Abstract
Increasing evidences have demonstrated that long noncoding RNAs (LncRNAs) play a significant role in the development of tumor. However, the role of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) in uveal melanoma remains unknown. In this study, we demonstrated that the expression of MALAT1 was upregulated in the uveal melanoma tissues compared to normal tissues. Among them, MALAT1 was upregulated in 72% (18/25) uveal melanoma tissues compared to their paired normal tissues. Knockdown of MALAT1 suppressed uveal melanoma cell proliferation, colony information, invasion and migration. Moreover, we showed that knockdown of MALAT1 promoted miR-140 expression and suppressed Slug and ADAM10 expression in the MUM-2C cell. In addition, we demonstrated that miR-140 was downregulated in the uveal melanoma tissues compared to normal tissues and cell lines. The expression level of MALAT1 was inversely correlated with the expression level of miR-140 in uveal melanoma tissues. These results suggested that MALAT1 served as an oncogenic LncRNA in the development of uveal melanoma.
Keywords: Long noncoding RNA, LncRNAs, MALAT1, uveal melanoma, mir-140
Introduction
Uveal melanoma is the most common primary intraocular malignancy in the adults, with an incidence about 0.7 per 100,000 [1-4]. Although development has been made in the therapy and diagnosis for the uveal melanoma, the 5-year survival rate still remains poor [5-7]. Most of the uveal melanoma patients have developed liver metastasis when diagnosed [8-10]. The molecular mechanisms of metastasis are still unknown [11,12]. Therefore, it is important to identify new prognostic factors and therapy targets for uveal melanoma.
Long noncoding RNAs (lncRNAs) are a new class of non-protein-coding RNAs longer than 200 nucleotides [13-15]. Accumulating evidences have suggested that lncRNAs play a crucial role in many cellular processes including cell development, growth, differentiation, invasion and apoptosis [16-21]. LncRNAs are deregulated in a lot of cancers such as gastric cancer, osteosarcoma, colorectal cancer, cervical cancer and hepatocellular carcinoma [22-26]. Moreover, lncRNAs can act as tumor suppressor genes or oncogenes in the tumor [27-29]. However, the expression and functional roles of lncRNAs in uveal melanoma development are still unknown.
In this study, we demonstrated that the expression metastasis associated lung adenocarcinoma transcript 1 (MALAT1) was upregulated in uveal melanoma tissues and cell lines. Moreover, knockdown of MALAT1 expression suppressed uveal melanoma cell proliferation, colony information, invasion and migration partly through modulating miR-140 expression.
Materials and methods
Tissue samples and cell line cultured and transfection
Uveal melanoma tissues and their paired normal tissues were collected from uveal melanoma patients and immediately kept in the liquid nitrogen. The uveal melanoma cell lines (MUM-2C, OCM-1A, MUM-2B and C918) and one melanocyte cell line (D78) were purchased from purchase Chinese Academy of Sciences (Beijing, China). The D78, OCM-1A and MUM-2C were cultured in the DMEM medium, and C918 and MUM-2B were maintained in RPMI 1640. Cell transfection was performed by using Lipofectamine 2000 (Invitrogen, USA) following to the manufacturer’s information.
RNA extraction and qPCR analysis
Total RNA was isolated from the cells or tissues using TRIzol kit (Invitrogen, USA) following to the manufacturer’s information. Quantitative Real-time PCR (qPCR) was performed to detect the expression of miR-140 and MALAT1 according to manufacturer’s protocol on the iQ5 qPCR System (Bio-Rad, CA). U6 and GAPDH were used as control for miRNA and mRNA expression, respectively.
Western blot assay
The protein was extracted from cells or tissues and the protein concentration was determined by using BCA kit following to the manufacturer’s information. Total protein was separated by 12% SDS-PAGE and transferred to PVDF (polyvinylidene fluoride) membrane. The membrane was blocked with non-fat milk and then inculcated with the primary antibody. The primary antibody was shown as following: PCNA, ki-67, Slug and ADAM10 (Abcam, USA).
