Abstract
Myocardial infarction could result in high morbidity and mortality and heart diseases of children have becoming prevalent. Functions of spermine administration on cardiomyocytes remain unknown. The present study was designed to investigate the role of spermine pretreatment on myocardial ischemia/reperfusion injury (IRI). A cell model of simulated ischemia/reperfusion injury was established by incubating neonatal Sprague-Dawley rat cardiomyocytes in ischemia medium and re-cultured in normal medium. Of note, spermine pretreatment significantly reduced apoptosis and increased viability of immature cardiomyocytes. Spermine pretreatment enhanced autophagic flux as determined by confocal microscopy and transmission electron microscopy. Furthermore, proteins of mammalian target of rapamycin (mTOR) pathway were significantly reduced in response to spermine pretreatment during IRI, while proteins related to autophagy were up-regulated. The cell viability was enhanced and apoptosis decreased by rapamycin after spermine pretreatment, while these were reversed by 3-methyladenine. However, when immature cardiomyocytes were pretreated with rapamycin or 3-methyladenine, followed by IRI and spermine administration, no significant changes of viability and apoptosis were observed. In conclusion, this study suggests that spermine is a potential novel approach for preventing IRI, especially in children.
Keywords: Spermine, ischemia/reperfusion injury, autophagy
Introduction
Myocardial infarction (MI) caused by coronary artery occlusion has become a leading cause of morbidity and mortality worldwide in humans [1]. The major risk factors of heart diseases include high-fat diet, diabetes, smoking, high blood pressure and genetic vulnerability of individual [2,3]. Timely myocardial reperfusion using either thrombolytic therapy or primary percutaneous coronary intervention can effectively limit myocardial infarct size, preserve left-ventricular systolic function and reduce the onset of heart failure [4]. However, myocardial reperfusion can lead to the production of reactive oxygen intermediates and calcium overload, resulting in irreversible injury such as cardiomyocyte death and heart dysfunction, which is known as ischemia/reperfusion injury (IRI) [5]. Of note, congenital heart disease (CHD) is the most prevalent birth defect and a major cause of children’s deaths, which leads to the longest hospital stays, highest mortality rates, and greatest average hospital charges in children [6]. Although many therapies have significantly reduced the mortality of heart disease of children, new effective therapies remain to be investigated.
Polyamines, such as putrescine, spermidine and spermine, are aliphatic cations that interact with nucleic acids and proteins, which function as modulators for cell growth, cell differentiation, and synthesis of DNA, RNA and proteins [7]. Cellular levels of polyamines are tightly controlled via synthesis, metabolism, and transport. Polyamines are catabolized by back-conversion through acetylation and oxidation was mediated by spermidine/spermine N1-acetyltransferase (SSAT) and N1-acetylpolyamine oxidase (APAO), respectively, or oxidation of spermine by spermine oxidase (SMO) [8]. Once the metabolism of polyamines is disrupted, many cellular processes are affected, including transcription, translation, gene expression regulation, autophagy and stress resistance [9].
Recent studies have demonstrated that polyamines are highly connected to tissue ischemia injury, such as cerebral ischemia, kidney ischemia/reperfusion injury and myocardial infarction. In a hypoxia plus serum deprivation condition, the activity of ornithine decarboxylase, rate limiting enzyme of putrescine, is rapidly and transiently induced, leading to increased putrescine level in H9c2 cardiomyoblasts. While, blockade of the activity of ornithine decarboxylase results in reduced cell apoptosis, indicating a role of putrescine in promoting cell apoptosis in that ischemia model [10]. Compared with control subjects, levels of glutamate, ornithine, spermine, and spermidine, all related to arginine metabolism, were elevated in stage C heart failure patients [11]. In an animal model of stroke, the levels of spermidine and spermine are reduced in infracted tissues compared to normal tissues, suggesting activated polyamine catabolism in infracted area [12]. Spermine is reported to induce a concentration-dependent increase in [Ca2+]i in isolated ventricular myocytes from rats [13]. However, the exact function of spermine in heart diseases of children remains obscure.
Emerging evidences have shown that spermine is related to cell apoptosis and autophagy. Spermine mediates the release of cytochrome c from mitochondria to the cytosol, which is a critical step in apoptosis and correlated to the activation of caspase cascade [14]. Conversely, another report showed that pretreatment with spermine significantly inhibited cell apoptosis, suppressed the expression of caspases-3 and -9 [15]. In non-cardiomyocytes, spermine increases autophagy by directing binding to the promoters of p53 and p21 proteins [16]. Since apoptosis and autophagy are deeply involved in I/R injury, and the effect of spermine on immature myocardium remains completely unknown, we designed the present study to investigate potential effects of pretreatment with spermine on apoptosis and autophagy in a model of simulated ischemia/reperfusion injury of cultured neonatal rat cardiomyocytes. Our data revealed that spermine might serve as a protective agent for myocardial IR injury in children.
