Abstract
Presently, there have been a lot of documents confirmed that aflatoxin B1 could promote the incident rate of hepato-cellular carcinoma, but the specific mechanism is not completely clear. Some evidences showed that it might relate to oxidative stress and inflammatory reaction. So the rat hepato-cellular carcinoma model was applied in this study for being discussed. Aflatoxin B1 was applied for inducing the rats to produce hepato-cellular carcinoma model to evaluate the expression of histopathology and glutathione transferase. At the same time, we also detected the expression of antioxidase, pro-inflammatory cytokine, proliferating cell nuclear antigen and etc in rat hepato-cellular carcinoma tissues. The histo-pathological results showed that the necrosis of liver cells could be observed after being induced by Aflatoxin B1 for 4 weeks. We could observe obvious hepato-cellular carcinoma in 10th week. The level of reactive oxygen species in liver cancer rose obviously, and the activity of antioxidant enzymes reduced. At the same time, the expression level of pro-inflammatory cytokine, TNFα, IL-1α, proliferating cell nuclear antigen and etc all increased significantly. In conclusion, the histological characteristics of hepato-cellular carcinoma could be induced by aflatoxin B1, and the progression of hepato-cellular carcinoma related closely to inflammatory reaction.
Keywords: Superoxide, oxidative stress, inflammatory factor, hepatocellular carcinoma
Introduction
Aflatoxin B1 (AFB1) is the production of aspergillus enzyme fungi and parasitic enzyme, which is regarded as the potential carcinogens. It has been confirmed to relate closely with the morbidity of hepato-cellular carcinoma (HCC). AFB1 can produce the intermediate medium for genetic toxicity by hepatic metabolism. Epoxide produces DNA addition compounds which can induce GC-TA variation by combining the guanine DNA of double stranded-N7. Except the inherent toxicity, the secondary mechanism which is produced by necrosis of liver cells also plays an important role in HCC development [1-3]. Oxidative stress is the main inhibitory factor of liver cells necrosis, which can also aggravate HCC development with a series of accompanied inflammatory cytokines. The decreased level of anti - oxidase in cancer cells in cancer cells can lead oxidative stress, mainly includes superoxide dismutase (SOD), scavenger enzymes, glutathione peroxidase and etc. But SOD is more closely related, which is the key step for catalytic oxidation pathway [4]. In a series of of pro-inflammatory cytokines, a part of factors can promote the compensatory proliferation of liver cells, which can lead to the malignant transformation of liver tissue easily. And TNF-α plays an important role in tumor formation. Some researches applied TNF-deficient mice in the experiment, the results showed that this factor processes a certain protective effect in tumor formation; while IL-1α can promote the metastasis of tumor cells by regulating the level of inflammation [5-7]. So in this study, we applied hepato-cellular carcinoma model which was induced by aflatoxin for researching the effect of peroxide and inflammatory cytokines on cancer tissue cells, which provides a certain reference value for clinical diagnosis studies.
Materials and methods
Construction of hepato-cellular carcinoma for a rat model
There were 40 (18 to 20-week old) male SB rats, weighting from 200 g to 220 g, which were bought from Beijing Weitong Lihua Experimental Animal Technology Co. Ltd.. All the rats were raised under standard animal feeding condition. Referring to the methods reported in the literature, dissolved AFB1 in DMSO. Injected 1 mg/kg AFB1 to abdominal cavity for the experimental group (20 rats), only gave equivalent dose of DMSO to the control group. All the operations of this study were informed and agreed by the ethics committee of our hospital.
Histo-pathological examination of liver
Executed the rats both in experimental group and control group and obtained the liver tissues. Cut the tissues into slices, the thickness was 0.3-0.5 cm and fixed them in Bouin’s fixation fluid. Transferred tissue blocks into 70% alcohol for the deprivation of body fluids. Finally embedding by ceresin wax, the obtained sections were stained with hematoxylin eosin (HE) staining. Put the slides on DPX and analyzed them by Leica 2000 microscope.