Cell proliferation and colony information
Cell proliferation was detected using MTT assay. 20 ml MTT solution (Sigma, USA) was put to each well and the cells were continued to incubate for 4 h. The absorbance was detected at 490 nm in the Thermo (Thermo, USA). Cells were cultured in the 6-well plates and incubated for 2 weeks. Then, the cell colonies were fixed and stained with 10% crystal violet and counted.
Cell migration and invasion assays
Wound healing analysis was performed to measure the cell migration. Cells were cultured in the 6-well plate and seed to 100% confluence. The wound was created using a pipette tip and then the cells continue to culture for 48 hours. The closure rate was measured and described by a percentage. For cell invasion, the Transwell coated with Matrigel was used. Cells were placed on the upper chamber and 10% FBS was given to the lower chamber. Cells were culture for 48 hours and then cells invaded to the membrane were measure with crystal violet.
Statistical analysis
Data was shown as the mean ± SD (standard deviation). Difference between two groups was used by Student’s t-test and one-way ANOVA was performed to measure the differences between more than two samples. P<0.05 was considered as significant difference.
Results
MALAT1 expression was increased in uveal melanoma tissues and cell
We found that MALAT1 was upregulated in uveal melanoma tissues compared to normal tissues (Figure 1A). Among them, MALAT1 expression was upregulated in 72% (18/25) uveal melanoma tissues compared to their paired normal tissues (Figure 1B). In addition, we demonstrated that MALAT1 was upregulated in uveal melanoma cell lines (MUM-2C, OCM-1A, MUM-2B and C918) compared to melanocyte cell line (D78).
Figure 1.
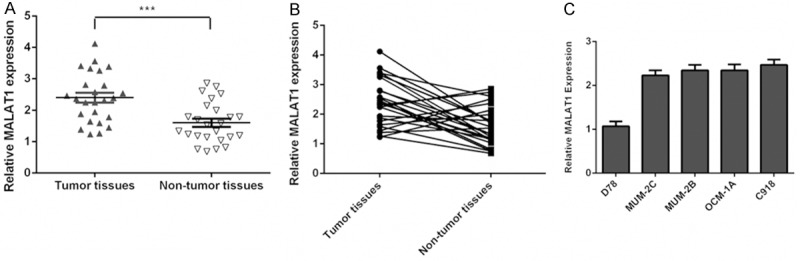
MALAT1 expression was increased in uveal melanoma tissues and cell. A. The expression of MALAT1 in the uveal melanoma tissues and normal tissues was measured using qRT-PCR. B. MALAT1 expression was upregulated in the 72% (18/25) uveal melanoma tissues compared to their paired normal tissues. C. The expression of MALAT1 in the uveal melanoma cell lines (MUM-2C, OCM-1A, MUM-2B and C918) and one melanocyte cell line (D78) was detected using qRT-PCR. ***p<0.001.
Inhibition of MALAT1 decreased uveal melanoma cell proliferation and colony formation
The expression level of MALAT1 was decreased in MUM-2C cells after transfected with siMALAT1 (Figure 2A). Inhibition of MALAT1 suppressed uveal melanoma cell proliferation (Figure 2B). In addition, siMALAT1 inhibited the mRNA expression of PCNA in MUM-2C cells (Figure 2C). PCNA was downregulated in MUM-2C cells after transfected with siMALAT1 (Figure 2D). Moreover, the mRNA expression of ki-67 was downregulated in the MUM-2C cells after transfected with siMALAT1 (Figure 2E). SiMALAT1 inhibited the protein expression of ki-67 in MUM-2C cells (Figure 2F). We also demonstrated that inhibition of MALAT1 suppressed MUM-2C cell colony formation (Figure 2G).
Figure 2.
