or with conditioned media from PLGF-treated MS1 cells. MS1 cells were cultured alone, or with PLGF (10 ng/ml, Sigma-Aldrich), or with conditioned media from proliferating beta-cells triggered by conditioned media from PLGF-treated MS1 cells. Two days after culture, beta-cells were analysed for proliferation by a 24-hours’ BrdU incorporation at a dose of 1 µmol/l, or cell number in a MTT assay. MS1 cells were analysed for proliferation by a 2-hours’ BrdU incorporation, or cell number in a MTT assay.
MTT assay
For assay of cell number, the beta-cells or MS1 cells (104) in 24-well-plate were subjected to a Cell Viability Kit (MTT, Roche, Nutley, NJ, USA) for 8 or 4 days, respectively, according to the manufacturer’s instruction.
Immunocytochemistry and immunohistochemistry
Pregnant mouse pancreases were fixed with 4% paraformaldehyde for 12 hours, and cultured mouse beta-cells or MS1 cells were fixed with 4% paraformaldehyde for 4 hours. Immunostaining for BrdU, CD31, insulin, GFP (to determine total beta-cells), and/or DAPI (nuclear staining to determine total MS1 cells, Sigma-Aldrich) was performed. Primary antibodies are guinea pig polyclonal anti-insulin (DAKO, Carpinteria, CA, USA), mouse monoclonal anti-CD31 (Becton-Dickinson Biosciences, San Jose, CA, USA), rabbit polyclonal anti-GFP and rat polyclonal anti-BrdU (Abcam, Cambridge, MA, USA). Antigen retrieval by incubation of the slides with 1 mol/l HCl at room temperature for 45 minutes was performed for BrdU staining. Secondary antibodies were Cy3- and Cy2- conjugated antibodies for corresponding species (Jackson ImmunoResearch Labs, West Grove, PA, USA).
Quantification of cell proliferation
Each experimental condition contains 5 repeats. In vitro, GFP staining (in MIP-GFP mice) was used to identify beta-cells, and DAPI staining was used to identify MS1 cells. In vivo, insulin staining was used to identify beta-cells, and CD31 staining was used to identify endothelial cells. With the help of insulin, islet endothelial cells can be determined. The quantification of BrdU+ beta-cells, BrdU+ endothelial cells and BrdU+MS1 cells was based on at least 1000 cells in each sample.
Statistics
All values are depicted as mean ± standard deviation from at least 5 individuals and are considered significant if p < 0.05. All data were statistically analysed using unpaired student’s T test. The figures were generated in Prism 6.0 (GraphPad Software Inc, La Jolla, CA, USA).
Results
Gestational beta-cell proliferation precedes proliferation of islet endothelial cells
Recently, we reported that impairment in gestational beta-cell mass growth may result from PE-associated reduction in PLGF, and may lead to development of GDM [18]. Moreover, the PLGF-induced beta-cell proliferation during gestation appears to be mediated by islet endothelial cells, and involves activation of PI3k/Akt signalling pathway in beta-cells. However, the exact underlying mechanisms remain unclear. Thus, we examined the proliferation of beta-cells and islet endothelial cells in the mouse pancreas at different time points during gestation after a 2 hours’ BrdU labelling. Insulin staining was used to identify beta-cells, and CD31 staining was used to identify endothelial cells, as shown in a representative image. With the help of insulin, islet endothelial cells can be determined (Figure 1A). We found that the proliferating beta cells increased since initiation of the gestation, peaked at G11, and then decreased (Figure 1B). However, the proliferating islet endothelial cells only increased since the middle of the gestation (G13), peaked at G15, and then decreased afterwards (Figure 1C). These data suggest that gestational beta-cell proliferation precedes proliferation of islet endothelial cells. Thus, although PLGF activates islet endothelial cells, which in turn promote beta-cell proliferation, it seems that PLGF may not increase the proliferation of islet endothelial cells directly and immediately. We hypothesize that proliferating beta-cells may trigger the proliferation of islet endothelial cells.
Figure 1.
