SUMMARY
The kinases RIPK1 and RIPK3 and the pseudo-kinase MLKL have been identified as key regulators of the necroptotic cell death pathway, although a role for MLKL within the whole animal has not yet been established. Here, we have shown that MLKL deficiency rescued the embryonic lethality caused by loss of Caspase-8 or FADD. Casp8−/−Mlkl−/− and Fadd−/−Mlkl−/− mice were viable and fertile but rapidly developed severe lymphadenopathy, systemic autoimmune disease and thrombocytopenia. These morbidities occurred more rapidly and with increased severity in Casp8−/−Mlkl−/− and Fadd−/−Mlkl−/− mice compared to Casp8−/−Ripk3−/− or Fadd−/−Ripk3−/− mice, respectively. These results demonstrate that MLKL is an essential effector of necroptosis in embryos caused by loss of Caspase-8 or FADD. Furthermore, they suggest that RIPK3 and/or MLKL may exert functions independently of necroptosis. It appears that non-necroptotic functions of RIPK3 contribute to the lymphadenopathy, autoimmunity and excess cytokine production that occur when FADD or caspase-8 mediated apoptosis is abrogated.
Keywords: necroptosis, MLKL, RIPK3, RIPK1, apoptosis, autoimmunity, lymphadenopathy
INTRODUCTION
Programmed necrosis or necroptosis is a form of regulated cell death that is thought to contribute to the host defense against viruses, and defects in this process may play a role in certain pathologies (Chan et al., 2014; Pasparakis and Vandenabeele, 2015). Similar to the death receptor induced apoptotic pathway, necroptosis can be triggered by certain members of the tumor necrosis factor (TNF) superfamily (e.g. TNF, FASL), and certain other extracellular and intracellular signals. TNF-induced necroptosis depends on receptor interacting protein kinase-1 (RIPK1) (Holler et al., 2000) and RIPK3 (Cho et al., 2009; He et al., 2009; Zhang et al., 2009). RIPK1 and RIPK3 interact through their RIP homotypic interaction motif (RHIM) and form a cytosolic complex required for induction of necroptosis (Cho et al., 2009; He et al., 2009; Vandenabeele et al., 2010). The pseudo-kinase, mixed lineage kinase domain-like (MLKL), is also essential for TNF-induced necroptosis in cells in culture. MLKL is a substrate of RIPK3 (Sun et al., 2012; Zhao et al., 2012) and together with RIPK1 and RIPK3 forms the necrosome. RIPK3-mediated phosphorylation triggers a conformational change in MLKL leading to the exposure of its N-terminal 4-helical bundle domain (Murphy et al., 2013; Orozco et al., 2014; Tanzer et al., 2015; Wu et al., 2014). This promotes oligomerization of MLKL and translocation to the plasma membrane (Cai et al., 2014; Chen et al., 2014; Dondelinger et al., 2014; Hildebrand et al., 2014; Su et al., 2014; Wang et al., 2014) where it causes, directly or indirectly, cell rupture with consequent release of the cellular content.
Recent evidence suggests that under certain conditions RIPK3 can initiate necroptosis independently of RIPK1 (Cook et al., 2014; Dillon et al., 2014; Kaiser et al., 2014; Moujalled et al., 2013; Orozco et al., 2014; Rickard et al., 2014; Wu et al., 2014). Similarly, in fibroblasts and mouse macrophages, Toll-like receptor (TLR) signaling proceeds independent of RIPK1 or its kinase activity, but it requires RIPK3 (He et al., 2011; Kaiser et al., 2013). Moreover, RIPK1 and RIPK3 can also regulate “death ligand” (e.g. TNF, FASL) induced apoptosis through activation of Caspase-8. For example, the kinase-inactive RIPK3 mutant D161N triggers apoptosis following assembly of an alternative Caspase-8-activating complex (Mandal et al., 2014; Newton et al., 2014). Ripk3D161N/D161N mutant mice are viable and rescue the embryonic lethality caused by loss of Caspase-8 (Mandal et al., 2014; Newton et al., 2014). However, not all kinase-inactive mutants of RIPK3 trigger apoptosis and additional factors are likely to be involved in determining the nature of the cell death that is triggered. In addition to its regulation of necroptotic and apoptotic cell death, RIPK3 can promote inflammation through its impact on cytokine and chemokine production in response to a number of stimuli (Kang et al., 2013; Lawlor et al., 2015; Vince et al., 2012; Young et al., 2007). In contrast, MLKL is so far only known to play a role in necroptosis (Allam et al., 2014; Murphy et al., 2013; Rickard et al., 2014).
To date, a role of MLKL in necroptosis within the whole animal has not been demonstrated. Here, we report that loss of MLKL prevented the embryonic lethality caused by loss of Caspase-8 or FADD (this embryonic lethality is due to excess necroptosis). Various cell types from Casp8−/−Mlkl−/− and Fadd−/−Mlkl−/− mice were resistant to diverse necroptotic cell death stimuli. Casp8−/−Mlkl−/− and Fadd−/−Mlkl−/− mice rapidly developed pronounced splenomegaly and lymphadenopathy, with a marked increase in CD3+CD4−CD8−B220+ T cells, resembling the abnormalities observed in animals lacking functional FASL or FAS. Compared with Casp8−/−Ripk3−/− or Fadd−/−Ripk3−/− mice, the Casp8−/−Mlkl−/− or Fadd−/−Mlkl−/− mice displayed an increased severity of lymphadenopathy and autoimmune manifestations. Thus, our data suggest that RIPK3 and MLKL differ in their contribution to lymphadenopathy and autoimmune disease caused by loss of Caspase-8 or FADD and this can be explained by their possible roles independent of necroptosis.
RESULTS
MLKL deficiency prevents the embryonic lethality caused by loss of Caspase-8 or FADD
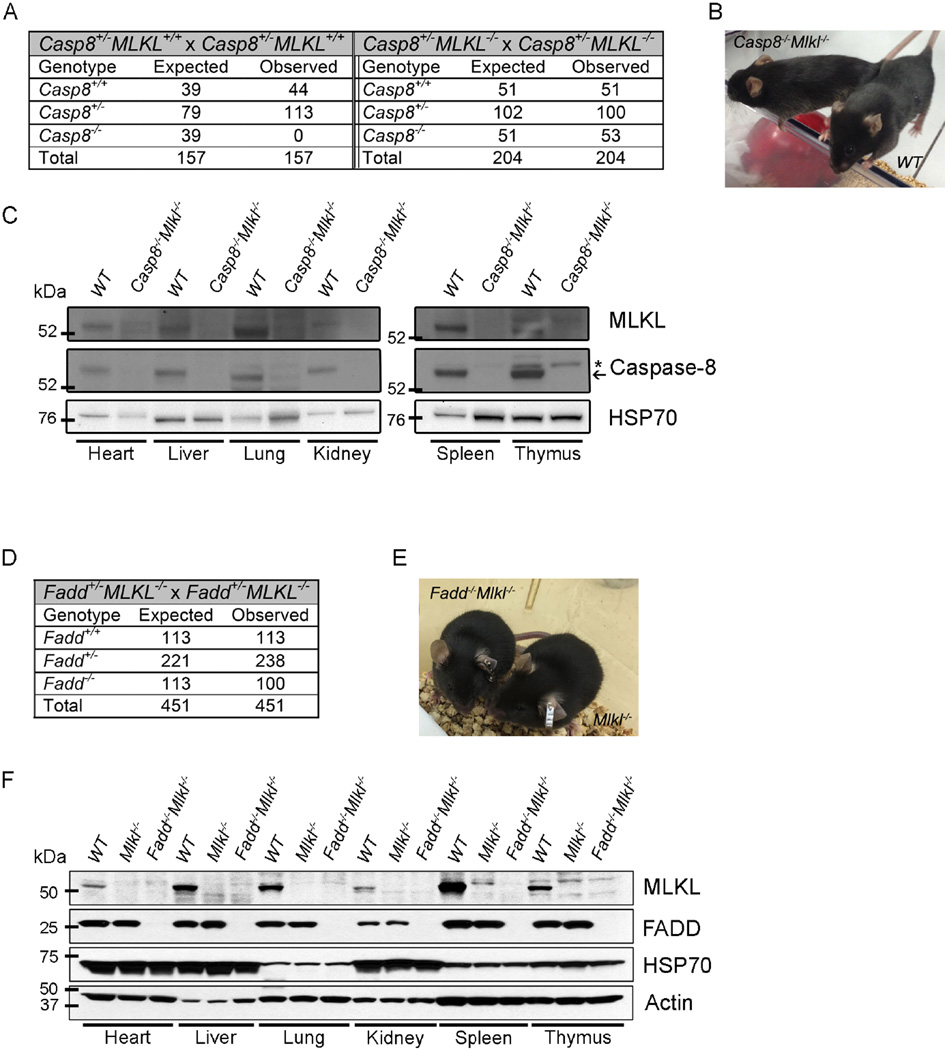
Loss of Caspase-8 or FADD causes embryonic lethality at ~E10.5 due to defects in vascular development (Varfolomeev et al., 1998; Yeh et al., 1998). Previously it has been shown that concomitant loss of RIPK1 or RIPK3 overcomes the embryonic lethality caused by loss of Caspase-8 or FADD by preventing abnormal necroptosis (Dillon et al., 2012; Kaiser et al., 2011; Oberst et al., 2011; Zhang et al., 2011). Recent studies have shown that the kinase activity of RIPK3 is required for the prevention of the embryonic lethality caused by the necroptosis elicited by loss of Caspase-8 or FADD (Mandal et al., 2014; Newton et al., 2014). To investigate the key role of MLKL, an important substrate for RIPK3 kinase activity, in necroptosis in a physiological context, we generated Casp8−/−Mlkl−/− double deficient mice (on a C57BL/6 background) by serial intercrossing of Casp8+/− and Mlkl+/− animals. Intercrosses of Casp8+/−Mlkl−/− mice yielded Casp8−/−Mlkl−/− offspring at the expected Mendelian ratio but parallel intercrosses of Casp8+/−Mlkl+/− or Casp8+/−Mlkl+/+ mice produced no viable Casp8−/−Mlkl+/− or Casp8−/−Mlkl+/+ offspring (Figure 1A). Adult Casp8−/−Mlkl−/− mice were viable and generated ostensibly normal offspring (Figure 1A and B). Immunoblot analysis of cell lysates confirmed that both MLKL and Caspase-8 were absent in tissues of Casp8−/−Mlkl−/− mice (Figure 1C). Similarly, intercrosses of Fadd+/−Mlkl−/− mice yielded Fadd−/−Mlkl−/− offspring at the expected Mendelian ratio (Figure 1D). cFLIPL (encoded by Cflar) functions to inhibit Caspase-8-mediated apoptosis and forms an enzymatically active heterodimer with Caspase-8 to prevent necroptosis (Oberst et al., 2011). Ablation of cFLIP results in the same early embryonic lethality (driven by excess necroptosis) observed in Caspase-8- or FADD-deficient animals (Yeh et al., 2000). While deletion of RIPK3 does not promote survival of cFLIP-deficient mice, Cflar−/−Fadd−/−Ripk3−/− mice are present at the expected Mendelian ratios at weaning (Dillon et al., 2012). We found that embryonic lethality caused by loss of cFLIP was similarly rescued by breeding into the Fadd−/−Mlkl−/− background (Figure S1A).