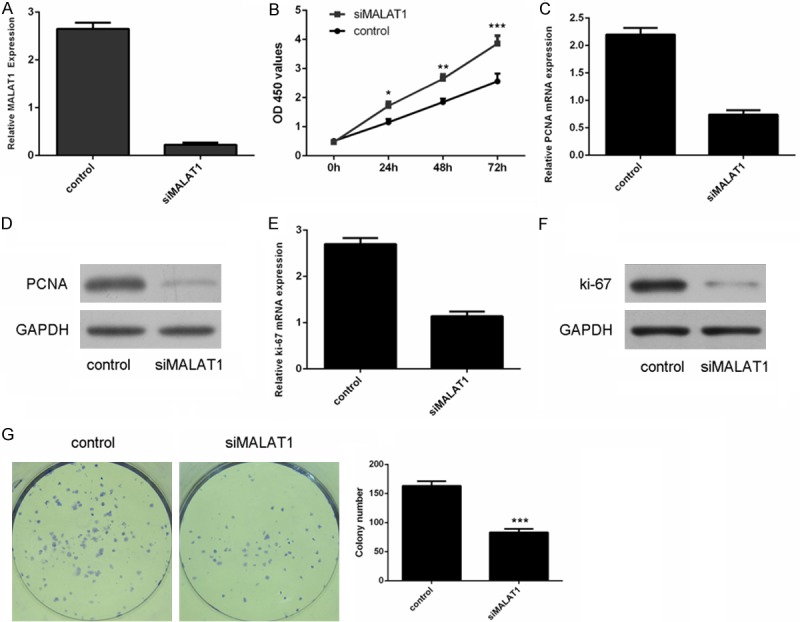
Inhibition of MALAT1 decreased the uveal melanoma cell proliferation and colony formation. A. Relative expression of MALAT1 in the MUM-2C cells after transfected with MALAT1 siRNA. B. Knockdown of MALAT1 suppressed the MUM-2C cells proliferation. C. Knockdown of MALAT1 inhibited the mRNA expression of PCNA. D. The protein expression of PCNA was detected using western blot. E. Knockdown of MALAT1 inhibited the mRNA expression of ki-67. F. The protein expression of ki-67 was detected using western blot. G. Knockdown of MALAT1 suppressed the MUM-2C cells colony formation. *p<0.05, **p<0.01, ***p<0.001.
Inhibition of MALAT1 suppressed uveal melanoma cell migration and invasion
To measure the role of MALAT1 on the invasive and migratory capability, transwell chamber and wound healing assay were done in the MUM-2C cell. We demonstrated that knockdown of MALAT1 inhibited MUM-2C cell invasion (Figure 3A). Moreover, knockdown of MALAT1 suppressed cell migration of the MUM-2C cell (Figure 3B).
Figure 3.

Knockdown of MALAT1 suppressed the uveal melanoma cell migration and invasion. A. Knockdown of MALAT1 suppressed the uveal melanoma cell invasion. B. Knockdown of MALAT1 suppressed the uveal melanoma cell migration. ***p<0.001.
MALAT1 negatively regulated miR-140 expression
We also confirmed that knockdown of MALAT1 can promote the miR-140 expression in the MUM-2C cell (Figure 4A). Moreover, inhibition of MALAT1 could suppress the expression of Slug in MUM-2C cell (Figure 4B and 4C). qRT-PCR analysis demonstrated that inhibition of MALAT1 could suppress the expression of ADAM10 in the MUM-2C cell (Figure 4D and 4E).
Figure 4.
