Abstract
Cerebral ischemia is the major causes the neuronal damages throughout the world. Present investigation evaluates the neuroprotective effect of (SS) in cerebral ischemic rat. All the rats were separated in to four group such as control group, ischemia/reperfusion (I/R) group and Spatholobus suberectus (100 and 200 mg/kg, p.o.) treated group which receives extract for 15 days prior to I/R. At the end of protocol all the rats were sacrificed and brain was isolated for the biochemical estimation. Further, oxidative stress was estimated by measuring the level of malondialdehyde (MDA), nitric oxide (NO), superoxide dismutase (SOD) and glutathione peroxidase (GPX) in the brain tissue. Moreover other parameters like cytokine (IL-10 and TNF-α), nuclear factor kappa B p65 (NF-κB), caspase 3, brain ATP level and DNA damage by comet assay was estimated in the brain tissues of cerebral ischemic rats. Result of the study suggested that treatment with Spatholobus suberectus significantly (P<0.01) decreases the MDA and NO level and increases in the activity of SOD and GPX in the brain tissues of cerebral ischemic rats compared to I/R rats. Moreover, treatment with SS significantly increases the expressions of IL-10 and brain ATP and decreases the expressions of TNF-α, caspase 3 and NF-κB in the brain tissues of cerebral ischemic rats compared to I/R rats. Comet assay also postulates that SS treated rats brain shows less DNA damage than ischemic rats. Present study concludes the neuroprorective effect of Spatholobus suberectus in cerebral ischemic rats by its antioxidant, anti apoptotic and anti-inflammatory activity.
Keywords: Spatholobus suberectus, cerebral ischemia, neuroprotection, antioxidant, apoptosis
Introduction
Cerebral stroke is the major causes of death and physical disability across the world [1]. Brain is a highly perused organ which requires 25% of blood pump by heart and thus any hindrance in the flow of blood may produce cerebral ischemia and neurological deficits [2]. Reported studies suggested that in cerebral ischemia oxidative stress and inflammation induces apoptosis and there by causes neurological defects [3]. Increased oxidative stress is related to depletion of ATP, alteration in iron homeostasis, dysfunction of mitochondria, neurotoxicity and changes in intracellular calcium in brain tissue which results in neuronal damage [4]. Moreover, all these condition increases the production of reactive oxygen species (ROS) and thus damages the brain tissues through oxidative damage [5].
Drugs were used for the management of cerebral ischemia; in long term therapy causes cerebral hemorrhages and bleeding [6,7]. Thus in recent years more stress is given on use of alternative and natural medicine for the management of cerebral ischemia. There are various herbal plants that show promising effect in the management of cerebral infraction/ischemia and protect the neuronal function.
Spatholobus suberectus is a medicinal plant (Leguminosae) traditionally used in China for the management of anemia, rheumatism, abnormality in menstruation and other disorders [8-10]. Stem of Spatholobus suberectus contain several chemical consistent like eriodictyol, dihydroquercetin, butin, neoisoliquiritigenin, plathymenin, dihydrokaempferol and liquiritigenin [11]. Spatholobus suberectus stem extract reported to posses various medicinal activities such as anti-inflammatory, anti platelet, anti rheumatic and antioxidant activity.
Material and methods
Plant extract
Spatholobus suberectus was collected from the local vendor and authenticated by Institute of Medicinal Plant Development, Beijing. Stem of S. suberectus was dried and boiled in distilled water for a specific duration of time. Thereafter extract was filtered by passing it through 150 µm and lyophilized. This powder of extract was reused by dissolving it in water and centrifuged at 10000 RPM for the duration of 5 min. This solution was filter and used for the study.
Animals
Healthy male wistar rats (250-300 g) at about 8 weeks of age were used for the pharmacological screening in the present study. The animals were housed at 25±2°C temperature, 12 h light/dark cycle and 60±5% of relative humidity. Rats were feed with standard diet and water ad libitum. Protocols of the present investigation for all the animal studies were approved by the Institutional Animal Ethical Committee (39/2015).
Experimental protocol
All the rats were separated into four different groups each group carries 10 rats such as sham operated group (control), ischemia/reperfusion group which receives saline solution for 15 days (I/R group) and S. suberectus (SS) treated group which receives extract of SS at a dose of 100 and 200 mg/kg, p.o. for the duration of 15 days I/R rats. At the end of protocol all the rats were exposed to cerebral ischemia for 1 hr and thereafter for 1 hr for reperfusion [12].
