Abstract
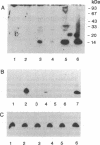
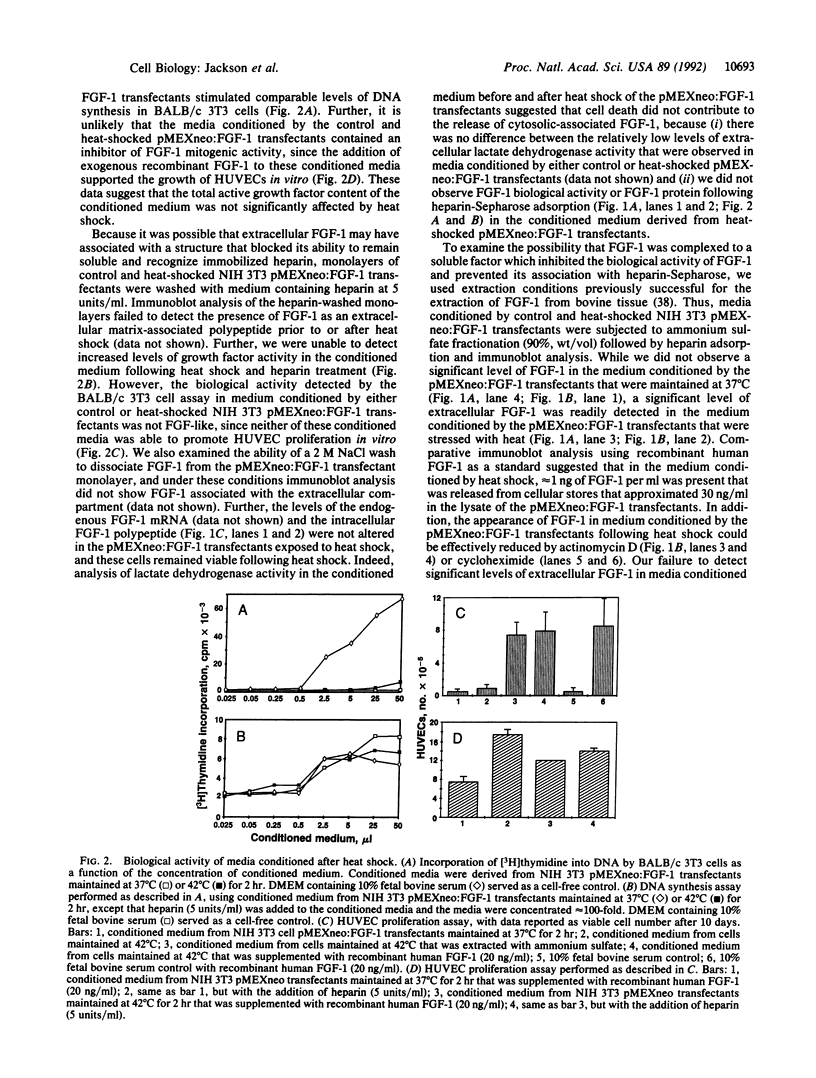
Fibroblast growth factor 1 (FGF-1) is a potent angiogenic and neurotrophic factor whose structure lacks a classical signal sequence for secretion. Although the initiation of these biological activities involves the interaction between FGF-1 and cell surface receptors, the mechanism responsible for the regulation of FGF-1 secretion is unknown. We report that murine NIH 3T3 cells transfected with a synthetic gene encoding FGF-1 secrete FGF-1 into their conditioned medium in response to heat shock. The form of FGF-1 released by NIH 3T3 cells in response to increased temperature (42 degrees C, 2 hr) in vitro is not biologically active and does not associate with either heparin or the extracellular NIH 3T3 monolayer matrix. However, it was possible to derive biologically active FGF-1 from the conditioned medium of heat-shocked NIH 3T3 cell transfectants by ammonium sulfate fractionation. The form of FGF-1 exposed by ammonium sulfate fractionation is similar in size to cytosolic FGF-1 and can bind and be eluted from immobilized heparin similarly to the recombinant human FGF-1 polypeptide. Further, the release of FGF-1 by NIH 3T3 cell transfectants in response to heat shock is reduced significantly by both actinomycin D and cycloheximide. These data indicate that increased temperature may upregulate the expression of a factor responsible for the secretion of FGF-1 as a biologically inactive complex that requires an activation step to exhibit the biological activity of the extracellular polypeptide mitogen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Brunner G., Gabrilove J., Rifkin D. B., Wilson E. L. Phospholipase C release of basic fibroblast growth factor from human bone marrow cultures as a biologically active complex with a phosphatidylinositol-anchored heparan sulfate proteoglycan. J Cell Biol. 1991 Sep;114(6):1275–1283. doi: 10.1083/jcb.114.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugler B., Amalric F., Prats H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol Cell Biol. 1991 Jan;11(1):573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Mehlman T., Friesel R., Johnson W. V., Maciag T. Multiple forms of endothelial cell growth factor. Rapid isolation and biological and chemical characterization. J Biol Chem. 1985 Sep 25;260(21):11389–11392. [PubMed] [Google Scholar]
- Chiang H. L., Terlecky S. R., Plant C. P., Dice J. F. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989 Oct 20;246(4928):382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Currie R. W. Effects of ischemia and perfusion temperature on the synthesis of stress-induced (heat shock) proteins in isolated and perfused rat hearts. J Mol Cell Cardiol. 1987 Aug;19(8):795–808. doi: 10.1016/s0022-2828(87)80390-5. [DOI] [PubMed] [Google Scholar]