Materials and methods
Reagents
Spermine was bought from Sigma. Terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling (TUNEL) kit was purchased from Roche. Anti-Bax, Bcl-2, mTOR, p62, LC3-I and LC3-II were from Abcam. The other antibodies used in this study were all from Cell Signaling Technology.
Primary cell culture
Primary cultures of neonatal rat cardiomyocytes in this study was performed as described elsewhere [17]. In brief, neonatal Sprague Dawley rats of 1 to 2 day old were sacrificed by anesthesia with 2% isoflurane inhalation. Hearts were minced and used for isolating single cells with a 0.25% solution of crude trypsin. The planting density of cardiomyocytes was 5×104 cells/cm2 for monolayer formation. Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (Gibco) and cultured in humidified air containing 5% CO2 at 37°C. 2 μM fluorodeoxyuridine was added to the medium to prevent the proliferation of non-myocytes.
Simulated ischaemia/reperfusion
Cells were placed to serum-free DMEM 2 h and then treated with ischaemic buffer solution (118 mM NaCl, 24 mM NaHCO3, 1 mM NaH2PO4·H2O, 2.5 mM CaCl2·2H2O, 0.5 mM sodium EDTA·2H2O, 20 mM sodium lactate, 16 mM KCl, pH 6.2). After pre-gassing with 95% N2 and 5% CO2 for at least 5 min, the ischaemic buffer solution was added to the cells. Cells were then placed in a sealed chamber containing the deoxygenation reagent which resulted in the consumption of O2 and the production of CO2. This Anaero Pack system (Mitsubishi Gas Chemical Co. Inc., Tokyo, Japan) provided near-anaerobic conditions, with an O2 concentration of <1% and a CO2 concentration of ~5% within 1 h of incubation at 37°C. The cells were exposed to these conditions for 2 h, and incubated in normal culture conditions (reperfusion) for 24 h [18].
Western blot
Western blot was performed following the standard procedures. Briefly, cells were homogenized in lysis buffer (100 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% SDS and 1% Triton X-100) containing protease inhibitors on ice. Next, 30 μg of each lysate was separated by SDS-PAGE, followed by transfer to PVDF membrane. After block with 5% non-fat milk, PVDF membrane was subjected to primary antibodies at 4°C overnight. Then, the membrane was incubated with indicated secondary antibodies for 2 h at room temperature, and visualized using enhanced chemiluminescence detection kit. Monoclonal antibody against β-actin was used as loading control. The signals of various bands were analyzed using the Image J software (National Institute of Health, Bethesda, MD).
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed to detect the formation of autophagosome in cultured cells. Cells were harvested and fixed 2.5% glutaraldehyde overnight at 4°C, and post-fixed in 1% buffered osmium tetroxide. The specimens were dehydrated in series of ethanol, embedded in epoxy resin and examined under a transmission electron microscope (H800; Hitachi).
Cell apoptosis
Cell apoptosis was detected using Annexin V/PI staining kit (BD Pharminogen) according to the manufacturer’s instructions. Cells were washed twice with cold PBS and then resuspended in 1X Binding Buffer at a concentration of 1×106 cells/ml. 100 µl of the solution (1×105 cells) was transferred to a 5 ml culture tube. 5 µl of FITC Annexin V and 5 µl PI were added into the tube. Cells were incubated for 15 min at RT (25°C) in the dark. 400 µl of 1X Binding Buffer was added to each tube. The apoptosis was analyzed by flow cytometry within 1 h. Unstained cells, cells stained with FITC Annexin V (no PI) and cells stained with PI (no FITC Annexin V) were used for setting up compensation and quadrants.
Cell viability examination
Cells were plated into 96-well plates (5×103 cells per well). Cell viability was assessed by cell-counting kit-8 assay (CCK-8, Beyotime Institute of Biotechnology). 10 μL of CCK8 was added to the cells, and the viability of cells was measured at 450 nm using an ELISA reader (Thermo) according to the manufacturer’s instructions. Three independent experiments were performed in quintuplicate.
Statistical analysis
Data were represented as mean ± standard errors of mean (SEM). All statistical analyses were performed by using SPSS (ver. 13.0) software. Comparisons between two groups were analyzed with Student’s t-test. P value of < 0.05 was considered statistically significant.