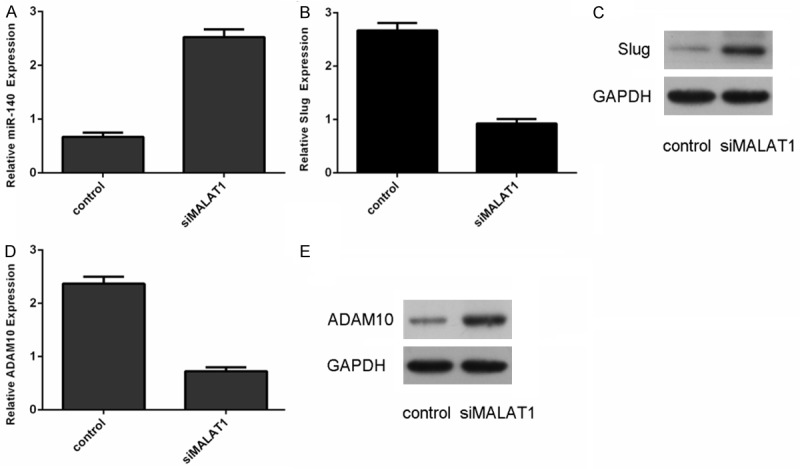
MALAT1 negatively regulated miR-140 expression. A. Knockdown of MALAT1 suppressed the miR-140 expression in the MUM-2C cells. B. Inhibition of MALAT1 inhibited the mRNA expression of Slug in the MUM-2C cells. C. Knockdown of MALAT1 suppressed the protein expression of Slug in the MUM-2C cells. D. Inhibition of MALAT1 inhibited the mRNA expression of ADAM10 in the MUM-2C cells. E. Knockdown of MALAT1 suppressed the protein expression of ADAM10 in the MUM-2C cells.
MiR-140 was downregulated in uveal melanoma tissues and cell
We found that miR-140 was downregulated in the uveal melanoma tissues compared to normal tissues (Figure 5A). Among them, miR-140 expression was upregulated in the 68% (17/25) uveal melanoma tissues compared to their paired normal tissues (Figure 5B). The expression level of miR-140 was inversely correlated with the MALAT1 expression level in uveal melanoma tissues (Figure 5C). In addition, we demonstrated that the expression of miR-140 was downregulated in uveal melanoma cell lines (MUM-2C, OCM-1A, MUM-2B and C918) compared to one melanocyte cell line (D78) (Figure 5D).
Figure 5.
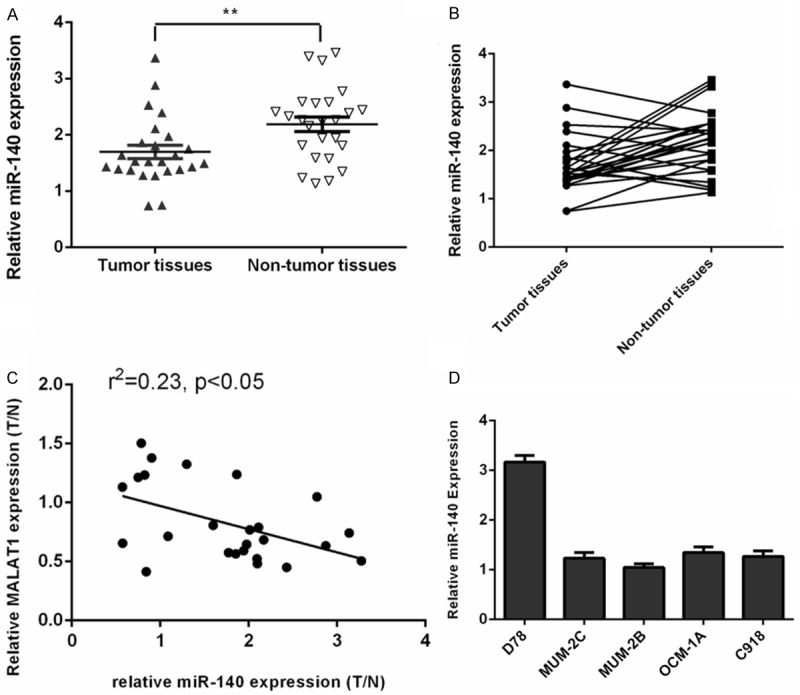
miR-140 was downregulated in uveal melanoma tissues and cell. A. The expression of miR-140 in the uveal melanoma tissues and normal tissues was measured using qRT-PCR. B. miR-140 expression was upregulated in the 68% (17/25) uveal melanoma tissues compared to their paired normal tissues. C. The expression level of miR-140 was inversely correlated with the MALAT1 expression level in uveal melanoma tissues. D. The expression of miR-140 in the uveal melanoma cell lines (MUM-2C, OCM-1A, MUM-2B and C918) and one melanocyte cell line (D78) was detected using qRT-PCR. **p<0.01.
