Abstract
Glioblastoma is a highly malignant cancer of glioma cells. Present study investigates the anti proliferative activity of granatin B on glioma cell by inducing apoptosis. In this study Glioma cell (U87) was used on which anti proliferative activity of granatin B (0, 20, 40 & 80 µM) assessed by 3-(4, 5-dimethylthylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay. Thereafter Apoptosis of glioma cell was assessed by apoptosis detection kit suing flow cytometer, DAPI staining and by estimating the activity of caspase 3 & 9 using caspase 3 & 9 kit. Expression of MMP9 protein was determined through gelatin zymography. Possible mechanism of apoptosis induction was proved by estimating the effect of granatin B with MMP9 agonist on cell proliferation, caspase 3 activity & MMP9 expression on glioblastoma cell. Result of the study suggested that granatin B significantly decreases the cell proliferation of glioma cell compared to 0 µM treated group. It was also observed that treatment with granatin B significantly induces apoptosis and increases the activity of caspase 3 & 9 protein compared to 0 µM treated group. Expression of MMP9 protein was also decreases with granatin B treatment of glima cell. MMP9 agonist significantly reverses the effect of granatin B on cell proliferation, caspase 3 and expression of MMP9 protein in glima cell. Present study concludes the anticancer activity of granatin B on glioblastoma cell by inducing apoptosis.
Keywords: Granatin B, glioblastoma, apoptosis, caspase 3 & 9
Introduction
Cancer of glioma cell is the commonest type of brain tumor having high death rate in adult which commonly known as glioblastoma [1]. It is an aggressive type of cancer which includes characteristic symptoms such as headache, changes in mood and behavior, and other symptoms resembles to the strokes. Currently available therapy for the management of glioblastoma includes adjuent chemotherapy and radiotherapy after surgical resection. Still it was observed that life expectancy is not more than 5 years after the diagnosis of glioblastoma [2]. Moreover, the median survival of patient diagnosed with glioblastoma is just for 14 months [3]. Thus, an extensive research is going on for the development of effective therapy to control glioblasmota progression.
Reported literature suggested that Punica granatum having antimicrobial, antioxidant, anti inflammatory and cancer [4-6]. Granatin B is naturally isolated ellagitannin form Punica granatum [7]. Granatin B is a carbonic anhydrase inhibitor as per the previous reports [8]. Thus on the basis these medicinal properties this study evaluates the anticancer activity of granatin B on glioma cells.
Material and methods
Cell and culture media
Glioblastoma (U87) cell lines were procured from Shanghai Institutes for Biological Sciences, Shanghai, China. In a 5% CO2 humidified incubator at 37°C cells lines were incubated. DMEM medium was used with (10% v/v) fetal bovine serum as a culture medium for the present study. Medium used in this study was replaced at a specific interval of time and cell were trypsinized routenly till it grows up to 80-90% of confluency. Granatin B was procured from Chromadex Inc. (Santa Ana, CA).
Estimation of cell viability by MTT assay
MTT assay was used to asses cellular viability. In which cell (5×103 cells/well) were placed at 96 well plate and treated with granatin B at a different doses for the period of the duration of 3 days. MTT assay was performed as described by Xiao et al. MTT (50 µl) was added to the well plate and keep it for incubation for 4 hr. at 37°C. Thereafter from the reaction mixture supernatant was washed out and subsequently dimethylsulfoxide (200 µl) was added to all the well plate at room temperature. The absorbance was estimated at 570 nm wavelength [9].
Estimation of apoptosis
Apoptosis of cell was assessed by apoptosis assay in which U87 cancerous cells (1×106 cell/well) placed for 1 day in 6 well plates. Cell plates were treated with granatin B at different concentrations such as 20, 40 & 80 µM for the period of the duration of 2 days. Apoptosis of cells were estimated as per the instruction given by manufacturer (BD biosciences) by using apoptosis detection kit. In all the well plate Annexin V-FITC of 5 µl was added and kept it for 10 min at 37°C for incubation and thereafter propidium iodide (PI) at a volume of 5 µl was added to it. Flowcytometer was used for the estimation of cell apoptosis [10].
DAPI staining assay
Fluorescence microscope (Olympus Corp., Japan) was used for the determination of apoptotic cell in which U87 cancerous cells (1×106 cell/well) placed for 1 day in 6 well plates. Cell plates were treated with granatin B at different concentrations such as 20, 40 & 80 µM for the period of the duration of 2 days. All the cells were stained by using DAPI stain (1 µg/ml) [9].