Results
Spermine inhibits immature cardiomyocyte apoptosis and enhances cell viability
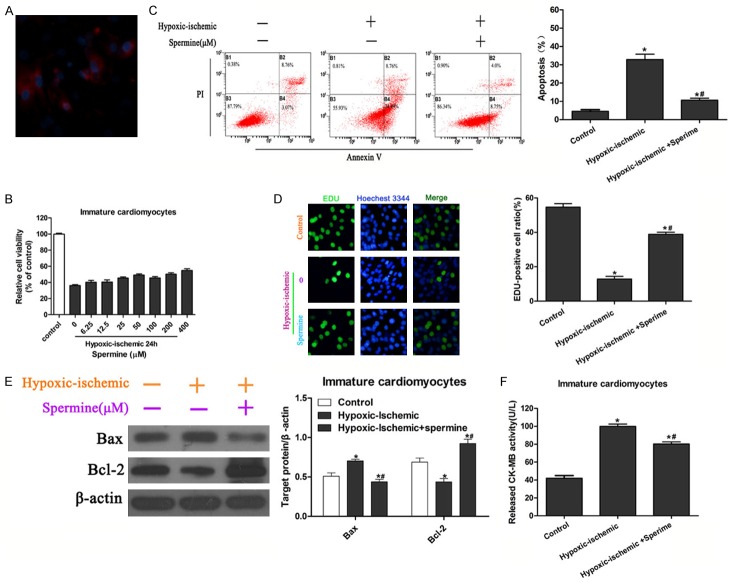
Therapeutic reagents for heart diseases of children are limited. Therefore, we isolated immature cardiomyocytes from neonatal rats and confirmed cell type by immunofluorescent assay with anti-myoactin antibody and DAPI (Figure 1A). To characterize the potential role of spermine during myocardial infarction, an ischemia/reperfusion injury model of cultured cells were established by hypoxia in a serum- and glucose-free medium, followed by reoxygenation in normal culture medium. The appropriate concentration of spermine was determined by pretreatment of cell with different concentrations of spermine and hypoxia for 24 h. Cell viability in hypoxia/ischemia condition was obviously lower compared to that in normal culture condition (Figure 1B). In addition, cell viability showed a dose-dependent manner with pretreatment of different concentrations of spermine except for a mild decrease under pretreatment of 100 μM spermine (Figure 1B). The 50 μM spermine was used for the followed experiment, as it showed a relatively high protective effect on cell viability (Figure 1B). The effect of spermine was checked by cell apoptosis and proliferation assay. Cell apoptosis was detected with Annxin V/PI staining. As expected, hypoxia/ischemia resulted in a significant increase of cell apoptosis. Of note, pretreatment with 50 μM spermine reduced the apoptosis of immature cardiomyocytes which was induced by hypoxia/ischemia treatment (Figure 1C). Furthermore, cell proliferation was performed by EDU incorporation assay. Hypoxia/ischemia treatment in immature cardiomyocytes exhibited a significant decrease of EDU-positive cells compared to normal culture and pretreatment with spermine significantly enhanced cell proliferation in hypoxia/ischemia group, as revealed by an increase in EDU-positive cells, which suggested a protective role of spermine on immature myocardium under hypoxia/ischemia induced injury (Figure 1D).
Figure 1.
Effect of spermine pretreatment on viability and apoptosis of immature cardiomyocytes exposed to hypoxia/ischemia. A. Identification of immature cardiomyocytes with anti-myoactin by immunofluorescent staining. Cells were counter stained with DAPI. B. Immature cardiomyocytes were pretreated with spermine at 0, 6, 25, 12, 5, 25, 50, 100, 200 and 400 μM and cell viability was determined by CCK-8 assay after hypoxia/ischemia for 24 h. Cells cultured in normal condition were used as control. Concentration of spermine for the following pretreatment was 50 μM. C. Representative apoptotic cells by flow cytometry after Annexin V/PI staining (left panels). Percentages of apoptotic cells were analyzed (right panels). *P<0.05 vs Control; #P<0.05 vs IRI. D. Representative immunofluorescent images of immature cardiomyocytes (left panels). Proliferating cells were stained with EDU, and total cells were stained with Hoechest 3344. Ratios of EDU-positive cells in total cells were calculated and analyzed (right panels). *P<0.05 vs Control; #P<0.05 vs IRI. E. Western blot shows the effects of spermine pretreatment on Bcl-2, Bax expression. Quantitation for Bax and Bcl-2 were performed from three independent experiments. *P<0.05 vs Control; #P<0.05 vs IRI. F. CK-MB levels were determined with commercialized kit and analyzed from three independent experiments. *P<0.05 vs Control; #P<0.05 vs IRI.