Figure 1. Casp8−/−Mlkl−/− mice are viable.
(A) Expected and observed frequency of mice of the indicated genotypes in offspring from crosses of mice with the indicated genotypes.
(B) Photograph of a 14-week-old Casp8−/−Mlkl−/− mouse bred from a double deficient cross alongside a wild-type (WT) mouse.
(C) Immunoblotting for Caspase-8, MLKL and HSP70 (loading control) from solid organs (left panel) and lymphoid tissues (right panel) of mice of the indicated genotypes.
(D) Expected and observed frequency of mice of the indicated genotypes in offspring from crosses of mice with the indicated genotypes.
(E) Photograph of an 8-week-old Fadd−/−Mlkl−/− mouse alongside a littermate control mouse.
(F) Immunoblotting for FADD, MLKL, Actin and HSP70 (last two used as loading controls) from solid organs and lymphoid tissues of mice of the indicated genotypes.
See also Figure S1.
Ripk1−/−Fadd−/− mice show early postnatal lethality (Zhang et al., 2011), while Ripk1−/−Fadd−/−Ripk3−/− mice survive into adulthood (Dillon et al., 2014). We found that Ripk1−/−Fadd−/−Mlkl−/− mice were similarly healthy (Figure S1C). Fadd−/−Mlkl−/−, Cflar−/−Fadd−/−Mlkl−/− and Ripk1−/−Fadd−/−Mlkl−/− mice were indistinguishable from their control littermates in early adulthood (Figure 1E, S1B, and S1D). Immunoblot analysis of cell lysates confirmed that both MLKL and FADD were absent in all tissues of Fadd−/−Mlkl−/− mice tested (Figure 1F). Collectively, these results show that loss of MLKL prevents the embryonic lethality caused by loss of Caspase-8, FADD, the embryonic lethality seen in mice lacking FADD and cFLIP, or the early post-natal lethality observed in animals deficient in FADD and RIPK1.
MLKL deficient cells are resistant to diverse necrotic cell death stimuli
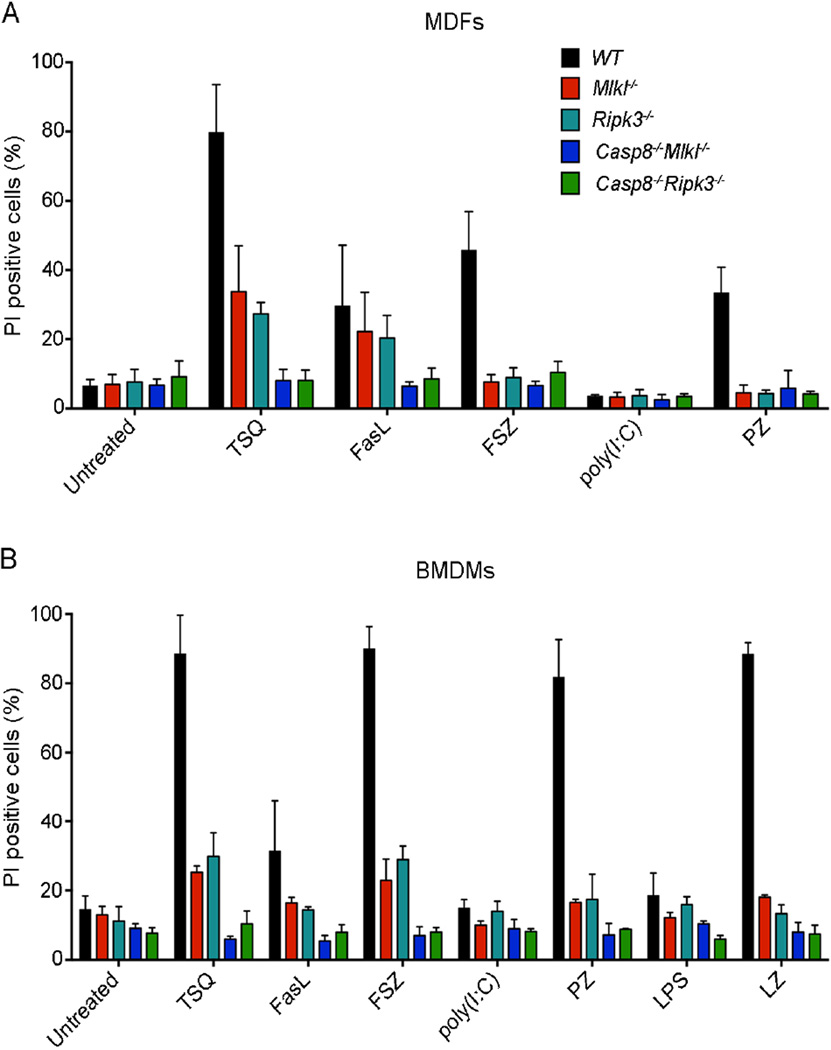
The role of MLKL in TNF-induced necroptosis was first demonstrated using siRNA mediated silencing or a chemical inhibitor (Sun et al., 2012; Zhao et al., 2012), and more recently by using cultured cells from Mlkl−/− mice (Murphy et al., 2013; Wu et al., 2013). To study the contribution of MLKL to diverse necroptotic cell death stimuli, we generated Casp8−/−Mlkl−/− mouse dermal fibroblasts (MDFs) and bone-marrow-derived macrophages (BMDMs) and treated them in vitro with TNF, FASL and ligands for TLR3 or TLR4. We also generated Casp8−/−Ripk3−/− MDFs and bone marrow derived macrophages (BMDMs) and used them as a positive control for resistance to necroptosis. We found that Casp8−/−Mlkl−/− MDFs and BMDMs were resistant to all necroptotic stimuli and responded in a similar way to Casp8−/−Ripk3−/− MDFs and BMDMs (Figure 2A and B). Similarly, primary early passage Fadd−/−Mlkl−/− mouse embryonic fibroblasts (MEFs) were resistant to both, necroptosis induced by TNF plus the pan-caspase inhibitor Z-VAD-FMK (zVAD) as well as death receptor induced apoptosis induced by TNF plus cycloheximide (CHX), although CHX treatment alone was somewhat toxic in these cells (Figure S2A and B). These results demonstrate that MLKL is critical for necroptotic cell death triggered by diverse upstream stimuli.
Figure 2. Cells from Casp8−/−Mlkl−/− mice are resistant to diverse necroptotic cell death stimuli.