Caspase-3 and caspase-9 activation assay
U87 cancerous cells (1×106 cell/well) placed for 1 day in 6 well plates. Cell plates were treated with granatin B at different concentrations such as 20, 40 & 80 µM for the period of the duration of 2 days. Activity of caspase 3 & 9 was estimated by as per the instructions of caspase 3 & 9 kit (Tiangen Biotech). All the glioma cells were incubated for 30 min by keeping it with cell lysis buffer. BCA protein reagent was used for the estimation of concentration of protein. Estimation of caspase 3 & 9 was achived using microplate spectrophotometer (Bio Rad lab., CA, USA) at a wavelength of 405 nm [9].
Real time PCR
U87 cancerous cells (1×106 cell/well) placed for 1 day in 6 well plates. Cell plates were treated with granatin B at different concentrations such as 20, 40 & 80 µM for the period of the duration of 2 days. RT PCR was used by assessment of expression of MMP9 of the cell. Extraction of total RNA was achieved from cell and thereafter expression of MMP 9 protein was estimated through a sequence detection system ABI 7300 HT (Applied Biosystems, Foster, USA).
Estimation of MMP 9 protein expression by zymography
U87 cancerous cells (1×106 cell/well) placed for 1 day in 6 well plates. Cell plates were treated with granatin B at different concentrations such as 20, 40 & 80 µM for the period of the duration of 2 days. Supernatant of culture media (20 µl) was added at an equal amount with buffer (sodium dodecylsulfate). Gel electrophoresis was used for this study in which after electrophoresis get was incubated at 37°C for the duration of 12 hr. thereafter gel was stained with Coomassie brilliant blue R-250.
Result
Evaluation of cell proliferation
Effect of granatin B on the glioma cells proliferation by MTT assay as shown in Figure 1. It was observed that granatin B at different dosage inhibits the glioma cells proliferation and this inhibition of proliferation was achieved in a dose and time dependent manner. There was significant decrease in the cell proliferation in granatin B (40 and 80 μM) after 2 and 3rd day of treatment compared to 0 μM treated group.
Figure 1.
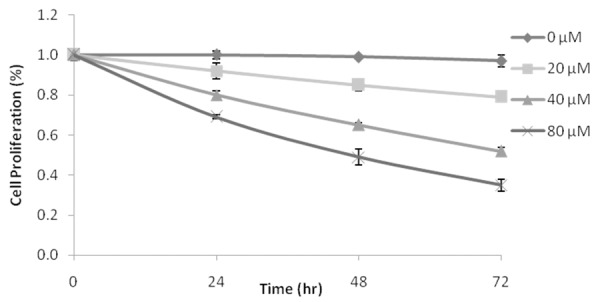
Effect of granatin B on the proliferation of glioma cells by MTT assay. **P<0.01 Vs granatin B 0 μM (n=3).
Figure 2 shows the effect of granatin B on apoptosis induction of glioma cancerous cells by Annexin V-FITC detection kit and through DAPI staining. It was observed that treatment with granatin B at 20, 40 & 80 μM significantly (P<0.01) induces the apoptosis of glioblastoma cells compared to 0 μM treated groups as shown in Figure 2A and 2B. Moreover, DAPI staining reveal the effect granatin B on cell apoptosis, as it significantly increases the apoptosis in a dose dependent manner compare to 0 μM treated group as showin in Figure 2C.
Figure 2.