Liver tissue extraction and biochemical assay
The composition of extraction buffer were 1 M HEPES, 3 M KCl, 1 mM EDTA, 10% Triton X-100, 1 mM DTT, 0.1 mM PMSF and 0.2 mM benzamidine. Collected liquid supernatant as cytoplasmic part after centrifuging for 10min under the condition of 4°C and 14000 ×g. Suspended the debris in extract once again and collected sediments by centrifugation, repeated this step for 3 times. The sediments suspended in the nucleus and extracted the solution for 20 min. Centrifuged for 10 min under the same condition, the obtained liquid supernatant was applied for PCNA detection. Reactive oxygen species (ROS) detection: diluted 1-2% liver tissue extracts in PBS buffer solution by NBT reduction method. Added NBT-PBS (1 mg NBT/ml) solution and incubated for 4-6 h under the condition of 37°C. The sediments were washed by carbinol for 3 times after centrifugation and dissolved in the mixture of 2 ml KOH and DMSO. Measured the absorbance value under 630 nm. Obtained OD value and compared it with the standard sample values constructed by NBT.
Total glutathione detection: mixed 0.1 ml liver extract, 1.5 ml Tris buffer solution (0.2 M) and 0.1 ml 5,50 2-nitrobenzoic acid (DTNB, 0.01 m), the pH value was adjusted to 8.2, added the 10 ml mixture into carbinol and incubated for 30 min, obtained 412 nm liquid supernatant after centrifuging and got absorbance value.
Antioxidant enzyme Native PAGE and GPx analysis
For SOD analysis, the extract contained 60 Ig protein, then formed SOD active strip after 7.5% non-degenerated PAGE. Scanned gelatum to make sure SOD1 stripe (migrated to the direction of anode). Follow the same steps, analyzed catalase and GPx. The gel was incubated in a mixture of hydrogen peroxide and formed stripe contrast with blue background after electrophoresis. Quantitative analysis of enzyme band was processed by gel optical density software AlphaImager 2200. Analysis of glutathione peroxidase (GPx; EC: 1.11.1.9) was that a part of liver homogenate cytoplasm contained 40 Ig protein and added them into 1 ml assay buffer, it contained 100 mM Tris-HCl (pH 7.2), 3 mM EDTA, 1 mM sodium azide, 0.25 mM H2O2, 0.5 mM NADPH, 0.17 mM GSH and 1 unit of glutathione reductase. Recorded absorbance value under 340 nm. One unit of GPx was defined as 1 μmol NADP+ produced in one minute under the condition of 25°C, the data presented as U/mg protein.
Semi quantitative RT-PCR
Abstracted total RNA from liver tissues by TRI kit according to product specification. 2 μg RNA was applied for the synthesis of cDNA. Used random primer and revertAid first strand cDNA to synthetizing kit (MBI fermentas); PCR reaction mixture contained 1×Taq polymerase buffer, 0.2 mM dNTPs, 1 uTaq polyase and 10 pmol primer. The amplification products was analyzed by 2% agarose gel electrophoresis and ethidium bromide staining. The specific sequence of primers was TNF-α: (forward primer 5’-CTCCCAGAAAAGCAA GCAA-3’; Reverse primer 5’-CGAG CAGGAATGAGAAGAGG-3’), IL-1α: (forward primer 5’-AATCCTCTGAGCTTGCCAGG-3’; Reverse primer 5’-GAGGGCAAAA GACTGACCCA-3’) and β-αctin: (forward primer 5’-TCTACAATGAGCTGCGTGTG-3’; Reverse primer 5’-ATGTCACGC ACGATTTCCC-3’).
Western blot analysis
The samples that contained 60 Ig protein were processed by 10% SDS-PAGE, then transferred protein bands to nitrocellulose filter and detected SOD1 (1:1000) and anti-PCNA (1:500). After that, used biotin second antibody (1:5000) to detect and applied ECL SuperSignal West Pico kit for detecting protein bands. Monoclonal b- actin peroxidase antibody (1:10000) and nuclear protein B antibody (1:5000) were regarded as load control.
Data statistics and analysis
The experimental data were showned as x±SD, and processed factor analysis of variance. Then detected the significance of differences by post hoc Tukey, if P<0.05, it means that the difference has statistical significance.