(A and B) Primary MDFs (A) and BMDMs (B) from the indicated genotypes were treated with TNF (100 ng/mL), FASL (10 ng/mL), poly(I:C) (25 µg/mL) or LPS (25 µg/mL) in the presence or absence of the caspase inhibitor Q-VD-OPh (25 µM), z-VAD-FMK (20 µM) or SMAC mimetic (CpdA: 500 nM) or were left untreated for 24 h. Cell viability was determined by staining with propidium iodide (PI) and flow cytometric analysis. Data are presented as mean ± SEM (n = 3 for each genotype). *P<0.05, **P<0.001, ***P<0.0001.
See also Figure S2.
Casp8−/−Mlkl−/− and Fadd−/−Mlkl−/− mice develop progressive lymphadenopathy
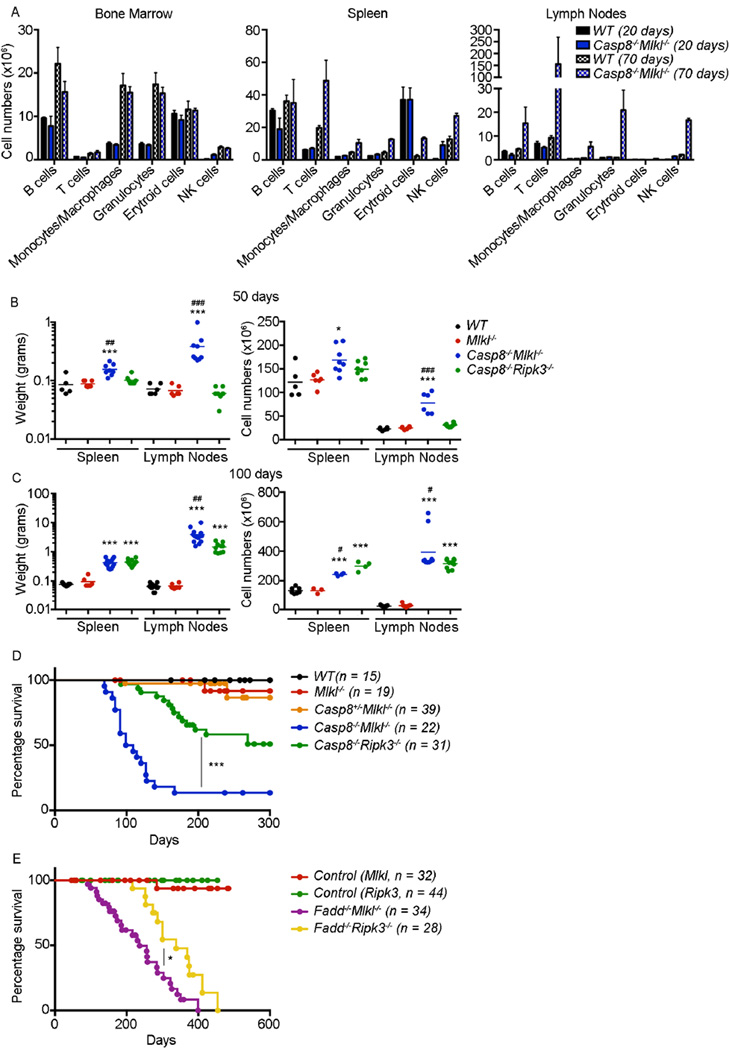
Caspase-8 is essential for FAS-mediated apoptosis (Salmena et al., 2003; Varfolomeev et al., 1998) and mice lacking Caspase-8, if protected from embryonic lethality by concomitant loss of RIPK3, develop lymphadenopathy (Kaiser et al., 2011; Oberst et al., 2011; Zhang et al., 2011), resembling that caused by defects in FAS-mediated apoptosis in mice (O’Reilly et al., 2009; Watanabe-Fukunaga et al., 1992) and humans (ALPS) (Rieux-Laucat et al., 1995). Casp8−/−Mlkl−/− mice developed normally and the analysis of the immune cells in bone marrow, spleen and lymph nodes at 20 days of age revealed no differences when compared to wild-type mice (Figure 3A and S3A). However, at 70 days of age the numbers of the different immune subsets were higher in spleen and lymph nodes, although they were normal in the bone marrow (Figure 3A and S3A). Casp8−/−Mlkl−/− mice developed progressive lymphadenopathy. After only 50 days the splenic and lymph node weights from Casp8−/−Mlkl−/− mice were increased 2- to 4-fold compared to those from wild-type, Mlkl−/− and Casp8+/−Mlkl−/− littermates (Figure 3B). At 100 days of age the spleen weights of the Casp8−/−Mlkl−/− mice were increased ~5-fold and the lymph nodes ~50-fold compared to those from wild-type, Mlkl−/− and Casp8+/−Mlkl−/− controls (Figure 3C and S3B). The lymphadenopathy in Casp8−/−Mlkl−/− mice appeared more severe than that previously reported in Casp8−/−Ripk3−/− mice (Kaiser et al., 2011; Oberst et al., 2011). However, this difference might be explained by the use of mice with a different genetic background (C57BL/6 vs mixed background). To investigate this rigorously, we compared our Casp8−/−Ripk3−/− mice on a C57BL/6 background to our Casp8−/−Mlkl−/− mice on the same genetic background. At 100 days of age spleens from both strains displayed a similar size but the lymph nodes were significantly enlarged (2- to 6-fold) in the Casp8−/−Mlkl−/− compared to the Casp8−/−Ripk3−/− mice (Figure 3C). In younger, 50 day old Casp8−/−Ripk3−/− mice, the lymphoid organs appeared overtly normal, whereas in similarly aged Casp8−/−Mlkl−/− mice the lymphoid organs were already significantly enlarged between 2- to 5-fold (Figure 3B and C). Thymus size was not altered in mice of any of the genotypes examined (Figure S3C). In accordance with the accelerated development of lymphadenopathy, the Casp8−/−Mlkl−/− mice died significantly earlier compared to the Casp8−/−Ripk3−/− mice (Figure 3D). Fadd−/−Mlkl−/− mice also developed lymphadenopathy (Figure S3D) and died significantly earlier compared to the Fadd−/−Ripk3−/− mice (Figure 3E). The median survival was longer in the two Fadd compound mutant mice as compared to the Casp8−/−Mlkl−/− and Casp8−/−Ripk3−/− mice (Figure 3D and E). We attribute this to the different facilities (and thus possible differences in microbiota) in which these animals were bred and maintained. It is also important to note, however, that unlike the Casp8−/−Mlkl−/− and Casp8−/−Ripk3−/− mice, the Fadd−/− animals were not fully backcrossed and retained some 129SV strain contribution. However, C57BL/6lpr/lpr and 129SVlpr/lpr mice showed comparable kinetics of disease, suggesting that the differences in disease observed between the Fadd−/−Mlkl−/− and Fadd−/−Ripk3−/− mice were due to differences in the presence or absence of RIPK3 and MLKL, as seen in the Caspase-8 and MLKL double deficient animals.
Figure 3. Casp8−/−Mlkl−/− mice develop progressive severe lymphadenopathy.
(A) Cross section of immune cells in bone marrow, spleen and lymph nodes from mice of the indicated genotypes at 20 days and 70 days of age (n = 3). Different cell subsets were determined by flow cyotmetry using the following markers: B cells (B220+ or CD19+), T cells (CD3+), Monocytes and Macrophages (MAC-1+), Granulocytes (GR-1+), Erythroid cells (TER119+) and NK cells (NK1.1+ and NKp46+). Data represent mean ± SD (n = 3). (B and C) Weight and total cell numbers of the lymph nodes (axillary, brachial, inguinal and mesenteric) and spleen from mice of the indicated genotypes at, (A) 50 days, and (B) 100 days. *P<0.05, ***P<0.0001 compared to WT mice; #P<0.05, ##P<0.01, ###P<0.0001 compared to Casp8−/−Ripk3−/− mice.
(D) Kaplan-Meyer survival curves for WT (n = 15), Mlkl−/− (n = 19), Casp8+/−Mlkl−/− (n = 39), Casp8−/−Mlkl−/− (n = 22) and Casp8−/−Ripk3−/− (n = 31) mice.
(E) Kaplan-Meyer survival curves for control (MLKL) (n=32, FaddwtMlkl−/− or Fadd+/−Mlkl−/−), Fadd−/−Mlkl−/− (n = 34), control (RIP3) (n=44, FaddwtRipk3−/− or Fadd+/−Ripk3−/−), and Fadd−/− Ripk3−/− (n = 28) mice. Survival of mice of different genotypes was compared by log-rank test. **P<0.001, ***P<0.0001.
See also Figure S3.