To investigate the molecular changes induced by spermine, we analyzed the expression of pro-apoptosis factor Bcl-2 and anti-apoptosis protein Bax by Western blot. Compared with control cells, the expression of Bax was significantly decreased and Bcl-2 increased in hypoxia/ischemia cells (P<0.05), and spermine reversed these changes (P<0.05 vs IRI group) (Figure 1E).
Creatine kinase MB (CK-MB) serves as diagnostic marker of myocardial tissue injury [19]. In this study, we measured the levels of CK-MB in the supernatant of cultured immature myocardium by enzyme-linked immunosorbent assay (ELISA). It should be noted that levels of CK-MB showed significant increase in IRI group compared to the control group (P<0.05), and that pretreatment with spermine significantly attenuated the level of CK-MB compared to IRI group only (P<0.05 vs IRI group) (Figure 1F). As the levels of CK-MB reflected the severity of heart injury, our data suggested that spermine had a pre-protective effect during the process of ischemia/reperfusion injury.
Spermine increases immature cardiomyocyte autophagy by mTOR pathway
Autophagy is an essential homeostatic process which is critically important for maintain health and dysregulated in heart diseases [20]. Herein, we evaluated the effect of spermine on cardiomyocyte autophagy. Light chain 3s (LC 3s) are specific markers for autolysosomes, thus we examined the effect of spermine on the LC 3s co-localization in immature cardiomyocytes in response to hypoxia/ischemia. In normal control, LC3 was diffused cytosolic and nuclear, whereas in hypoxia/ischemia condition, colocalization of RFP-LC3 with GFP-LC3 was observed, indicating the autolysosome formation (Figure 2A). As a result, spermine treatment significantly enhanced the formation of autolysosome in immature cardiomyocytes during hypoxia/ischemia injury (Figure 2A). Similar results were obtained by transmission electron microscopy (TEM) examination that spermine significantly resulted in increased number of autolysosomes (Figure 2B). These results indicated that spermine played pro-autophagic role in immature cardiomyocytes.
Figure 2.
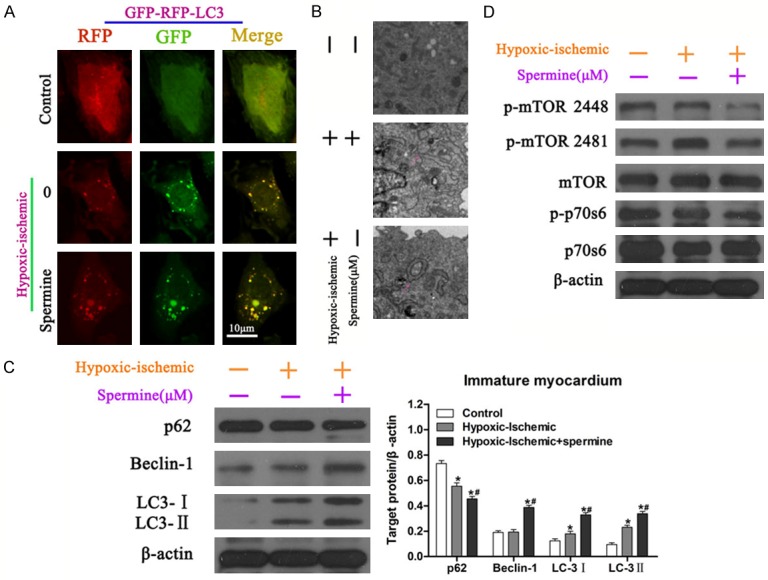
Effect of spermine pretreatment on autophagy induction in immature cardiomyocytes. A. Immature cardiomyocytes in different conditions were co-transfected with RFP-LC3 and GFP-LC3, and subjected to confocol microscopy. The yellow dots indicate autolysomes. B. Autophagic vacuoles exposed to different treatments were examined by transmission electron microscopy. Arrows indicate autolysomes (double membrane structure, also see the inserts). C. Immature cardiomyocytes in response to different conditions were lysed and used to Western blot. Western blot analysis of cell lysates was performed using antibodies as indicated. Protein expressions of p-mTOR 2448, p-mTOR 2481, mTOR, p-p70s6, p70s6 were analyzed. The expression of ß-actin was used as loading control. *P<0.05 vs Control; #P<0.05 vs IRI. D. Western blot analysis of cell lysates was performed using p62, Beclin-1, LC3-I, LC3-II and ß-actin (left panels). Quantization of indicated proteins was analyzed with normalization to ß-actin (right panels). *P<0.05 vs Control; #P<0.05 vs IRI.