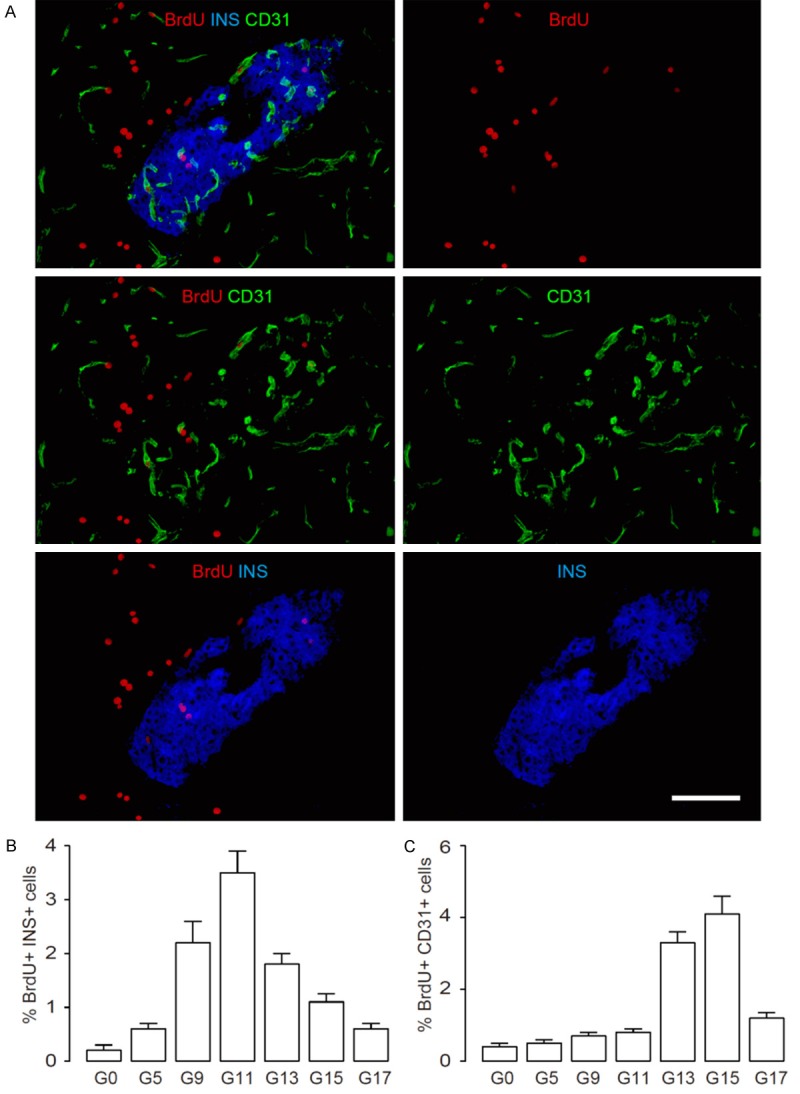
Gestational beta-cell proliferation precedes proliferation of islet endothelial cells. (A-C) We examined the proliferation of beta-cells and islet endothelial cells in the mouse pancreas at different time points during gestation by 2 hours’ BrdU labelling. Insulin (INS) staining was used to identify beta-cells, and CD31 staining was used to identify endothelial cells, as shown in a representative image (A). (B) Percentage of BrdU+ INS+ cells at different time points after gestation. (C) Percentage of BrdU+ CD31+ islet endothelial cells (within islets based on INS staining) at different time points after gestation. N=5. Scale bar is 50 µm.
PLGF itself does not increase proliferation of endothelial cells
To examine whether this hypothesis may be correct, we treated mouse islet endothelial cells, MS1, with or without PLGF (10 ng/ml), to examine the direct effects of PLGF on MS1 cell proliferation after a 2 hour’s BrdU labelling (Figure 2A). We found that PLGF itself did not increase proliferation of MS1 cells, by representative immunocytochemistry images (Figure 2B), and by quantification (Figure 2C). Moreover, PLGF treatment did not increase MS1 cell number in a MTT assay (Figure 2D). These data suggest that PLGF itself does not increase proliferation of endothelial cells.
Figure 2.