Cerebral ischemia/reperfusion (I/R) induction
All the rats were anesthetized by an i.p. of chloral hydrate (360 mg/kg) and subjected them to ischemia. In which by a incision in the neck, left and right carotid arteries were exposed and ischemic condition was developed by applying clamp to carotid arteries for the duration of 1 hr. thereafter circulation was restored by declamping the arteries and reperfusion was permitted for the period of 1 hr. moreover during the period of study temperature was maintained at 37°C to avoid hypothermia [12].
Preparation of tissue homogenate
At the end of protocol all the rats were sacrificed by cervical dislocation. The brain of each rat was isolated and homogenized in both ice cold solution and phosphate buffer solution for biochemical estimation.
Estimation of oxidative stress parameters
In brain homogenate lipid peroxide level was estimated in terms of MDA level. In which absorbance was estimated at 535 and 520 nm by using UV spectrophotometer. The concentration of MDA was estimated by calculation the differences in the absorbance and plotted it on standard curve [13].
Estimation of nitric oxide (NO) was done as per the method described by Miranda et al. in which the reduction of nitrate to nitrite was estimated at 540 nm [14].
Superoxide dismutase (SOD) was estimated in the brain tissue of STZ treated AD rats by using riboflavin sensitized method. The alteration in absorbance was observed for 4 min at 460 nm [15].
Activity of glutathione peroxidase (GPX) enzyme was estimated as per the method described by Paglia and Valentine. In this assay activity of enzyme is directly proportional to decrease in the optical density at 340 nm [16].
Estimation of cytokines in tissue homogenate
Cytokines such as TNF-α and IL-10 were estimated in the tissue homogenate by using ELISA kits (RayBiotech, Inc., USA). The absorbance for the reactant solution was estimated at 450 nm [17,18].
Estimation of nuclear factor-κB p65 (NF-κB p65) in tissue homogenate
NF-κB p65 was estimated in the brain tissue homogenate by using ELISA kit. In this assay cell membranes were busted out by using freeze thaw cycle and then by centrifuging it at 5000 RPM for the period of 5 min at 2°C. Supernatant of the homogenate was separated and used it immediately for the assay. Optical density of the resultant solution was estimated with in 5 min at 450 nm [19].
Estimation of caspase 3 in tissue homogenate
Caspase 3 level was determined by using a ELISA kit. The absorbance of final solution was estimated at 450 nm by microplate reader [20].
Estimation of ATP in tissue homogenate
ATP level in the brain tissue was estimated by ELISA kit and the intensity of resultant solution was determined at 450 nm [21].
Estimation of DNA damage
Damage of DNA was estimated by Comet assay method. In this assay brain sample was crushed and suspended it with PBS and stirred for the duration of 5 min. the filtered sample of 100 µl of cell suspension was mixed with 600 µl of agarose and thereafter spreading this mixture on a slide which was pre coated with agarose. These slides were kept at 4°C to get solidify and then place it in a lysing solution for the period of 60 min. Slides were placed in electrophoresis chamber, which was filled with alkaline buffer and to allow for 20 min to unwind the DNA. Electrophorasis was allowed for the duration of 20 min at 180 mA and 25 V. Slides were washed with 0.4 M Tris HCl buffer. Thereafter staining was done by adding ethidium bromide solution on each slide and pattern of DNA fragment for each sample was observed through fluorescence microscope. The Comet 5 image software (Kinetic Imaging Ltd., UK) was used for the estimation of cellular DNA damage [22].
Statistical analysis
All the values of these experiments were articulated as mean ± SEM and the data was statistically analyzed by one-way ANOVA and thereafter applied to Dunnett post hoc test. P<0.05 was considered statistically significant.
Result
Effect of S. suberectus on parameters of oxidative stress
Effect of aqueous extract of stem of S. suberectus on oxidative stress parameters was shown in Table 1. It was observed that level of MDA and NO significantly increases up to 7.31 and 9.82 µM/ 100 mg of wet tissue respectively in the brain tissues of I/R group rats compared to control group rats. Whereas, the level of SOD and GPX found to be decreased upto 0.86 and 79.1 U/ 100 mg of wet tissue respectively compared to control group of rats. Treatment with S. suberectus significantly decreases (P<0.01) the MDA and NO level in the brain tissues of ischemic rats compared to I/R group. Moreover activity of SOD and GPX increases significantly (P<0.01) compared to I/R group.
Table 1.