Casp8−/−Mlkl−/− and Fadd−/−Mlkl−/− mice accumulate CD3+CD4−CD8−B220+ T cells
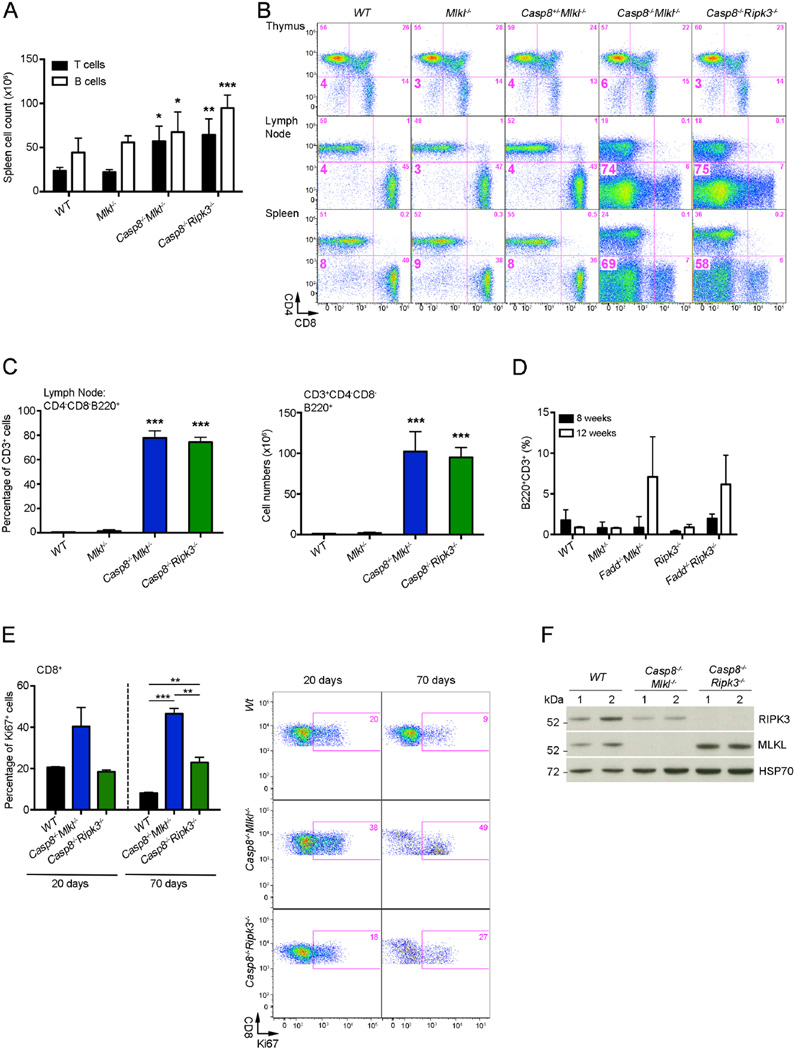
Consistent with their splenomegaly and lymphadenopathy, Casp8−/−Mlkl−/− mice had abnormally increased numbers of leukocytes in these lymphoid tissues. These cells were mostly CD3+ T cells but the numbers of CD19+ B cells were also increased (Figure 4A). The Casp8−/−Mlkl−/− mice had normal distribution of T cell sub-populations in the thymus, but with increasing age they accumulated large numbers of ‘unusual’ CD3+CD4−CD8−B220+ Double Negative (DN) T cells in their secondary lymphoid organs (Figure 4B and C). Similarly, Fadd−/−Mlkl−/− and Fadd−/−Ripk3−/− mice also accumulated increased numbers of such DN T cells (Figure 4D). Mice and humans with defects in FAS-mediated apoptosis (e.g. Faslpr/lpr, Casp8−/−Ripk3−/−, and Fadd−/−Ripk3−/− mutant mice) accumulate large numbers of double negative (DN) T cells that are thought to be descendants of previously activated CD3+CD4−CD8+ T cells that have failed to undergo programmed cell death (Dillon et al., 2012; Kaiser et al., 2011; O’Reilly et al., 2009; Oberst et al., 2011; Siegel et al., 2000). We studied the proliferation of CD3+CD8+ T cells from 20 and 70 days old mice by flow cytometric analysis of Ki67 expression. At 20 days of age the percentages of Ki67+ cells were slightly higher in Casp8−/−Mlkl−/− mice compared to Casp8−/−Ripk3−/− and wild-type mice (Figure 4E). At 70 days of age, increased percentages and numbers of Ki67+ CD3+CD8+ T cells were found in both Casp8−/−Mlkl−/− and Casp8−/−Ripk3−/− mice compared to wild-type controls. Moreover, the proliferation rates (Ki67+ cells) of Casp8−/−Mlkl−/− CD3+CD8+ T cells were higher compared to Casp8−/−Ripk3−/− CD3+CD8+ T cells (Figure 4E). Immunoblot analysis revealed that the DN T cells that had accumulated in the lymph nodes of Casp8−/−Mlkl−/− and Casp8−/−Ripk3−/− expressed similar amounts of MLKL and RIPK3 (unless the corresponding gene was deleted) compared to the normal T cells from wild-type mice (Figure 4F). Casp8−/−Mlkl−/− and Casp8−/−Ripk3−/− mice had significantly lower numbers of circulating lymphocytes compared with control mice (Figure S4A), possibly because many of them were trapped in the enlarged lymph nodes and spleen. Lower numbers of circulating lymphocytes were also found in Fadd−/−Mlkl−/− mice (Figure S4B).
Figure 4. Casp8−/−Mlkl−/− mice accumulate ‘unusual’ B220+CD3+CD4−CD8− T-cells.
(A) Numbers of B and T cells recovered from spleens from mice of the indicated genotypes. Values represent mean ± SD (n = 3–5 mice/genotype), *P<0.05, **P<0.001, ***P<0.0001.
(B) Representative flow cytometric images of the ‘unusual’ DN T cells (numbers indicate percentage of cells in each quadrant) (n=4).
(C) The percentages and numbers of the ‘unusual’ DN T cells in the lymph nodes of mice of the indicated genotypes were measured by flow cytometric analysis. Values represent mean ± SD (n = 3–5 mice/genotype), *P<0.05, **P<0.001, ***P<0.0001.
(D) The percentages and numbers of the ‘unusual’ DN T cells in the peripheral blood of mice of the indicated genotypes were measured by flow cytometric analysis. Values represent mean ± SD (n = 3–5 mice/genotype).
(E) Proliferation of CD3+CD8+ T cells from mice of the indicated genotypes was determined by staining for Ki67 after fixation and permeabilization. Percentages of Ki67+ cells in 20 days and 70 days old mice of the indicated genotypes (n = 3) (left) and representative flow cytometric images of the CD3+CD8+ T cells (numbers indicate percentage of cells in each quadrant) (n = 3) (right) are shown. Data are presented as mean ± SD, for each genotype **P<0.001, ***P<0.0001.
(F) Immunoblotting for RIPK3, MLKL and HSP70 (loading control) in extracts from purified DN T cells (Casp8−/−Mlkl−/− and Casp8−/−Ripk3−/− mice) or total T cells (wild-type mice). Two mice per genotype were used.
See also Figure S4.
Casp8−/−Mlkl−/− and Fadd−/−Mlkl−/− mice develop autoimmune disease and thrombocytopenia
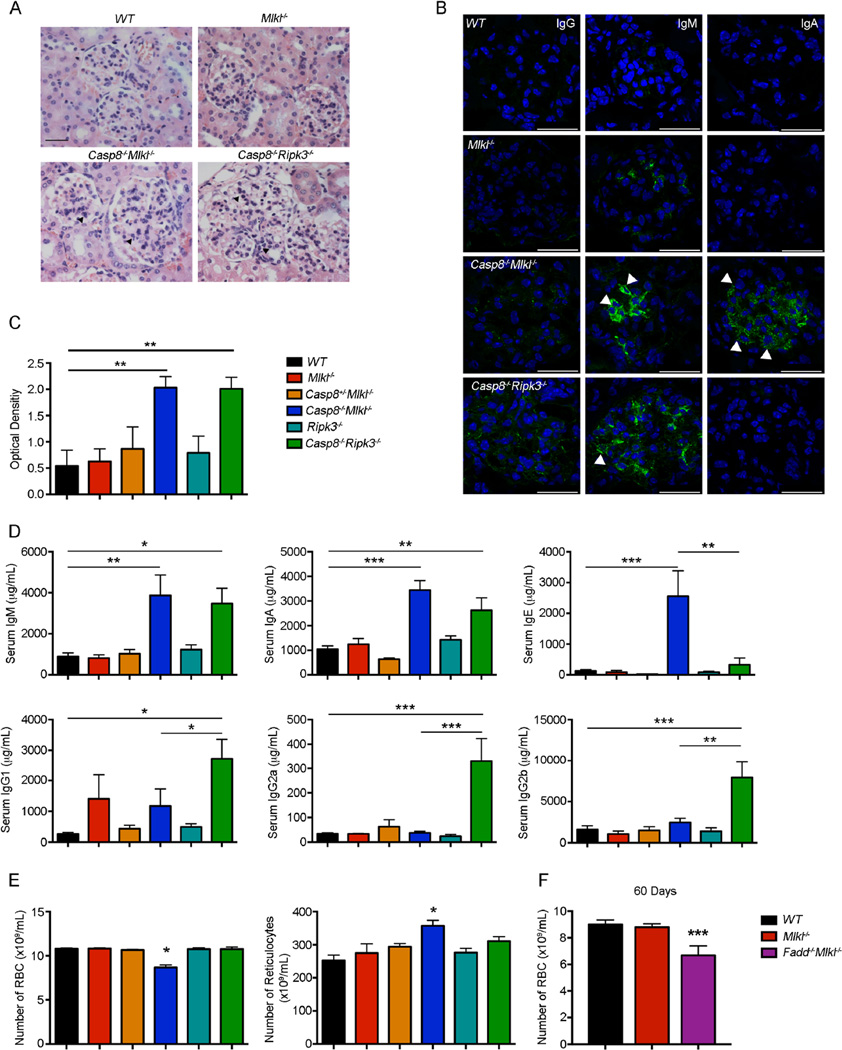
The development of autoimmune glomerulonephritis has been well documented in FASL-or FAS-deficient mice and humans (O’Reilly et al., 2009; Rieux-Laucat et al., 1995; Watanabe-Fukunaga et al., 1992). We therefore examined whether Casp8−/−Mlkl−/− mice also develop glomerulonephritis. Kidneys from 100-day-old Casp8−/−Mlkl−/− mice showed signs of early glomerulonephritis, characterized by glomerular enlargement, and leukocyte infiltration (Figure 5A). This pathology was also evident in the kidneys from age-matched Casp8−/−Ripk3−/− mice but was not seen in the parental Mlkl−/− or in wild-type mice (Figure 5A). Immunostaining and confocal microscopy revealed moderate IgM and IgA and mild IgG deposition in the renal glomeruli of diseased Casp8−/−Mlkl−/− mice, whereas in diseased Casp8−/−Ripk3−/− mice the Ig deposition consisted primarily of IgM, to a lesser extent IgG but not IgA (Figure 5B). ELISA analysis revealed that the concentration of antinuclear auto-antibodies (ANA) in the sera of Casp8−/−Mlkl−/− and Casp8−/−Ripk3−/− mice was similarly elevated compared to control mice (Figure 5C). Moreover, mice lacking MLKL and Caspase-8 had abnormally elevated concentrations of IgM, IgA and IgE, whereas in mice lacking RIPK3 and Caspase-8 the concentrations of all Ig isotypes, with the exception of IgE, were elevated (Figure 5D).