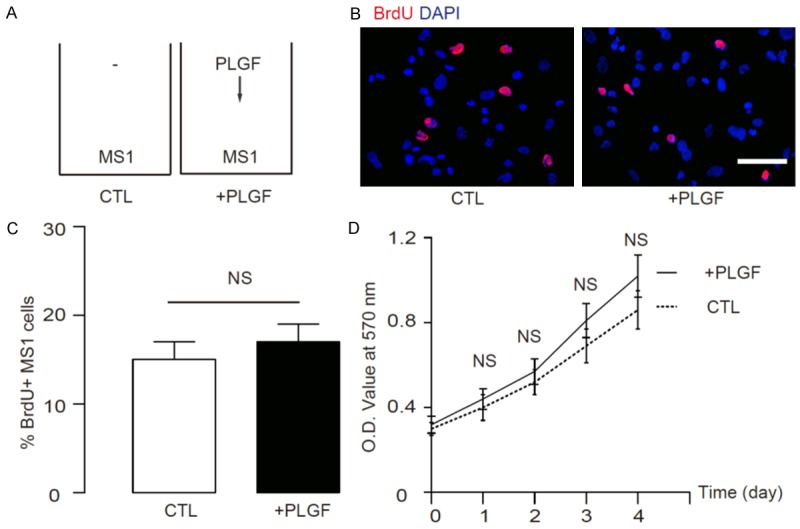
PLGF itself does not increase proliferation of endothelial cells. (A) We treated mouse islet endothelial cells, MS1, with or without PLGF (10 ng/ml), to examine the direct effects of PLGF on MS1 cell proliferation by 2 hour’s BrdU incorporation. (B, C) We found that PLGF itself did not increase proliferation of MS1 cells, by representative immunocytochemistry images (B), and by quantification (C). (D) PLGF treatment did not increase MS1 cell number in a MTT assay. N=5. NS: non-significant. Scale bar is 20 µm.
PLGF activates endothelial cells to release growth factors to augment beta-cell proliferation
In order to figure out whether PLGF-activated endothelial cells may induce beta-cell proliferation in a paracrine-manner, we cultured purified beta-cells from MIP-GFP mice in conditioned media (CM) from PLGF-treated MS1 (Figure 3A). We found that these CM significantly increased beta-cell proliferation by a 24 hours’ BrdU labelling, shown by representative immunocytochemistry images (Figure 3B), and by quantification (Figure 3C). Moreover, these CM from PLGF-treated MS1 cells significantly increased beta-cell number in a MTT assay (Figure 3D). These data suggest that PLGF-activated endothelial cells may release grow factors to promote beta-cell proliferation.
Figure 3.
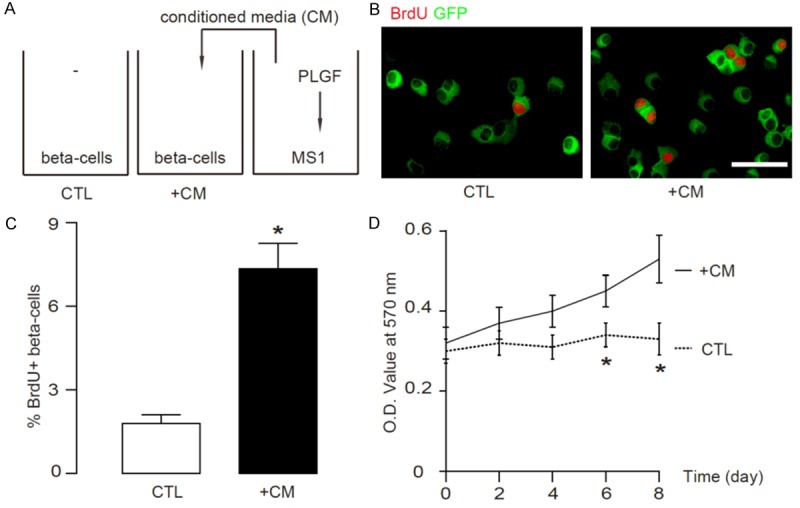
PLGF activates endothelial cells to release growth factors to augment beta-cell proliferation. (A) We cultured purified beta-cells from MIP-GFP mice in conditioned media (CM) from PLGF-treated MS1 cells. (B, C) We found that these CM significantly increased beta-cell proliferation by a 24 hours’ BrdU incorporation, by representative immunocytochemistry images (B), and by quantification (C). (D) These CM from PLGF-treated MS1 cells significantly increased beta-cell number in a MTT assay. N=5. *p < 0.05. Scale bar is 20 µm.