Results
Histo-pathological evaluation
Histo-pathological evaluation is always used in diagnosis and clinical staging of hepato-cellular carcinoma. In this study, we recorded and analyzed the morphology of liver and corresponding histological changes at different time points after AFB1 treatment. Compared with control group, the surface of rat liver tissue was slightly rough which was treated by AFB1 in the 4th week and this condition became more obvious in the 6th week, we could find several tumor nodules in the 10th week. The formation of focus of altered hepatocytes (FAH) was regarded as tumor-like lesions during HCC developing process. Leaflet mechanism of normal liver tissues and arrangement of hepatic cells could be found in the control group. After using AFB1 for 4 weeks, we could find partial necrosis of the liver cells arranged in disorder, some areas of cytoplasmic staining were different in the 6th week, which was expressed as mixed cell focus (MCF) and always formed FAH. Obvious compression of leaflet structure could be found in the 10th week and compact liver cell mass existed in discrete FAH region could be found in lymphocyte infiltration slices (Figure 1).
Figure 1.
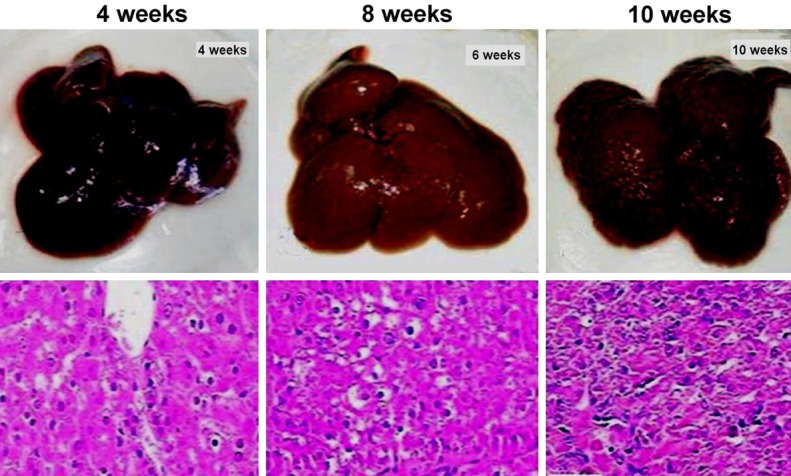
Histopathological changes of hepatocellular carcinoma induced by AFB1 at different time for experimental group.
Activities of antioxidant enzymes
In order to confirm whether AFB1 toxicity can influence the oxidase in the liver, we detected oxidase activity of experimental group. SOD1 was the key enzyme in the pathway of cell oxidase, the activity of this enzyme which was treated by AFB1 in experimental group decreased obviously (P<0.01). It was consistent with the decreased expression of liver SOD1 protein (Figure 2A, P<0.01). H2O2 was the product of SOD, compared with control group, we could find out that catalase activity of experimental group decreased obviously after carrying a further metabolism by scavenger enzymes and glutathione peroxidase (P<0.01). GPx1 and GPx2 in GPx subtype were believed to be closely related to the development of tumor, the activity level of GPx1 in experimental group decreased obviously (P<0.01), while the activity of GPx2 was significantly enhanced (P<0.05). Besides that, to distinguish the biological relevance of GPx changes, total GPx activity spectrophotometric detection of liver extract showed that the level of experimental group was lower than that of control group (P<0.05) (Figure 2B).
Figure 2.
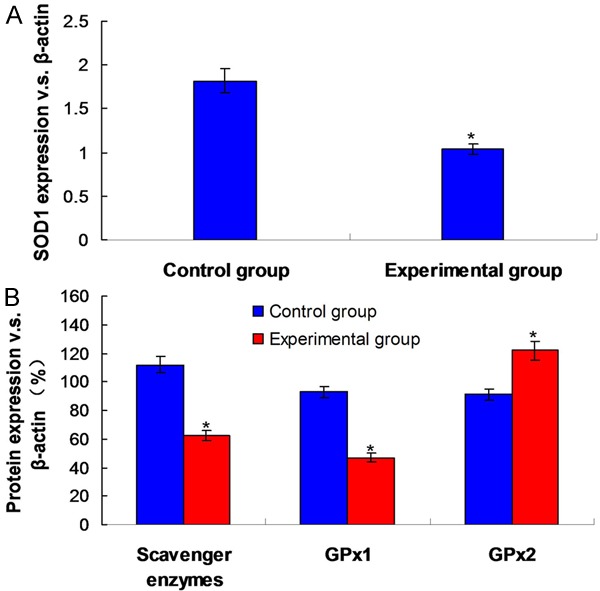
Protein expression and activities of antioxidant enzymes. A. Activity and expression of SOD1. B. Activity and expression of scavenger enzymes and GPx. *P<0.05 means the difference compared to the Control group.