- Currie R. W., White F. P. Trauma-induced protein in rat tissues: a physiological role for a "heat shock" protein? Science. 1981 Oct 2;214(4516):72–73. doi: 10.1126/science.7280681. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H., Mehta H. B., Barrieux A., Guth B. D., Neeley W. E., Ross J., Jr Ischemia of the dog heart induces the appearance of a cardiac mRNA coding for a protein with migration characteristics similar to heat-shock/stress protein 71. Circ Res. 1986 Jul;59(1):110–114. doi: 10.1161/01.res.59.1.110. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. Molecular basis of fever in humans. Am J Med. 1982 May;72(5):799–819. doi: 10.1016/0002-9343(82)90548-4. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Forough R., Engleka K., Thompson J. A., Jackson A., Imamura T., Maciag T. Differential expression in Escherichia coli of the alpha and beta forms of heparin-binding acidic fibroblast growth factor-1: potential role of RNA secondary structure. Biochim Biophys Acta. 1991 Nov 11;1090(3):293–298. doi: 10.1016/0167-4781(91)90192-o. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991 Jul 26;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Frey A. B., Levine A. J. Immunological evidence for the association of p53 with a heat shock protein, hsc70, in p53-plus-ras-transformed cell lines. Mol Cell Biol. 1987 Aug;7(8):2863–2869. doi: 10.1128/mcb.7.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T., Maciag T. Cyclooxygenase gene expression is down-regulated by heparin-binding (acidic fibroblast) growth factor-1 in human endothelial cells. J Biol Chem. 1991 Dec 15;266(35):24059–24063. [PubMed] [Google Scholar]
- Imamura T., Engleka K., Zhan X., Tokita Y., Forough R., Roeder D., Jackson A., Maier J. A., Hla T., Maciag T. Recovery of mitogenic activity of a growth factor mutant with a nuclear translocation sequence. Science. 1990 Sep 28;249(4976):1567–1570. doi: 10.1126/science.1699274. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Jurivich D. A., Sistonen L., Kroes R. A., Morimoto R. I. Effect of sodium salicylate on the human heat shock response. Science. 1992 Mar 6;255(5049):1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- Kandel J., Bossy-Wetzel E., Radvanyi F., Klagsbrun M., Folkman J., Hanahan D. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell. 1991 Sep 20;66(6):1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Koskinen P. J., Sistonen L., Evan G., Morimoto R., Alitalo K. Nuclear colocalization of cellular and viral myc proteins with HSP70 in myc-overexpressing cells. J Virol. 1991 Feb;65(2):842–851. doi: 10.1128/jvi.65.2.842-851.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. B., Smith C. W., Eskin S. G., McIntire L. V. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990 Jan 1;75(1):227–237. [PubMed] [Google Scholar]
- Maher P. A., Pasquale E. B. Heat shock induces protein tyrosine phosphorylation in cultured cells. J Cell Biol. 1989 Jun;108(6):2029–2035. doi: 10.1083/jcb.108.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarelli F., Raulais D., Courtois Y. Fibroblast growth factor phosphorylation and receptors in rod outer segments. EMBO J. 1989 Aug;8(8):2265–2273. doi: 10.1002/j.1460-2075.1989.tb08351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Mutational analysis of the human HSP70 protein: distinct domains for nucleolar localization and adenosine triphosphate binding. J Cell Biol. 1989 Nov;109(5):1947–1962. doi: 10.1083/jcb.109.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski K. L., Welch W. J., Morimoto R. I. Cell cycle-dependent association of HSP70 with specific cellular proteins. J Cell Biol. 1989 Feb;108(2):413–423. doi: 10.1083/jcb.108.