Effect of spermine on protein expression related to autophagy was investigated. Western blot experiment showed that spermine significantly up-regulated protein expressions of beclin-1, LC3-I and LC3-II, and down-regulated p62 expression, suggesting an increased autophagy (P<0.05) (Figure 2C). The most straightforward path of autophagy requires the inhibition of a cytoplasmic kinase receptor, the mammalian target of rapamycin (mTOR) [21]. Our Western blot analysis showed that spermine pretreatment reduced the phosphorylation of mTOR and p70s6 in immature cardiomyocytes in response to hypoxia/ischemia [22]. These data confirmed the role of spermine on increasing autophagy at molecular levels.
Effect of autophagy activator and inhibitor on the role of spermine pretreatment in IRI
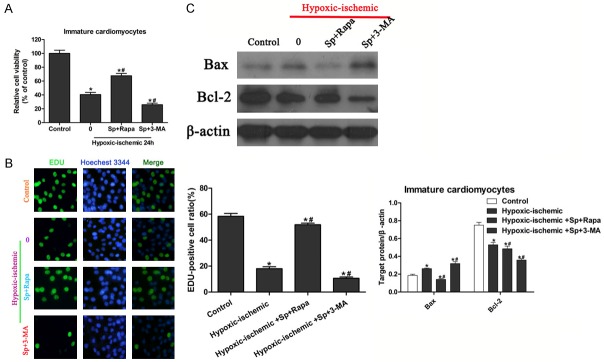
To further confirm the pro-autophagic effect of spermine in immature cardiomyocytes, autophagy activator, rapamycin, a specific inhibitor of mTOR, and inhibitor, 3-methyladenine (3-MA) were used. Immature cardiomyocytes were pretreated with spermine, followed by cultured under hypoxia/ischemia condition, and then treated with either rapamycin or 3-MA. Cell viability was measured used CCK-8 assay and results showed that exposure to hypoxia/ischemia decreased cell viability compared with control cells (Figure 3A). Pretreatment with spermine, and addition of rapamycin to injured immature cardiomyocytes significantly enhanced cell viability, while 3-MA showed the opposite effect (Figure 3A). These data indicated that pretreatment with spermine promoted cell viability via autophagic pathway. Cell viability with EDU incorporation assay showed similar results, where immature cardiomyocytes pretreated with spermine, followed by IRI and rapamycin administration showed significant more EDU-positive cells compared to the other groups (Figure 3B). Our data indicated that rapamycin could protect immature cardiomyocytes during IRI after pretreatment with spermine, whereas 3-MA had contrary effect.
Figure 3.
Effect of rapamycin and 3-MA treatment after spermine pretreatment on viability and apoptosis of immature cardiomyocytes exposed to hypoxia/ischemia. A. Cell viability was determined with CCK-8 assay in different conditions. *P<0.05 vs Control; #P<0.05 vs IRI. B. Representative immunofluorescent images of immature cardiomyocytes subjected to different conditions (left panels). Proliferating cells were stained with EDU, and total cells were stained with Hoechest 3344. Ratios of EDU-positive cells in total cells were calculated and analyzed (right panels). *P<0.05 vs Control; #P<0.05 vs IRI. C. Western blot was performed to evaluate Bcl-2, Bax expression in different conditions (upper panels). Quantization for Bax and Bcl-2 were normalized to ß-actin (bottom panels). *P<0.05 vs Control; #P<0.05 vs IRI.
Furthermore, protein expression related to cell apoptosis was determined. Western blot showed that rapamycin administration after the pretreatment with spermine significantly decreased the expression level of Bax, an anti-apoptosis protein, compared to either control or IRI group (P<0.05), while 3-MA had opposite effect (Figure 3C). The expression level of Bcl-2, a pro-apoptosis protein, was significantly lower in 3-MA treated group compared to the other groups (Figure 3C). Together, these results showed that rapamycin could increase call viability and protect cell from IRI after pretreatment with spermine.
Effect of spermine on cardiomyocytes viability during IRI after pretreatment with rapamycin or 3-MA
To investigate whether the time of treatment influenced the protective effect of rapamycin or damage role of 3-MA combined with spermine during IRI, immature cardiomyocytes were pretreated with either rapamycin or 3-MA, followed by IRI and administration with spermine. Surprisingly, compared to IRI group, no significant difference were observed in rapamycin+spermine IRI group and 3-MA+spermine IRI group (Figure 4A). Similar results were shown by EDU incorporation assay (Figure 4B). These results implying that the protective effect of rapamycin or damage effect of 3-MA depended on the pretreatment with spermine. Protein expressions of Bax and Bcl-2 were examined. As expected, pretreatment with rapamycin or 3-MA, followed by IRI and spermine treatment showed similar expression levels of Bax and Bcl-2 compared to IRI group, suggesting the effect of rapamycin or 3-MA was abolished by changing administration time point (Figure 4C).
Figure 4.