Discussion
In this study, we demonstrated that MALAT1 was upregulated in the uveal melanoma tissues compared to normal tissues. Among them, MALAT1 expression was upregulated in the 72% (18/25) uveal melanoma tissues compared to their paired normal tissues. Knockdown of MALAT1 suppressed uveal melanoma cell proliferation, colony information, invasion and migration. Moreover, we showed that knockdown of MALAT1 promoted miR-140 expression and suppressed the Slug and ADAM10 expression in the MUM-2C cell. In addition, we demonstrated that miR-140 was downregulated in uveal melanoma tissues compared to normal tissues and cell lines. The expression level of MALAT1 was inversely correlated with the expression level of miR-140 expression in uveal melanoma tissues. These results suggested that MALAT1 played an oncogenic role in the development of uveal melanoma.
MALAT1, also named as NEAT2 (non-coding nuclearenriched abundant transcript 2), is a highly conserved lncRNA with about 8000-nt in length [14,23,28,30]. Previous studies demonstrated that MALAT1 expression was upregulated in many tumors such as bladder cancer, gastric cancer, osteosarcoma and pancreatic cancer [26,31-33]. For example, Pang et al [34]. demonstrated that MALAT1 expression was higher in the pancreatic cancer than in non-cancerous tissues and correlated with tumor size, clinical stage, distant metastasis and lymph node metastasis. Jin et al [35]. showed that MALAT1 expression was increased in the triple-negative breast cancer tissues. Knockdown of MALAT1 suppressed cell motility, proliferation, and increased cell apoptosis. Downregulation of MALAT1 promoted expression of miR-1 in breast cancer cell. Zhang et al [36]. demonstrated that MALAT1 expression was upregulated in clear cell renal cell carcinoma (ccRCC) tissues ad cells. Knockdown of MALAT1 inhibited renal cancer cell migration, proliferation and invasion. However, the role of MALAT1 still remains unknown. In this study, we demonstrated that MALAT1 was upregulated in the uveal melanoma tissues compared to normal tissues. Among them, MALAT1 expression was upregulated in the 72% (18/25) uveal melanoma tissues compared to their paired normal tissues. Knockdown of MALAT1 suppressed the uveal melanoma cell proliferation, colony information, invasion and migration. These data suggested that MALAT1 plays an oncogene LncRNA in the uveal melanoma.
Previous study revealed that knockdown of MALAT1 promoted miR-140 expression in the glioma cells [37]. In line with this data, we also showed that knockdown of MALAT1 could promote miR-140 expression in the MUM-2C cell. Increasing studies have suggested that miR-140 acts an important role in many tumors including non-small cell lung cancer, colorectal cancer, ovarian cancer and esophageal cancer [38-41]. Moreover, Li et al [41]. demonstrated that miR-140 was downregulated in esophageal cancer tissues and knockdown of miR-140 increased esophageal cancer cell invasion through targeting Slug expression. Kai et al [42]. showed that miR-140-5p could inhibit tongue squamous cell carcinoma invasion and migration by directly targeting ADAM10 expression. In our study, we revealed that miR-140 was downregulated in the uveal melanoma tissues and cell lines. The expression level of MALAT1 was inversely correlated with miR-140 expression level in uveal melanoma tissues. In addition, knockdown of MALAT1 promoted the expression of Slug and ADAM10, which were the direct target genes of miR-140. These data suggested that MALAT1 suppressed the uveal melanoma cell proliferation and invasion through modulating miR-140 expression.