Conditioned media from the proliferating beta-cells stimulated by PLGF-treated MS1 cells in turn promote proliferation of endothelial cells
Next, we examined the effects of proliferating beta-cells on the endothelial cells. Conditioned media were taken from the proliferating beta cells stimulated by PLGF-MS1 conditioned media. These conditioned media were termed as CM-b (Figure 4A). MS1 cells were then treated with/without CM-b (Figure 4A) for 2 days and their proliferation was measured by a 2 hour’s BrdU labelling. We found that CM-p significantly increased MS1 cell proliferation, by representative immunocytochemistry images (Figure 4B), and by quantification (Figure 4C). Moreover, these CM-p from the proliferating beta-cells stimulated by PLGF-treated MS1 media significantly increased endothelial cell number in a MTT assay (Figure 4D). These data suggest that proliferating beta-cells in turn promote proliferation of endothelial cells.
Figure 4.
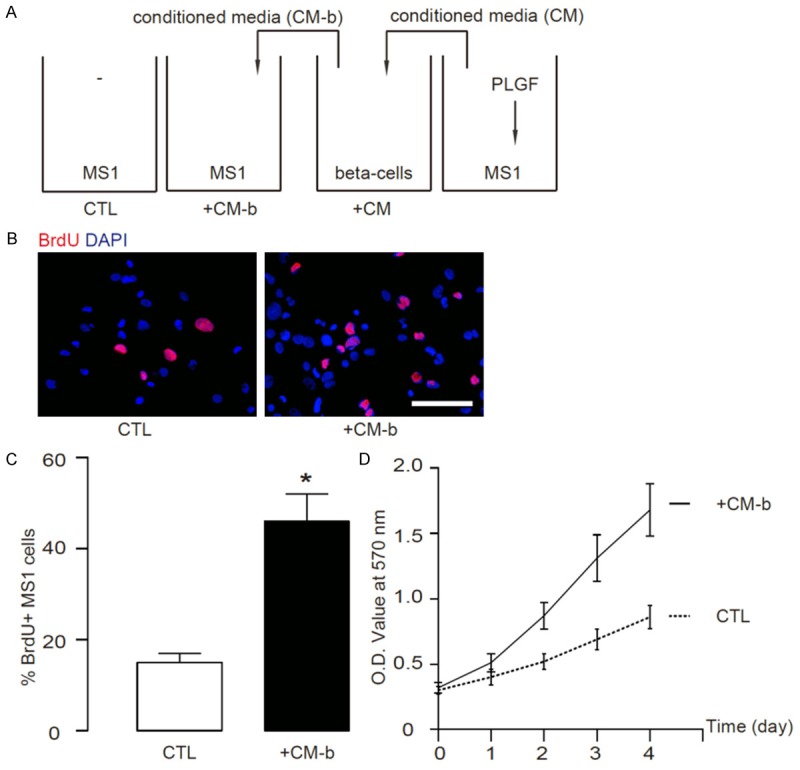
Conditioned media from the proliferating beta-cells stimulated by PLGF-treated MS1 cells in turn promote proliferation of endothelial cells. (A) Conditioned media were taken from the proliferating beta cells stimulated by PLGF-MS1 conditioned media. These conditioned media were termed as CM-b. MS1 cells were then treated with/without CM-b for 2 days and their proliferation was measured by 2 hour’s BrdU incorporation. (B, C) We found that CM-p significantly increased MS1 cell proliferation, by representative immunocytochemistry images (B), and by quantification (C). (D) These CM-p from the proliferating beta-cells stimulated by PLGF-treated MS1 cells significantly increased endothelial cell number in a MTT assay. N=5. *p < 0.05. Scale bar is 20 µm.
Hence, we have proposed a model based on the findings in the current study and our previous work. PLGFs play a key role in gestation. PLGF activates islet endothelial cells that release growth factors to promote beta-cell proliferation. Proliferating beta-cells release endothelial cell growth factors to increase proliferation of islet endothelial cells. The growth of beta-cells and islet endothelial cells results in growth of islets. Reduction of PLGF impairs this regulation axis, resulting in decreased gestational islet growth and development of GDM (Figure 5).
Figure 5.