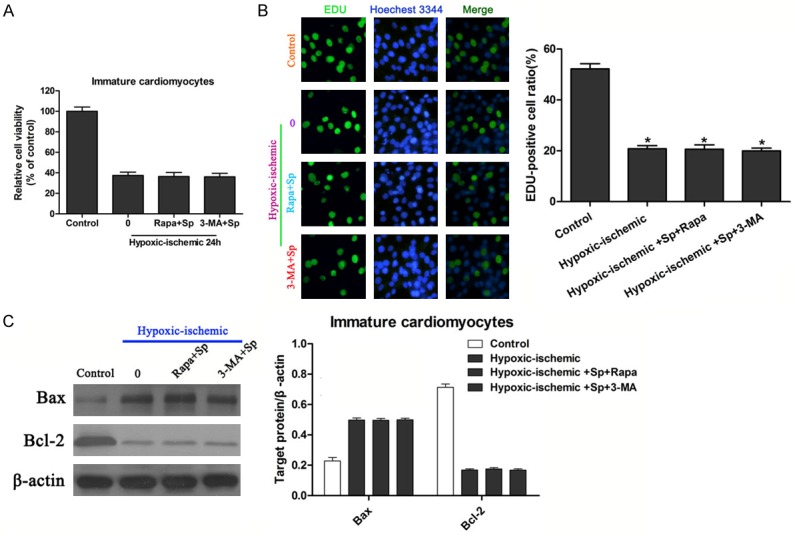
Effect of spermine treatment after rapamycin or 3-MA pretreatment on viability and apoptosis of immature cardiomyocytes exposed to hypoxia/ischemia. A. Cell viability was determined with CCK-8 assay in different conditions. *P<0.05 vs Control; #P<0.05 vs IRI. B. Proliferation of immature cardiomyocytes subjected to different conditions was analyzed with EDU incorporation assay (left panels). Proliferating cells were stained with EDU, and total cells were stained with Hoechest 3344. Percentages of EDU-positive cells were calculated and analyzed (right panels). *P<0.05 vs Control; #P<0.05 vs IRI. C. Bcl-2, Bax expression in different conditions were analyzed with Western blot (left panels). Quantization for Bax and Bcl-2 were normalized to ß-actin (right panels). *P<0.05 vs Control; #P<0.05 vs IRI.
Discussion
In the present study, we observed that pretreatment with spermine could significantly enhance cell viability and decrease cell apoptosis of immature cardiomyocytes under hypoxia/ischemia condition. The cardioprotective effect of spermine was mediated by autophagy. Furthermore, use of rapamycin after pretreatment with spermine significantly attenuated the injury of immature cardiomyocytes induced by hypoxia/ischemia; whereas administration of 3-MA diminished the protective role of spermine. In addition, when immature cardiomyocytes were pretreated with rapamycin or 3-MA, followed by IRI and spermine therapy, cells showed no significant differences in viability or apoptosis, abolishing the effect of rapamycin, 3-MA and spermine. Our study revealed a promising new therapy for reducing heart injury of children, pretreatment with spermine, and use of rapamycin when disease develops.
Autophagy is a conserved catabolic process that cellular components are transported to and degraded in the lysosomes [22]. Emerging evidences have showed that autophagy is involved in the pathogenesis of acute and chronic ischemia heart injury and heart failure [23,24]. Many studies demonstrate that in ischemic-reperfused cardiomyocytes autophagy is significantly up-regulated, suggesting a role of autophagy in the process of IR injury [25]. It is evident that modification of the autophagic response via various ways is related to the heart function remodeling. However, we raised the question that whether autophagy is beneficial or detrimental to cardiomyocytes. Yutaka Matsui and his colleagues demonstrated that autophagy activation clearly reduced the myocardial infarct size in a mouse model of I/R injury [26]. However, they also showed that excessive autophagy could promote cardiomyocytes death, suggesting a harmful role of excessive autophagy [26]. They concluded that autophagy played distinct roles during ischemia and reperfusion: autophagy may be protective during ischemia, whereas it may be detrimental during reperfusion [26]. In the current study, we found that pretreatment with spermine could induce autophagy, as revealed by increase of autolysosome, elevated expression of autophagic proteins LC3-I, LC3-II and beclin1. We further observed that induction of autophagy using rapamycin strengthened the pre-protective role of spermine during IRI.
Canonical autophagy, also known as macroautophagy, can be triggered in different ways. The most straightforward path requires the inhibition of a cytoplasmic kinase receptor, the mammalian target of rapamycin (mTOR) which contains two functionally distinct protein-mTOR complexes (mTORC1 and mTORC2) [27]. Here, we found that the mTOR pathway was inhibited after pretreating immature cardiomyocytes with spermine. Western blot data demonstrated phosphorylated mTOR (Ser 2448, 2481) and phosphorylated p70s6 were significantly down-regulated when immature cardiomyocytes were pretreated with spermine, followed by IRI.