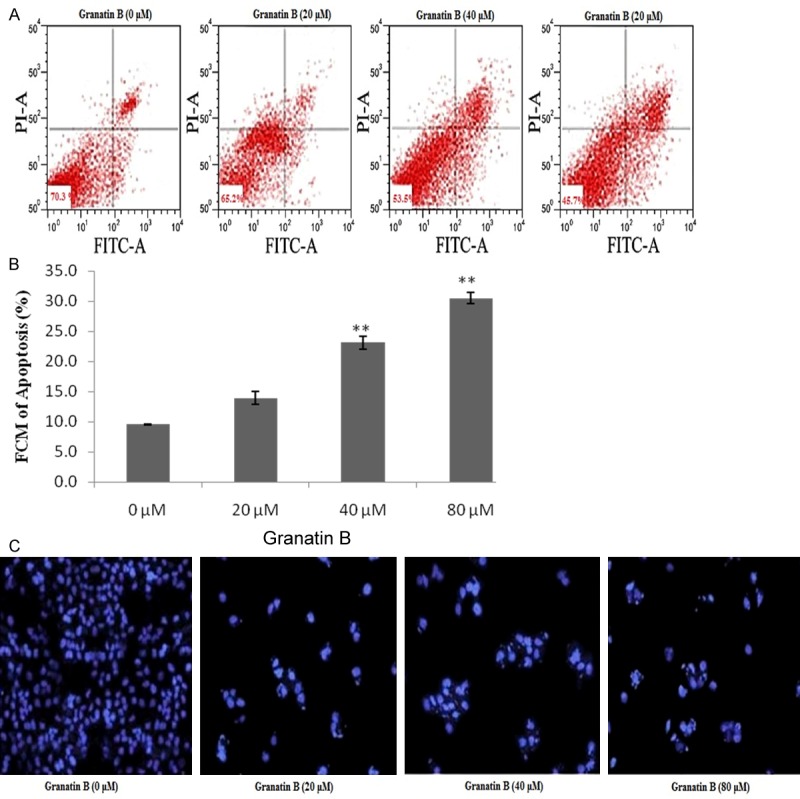
Effect of granatin B on apoptosis induction of glioma cells (U87). A. Apoptosis of glioma cell analyzed by Flow cytometric. B. Apoptosis level of glioma cells. C. Gliomacell apoptosis by DAPI staining. **P<0.01 Vs granatin B 0 μM.
Evaluation of activity of caspase 3 & 9
Activity of caspase 3 & 9 were estimated by using caspase 3 & 9 assay. It was observed that the activity of caspase 3 & 9 significantly (P<0.05, P<0.01) induced in the granatin B at a dose of 20, 40 & 80 μM treated group compared to 0 μM treated group. Activity of caspase 3 & 9 found to be increases in a dose dependent manner as shown in Figure 3A and 3B.
Figure 3.
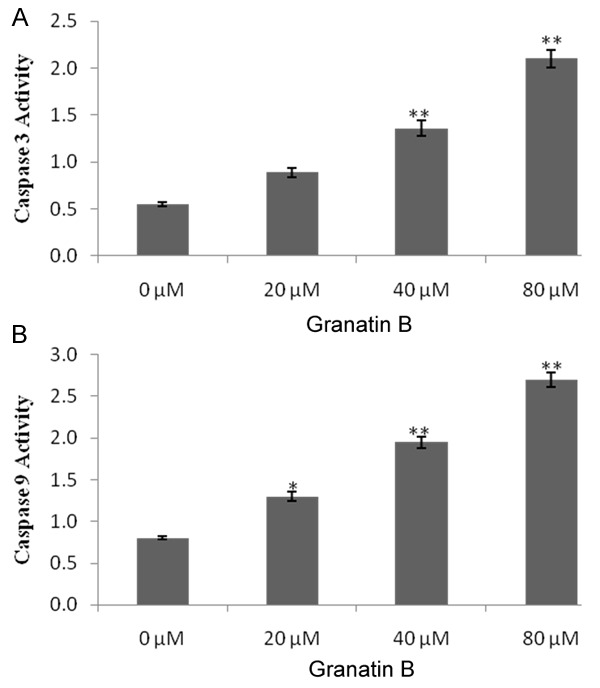
Effect of granatin B on activation of caspase 3 & 9 in glioma cancerous cells. A. Activity of caspase 3. B. Activity of caspase 9. *P<0.05, **P<0.01 Vs granatin B 0 μM.
Effect Of granatin B on MMP 9 protein expression
Expression of MMP9 protein was estimated by zymographic analysis in the given study. Granatin B treated group (20, 40 & 80 μM) shows the significant decreased (P<0.05, P<0.01) in the protein expression of MMP9 compared to 0 μM as shown in Figure 4. It was observed that this decreased in the protein expressions of MMP9 in dose dependent manner.
Figure 4.
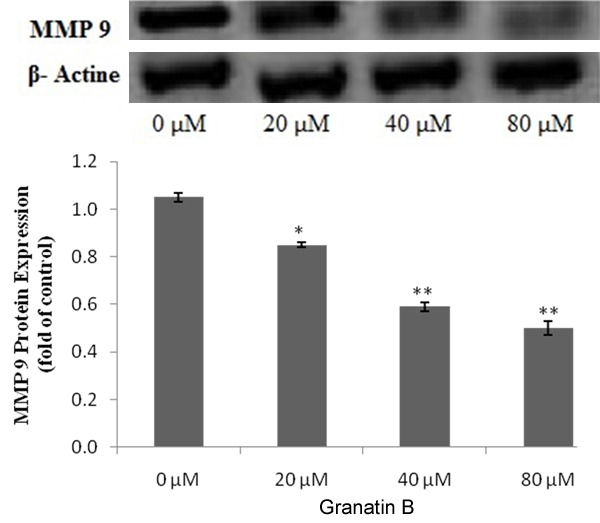
Effect of Granatin B on protein expression of MMP9 in glioma cancerous cell. *P<0.05, **P<0.01 Vs granatin B 0 μM.