Figure 5. Casp8−/−Mlkl−/− mice develop autoimmune glomerulonephritis.
(A) Representative H&E-stained sections of the kidneys from mice of the indicated genotypes were examined for pathological changes, such as hyper-cellularity, glomerular enlargement or leukocyte infiltration. Kidneys from 6 mice of each genotype were analyzed. Magnification × 40.
(B) Representative confocal photomicrographs of frozen sections (kidneys from 3 mice of each genotype analyzed) stained for the presence of IgA-, IgG- or IgM-containing immune complexes (green) in glomeruli. Nuclei are revealed by staining with DAPI (blue). Scale bars represent 25 µm.
(C and D) Sera of mice of the indicated genotypes were collected and the concentrations of (C) ANA or (D) total antibodies of the different Ig isotypes were quantified by ELISA (age 100 days). Values in graphs represent mean ± SEM (n = 6–13 mice/genotype), *P<0.05, **P<0.001, ***P<0.0001.
(E) RBC and reticulocyte numbers were measured in blood samples of mice of the indicated genotypes. Data represent mean ± SD (n = 9–53 mice/genotype), *P<0.05, **P<0.001, ***P<0.0001.
(F) RBC numbers were measured in blood samples of mice of the indicated genotypes at 60 days of age. Data represent mean ± SD (n = 5 mice/genotype).
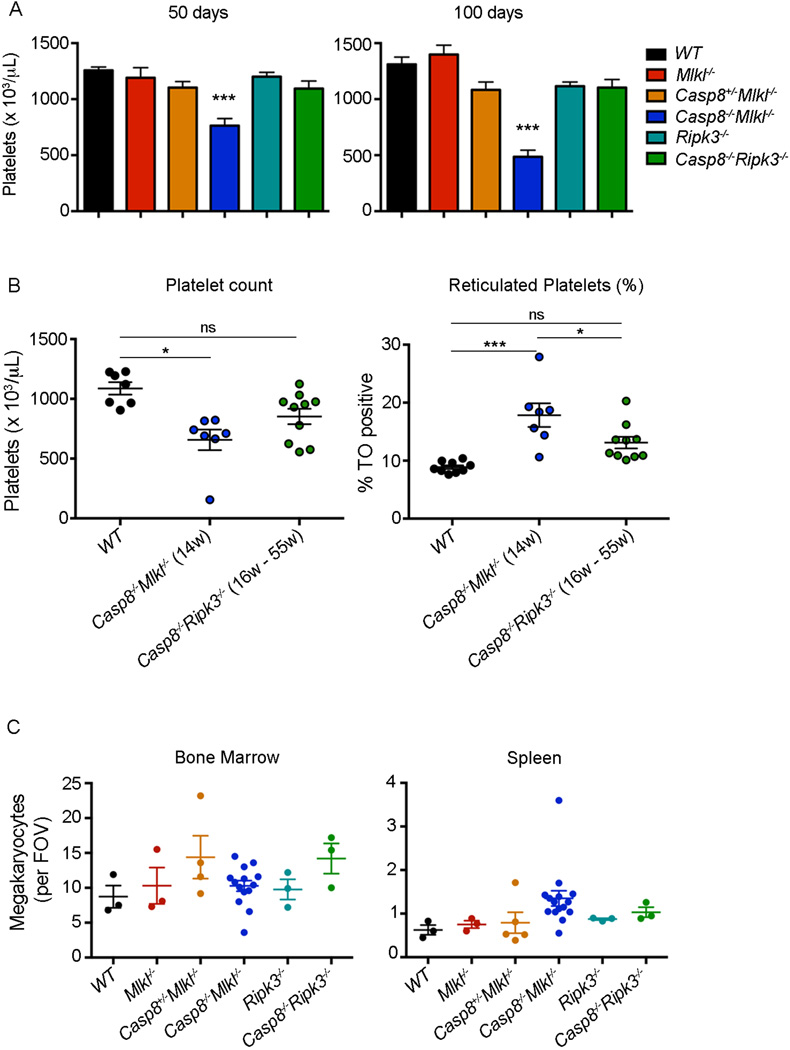
Red blood cell (RBC) numbers were abnormally low in diseased Casp8−/−Mlkl−/− mice and, accordingly, reticulocytes were significantly higher in these animals (Figure 5E). Casp8−/−Mlkl−/− mice also presented with low platelet counts at 50 days of age and the thrombocytopenia became more severe as they became diseased. These abnormalities were not observed in age-matched Casp8−/−Ripk3−/− mice (Figure 6A). Accordingly, the percentages of reticulated platelets were higher in Casp8−/−Mlkl−/− mice than in control mice (Figure 6B). Megakaryocyte numbers and morphology were normal in the bone marrow, albeit somewhat increased in the spleens of Casp8−/−Mlkl−/− mice, in comparison with WT controls (Figure 6C). Reduced RBC and platelet counts were also seen in Fadd−/−Mlkl−/− as early as 60 days (Figures 5F and data not shown). These results show that combined loss of MLKL and Caspase-8 or MLKL and FADD causes autoimmune pathology that is more severe compared to mice lacking RIPK3 and Caspase-8.
Figure 6. Casp8−/−Mlkl−/− mice have abnormally low platelet counts.
(A) Platelet counts in mice of the indicated genotypes at 50 days (left) and 100 days (right) of age. Data represent mean ± SD (n = 6–19 mice/genotype), *P<0.05, **P<0.001, ***P<0.0001.
(B) Platelet counts (left) and percentages of reticulated platelets (right) in mice of the indicated genotypes and age. Data represent mean ± SEM (n = 7–10 mice/genotype), *P<0.05, **P<0.001, ***P<0.0001.
(C) Megakaryocyte counts in H&E-stained sections of bone marrow (left) and spleen (right). Data represent mean per field of view (FOV; × 200) from 10 fields per individual mouse (n = 3–14 mice/genotype).