Effect of S. suberectus on parameters of oxidative stress in the brain tissue of ischemic rats
| Sr. No. | Group | MDA (µM/100 mg of wet tissue) | NO (µM/100 mg of wet tissue) | SOD (U/100 mg of wet tissue) | GPX (U/100 mg of wet tissue) |
|---|---|---|---|---|---|
| 1. | Control | 2.49±0.26 | 9.82±0.85 | 2.71±0.27 | 179.4±12.52 |
| 2. | I/R group | 7.31±0.61@ | 18.6±1.15@ | 0.86±0.09@ | 79.1±8.31@ |
| 3. | I/R + SS (100 mg/kg) | 5.27±0.38* | 15.9±0.91* | 1.59±0.11** | 98.8±7.75** |
| 4. | I/R + SS (200 mg/kg) | 3.75±0.31** | 13.5±0.73** | 2.15±0.18** | 126.9±9.41** |
Result of the study expressed as means ± SD (n=10);
P<0.01 than control;
P<0.05 than I/R group;
P<0.01 than I/R group.
Effect of S. suberectus on cytokines
As shown in Figure 1. Cerebral ischemia significantly decreases the level of IL-10 and increases the TNF-α in the brain tissues of I/R group compared to control group. There were significant increase in the level of IL 10 and decrease in TNF-α in the brain tissue of SS treated group compared to I/R group of rats.
Figure 1.
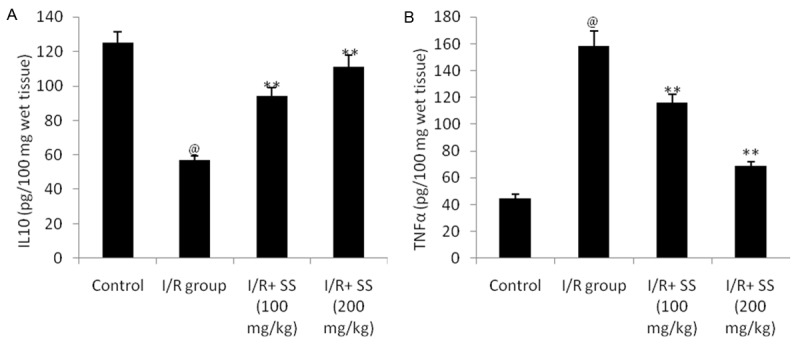
Effect of S. suberectus on the level of cytokines in the brain tissue of ischemic rats. Result of the study expressed as means ± SD (n=10), @P<0.01 than control; **P<0.01 than I/R group.
Effect of S. suberectus on the level of NF-κB p65
Effect of S. suberectus extract on the level of NF-κB p65 in the brain tissue of cerebral ischemic rats was shown in Figure 2. The result suggested that treatment with S. suberectus extract significantly decreases the NF-κB p65 level in the brain tissue of cerebral ischemia rats compared to I/R group of rats. This decrease in the NF-κB p65 level with with S. suberectus extract treatment was found to be in a dose dependent manner.
Figure 2.
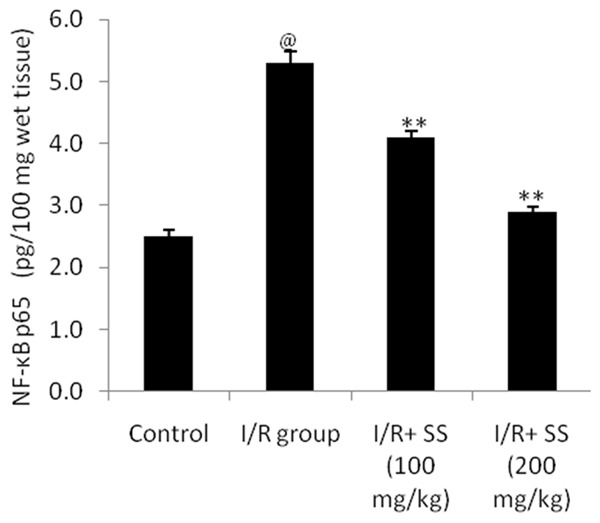
Effect of S. suberectus on the level of NF-κB p65 in the brain tissue of ischemic rats. Result of the study expressed as means ± SD (n=10), @P<0.01 than control; **P<0.01 than I/R group.
Effect of S. suberectus on the level of Caspase-3
Estimation of caspase 3 level in the brain tissue of ischemic rat was illustrated in Figure 3. There were significant (P<0.01) decreases in the caspase 3 in S. suberectus extract treated cerebral ischemic rats compared to I/R group.