In conclusion, we demonstrated that MALAT1 was upregulated in the uveal melanoma tissues and cell lines. Moreover, knockdown of MALAT1 suppressed the uveal melanoma cell proliferation, colony information, invasion and migration partly through modulating miR-140 expression.
Acknowledgements
This work was supported by the Postdoctoral scientific research developmental fund of Heilongjiang Province (No.LBH-Q14126).
References
- 1.Sun L, Wang Q, Gao X, Shi D, Mi S, Han Q. MicroRNA-454 functions as an oncogene by regulating PTEN in uveal melanoma. FEBS Lett. 2015;589:2791–2796. doi: 10.1016/j.febslet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, Bian G, Meng Z, Dang G, Shi D, Mi S. MiR-144 Inhibits Uveal Melanoma Cell Proliferation and Invasion by Regulating c-Met Expression. PLoS One. 2015;10:e0124428. doi: 10.1371/journal.pone.0124428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Li Z, Yu X, Shen J, Jiang Y. MicroRNA dysregulation in uveal melanoma: a new player enters the game. Oncotarget. 2015;6:4562–4568. doi: 10.18632/oncotarget.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato T, Han F, Yamamoto A. The biology and management of uveal melanoma. Curr Oncol Rep. 2008;10:431–438. doi: 10.1007/s11912-008-0066-z. [DOI] [PubMed] [Google Scholar]
- 5.Patel SP. Latest developments in the biology and management of uveal melanoma. Curr Oncol Rep. 2013;15:509–516. doi: 10.1007/s11912-013-0348-y. [DOI] [PubMed] [Google Scholar]
- 6.Pereira PR, Odashiro AN, Lim LA, Miyamoto C, Blanco PL, Odashiro M, Maloney S, De Souza DF, Burnier MN Jr. Current and emerging treatment options for uveal melanoma. Clin Ophthalmol. 2013;7:1669–1682. doi: 10.2147/OPTH.S28863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoenfield L. Uveal melanoma: A pathologist’s perspective and review of translational developments. Adv Anat Pathol. 2014;21:138–143. doi: 10.1097/PAP.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 8.Shildkrot Y, Thomas F, Al-Hariri A, Fry CL, Haik BG, Wilson MW. Socioeconomic factors and diagnosis of uveal melanoma in the mid-southern United States. Curr Eye Res. 2011;36:824–830. doi: 10.3109/02713683.2011.593109. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Rahman MH, Boru G, Massengill J, Salem MM, Davidorf FH. MET oncogene inhibition as a potential target of therapy for uveal melanomas. Invest Ophthalmol Vis Sci. 2010;51:3333–3339. doi: 10.1167/iovs.09-4801. [DOI] [PubMed] [Google Scholar]
- 10.Gardner FP, Serie DJ, Salomao DR, Wu KJ, Markovic SN, Pulido JS, Joseph RW. c-MET expression in primary and liver metastases in uveal melanoma. Melanoma Res. 2014;24:617–620. doi: 10.1097/CMR.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 11.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Eschelman DJ, Gonsalves CF, Sato T. Transhepatic therapies for metastatic uveal melanoma. Semin Intervent Radiol. 2013;30:39–48. doi: 10.1055/s-0033-1333652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Yu X, Shen J. Long non-coding RNAs: emerging players in osteosarcoma. Tumour Biol. 2015 doi: 10.1007/s13277-015-4749-4. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimoto R, Mayeda A, Yoshida M, Nakagawa S. MALAT1 long non-coding RNA in cancer. Biochim Biophys Acta. 2016;1859:192–9. doi: 10.1016/j.bbagrm.