To confirm protective effect of autophagy activation, autophagy induction and inhibition reagents, rapamycin and 3-MA were used. Rapamycin treatment significantly enhanced the pre-protective role of spermine, as evidenced by elevated cell viability and EDU-positive cells. By contrast, the autophagy inhibitor 3-MA treatment attenuated or even reversed the pre-protective role of spermine under hypoxia/.ischemia condition detected by CCK-8 test and EDU incorporation assay. In addition, the Western blot results of anti-apoptotic protein Bax, and pro-apoptotic protein Bcl-2, further confirmed the role of rapamycin and 3-MA in the pre-protective effect of spermine during IRI. These data supported the hypothesis that the spermine induced autophagy showed a beneficial effect on immature cardiomyocytes during the hypoxia/ischemia injury.
Pediatric heart diseases have account for a big part of heart diseases. However, efficient treatment and underlying mechanisms remain to be elucidated. In this study, we used neonatal rat cardiomyocytes to establish a model of simulated ischemia/reperfusion injury (IRI), and searched for appropriate agents to protect the cardiomyocytes from IRI. Polyamines are known to be essential for growth and development in prokaryotes and eukaryotes. Notably, spermine synthesized from putrescine is reported to control various cellular processes, including DNA stability, transcription and translation [28]. Very little is known about the involvement of spermine in induction of autophagy in cardiac myocytes. We examined the role of pretreatment with spermine in immature cardiomyocytes exposed to IRI. The results showed that spermine pretreatment significantly increased cell viability and decreased cell apoptosis; moreover, autophagy was induced by pretreatment with spermine. With autophagy activator and inhibitor, we discovered that spermine-induced autophagy showed protective role for immature cardiomyocytes against IRI. We wondered if the treatment time of spermine and rapamycin influenced the final effect. To test this, we pretreated cells with rapamycin, or 3-MA, followed by IRI, and spermine treatment. Surprisingly, the effect of rapamycin, or 3-MA and spermine was abolished. No significant differences of cell viability or EDU-positive cells were observed among the three treated groups. Western blot results also demonstrated no obvious changes of Bax and Bcl-2. Out data revealed that spermine showed pre-protective role for immature cardiomyocytes against IRI and autophagy activator, rapamycin, could enhance the effect of spermine.
In conclusion, spermine has pre-protective effect for immature cardiomyocytes by inducing autophagy, which can be further strengthened by rapamycin. Our study suggests that pretreatment with spermine is a potential novel approach for therapy of children myocardial injury.
Acknowledgements
This work was supported by a grant from National Natural Science Foundation of China (NO. 81200129) and a grant from the Health and Family Planning Commission of Zhejiang Province (No. 2013RCB007).
References
- 1.Fox CS, Coady S, Sorlie PD, D’Agostino RS, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 2.Boren J, Matikainen N, Adiels M, Taskinen MR. Postprandial hypertriglyceridemia as a coronary risk factor. Clin Chim Acta. 2014;431:131–142. doi: 10.1016/j.cca.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Sayols-Baixeras S, Lluis-Ganella C, Lucas G, Elosua R. Pathogenesis of coronary artery disease: focus on genetic risk factors and identification of genetic variants. Appl Clin Genet. 2014;7:15–32. doi: 10.2147/TACG.S35301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frohlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ. Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J. 2013;34:1714–1722. doi: 10.1093/eurheartj/eht090. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Zeng Z, Li Q, Xu Q, Xie J, Hao H, Luo G, Liao W, Bin J, Huang X, Liao Y. Inhibition of microRNA-497 ameliorates anoxia/reoxygenation injury in cardiomyocytes by suppressing cell apoptosis and enhancing autophagy. Oncotarget. 2015;6:18829–18844. doi: 10.18632/oncotarget.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei H, Roscigno CI, Hanson CC, Swanson KM. Families of children with congenital heart disease: A literature review. Heart Lung. 2015;44:494–511. doi: 10.1016/j.hrtlng.