Figure 3.
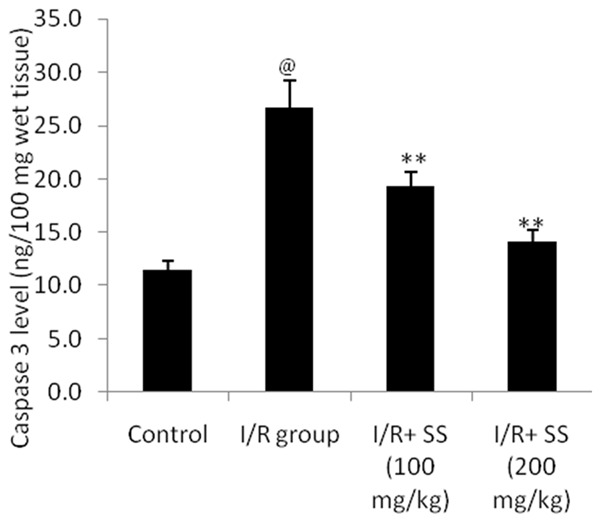
Effect of S. suberectus on the level of caspase 3 in the brain tissue of ischemic rats. Result of the study expressed as means ± SD (n=10), @P<0.01 than control; **P<0.01 than I/R group.
Effect of S. suberectus on the level of ATP
Effect of aqueous extract of S. suberectus on the level of ATP in cerebral ischemic rat was shown in Figure 4. It was observed that the level of ATP in the brain tissue of S. suberectus treated cerebral ischemic rats significantly increases (P<0.01) than I/R group of rats. This decrease in the ATP level was found to be in a dose dependent manner.
Figure 4.
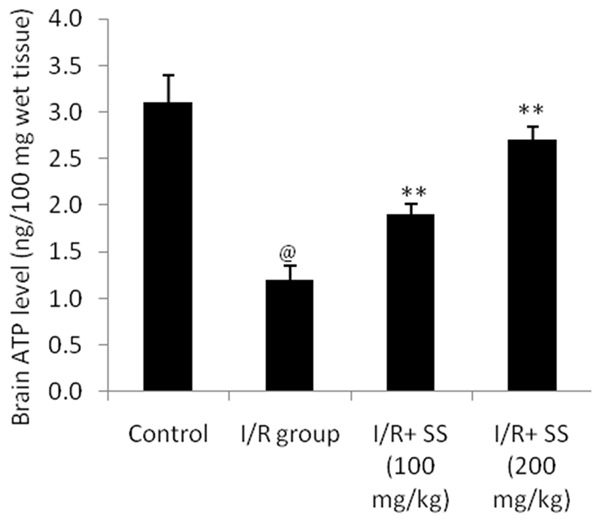
Effect of S. suberectus on the level of ATP in the brain tissue of ischemic rats. Result of the study expressed as means ± SD (n=10), @P<0.01 than control; **P<0.01 than I/R group.
Effect of S. suberectus on the extent of DNA damage
Figure 5 shows that the effect of S. suberectus on DNA damage estimated by comet assay in the brain tissues of ischemic rats. Extent of DNA damage was estimated by determining % of tailed cell, % of untailed cell, length of tail, DNA tail and tail moment of extracted cellular DNA. It was observed that treatment with S. suberectus significantly decreases the % of tailed cell and increases the % untailed cell in cerebral ischemic rats compared to I/R group. Moreover, parameters like tail length, DNA tailing and tail moment were found to be significantly (P<0.01) decreases in S. suberectus treated group compared to I/R treated group of rats.
Figure 5.