Effect of granatin B and MMP 9 agonist on the protein expression of MMP 9
MMP 9 agonist reverses the effect of granatin B on caspase activity, MMP9 proteins expression & cell proliferation in glioma cells as shown in Figure 5. It was observed that caspase 3 activity significantly reversed in GRN+ agonist treated group compared GRN (40 µM) treated groups (Figure 5A). Protein expressions of MMP9 and cell proliferation were significantly decreases (P<0.01) in GRN (40 µM) treated group compared to control group. Whereas GRN (40 µM) co administered with MMP9 agonist significantly reverse the decreased activity of Protein expressions of MMP9 and cell proliferation of glioma cells (Figure 5B and 5C).
Figure 5.
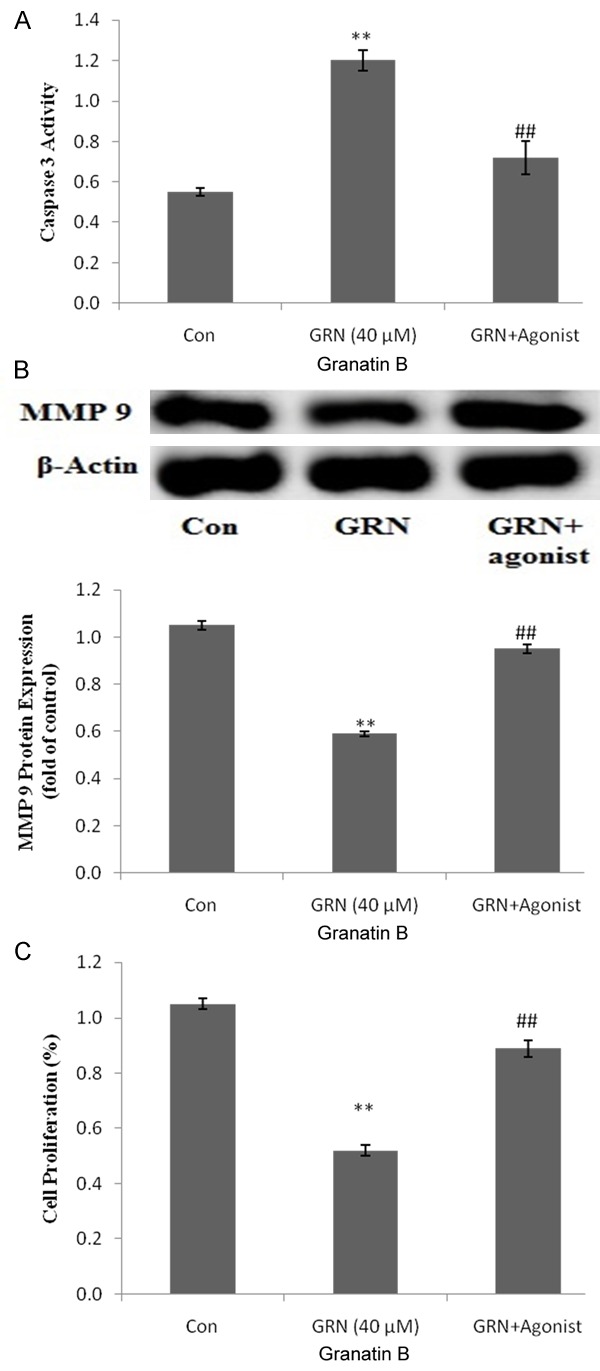
Effect of MMP 9 agonist on the activity of Granatin B in glioma cancerous cell. A. Caspase activity 3. B. Protein expression of MMP9. C. Cell proliferation. **P<0.01 Vs Control, #P<0.01 Vs GRN (40 µM).
Discussion
Glioblastoma is an aggressive type of neuronal cancer and its uncontrolled proliferation limits clinical treatment to it [11]. This study investigates the anticancer activity of granatin B on glioblastoma cell lines. Granatin B has a strong carbonic anhydrase activity and the herbs contain this ellagitannin posses anti inflammatory and antioxidant activity [5,6,8]. Previously reported study suggested that activity of carbonic anhydrase inhibitors found to inhibit the glioblastoma cancer by decreasing apoptosis [12]. It was found that granatin B significantly decreases (P<0.01) the cell proliferation and induces the apoptosis of glioma cells. granatin B reduces the glioma cell proliferation by inducing apoptosis and thereby it posses anti cancer activity.