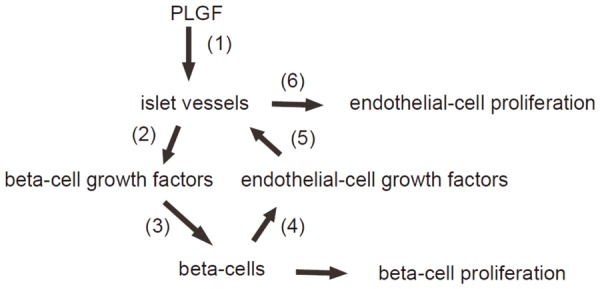
Schematic of the model. PLGFs play a key role in gestation. PLGF activates islet endothelial cells that release growth factors to promote beta-cell proliferation. Proliferating beta-cells release endothelial cell growth factors to increase proliferation of islet endothelial cells. The growth of beta-cells and islet endothelial cells results in growth of islets. Reduction of PLGF impairs this regulation axis, resulting in decreased gestational islet growth and development of GDM.
Discussion
PE and GDM are two common complications in pregnant women and are responsible for majority of maternal and fatal mortality. Moreover, the frequent co-occurrence of these two complications in the same patient implies a causal relationship. Although the molecular pathogenesis of the two diseases has been extensively studied, no conclusive results have so far been achieved.
Recently, it was reported that reduced serum PLGF levels in pregnant women may be associated with the onset of both PE and GDM. A strong correlation between serum PLGF levels and presence of GDM has been found. In L-NAME mouse model for human PE, which induces all PE-like features and a reduction of PLGF level, impairment of gestational growth of beta-cell mass was detected. Moreover, the increases in beta-cell proliferation in pregnant mice were significantly reduced by reduced PLGF in this model, which is substantialized in a gain-of-function experiment, in which exogenous PLGF corrected the impairment in beta-cell mass growth and PE-associated features [18]. Further, in a follow-up study, PLGF was found to activate islet endothelial cells through its binding to VEGFR1 in endothelial cells, and activated endothelial cells may secrete trophic factors to promote proliferation of the beta-cells in a PI3k/Akt-signalling-dependent manner [19]. However, there are still several remaining questions. How are all these molecular events organized? Do the islet endothelial cells proliferate immediately after activation and does their proliferation precede beta-cell proliferation? Do beta-cells also affect islet endothelial cells in turn?
To address these questions, we studied the proliferating beta-cells and islet endothelial cells during gestation. Interestingly, we found that the increases in cell proliferation occurred earlier in beta-cells than in islet endothelial cells during gestation. These data do not support the hypothesis that PLGF directly increase islet endothelial cell proliferation, otherwise the cell proliferation should be detected earlier in islet endothelial cells, than in beta-cells. Hence, the proliferation of endothelial cells may be triggered by proliferating beta-cells. This hypothesis is highly possible, since previous studies have shown that islet endothelial cells and beta-cells have a close relationship and they affect the development, function and homeostasis of each other [8-16].
We then examined this possibility in vitro. We found that PLGF itself failed to induce proliferation of MS1 cells, consistent with our in vivo data. However, conditioned media from the PLGF-treated MS1 cells induced beta-cell proliferation, resulting in increases in beta-cell number, consistent with our previous report. Moreover, proliferation of MS1 cells significantly increased when MS1 cells were cultured in conditioned media from proliferating beta-cells activated with conditioned media from PLGF-treated MS1 cells. This model mimics what may have occurred in vivo during gestation.
Together, these data suggest that gestational PLGF may stimulate islet endothelial cells to release growth factors to promote beta-cell proliferation, and proliferating beta-cells in turn release growth factors for endothelial cells to increase proliferation of endothelial cells. Finally, the proliferation of both beta-cells and islet endothelial cells result in a relatively consistent cell composition of an islet, to allow the islet to properly function in response to metabolic need.
Together with previous reports, we propose a model in that PLGF plays a key role in gestation. Reduction in PLGF may induce vascular defects that affect cardiac endothelial cells to induce hypertension and other PE-like symptoms. Reduction in PLGF may also induce vascular defects that affect islet endothelial cells that are needed to release growth factors to augment beta-cell proliferation in a PI3k/Akt-signalling-dependent manner. The effects of PLGF on gestational beta-cell growth require an intimate crosstalk between beta-cells and islet endothelial cells. Targeting all these control points may help to prevent and treat PLGF-related PE and GDM.
Acknowledgements
This research was supported by a grant from Shanghai Three-year Plan for Developing Public Health System (GWIV-4) to Jian Shen.
Disclosure of conflict of interest
None.