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Pegg AE. Mammalian polyamine metabolism and function. Iubmb Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zahedi K, Barone S, Wang Y, Murray-Stewart T, Roy-Chaudhury P, Smith RD, Casero RJ, Soleimani M. Proximal tubule epithelial cell specific ablation of the spermidine/spermine N1-acetyltransferase gene reduces the severity of renal ischemia/reperfusion injury. PLoS One. 2014;9:110–121. doi: 10.1371/journal.pone.0110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Zhang H, Xue G, Zhang L, Zhang W, Wang L, Lu F, Li H, Bai S, Lin Y, Lou Y, Xu C, Zhao Y. Exercise training preserves ischemic preconditioning in aged rat hearts by restoring the myocardial polyamine pool. Oxid Med Cell Longev. 2014;2014:457429. doi: 10.1155/2014/457429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tantini B, Fiumana E, Cetrullo S, Pignatti C, Bonavita F, Shantz LM, Giordano E, Muscari C, Flamigni F, Guarnieri C, Stefanelli C, Caldarera CM. Involvement of polyamines in apoptosis of cardiac myoblasts in a model of simulated ischemia. J Mol Cell Cardiol. 2006;40:775–782. doi: 10.1016/j.yjmcc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Cheng ML, Wang CH, Shiao MS, Liu MH, Huang YY, Huang CY, Mao CT, Lin JF, Ho HY, Yang NI. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol. 2015;65:1509–1520. doi: 10.1016/j.jacc.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Park MH, Igarashi K. Polyamines and their metabolites as diagnostic markers of human diseases. Biomol Ther (Seoul) 2013;21:1–9. doi: 10.4062/biomolther.2012.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Xu C, Zhao W, Zhang J, Cao K, Yang B, Wu L. Calcium and polyamine regulated calcium-sensing receptors in cardiac tissues. Eur J Biochem. 2003;270:2680–2688. doi: 10.1046/j.1432-1033.2003.03645.x. [DOI] [PubMed] [Google Scholar]
- 14.Stefanelli C, Stanic’ I, Zini M, Bonavita F, Flamigni F, Zambonin L, Landi L, Pignatti C, Guarnieri C, Caldarera CM. Polyamines directly induce release of cytochrome c from heart mitochondria. Biochem J. 2000;347:875–880. [PMC free article] [PubMed] [Google Scholar]
- 15.Han L, Xu C, Jiang C, Li H, Zhang W, Zhao Y, Zhang L, Zhang Y, Zhao W, Yang B. Effects of polyamines on apoptosis induced by simulated ischemia/reperfusion injury in cultured neonatal rat cardiomyocytes. Cell Biol Int. 2007;31:1345–1352. doi: 10.1016/j.cellbi.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Chae YB, Kim MM. Activation of p53 by spermine mediates induction of autophagy in HT1080 cells. Int J Biol Macromol. 2014;63:56–63. doi: 10.1016/j.ijbiomac.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Aplin M, Christensen GL, Schneider M, Heydorn A, Gammeltoft S, Kjolbye AL, Sheikh SP, Hansen JL. The angiotensin type 1 receptor activates extracellular signal-regulated kinases 1 and 2 by G protein-dependent and -independent pathways in cardiac myocytes and langendorff-perfused hearts. Basic Clin Pharmacol Toxicol. 2007;100:289–295. doi: 10.1111/j.1742-7843.2007.00063.x. [DOI] [PubMed] [Google Scholar]
- 18.Li HZ, Han LP, Jiang CM, Li H, Zhao YJ, Gao J, Lin Y, Ma SX, Tian Y, Yang BF, Xu CQ. Effect of dopamine receptor 1 on apoptosis of cultured neonatal rat cardiomyocytes in simulated ischaemia/reperfusion. Basic Clin Pharmacol Toxicol. 2008;102:329–336. doi: 10.1111/j.1742-7843.2007.00177.x. [DOI] [PubMed] [Google Scholar]
- 19.Rao PS, Cohen MV, Mueller HS. Production of free radicals and lipid peroxides in early experimental myocardial ischemia. J Mol Cell Cardiol. 1983;15:713–716. doi: 10.1016/0022-2828(83)90260-2. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Sun HQ, Zhu X, Zhang L, Albanesi J, Levine B, Yin H. GABARAPs regulate PI4P-dependent autophagosome:lysosome fusion. Proc Natl Acad Sci U S A. 2015;112:7015–7020. doi: 10.1073/pnas.1507263112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rigacci S, Miceli C, Nediani C, Berti A, Cascella R, Pantano D, Nardiello P, Luccarini I, Casamenti F, Stefani M. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: a mechanistic insight. Oncotarget. 2015;6:35344–35357. doi: 10.18632/oncotarget.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa R, Morrison A, Wang J, Manithody C, Li J, Rezaie AR. Activated protein C modulates cardiac metabolism and augments autophagy in the ischemic heart. J Thromb Haemost. 2012;10:1736–1744. doi: 10.1111/j.1538-7836.2012.04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 26.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 27.Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12:437–452. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- 28.Kaeberlein M. Spermidine surprise for a long life. Nat Cell Biol. 2009;11:1277–1278. doi: 10.1038/ncb1109-1277. [DOI] [PubMed] [Google Scholar]