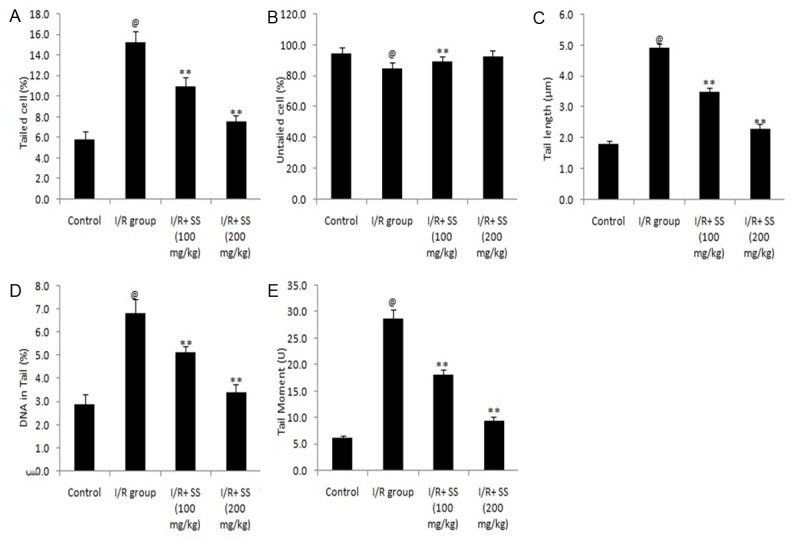
Effect of S. suberectus on DNA damage estimated by comet assay in the brain tissue of ischemic rats. Result of the study expressed as means ± SD (n=10), @P<0.01 than control; **P<0.01 than I/R group.
Discussion
Cerebral ischemia causes the degeneration of neuronal cells as it generates oxidative stress and increases the production of cytokines [23]. Naturally body produces few antioxidant molecules that detoxified the free radicals in normal condition and this harmony get disturb in pathological condition like cerebral ischemia. Here present investigation evaluates the neurprotective effect of Spatholobus suberectus in cerebral ischemia by its strong antioxidant and anti-inflammatory property.
Literature suggested that ischemia increases free radical and reactive oxygen species which exceeded to the production of antioxidant and thus overproduced oxidative species damages nucleic acid and protein [24]. All this result in the DNA fragmentation, damages the cell membrane and its functions. The stem of Spatholobus suberectus contain various natural compounds such as dihydroquercetin, butin, neoisoliquiritigenin, plathymenin that posses strong antioxidant property [8]. Result of the study suggested that treatment with aqueous extract of stem of Spatholobus suberectus significantly decreases (P<0.05, P<0.01) the MDA and NO level and increases in the activity of SOD and GPX in the brain tissues of cerebral ischemic rats compared I/R group.
Moreover, level of NF-κB and cytokines (IL-10 and TNF-α) was estimated in the brain tissues of cerebral ischemic rats. Reported studies suggested that oxidative stress plays role in the activation of NF-κB and thereby enhances the expressions of pro inflammatory cytokines such as IL-10 and TNF-α [25]. These cytokine contribute in the injury in cerebral ischemia by activating the cellular adhesion molecule. These activated molecules increases the infiltration of leukocyte and cause increase in inflammation and oxidative stress, which enhances the cerebral damage. The drugs that blocks the activity of TNF-α significantly decreases the infract volume and brain injury in cerebral ischemia [26]. Results of our study suggested that there were significant (P<0.01) decrease in the expressions of TNF-α and increase in IL-10 in Spatholobus suberectus treated group of cerebral ischemic rats compared to I/R group.
Cerebral ischemia results in neuronal damage by activating the apoptosis mechanism and caspase 3 among the caspases plays vital role in the apoptosis [27]. Caspase 3 activation results in cleavage of actin and thereby losses control over the DNase activity. It also breaks the proteins that are important for cell stability and DNA repair and thus causes apoptosis [28]. It was observed that Spatholobus suberectus effectively inhibits the expressions of caspase 3 in cerebral ischemic rats in a dose dependent manner. Moreover, effect of Spatholobus suberectus on brain ATP was also observed in this study. Mitochondria, power house of cell produces ATP for the energy and any disturbance in the cellular blood flow impairs the oxidative phosphorylation and thus causes decrease in mitochondrial respiration and ATP production [10]. This decreased production of ATP enhances the apoptosis and neuronal damage in cerebral ischemia. Our study result suggested that treatment with Spatholobus suberectus significantly increases the brain ATP level and thus prevent the DNA damage in brain tissue of cerebral ischemic rats.
Conclusion
Present study concludes the neuroprorective effect of Spatholobus suberectus in cerebral ischemic rats by its antioxidant, anti apoptotic and anti-inflammatory activity.
Disclosure of conflict of interest
None.