References
- 1.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 2.Rao R, Sen S, Han B, Ramadoss S, Chaudhuri G. Gestational diabetes, preeclampsia and cytokine release: similarities and differences in endothelial cell function. Adv Exp Med Biol. 2014;814:69–75. doi: 10.1007/978-1-4939-1031-1_6. [DOI] [PubMed] [Google Scholar]
- 3.Schneider S, Freerksen N, Rohrig S, Hoeft B, Maul H. Gestational diabetes and preeclampsia--similar risk factor profiles? Early Hum Dev. 2012;88:179–184. doi: 10.1016/j.earlhumdev.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Dempsey JC, Butler CL, Williams MA. No need for a pregnant pause: physical activity may reduce the occurrence of gestational diabetes mellitus and preeclampsia. Exerc Sport Sci Rev. 2005;33:141–149. doi: 10.1097/00003677-200507000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Wen SW, Xie RH, Tan H, Walker MC, Smith GN, Retnakaran R. Preeclampsia and gestational diabetes mellitus: pre-conception origins? Med Hypotheses. 2012;79:120–125. doi: 10.1016/j.mehy.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–1544. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 7.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 8.Brissova M, Aamodt K, Brahmachary P, Prasad N, Hong JY, Dai C, Mellati M, Shostak A, Poffenberger G, Aramandla R, Levy SE, Powers AC. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes beta cell regeneration. Cell Metab. 2014;19:498–511. doi: 10.1016/j.cmet.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 10.Xiao X, Guo P, Chen Z, El-Gohary Y, Wiersch J, Gaffar I, Prasadan K, Shiota C, Gittes GK. Hypoglycemia reduces vascular endothelial growth factor a production by pancreatic Beta cells as a regulator of Beta cell mass. J Biol Chem. 2013;288:8636–8646. doi: 10.1074/jbc.M112.422949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao X, Prasadan K, Guo P, El-Gohary Y, Fischbach S, Wiersch J, Gaffar I, Shiota C, Gittes GK. Pancreatic duct cells as a source of VEGF in mice. Diabetologia. 2014;57:991–1000. doi: 10.1007/s00125-014-3179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 13.Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 14.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev. 2003;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 16.Cleaver O, Dor Y. Vascular instruction of pancreas development. Development. 2012;139:2833–2843. doi: 10.1242/dev.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao X, Gittes GK. Concise Review: New Insights Into the Role of Macrophages in beta-Cell Proliferation. Stem Cells Transl Med. 2015;4:655–658. doi: 10.5966/sctm.2014-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Ying H, Cai G, Guo Q, Chen L. Impaired proliferation of pancreatic beta cells, by reduced placental growth factor in pre-eclampsia, as a cause for gestational diabetes mellitus. Cell Prolif. 2015;48:166–74. doi: 10.1111/cpr.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Ying H, Cai G, Guo Q, Chen L. Pre-Eclampsia-Associated Reduction in Placental Growth Factor Impaired Beta Cell Proliferation Through PI3k Signalling. Cell Physiol Biochem. 2015;36:34–43. doi: 10.1159/000374051. [DOI] [PubMed] [Google Scholar]
- 20.Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic beta -cells. Am J Physiol Endocrinol Metab. 2003;284:E177–183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 21.Perides G, van Acker GJ, Laukkarinen JM, Steer ML. Experimental acute biliary pancreatitis induced by retrograde infusion of bile acids into the mouse pancreatic duct. Nat Protoc. 2010;5:335–341. doi: 10.1038/nprot.2009.243. [DOI] [PubMed] [Google Scholar]
- 22.Xiao X, Guo P, Prasadan K, Shiota C, Peirish L, Fischbach S, Song Z, Gaffar I, Wiersch J, El-Gohary Y, Husain SZ, Gittes GK. Pancreatic cell tracing, lineage tagging and targeted genetic manipulations in multiple cell types using pancreatic ductal infusion of adeno-associated viral vectors and/or cell-tagging dyes. Nat Protoc. 2014;9:2719–2724. doi: 10.1038/nprot.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao X, Chen Z, Shiota C, Prasadan K, Guo P, El-Gohary Y, Paredes J, Welsh C, Wiersch J, Gittes GK. No evidence for beta cell neogenesis in murine adult pancreas. J Clin Invest. 2013;123:2207–2217. doi: 10.1172/JCI66323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, Byers HR, Folkman J. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]