Casp8−Mlkl− derived T cells produce abnormally increased amounts of proinflammatory cytokines and chemokines
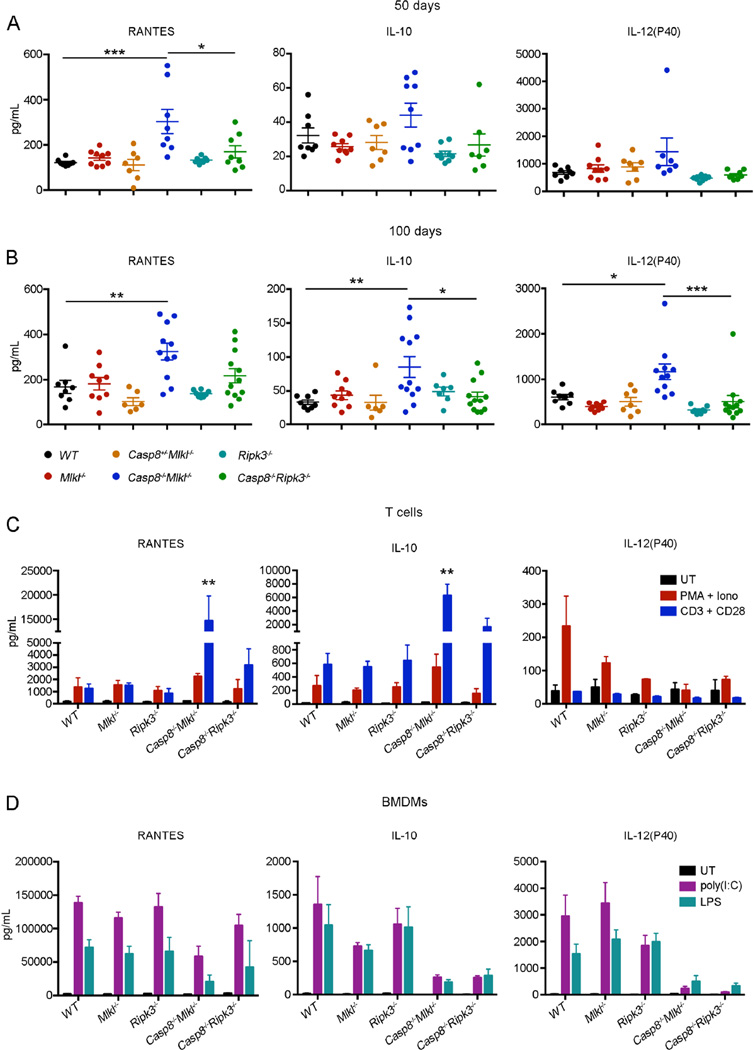
In both human autoimmune lymphoproliferative syndrome (ALPS) patients and FASL- or FAS-deficient mice, lymphadenopathy with accumulation of TCRβ+CD4−CD8−B220+ T cells is accompanied by abnormally increased amounts of certain pro-inflammatory cytokines (O’Reilly et al., 2009; Oliveira et al., 2010; Watanabe et al., 1995). We analyzed the serum concentrations of 23 cytokines and chemokines and found that RANTES (CCL5) was significantly elevated in Casp8−/−Mlkl−/− mice compared to Casp8−/−Ripk3−/− and wild-type mice at both 50 and 100 days of age (Figure 7A and B). The concentrations of IL-10 and IL-12p40 were slightly higher in young Casp8−/− Mlkl−/− mice and IL-10 increased significantly with age (Figure 7A and B). Other cytokines, including TNFα, IFN-γ, IL-6 or IL-1β, were normal in these animals (Figure S6A and B). Macrophages and T cells are two of the main subtypes of immune cells responsible for cytokine production. In order to determine the contribution of each of these two subsets to the abnormally increased concentrations of cytokines in Casp8−/−Mlkl−/− mice, we evaluated the production of cytokines by BMDMs and purified T cells after stimulation with LPS or poly(I:C) and PMA plus ionomycin or CD3 plus CD28 antibodies, respectively. Significantly higher amounts of RANTES, IL-10, MIP-1α and MIP-1β were detected in CD3 plus CD28 antibody stimulated T cells from Casp8−/−Mlkl−/− mice compared to T cells from Casp8−/− Ripk3−/− and WT mice (Figure 7C and S7). Stimulation of T cells with PMA and ionomycin led to a slight increase in the production of RANTES and IL-10 but no difference was observed in BMDMs (Figure 7C, D, S7 and S8). These results show that the abnormal serum concentrations of pro-inflammatory cytokines and chemokines are mainly due to the overproduction of these factors by T cells. Collectively, these findings indicate that Casp8−/−Mlkl−/− mice develop lymphadenopathy and autoimmune disease more rapidly and aggressively than Casp8−/−Ripk3−/− mice, because RIPK3 in T cells of the former enhances production of cytokines and chemokines that promote disease.
Figure 7. Casp8−/−Mlkl−/− mice have abnormally increased amounts of certain proinflammatory cytokines and chemokines.
(A and B) The concentrations of 23 cytokines and chemokines (indicated in Experimental Procedures) were measured in the sera of mice of the indicated genotypes by the multiplex system at, (A) 50 days when animals still appeared healthy, or (B) 100 days of age when Casp8−/−Mlkl−/− mice were showing clear signs of illness. The concentrations of RANTES, IL-10 and IL-12p40 are shown. Each dot represents a single mouse; the bar indicates the average; the error bars represent SEM. *P<0.05, **P<0.001, ***P<0.0001.
(C and D) 23 cytokines and chemokines were measured in the supernatants of purified T cells activated with PMA (2 ng/mL) plus ionomycin (10 ng/mL) or CD3 (10 µg/mL) plus CD28 (10 µg/mL) antibodies (C) or BMDMs stimulated with poly(I:C) (25 µg/mL) or LPS (25 µg/mL) (D) from mice of the indicated genotypes. The concentration of RANTES, IL-10 and IL-12p40 are shown. Data represent mean ± SD (n = 3 mice/genotype). **P<0.001 Casp8−/−Mlkl−/− mice compared to any of the other genotypes.
See also Figure S5–S7.
DISCUSSION
Necroptosis has emerged as an alternative programmed cell death process to apoptosis that may have critical functions in health and disease. We now know that this process requires RIPK1, RIPK3 and its substrate MLKL. Although the role of the pseudo-kinase, MLKL, downstream of RIPK3 in necroptosis has been validated in vitro, so far no evidence for its role in a physiological context has been provided. Casp8−/−Mlkl−/− double deficient mice were viable and matured into fertile adults, but they rapidly developed severe lymphadenopathy similar to FASL- or FAS-deficient, Casp8−/−Ripk3−/− and Fadd−/−Ripk3−/− mice as well as FAS-deficient humans (Dillon et al., 2012; Kaiser et al., 2011; O’Reilly et al., 2009; Oberst et al., 2011; Rieux-Laucat et al., 1995; Watanabe-Fukunaga et al., 1992). Casp8−/−Mlkl−/− mice succumbed significantly earlier than Casp8−/−Ripk3−/− mice due to a more rapid onset of severe lymphadenopathy and autoimmune pathology. This acceleration was mainly due to an increased proliferation rate of CD3+CD8+ T cells in the Casp8−/−Mlkl−/− mice. Moreover, Casp8−/−Mlkl−/− derived T cells secreted strikingly higher amounts of certain pro-inflammatory cytokines and chemokines, such as RANTES and IL-10, when stimulated with CD3 plus CD28 antibodies. RANTES and IL-10 were also elevated in the sera of Casp8−/−Mlkl−/− mice, most likely due to T cell-mediated secretion, while in Casp8−/−Ripk3−/− mice the amounts of these factors were unremarkable. The chemokine RANTES plays an essential role in inflammation by recruiting T cells, macrophages and eosinophils to inflammatory sites (Rot et al., 1992; Schall et al., 1990). Accordingly, RANTES has been implicated in a number of inflammatory diseases, such as asthma, atherosclerosis and rheumatoid arthritis (Pattison et al., 1996; Powell et al., 1996; Rathanaswami et al., 1993). It has recently been shown that HeLa cells that were protected from TNF induced cell death secreted large amounts of RANTES and certain other chemokines and cytokines (Kearney et al., 2013). Remarkably, in this setting IAP (Inhibitor of Apoptosis Protein) antagonism influenced TNF-induced production of RANTES and even led to spontaneous production of this chemokine (Wong et al., 2014). Although transient overexpression of RIPK1 in tumor cells enhances production of cytokines and chemokines, including RANTES, the silencing of RIPK1 using RNA interference does not diminish RANTES expression. (Kearney et al., 2013). Since in our mice neither IAPs nor RIPK1 expression has been altered, it appears likely that RIPK3 plays a critical role in driving the expression of RANTES.
Our study shows that Casp8−/−Mlkl−/− mice developed progressive thrombocytopenia as they became diseased. Low platelet counts can be caused by impaired production of platelets by megakaryocytes in the bone marrow, viral infection, splenic sequestration or increased platelet destruction (commonly mediated by auto-antibodies). Thus, the thrombocytopenia observed in the Casp8−/−Mlkl−/− mice might be explained by the enlarged spleen or the production of auto-antibodies. Indeed, autoimmune thrombocytopenia is a characteristic of human ALPS (Teachey et al., 2005). However, our Casp8−/−Ripk3−/− mice, which also present with enlarged spleens, high concentrations of auto-antibodies and other classical features of ALPS, did not develop thrombocytopenia. One explanation for why thrombocytopenia is only seen in Casp8−/−Mlkl−/− mice might be that RIPK3 is somehow critical for the production of anti-platelet auto-antibodies or that MLKL plays a role in platelet formation/clearance, independent of its function as a key mediator of necroptosis.