References
- 1.Moskowitz MA, Lo EH, Ladecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–98. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17:197–218. doi: 10.1016/j.pathophys.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Yousuf S, Atif F, Ahmad M, Hoda N, Ishrat T, Khan B, Islam F. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 2009;1250:242–53. doi: 10.1016/j.brainres.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 4.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 5.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 7.Diener HC, Bogousslavsky J, Brass LM. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischemic attack in high-risk patients (MATCH): randomized, double-blind, placebo controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee MH, Lin YP, Hsu FL, Zhan GR, Yen KY. Bioactive constituents of Spatholobus suberectus in regulating tyrosinase-related proteins and mRNA in HEMn cells. Phytochemistry. 2006;67:1262–70. doi: 10.1016/j.phytochem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Lam TL, Lam ML, Au TK, Ip DT, Ng TB, Fong WP, Wan DC. A comparison of human immunodeficiency virus type-1 protease inhibition activities by the aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2000;67:2889–2896. doi: 10.1016/s0024-3205(00)00864-x. [DOI] [PubMed] [Google Scholar]
- 10.Li RW, David Lin G, Myers SP, Leach DN. Antiinflammatory activity of Chinese medicinal vine plants. J Ethnopharmacol. 2003;85:61–67. doi: 10.1016/s0378-8741(02)00339-2. [DOI] [PubMed] [Google Scholar]
- 11.Yen KY. The illustrated Chinese materia medica. In: Yen KY, editor. The Illustrated Chinese Materia Medica. Taipei: SMC Publishing Inc.; 1992. [Google Scholar]
- 12.Seif-el-Nasr M, Fahim AT. Antioxidant effect of N omega-nitro-L-arginine methyl ester (LNAME) on global cerebral ischemia in a rat model. Arzneimittelforschung. 2001;51:628–32. doi: 10.1055/s-0031-1300092. [DOI] [PubMed] [Google Scholar]
- 13.Mihara M, Uchiyama M. Determination ofmalonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 14.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 15.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 16.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 17.Bonavida B. Immunomodulatory effect of tumor necrosis factor. Biotherapy. 1991;3:127–33. doi: 10.1007/BF02172085. [DOI] [PubMed] [Google Scholar]
- 18.Braun J, Yin Z, Spiller I, Siegert S, Rudwaleit M, Liu L, Radbruch A, Sieper J. Low secretion of tumor necrosis factor alpha, but no other Th1 or Th2 cytokines, by peripheral blood mononuclear cells correlates with chronicity in reactive arthritis. Arthritis Rheum. 1999;42:2039–44. doi: 10.1002/1529-0131(199910)42:10<2039::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Bell SA, Page S, Baumgartner B, Berking C, Haas M, Eisele T, Neumeier D, Brand K. Involvement of nuclear factor-kappaB in a murine model for the acute form of autoimmune-like toxic oil syndrome. Toxicol Appl Pharmacol. 1999;157:213–21. doi: 10.1006/taap.1999.8684. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–4. [PubMed] [Google Scholar]
- 21.Cailla H, Le Borgne De Kaouel C, Roux D, Delaage M, Marti J. Monoclonal antibodies to 5’- triphospho-(2’-5’)adenyladenosine oligonucleotides. Proc Natl Acad Sci U S A. 1982;79:4742–6. doi: 10.1073/pnas.79.15.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 23.Tang NY, Liu CH, Hsieh CT, Hsieh CL. The anti-inflammatory effect of paeoniflorin on cerebral infarction induced by ischemia-reperfusion injury in Sprague-Dawley rats. Am J Chin Med. 2010;38:51–64. doi: 10.1142/S0192415X10007786. [DOI] [PubMed] [Google Scholar]
- 24.Li YH, Jiang B, Zhang T, MuW M, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–50. [Google Scholar]
- 25.Lee MH, Lin YP, Hsu FL, Zhan GR, Yen KY. Bioactive constituents of Spatholobus suberectus in regulating tyrosinase-related proteins and mRNA in HEMn cells. Phytochemistry. 2006;67:1262–70. doi: 10.1016/j.phytochem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Li Z, Zhang X, Wang S, Zhu C, Miao J, et al. Protective effect of shikonin in experimental ischemic stroke: attenuated TLR4, p-p38-MAPK, NF-κB, TNF-α and MMP-9 expression, up-regulated claudin-5 expression, ameliorated BBB permeability. Neurochem Res. 2014;39:97–106. doi: 10.1007/s11064-013-1194-x. [DOI] [PubMed] [Google Scholar]
- 27.Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Han B, Ma X, Qi S. The effects of propofol on hippocampal caspase-3 and Bcl-2 expression following forebrain ischemia-reperfusion in rats. Brain Res. 2010;1356:11–23. doi: 10.1016/j.brainres.2010.08.012. [DOI] [PubMed] [Google Scholar]