ROS and glutathione level
The increased glutathione level was regarded to relate to the occurrence of cancer. Compared with control group, the ROS level of rats in experimental group present almost 1.5 times strengthening. Glutathione depletion was regarded as the marker of oxidative stress in cells, glutathione level in AFB1 treatment group decreased obviously (P<0.05).
Expression of inflammatory cytokines
In order to confirm whether Aflatoxin B1 could lead chronic inflammation of the liver, we detected and analyzed the expressions of TNF-α and IL-1α inflammatory cytokines. The results showed that compared with control group, the expressions of TNF-α (2 times; P<0.01) and IL-1α (3 times; P<0.01) increased significantly in experimental group. The increased expression of these two inflammatory cytokines could make the cells proliferate rapidly, PCNA was always used as the marker of cell proliferation, and the PCNA expression of rat liver nuclei which was treated by AFB1 increased obviously (3 times; P<0.01). Immuno-histochemical staining analysis showed that the number of PCNA positive cell nucleus increased significantly in rat FAH area of experimental group (P<0.01) (Figure 3).
Figure 3.
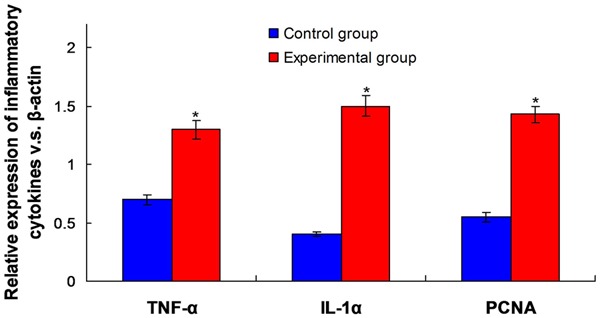
Observation for the different expression of inflammatory cytokines TNF-α, IL-1α and cell proliferation related factor PCNA (the ratio of β-actin) in control and experimental group. *P<0.05 means the statistical difference between groups.
Discussion
AFB1 is a common genetic toxicity factor, which is closely related to the incidence of hepato-cellular carcinoma in the population. At present, some studies showed that using aflatoxin could lead neoplastic lesion to exist in rat liver. While there is still a lack of relevant reports on the specific histo-pathological changes [9,10]. In this study, we found that pathology of liver tissue in rats showed disordered leaflet structure and necrotic cell formation in the 4th week after being treated by AFB1. It was clear that mixed cell focal region (MCF) could be found after staining, which was the location of liver tumor. The formation of MCF was very obvious in the 6th week, and we could find apparently compressed cell mass and widely infiltrated lymphocytes around the similar region in the 10th week. FAH cell presented significantly enhanced proliferating cell nuclear antigen (PCNA) and tumor progression marker (GST-pi). Thus it was further confirmed that during AFB1 induced progression of hepato-cellular carcinoma, such region was tumor lesion area. These results showed that the progress of FAH could be divided into different period by necrosis of hepatic cells in tissue sections and the formation of MCF. And the formation of FAH and HCC nodule in liver surface were consistent with histologic characteristics of the clinical patients who were in the III stage. So we considered that periodization was suitable for judging pathogenic mechanism of hepato-cellular carcinoma which was caused by AFB1.
At present, it was reported that a series of cytokines could be released during HCC development process, which was different from induced liver cancer. So it is necessary to find the markers of reliable AFB1 which could induce HCC specific markers [11,12]. From this aspect, GST-pi played an important role because it participated in the detoxification process of AFB1 epoxide, which was produced in liver after AFB1 poisoning. GST-pi positive tumor cells could also be found in rat liver tissues which were treated by AFB1, so we were able to judge the progression of liver cancer by the dynamic change of GST-pi expression. In this study, we found out that for the rats which were treated by AFB1, the expression of GST-pi showed an increasing trend from the 4th to 10th week, and histo-pathological observation showed that there was no obvious change in the expression of GST-pi which contained normal liver cells. We also found GST-pi level increased significantly in rat serum. Integrated current obtained results, GST-pi could be regarded as a effective marker and it could predict the formation of early hepato-cellular carcinoma which were induced by AFB1.