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Xiao H, Tang K, Liu P, Chen K, Hu J, Zeng J, Xiao W, Yu G, Yao W, Zhou H, Li H, Pan Y, Li A, Ye Z, Wang J, Xu H, Huang Q. LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200s in clear cell kidney carcinoma. Oncotarget. 2015;6:38005–38015. doi: 10.18632/oncotarget.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu X, Li Z. Long non-coding RNA HOTAIR: A novel oncogene (Review) Mol Med Rep. 2015;12:5611–5618. doi: 10.3892/mmr.2015.4161. [DOI] [PubMed] [Google Scholar]
- 17.Yu X, Li Z. Long non-coding RNA growth arrest-specific transcript 5 in tumor biology. Oncol Lett. 2015;10:1953–1958. doi: 10.3892/ol.2015.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–161. doi: 10.1093/hmg/ddq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li PF, Chen SC, Xia T, Jiang XM, Shao YF, Xiao BX, Guo JM. Non-coding RNAs and gastric cancer. World J Gastroenterol. 2014;20:5411–5419. doi: 10.3748/wjg.v20.i18.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tano K, Akimitsu N. Long non-coding RNAs in cancer progression. Front Genet. 2012;3:219. doi: 10.3389/fgene.2012.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konishi H, Ichikawa D, Yamamoto Y, Arita T, Shoda K, Hiramoto H, Hamada J, Itoh H, Fujita Y, Komatsu S, Shiozaki A, Ikoma H, Ochiai T, Otsuji E. Plasma MALAT1 Level Is Associated with Liver Damage and Predicts Development of Hepatocellular Carcinoma. Cancer Sci. 2016;107:149–54. doi: 10.1111/cas.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Bai HS, Deng Y, Fan L. High MALAT1 expression predicts a poor prognosis of cervical cancer and promotes cancer cell growth and invasion. Eur Rev Med Pharmacol Sci. 2015;19:3187–3193. [PubMed] [Google Scholar]
- 24.Qi P, Xu MD, Ni SJ, Huang D, Wei P, Tan C, Zhou XY, Du X. Low expression of LOC285194 is associated with poor prognosis in colorectal cancer. J Transl Med. 2013;11:122. doi: 10.1186/1479-5876-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Ma M, Liu W, Ding W, Yu H. Enhanced expression of long noncoding RNA CARLo-5 is associated with the development of gastric cancer. Int J Clin Exp Pathol. 2014;7:8471–8479. [PMC free article] [PubMed] [Google Scholar]
- 26.Cai X, Liu Y, Yang W, Xia Y, Yang C, Yang S, Liu X. Long noncoding RNA MALAT1 as a potential therapeutic target in osteosarcoma. J Orthop Res. 2015 doi: 10.1002/jor.23105. [DOI] [PubMed] [Google Scholar]
- 27.Tian X, Xu G. Clinical value of lncRNA MALAT1 as a prognostic marker in human cancer: systematic review and meta-analysis. BMJ Open. 2015;5:e008653. doi: 10.1136/bmjopen-2015-008653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao X, Zhao R, Chen Q, Zhao Y, Zhang B, Zhang Y, Yu J, Han G, Cao W, Li J, Chen X. MALAT1 might be a predictive marker of poor prognosis in patients who underwent radical resection of middle thoracic esophageal squamous cell carcinoma. Cancer Biomark. 2015;15:717–723. doi: 10.3233/CBM-150513. [DOI] [PubMed] [Google Scholar]