Caspase 3 & 9 is the member of cysteine-aspartic acid protease family which activates the process apoptosis [13]. It induces the intrinsic mitochondrial pathway and thereby induces the process of apoptosis [14]. In the given investigation granatin B significantly increases the activity of caspase 3 & 9.
MMP 2 and 9 are the types of metalloproteinase enzyme and it becomes a topic of interest for the research. Several studies suggested that MMP 2 & 9 inhibitor induces apoptosis of cancerous cells [15]. It was also observed that expression of MMP 9 protein significantly decreases with the granatin B treatment which was also reversed when co administered with MMP9 agonist. Hence it confirms that granatin B induces apoptosis by inhibiting expression of MMP 9 protein in glioma cell. Present study concludes the anticancer activity of granatin B in glioblastoma cell by inducing apoptosis. Study also suggested that it induces apoptosis by inhibiting the expression of MMP9 protein specifically.
Acknowledgements
The authors are thankful to the Jining No. 1 People’s Hospital, China for the financial support of the present research.
Disclosure of conflict of interest
None.
Authors’ contribution
Shao-Ping Wang, and Yan-Jun Zhong design and perform the given research work. Mei Yang and Jian Xue contributed in the interpretation of result.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Al-Zoreky NS. Antimicrobial activity of pomegranate (Punica granatum L. ) fruit peels. Int J Food Microbiol. 2009;134:244–248. doi: 10.1016/j.ijfoodmicro.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Chidambara Murthy KN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50:4791–5. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- 6.Lee CJ, Chen LG, Liang WL, Wang CC. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo . Food Chem. 2010;118:315–322. [Google Scholar]
- 7.Steinmetz WE. NMR assignment and characterization of proton exchange of the ellagitannin granatin B. Magn Reson Chem. 2010;48:565–570. doi: 10.1002/mrc.2615. [DOI] [PubMed] [Google Scholar]
- 8.Satomi H, Umemura K, Ueno A, Hatano T, Okuda T, Noro T. Carbonic anhydrase inhibitors from the pericarps of Punica granatum L. Biol Pharm Bull. 1993;16:787–790. doi: 10.1248/bpb.16.787. [DOI] [PubMed] [Google Scholar]
- 9.Xiao J, Liu L, Zhong Z, Xiao C, Zhang J. Mangiferin regulates proliferation and apoptosis in glioma cells by induction of microRNA-15b and inhibition of MMP-9 expression. Oncol Rep. 2015;33:2815–2820. doi: 10.3892/or.2015.3919. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, Pan Y, Ma H, Li W. Simvastatin inhibits proliferation and induces apoptosis in human lung cancer cells. Oncol Res. 2013;20:351–7. doi: 10.3727/096504013X13657689382897. [DOI] [PubMed] [Google Scholar]
- 11.Ma H, Zhang Y, Wang H, Han C, Lei R, Zhang L, Yang Z, Rao L, Qing H, Xiang J. Effect and mechanism of mitomycin C combined with recombinant adeno-associated virus type II against glioma. Int J Mol Sci. 2014;15:1–14. doi: 10.3390/ijms15010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cianchi F, Vinci MC, Supuran CT, Peruzzi B, De Giuli P, Fasolis G, Perigli G, Pastorekova S, Papucci L, Pini A, Masini E, Puccetti L. Selective inhibition of carbonic anhydrase IX decreases cell proliferation and induces ceramide-mediated apoptosis in human cancer cells. J Pharmacol Exp Ther. 2010;334:710–9. doi: 10.1124/jpet.110.167270. [DOI] [PubMed] [Google Scholar]
- 13.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 14.Harrington HA, Ho KL, Ghosh S, Tung KC. Construction and analysis of a modular model of caspase activation in apoptosis. Theor Biol Med Model. 2008;5:26. doi: 10.1186/1742-4682-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyormoi O, Mills L, Bar-Eli M. An MMP-2/MMP-9 inhibitor, 5a, enhances apoptosis induced by ligands of the TNF receptor superfamily in cancer cells. Cell Death Differ. 2003;1:558–69. doi: 10.1038/sj.cdd.4401209. [DOI] [PubMed] [Google Scholar]