At present, there are still not enough evidences that can confirm the genetic toxicity of tumor including AFB1 pathogens could cause the gene mutation in HCC pathological progression. And some studies reported that the secondary mechanism which was caused by necrosis of liver cells played a more important role in tumor progression [13-15]. So we selected peroxide-inflammatory reaction pathway to explain the relevant mechanism. Proliferation of tumor cells usually accompanied by continuous oxidative stress reaction, may be due to a further DNA damage and genetic instability in malignant transformation of tumor cells. AFB1 related to the damage of DNA, so oxidative stress could also be regarded as a predictor that could judge the pathological changes of HCC induced by AFB1. In this study, we found that the ROS level in the liver tissue of rats treated with AFB1 was consistent with reduced glutathione levels. So that we could confirm there was some correlation between tumor growth and oxidation conditions. At the same time, we found that the expression level of SOD1 in liver tissue of rats which was treated with AFB1 also decreased, and immuno-histochemical staining analysis indicated that FAH region was the most obvious decreased place. All of these phenomena reflected that SOD1 expression related to the generation of the central nodule in liver tumor. H2O2 that was produced by SOD activity finally completed metabolic degradation through catalase and GPx. In this study, we found that rat catalase level of experimental group decreased obviously. While GPx included multiple subtypes and it was still not clear that the correlation between it and specific mechanisms of tumor occurrence. Some studies showed that the enhanced GPx2 activity could inhibit MCF7 cell apoptosis and promote HT-29 adenocarcinoma cell proliferation [16]. So GPx subtype could provide a certain growth auxiliary conditions for HCC development which was induced by AFB1.
In all kinds of inflammatory cytokines, TNF-α played an important role in the development of tumor. Some reports showed that the rats that were lack of this factor could avoid the occurrence of tumor [17,18]. IL-α could promote the synthesis and secretion of TNF-α with oxidative stress, so these two kind of factors could be supplementary to each other and promote tumor genesis and progression [19]. In this study, we found that AFB1 could induce FAH tubercle to exist in liver, and SOD1 inhibit oxidative stress. Then the expression level of TNF-α and IL-α in rat liver increased obviously. So it reminded that the toxicity of AFB1 could promote the formation of tumor by oxidative stress -TNF-α/IL-α pathway. PCNA was the accessory protein of DNA synase, which expression level increased obviously in fast proliferating cell nucleus [20-22]. The expression of this factor increased significantly in rat liver which was treated by AFB1, which was basically the same with the result that PCNA positive cells mainly concentrated in FAH area.
In conclusion, pro-inflammatory cytokine and oxidative stress play an important role in the occurrence and the progression of hepato-cellular carcinoma which were induced by AFB1.
Disclosure of conflict of interest
None.