This is the first direct in vivo comparison between co-deletion of MLKL or RIPK3 in a Caspase-8 or FADD deficient background. An in-depth study of the response of Casp8−/−Mlkl−/− and Casp8−/−Ripk3−/− derived cells to a panel of necrotic cell death stimuli has revealed no difference in the response of cells from these two genotypes. Both murine models share most of the ALPS-like phenotype, however, an unexpected acceleration in the onset of this condition is seen in Casp8−/−Mlkl−/− mice. Although a role for MLKL beyond inducing cell death, particularly in the development of thrombocytopenia, is a possibility, we think it is more likely that RIPK3 may drive the biological mechanisms underpinning the observed differences, since in these mice the RIPK3 non-necroptotic activity is left intact. Our research demonstrates the key role of T cells in the development and progression of the lymphadenopathy and autoimmune disease in these mice and points towards RIPK3 non-necroptotic functions controlling, at least partially, lymphoid homeostasis and production of chemokines and cytokines following loss of Caspase-8 or FADD.
In conclusion, our results support the emergent notion that RIPK1, RIPK3 and MLKL are essential for necroptosis in vivo and in vitro but also have additional functions beyond inducing cell death, a concept that has to be taken into account when targeting these molecules for the treatment of human diseases.
EXPERIMENTAL PROCEDURES
Mice
The Casp8+/− (Ch’en et al., 2008; Salmena et al., 2003), Fadd−/− (Yeh et al., 1998), Cflar−/−(Yeh et al., 2000), Ripk3−/− (Newton et al., 2004) and Mlkl−/− (Murphy et al., 2013) mice have been described. The Casp8+/− mice were originally generated on a mixed C57BL/6x129SV background using 129SV derived ES cells and were then backcrossed onto a C57BL/6 background for >10 generations. The Ripk3−/− and Mlkl−/− mice were generated on an inbred C57BL/6 background using C57BL/6 derived ES cells. Mlkl−/− mice were crossed with Casp8+/− and the resulting Casp8+/−Mlkl+/− offspring were mated with Mlkl+/− mice to produce Casp8+/−Mlkl+/− mice. Fadd−/− and Cflar−/− mice were crossed onto the C57BL/6 background and analyzed for purity (Philip et al., 2014). Animal experiments were performed according to protocols approved by the Walter and Eliza Hall Institute of Medical Research Animal Ethics Committee and the St. Jude Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Animals.
Flow cytometry
Single cell suspensions of lymphoid organs were stained with fluorochrome or biotin conjugated antibodies to the following surface markers (produced in our laboratory except where indicated): CD4 (BioLegend, clone GK1.5), CD8 (clone 53.6.72), B220 (clone RA3–6B2), CD19 (clone 1D3), Mac-1 (clone M1/70), Gr-1 (clone RB6–8C5), NK1.1 (BD PharMingen, clone PK136), NKp46 (BD PharMingen, clone 29A1.4), Ter119 (clone Ter-119) and CD3 (clone 145-2C11). For cell proliferation analysis, intracellular staining with antibodies against Ki67 (BD PharMingen, clone B56, 30 min at 4°C) was performed after fixation and permeabilization using the reagents from the BD cytofix/cytoperm kit. Data from immunofluorescent staining of cells were acquired on an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar).
Histological and hematological analyses
For histological examination, tissues were fixed in 10% (v/v) formalin in phosphate buffered saline (PBS) and sections were prepared and stained with hematoxylin and eosin (H&E) using standard techniques. All photomicrographs were acquired using a ×10/NA 0.3, ×20/NA 0.50 or ×40/NA 0.75 objective lens attached to an Axioplan 2 microscope (Carl Zeiss, North Ryde, NSW, Australia). For differential cell counts, blood was collected into tubes containing EDTA (Sarstedt) and analyzed with an Advia 120 analyzer (Bayer). Alternatively, blood was collected via heparin-coated capillaries and peripheral blood counts were performed using a Forcyte hematology analyzer following the manufacturer’s instructions. Reticulated platelets were enumerated using staining with thiazole orange (Josefsson et al., 2012). Megakaryocytes were counted manually in sections of sternum and spleen stained H&E with a minimum of 10 high-power fields (x200) analyzed.
Measurement of cytokine and anti-nuclear auto-antibody (ANA) concentrations
Concentrations of cytokines and chemokines in cell supernatants and sera were measured by using the Bio-Plex Pro mouse cytokine 23-plex (Bio-Rad, Gladesville, NSW, Australia) and Bio-Plex Pro mouse cytokine Th17-plex magnetic bead immunoassays on a Bio-Rad Bio-Plex instrument, following the manufacturer’s instructions. The cytokines measured included: CD40L, IL-1α, IL-β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(P40), IL-12(p70), IL-13, IL-17A/F, IL-21, IL-22, IL-23(p19), IL-31, IL-33, eotaxin, G-CSF, GM-CSF, IFN-γ, KC (CXCL1), MCP-1, MIP-1α, MIP-1β, MIP-3α, RANTES and TNFα. Concentrations of ANA were measured by ELISA (The Binding, Site, Birmingham, UK) according to the manufacturer’s instructions.
Measurement of serum immunoglobulins
The concentartions of serum immunoglobulins were determined by ELISA using sheep anti-mouse Ig (total) antibodies (Silenus Laboratories, Australia) as a capture reagent and the assay was developed with mouse Ig isotype-specific goat antibodies conjugated to horseradish peroxidase (Southern Biotechnology). Purified myeloma proteins were used as standards (Sigma, MO, USA).
Immunofluorescent staining and confocal microscopy
To stain for immune complex deposits, the kidneys from aged mice were snap-frozen in isopentane, sectioned, acetone fixed, and blocked with PBS/2% FCS, followed by staining with FITC-coupled goat antibodies specific to mouse IgM, IgG or IgA (Southern Biotechnology, Birmingham, AL, USA). All immunofluorescent stainings were analyzed using a Leica laser scanning (SP8) confocal microscope.
Generation and treatment of cell lines
Mouse dermal fibroblasts were isolated from 3 mice of each genotype and immortalized by retroviral expression of SV40 large T antigen to generate 3 biologically independent cell lines. Cell death assays were carried out in 24-well plates, seeding 1 × 105 cells per well. Cells were treated with the broad-spectrum caspase inhibitors, Q-VD-OPh (25 µM) or Z-VAD-FMK (20 µM), or vehicle 30 min prior to addition of hTNF-Fc (100 ng/mL, (Bossen et al., 2006)), FASL (10 ng/mL (O’Reilly et al., 2009), poly(I:C) (25 µg/mL) and SMAC mimetic (500 nM Compound A, (Vince et al., 2007)). After 24 h, cells were harvested, stained with propidium iodide (100 ng/mL) and PI-positive cells were quantified by analysis in a BD FACSCalibur flow cytometer.
To generate BMDMs, cell suspensions from bone marrow were cultured for 6 days in DMEM supplemented with 10% FCS and 20% L929 conditioned medium (a source of M-CSF). On day 6 the resulting BMDMs were plated at 5 × 105 cells per well in a 24-well plate and 24 h later treated with TNF, FASL, poly(I:C), LPS (25 µM), Smac mimetic, Q-VD-OPh or Z-VAD-FMK and cell survival was monitored as described above. Culture supernatants were collected for further analysis.
Primary MEFs were generated as described (Dillon et al., 2014). Cells were treated with TNF plus CHX (for induction of apoptosis) or TNF plus Z-VAD-FMK (for induction of necroptosis). Cell death was monitored in real time using an Incucyte FLR or Zoom imaging systems (Essen Bioscience, Ann Arbor, MI, USA). In brief, cells were plated in medium containing 25 nM of the membrane impermeable dye Sytox Green (S7020; Invitrogen, Eugene, OR, USA). At the end point, all cells were stained with 100 nM of the membrane permeable dye Syto24 Green (S7559; Invitrogen). Cell death was quantified using the Incucyte image analysis software (Essen Bioescience). Data were expressed as percentages of the ratio between Sytox Green/Syto24 (% Sytox Green+) cells.
Immunoblotting
Single-cell suspensions were prepared from lymphoid tissues (lymph nodes, spleen, thymus, FACS sorted cells) and solid organs. Cells were washed twice in PBS and lysed in modified RIPA buffer (150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% sodium deoxycholate, 50 mM Tris-HCl pH 7.4) supplemented with complete protease inhibitor cocktail tablets (Roche) for 20 min on ice and clarified by centrifugation at 13,000 rpm for 10 min at 4° C. Protein concentration was measured using the Bio-Rad protein assay kit and 30–70 µg of total protein per extract were resolved on 4–12% Bis-Tris SDS-PAGE gels (Life Technologies or Bio-Rad), and then transferred to nitrocellulose membranes. Membranes were blocked in 5% low fat milk in Tris-buffered saline containing 0.1% (v/v) Tween-20 (TBST) for at least 1 h. Nitrocellulose membranes were probed with primary antibodies in 5% low fat milk in TBST and incubated for 4 h at room temperature or over night at 4°C. Western blotting was performed using antibodies as follows: rat monoclonal anti-MLKL (clone 3H1; (Murphy et al., 2013)), rabbit anti-MLKL (AP14272b, Abgent), rat monoclonal anti-caspase-8 (clone 3B10; (O'Reilly et al., 2004)), rabbit anti-RIPK3 (14401s, Cell Signaling), rabbit anti-FADD (Sc-6036, Santa Cruz) and, as a loading control, mouse monoclonal antibodies to HSP70 (N6, a kind gift from Dr R Anderson, Peter MacCallum Cancer Research Centre, Melbourne, VIC, Australia), mouse anti-HSP70 (14401s;Cell Signaling) or mouse anti-Actin (Sc-47778, Santa Cruz)). Bound primary antibodies were visualized using HRP-conjugated secondary antibodies against mouse, rat, or rabbit IgG (GE Healthcare and Jackson Immunoresearch) followed by use of the ECL detection system (GE Healthcare).