- 29.Huang MD, Chen WM, Qi FZ, Xia R, Sun M, Xu TP, Yin L, Zhang EB, De W, Shu YQ. Long non-coding RNA ANRIL is upregulated in hepatocellular carcinoma and regulates cell apoptosis by epigenetic silencing of KLF2. J Hematol Oncol. 2015;8:50. doi: 10.1186/s13045-015-0146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin C, Yan B, Lu Q, Lin Y, Ma L. The role of MALAT1/miR-1/slug axis on radioresistance in nasopharyngeal carcinoma. Tumour Biol. 2015 doi: 10.1007/s13277-015-4227-z. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, Zhang J, Huang H. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget. 2015;6:41045–55. doi: 10.18632/oncotarget.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Su L, Chen X, Li P, Cai Q, Yu B, Liu B, Wu W, Zhu Z. MALAT1 promotes cell proliferation in gastric cancer by recruiting SF2/ASF. Biomed Pharmacother. 2014;68:557–564. doi: 10.1016/j.biopha.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Fu X, Liu Y, Zhuang C, Liu L, Cai Z, Huang W. Synthetic artificial microRNAs targeting UCA1-MALAT1 or c-Myc inhibit malignant phenotypes of bladder cancer cells T24 and 5637. Mol Biosyst. 2015;11:1285–1289. doi: 10.1039/c5mb00127g. [DOI] [PubMed] [Google Scholar]
- 34.Pang EJ, Yang R, Fu XB, Liu YF. Overexpression of long non-coding RNA MALAT1 is correlated with clinical progression and unfavorable prognosis in pancreatic cancer. Tumour Biol. 2015;36:2403–2407. doi: 10.1007/s13277-014-2850-8. [DOI] [PubMed] [Google Scholar]
- 35.Jin C, Yan B, Lu Q, Lin Y, Ma L. Reciprocal regulation of Hsa-miR-1 and long noncoding RNA MALAT1 promotes triple-negative breast cancer development. Tumour Biol. 2016;37:7383–94. doi: 10.1007/s13277-015-4605-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhang HM, Yang FQ, Chen SJ, Che J, Zheng JH. Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumour Biol. 2015;36:2947–2955. doi: 10.1007/s13277-014-2925-6. [DOI] [PubMed] [Google Scholar]
- 37.Ma J, Wang P, Yao Y, Liu Y, Li Z, Liu X, Zhao X, Xi Z, Teng H, Liu J, Xue Y. Knockdown of long non-coding RNA MALAT1 increases the blood-tumor barrier permeability by up-regulating miR-140. Biochim Biophys Acta. 2015;1859:324–338. doi: 10.1016/j.bbagrm.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Dong W, Yao C, Teng X, Chai J, Yang X, Li B. MiR-140-3p suppressed cell growth and invasion by downregulating the expression of ATP8A1 in non-small cell lung cancer. Tumour Biol. 2015 doi: 10.1007/s13277-015-3452-9. [DOI] [PubMed] [Google Scholar]
- 39.Kong XM, Zhang GH, Huo YK, Zhao XH, Cao DW, Guo SF, Li AM, Zhang XR. MicroRNA-140-3p inhibits proliferation, migration and invasion of lung cancer cells by targeting ATP6AP2. Int J Clin Exp Pathol. 2015;8:12845–12852. [PMC free article] [PubMed] [Google Scholar]
- 40.Lan H, Chen W, He G, Yang S. miR-140-5p inhibits ovarian cancer growth partially by repression of PDGFRA. Biomed Pharmacother. 2015;75:117–122. doi: 10.1016/j.biopha.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Jiang G, Zhou J, Wang H, Gong Z, Zhang Z, Min K, Zhu H, Tan Y. Down-regulation of miR-140 induces EMT and promotes invasion by targeting Slug in esophageal cancer. Cell Physiol Biochem. 2014;34:1466–1476. doi: 10.1159/000366351. [DOI] [PubMed] [Google Scholar]
- 42.Kai Y, Peng W, Ling W, Jiebing H, Zhuan B. Reciprocal effects between microRNA-140-5p and ADAM10 suppress migration and invasion of human tongue cancer cells. Biochem Biophys Res Commun. 2014;448:308–314. doi: 10.1016/j.bbrc.2014.02.032. [DOI] [PubMed] [Google Scholar]