References
- 1.Xu Z, Chen Y, Gu D, Lee NP, Sun S, Gong W, Tan Y, Luk JM, Chen J. SOD2 rs4880 CT/CC genotype predicts poor survival for Chinese gastric cancer patients received platinum and based adjuvant chemotherapy. Am J Transl Res. 2015;7:401–410. [PMC free article] [PubMed] [Google Scholar]
- 2.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 3.Conklin KA. Cancer chemotherapy and antioxidants. J Nutr. 2004;134:3201S–3204S. doi: 10.1093/jn/134.11.3201S. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa Y, Takano T, Miyauchi A, Matsuzuka F, Yoshida H, Kuma K, Amino N. Decreased expression of glutathione peroxidase mRNA in thyroid anaplastic carcinoma. Cancer Lett. 2002;182:69–74. doi: 10.1016/s0304-3835(02)00069-1. [DOI] [PubMed] [Google Scholar]
- 5.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Ding WQ, Vaught JL, Yamauchi H, Lind SE. Differential sensitivity of cancer cells to docosahexaenoic acid-induced cytotoxicity: the potential importance of down-regulation of superoxide dismutase 1 expression. Mol Cancer Ther. 2004;3:1109–1117. [PubMed] [Google Scholar]
- 7.Ha HL, Shin HJ, Feitelson MA, Yu DY. Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol. 2010;16:6035–6043. doi: 10.3748/wjg.v16.i48.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angsubhakorn S, Get-Ngern P, Miyamoto M, Bhamarapravati N. A single dose-response effect of aflatoxin B1 on rapid liver cancer induction in two strains of rats. Int J Cancer. 1990;46:664–668. doi: 10.1002/ijc.2910460419. [DOI] [PubMed] [Google Scholar]
- 9.Brigelius-Flohe R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790:1555–1568. doi: 10.1016/j.bbagen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Singh KB, Trigun SK. Apoptosis of Dalton’s lymphoma due to in vivo treatment with emodin is associated with modulations of hydrogen peroxide metabolizing antioxidant enzymes. Cell Biochem Biophys. 2013;67:439–449. doi: 10.1007/s12013-011-9305-2. [DOI] [PubMed] [Google Scholar]
- 11.Dias MC, Rodrigues MA, Reimberg MC, Barbisan LF. Protective effects of Ginkgo biloba against rat liver carcinogenesis. Chem Biol Interact. 2008;173:32–42. doi: 10.1016/j.cbi.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Ravinayagam V, Jaganathan R, Panchanadham S, Palanivelu S. Potential Antioxidant Role of Tridham in Managing Oxidative Stress against Aflatoxin-B(1)-Induced Experimental Hepatocellular Carcinoma. Int J Hepatol. 2012;2012:428373. doi: 10.1155/2012/428373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin Y, Ekmekcioglu S, Liu P, Duncan LM, Lizee G, Poindexter N, Grimm EA. Constitutive aberrant endogenous interleukin-1 facilitates inflammation and growth in human melanoma. Mol Cancer Res. 2011;9:1537–1550. doi: 10.1158/1541-7786.MCR-11-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naiki-Ito A, Asamoto M, Hokaiwado N, Takahashi S, Yamashita H, Tsuda H, Ogawa K, Shirai T. Gpx2 is an overexpressed gene in rat breast cancers induced by three different chemical carcinogens. Cancer Res. 2007;67:11353–11358. doi: 10.1158/0008-5472.CAN-07-2226. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Koiri RK, Trigun SK. Acute and chronic hyperammonemia modulate antioxidant enzymes differently in cerebral cortex and cerebellum. Neurochem Res. 2008;33:103–113. doi: 10.1007/s11064-007-9422-x. [DOI] [PubMed] [Google Scholar]
- 16.Koiri RK, Trigun SK. Dimethyl sulfoxide activates tumor necrosis factoralpha-p53 mediated apoptosis and down regulates D-fructose-6-phosphate-2-kinase and lactate dehydrogenase-5 in Dalton’s lymphoma in vivo. Leuk Res. 2011;35:950–956. doi: 10.1016/j.leukres.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Pirisi M, Leutner M, Pinato DJ, Avellini C, Carsana L, Toniutto P, Fabris C, Boldorini R. Reliability and reproducibility of the edmondson grading of hepatocellular carcinoma using paired core biopsy and surgical resection specimens. Arch Pathol Lab Med. 2010;134:1818–1822. doi: 10.5858/2009-0551-OAR1.1. [DOI] [PubMed] [Google Scholar]
- 18.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 20.Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 21.Smela ME, Currier SS, Bailey EA, Essigman JM. The chemistry and biology of aflatoxin B(1): from mutational spectrometry to carcinogenesis. Carcinogenesis. 2001;22:535–545. doi: 10.1093/carcin/22.4.535. [DOI] [PubMed] [Google Scholar]
- 22.Shang WQ, Yu JJ, Zhu L, Zhou WJ, Chang KK, Wang Q, Li MQ. Blocking IL-22, a potential treatment strategy for adenomyosis by inhibiting crosstalk between vascular endothelial and endometrial stromal cells. Am J Transl Res. 2015;7:1782–1797. [PMC free article] [PubMed] [Google Scholar]