T cell purification
Total T cell populations were purified from lymph nodes by negative selection of other cell types using magnetic beads (BioMag, Qiagen) with antibodies against GR-1, MAC-1, CD 19, Ter1 19. DN T cells were purified by negative selection as above with the addition of antibodies against CD4 and CD8.
T cell stimulation
Purified total T cells were cultured in medium (RPMI 1640 supplemented with 10% FBS, L-glutamine, penicillin, streptomycin and 2-mercaptoethanol) with mIL-2 in flat-bottom plates coated with antibodies against CD3 (10 µg/mL, clone 1452c11) and CD28 (10 µ g/mL, clone 37.N.51) or on uncoated flat-bottom plates with phorbol-12-myristeacetate (PMA) (2 ng/mL) plus ionomycin (10 ng/mL). After 48 h, culture supernatants were collected for further analysis.
Statistical analysis
Data are presented as mean ± SD or mean ± SEM as indicated. Statistical analysis was performed using Student’s t-test, log rank (Mantel–Cox) test for animal survival curves, or one-way analysis of variance using Tukey’s comparison test to compare multiple groups where appropriate. To determine the statistical significance of perinatal survival, a chi-square test was used (Graph Pad).
Supplementary Material
Acknowledgments
We thank J. Lochland and C. Hall for genotyping, JG. Zhang for FasL, M. Yang for technical assistance and S. Hedrick (UC) for Casp8−/− mice. S.A-D. was supported by a Spanish Ministry of Education Postdoctoral Fellowship. M.C.T. was supported by a Victorian International Research Scholarship. This work was funded by grants and fellowships from the NHMRC (1016647, 1058344, 1079250, 1047672, 1025594, 1057905 and 1046984), the Australian Cancer Research Fund, the U.S. National Institutes of Health and the American Lebanese Syrian Associated Charities. This work made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS (361646).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures.
AUTHOR CONTRIBUTIONS
S.A.-D., D.R.G. and A.S. designed the study and wrote the manuscript; S.A.-D. and C.P.D. performed the experiments and analyzed the data; A.L. and L.A.O. performed ELISA autoimmune analyses and IF of kidney samples; M.C.T., N.L. and D.A.R performed cell death assays in cell lines; M.L. and E.C.J. performed platelet and megakaryocytes analysis; R.H., J.S. and W.S.A. provided mice and intellectual input.
REFERENCES
- Allam R, Lawlor KE, Yu EC, Mildenhall AL, Moujalled DM, Lewis RS, Ke F, Mason KD, White MJ, Stacey KJ, et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO reports. 2014;15:982–990. doi: 10.15252/embr.201438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. The Journal of biological chemistry. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nature cell biology. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'en IL, Beisner DR, Degterev A, Lynch C, Yuan J, Hoffmann A, Hedrick SM. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17463–17468. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FK, Luz NF, Moriwaki K. Programmed Necrosis in the Cross Talk of Cell Death and Inflammation. Annual review of immunology. 2014 doi: 10.1146/annurev-immunol-032414-112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell research. 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook WD, Moujalled DM, Ralph TJ, Lock P, Young SN, Murphy JM, Vaux DL. RIPK1- and RIPK3-induced cell death mode is determined by target availability. Cell death and differentiation. 2014;21:1600–1612. doi: 10.1038/cdd.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell reports. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell reports. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting-kinase-3-mediated pathway. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, Pierotti C, Garnier JM, Dobson RC, Webb AI, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:15072–15077. doi: 10.1073/pnas.1408987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature immunology. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- Josefsson EC, White MJ, Dowling MR, Kile BT. Platelet life span and apoptosis. Methods Mol Biol. 2012;788:59–71. doi: 10.1007/978-1-61779-307-3_5. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3 and MLKL. The Journal of biological chemistry. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Kearney CJ, Sheridan C, Cullen SP, Tynan GA, Logue SE, Afonina IS, Vucic D, Lavelle EC, Martin SJ. Inhibitor of apoptosis proteins (IAPs) and their antagonists regulate spontaneous and tumor necrosis factor (TNF)-induced proinflammatory cytokine and chemokine production. The Journal of biological chemistry. 2013;288:4878–4890. doi: 10.1074/jbc.M112.422410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D'Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. The Journal of experimental medicine. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Molecular cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moujalled DM, Cook WD, Okamoto T, Murphy J, Lawlor KE, Vince JE, Vaux DL. TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1. Cell death & disease. 2013;4:e465. doi: 10.1038/cddis.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Molecular and cellular biology. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly LA, Divisekera U, Newton K, Scalzo K, Kataoka T, Puthalakath H, Ito M, Huang DC, Strasser A. Modifications and intracellular trafficking of FADD/MORT1 and caspase-8 after stimulation of T lymphocytes. Cell death and differentiation. 2004;11:724–736. doi: 10.1038/sj.cdd.4401408. [DOI] [PubMed] [Google Scholar]
- O'Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–663. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, Rieux-Laucat F, Siegel RM, Su HC, Teachey DT, Rao VK. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–e40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco S, Yatim N, Werner MR, Tran H, Gunja SY, Tait SW, Albert ML, Green DR, Oberst A. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell death and differentiation. 2014;21:1511–1521. doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- Pattison JM, Nelson PJ, Huie P, Sibley RK, Krensky AM. RANTES chemokine expression in transplant-associated accelerated atherosclerosis. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1996;15:1194–1199. [PubMed] [Google Scholar]
- Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky-Dolfi MA, Zwack EE, Hu B, Fitzgerald L, Mauldin EA, Copenhaver AM, et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7385–7390. doi: 10.1073/pnas.1403252111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell N, Humbert M, Durham SR, Assoufi B, Kay AB, Corrigan CJ. Increased expression of mRNA encoding RANTES and MCP-3 in the bronchial mucosa in atopic asthma. The European respiratory journal. 1996;9:2454–2460. doi: 10.1183/09031936.96.09122454. [DOI] [PubMed] [Google Scholar]
- Rickard JA, O'Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. The Journal of experimental medicine. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes & development. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nature immunology. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- Su L, Quade B, Wang H, Sun L, Wang X, Rizo J. A plug release mechanism for membrane permeation by MLKL. Structure. 2014;22:1489–1500. doi: 10.1016/j.str.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Tanzer MC, Tripaydonis A, Webb AI, Young SN, Varghese LN, Hall C, Alexander WS, Hildebrand JM, Silke J, Murphy JM. Necroptosis signalling is tuned by phosphorylation of MLKL residues outside the pseudokinase domain activation loop. Biochem J. 2015 doi: 10.1042/BJ20150678. [DOI] [PubMed] [Google Scholar]
- Teachey DT, Manno CS, Axsom KM, Andrews T, Choi JK, Greenbaum BH, McMann JM, Sullivan KE, Travis SF, Grupp SA. Unmasking Evans syndrome: T-cell phenotype and apoptotic response reveal autoimmune lymphoproliferative syndrome (ALPS) Blood. 2005;105:2443–2448. doi: 10.1182/blood-2004-09-3542. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Science signaling. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, et al. IAP antagonists target cIAP1 to induce TNFαlpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Molecular cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Suda T, Hashimoto H, Nagata S. Constitutive activation of the Fas ligand gene in mouse lymphoproliferative disorders. The EMBO journal. 1995;14:12–18. doi: 10.1002/j.1460-2075.1995.tb06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wong WW, Vince JE, Lalaoui N, Lawlor KE, Chau D, Bankovacki A, Anderton H, Metcalf D, O'Reilly L, Jost PJ, et al. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood. 2014;123:2562–2572. doi: 10.1182/blood-2013-06-510743. [DOI] [PubMed] [Google Scholar]
- Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J, Chen W, Zhang Y, Zhou X, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell research. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H, Song Y, Chen X, Shao J, Han J. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell death and differentiation. 2014;21:1709–1720. doi: 10.1038/cdd.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WC, de la Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, Mak TW. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- Young DA, Hegen M, Ma HL, Whitters MJ, Albert LM, Lowe L, Senices M, Wu PW, Sibley B, Leathurby Y, et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis and rheumatism. 2007;56:1152–1163. doi: 10.1002/art.22452. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.