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Pain D., Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988 Dec;107(6 Pt 1):2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak T. S., Jr Synthesis of a stress protein following transient ischemia in the gerbil. J Neurochem. 1985 Nov;45(5):1635–1641. doi: 10.1111/j.1471-4159.1985.tb07236.x. [DOI] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Plouët J., Mascarelli F., Loret M. D., Faure J. P., Courtois Y. Regulation of eye derived growth factor binding to membranes by light, ATP or GTP in photoreceptor outer segments. EMBO J. 1988 Feb;7(2):373–376. doi: 10.1002/j.1460-2075.1988.tb02823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto N., Talarico D., Florkiewicz R., Rifkin D. B. Selective expression of high molecular weight basic fibroblast growth factor confers a unique phenotype to NIH 3T3 cells. Cell Regul. 1991 Sep;2(9):699–708. doi: 10.1091/mbc.2.9.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renko M., Quarto N., Morimoto T., Rifkin D. B. Nuclear and cytoplasmic localization of different basic fibroblast growth factor species. J Cell Physiol. 1990 Jul;144(1):108–114. doi: 10.1002/jcp.1041440114. [DOI] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Forough R., Maier J. A., Case J. P., Jackson A., Engleka K., Maciag T., Wilder R. L. Detection of high levels of heparin binding growth factor-1 (acidic fibroblast growth factor) in inflammatory arthritic joints. J Cell Biol. 1990 Apr;110(4):1417–1426. doi: 10.1083/jcb.110.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G., Garaci E., Amici C. Prostaglandins with antiproliferative activity induce the synthesis of a heat shock protein in human cells. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8407–8411. doi: 10.1073/pnas.86.21.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins. J Biol Chem. 1990 Jul 25;265(21):12111–12114. [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R., Hughes C. S. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4843–4847. doi: 10.1073/pnas.81.15.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. A., Haudenschild C. C., Anderson K. D., DiPietro J. M., Anderson W. F., Maciag T. Heparin-binding growth factor 1 induces the formation of organoid neovascular structures in vivo. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7928–7932. doi: 10.1073/pnas.86.20.7928. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Carbone A., Welch W. J. Medium tumor antigen of polyomavirus transformation-defective mutant NG59 is associated with 73-kilodalton heat shock protein. J Virol. 1987 Feb;61(2):405–410. doi: 10.1128/jvi.61.2.405-410.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Spector D., Welch W. Differential distribution of the adenovirus E1A proteins and colocalization of E1A with the 70-kilodalton cellular heat shock protein in infected cells. J Virol. 1988 Nov;62(11):4153–4166. doi: 10.1128/jvi.62.11.4153-4166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. P. Protein synthesis in rat telencephalon slices: high amounts of newly synthesized protein found in association with brain capillaries. Neuroscience. 1980;5(1):173–178. doi: 10.1016/0306-4522(80)90084-6. [DOI] [PubMed] [Google Scholar]
- White F. P. The synthesis and possible transport of specific proteins by cells associated with brain capillaries. J Neurochem. 1980 Jul;35(1):88–94. doi: 10.1111/j.1471-4159.1980.tb12492.x. [DOI] [PubMed] [Google Scholar]