Abstract
Circulating tumor cells (CTCs) are tumor cells that are separated from the primary site or metastatic lesion and disseminate in blood circulation. CTCs are considered to be part of the long process of cancer metastasis. As a 'liquid biopsy', CTC molecular examination and investigation of single cancer cells create an important opportunity for providing an understanding of cancer biology and the process of metastasis. In the last decade, we have seen dramatic development in defining the role of CTCs in lung cancer in terms of diagnosis, genomic alteration determination, treatment response and, finally, prognosis prediction. The aims of this review are to understand the basic biology and to review methods of detection of CTCs that apply to the various types of solid tumor. Furthermore, we explored clinical applications, including treatment monitoring to anticipate therapy resistance as well as biomarker analysis, in the context of lung cancer. We also explored the potential use of cell-free circulating tumor DNA (ctDNA) in the genomic alteration analysis of lung cancer.
Keywords: Neoplastic Cells, Circulating; DNA, Neoplasm; Lung Neoplasms; Biology; Methods
INTRODUCTION
Lung cancer remains the main cause of mortality among patients with cancer worldwide.1,2 Among American patients with cancer, it is estimated that lung cancer is responsible for the mortality of 28% male and 26% female patients.3 Efforts have been made to detect lung cancer as early as possible by methods such as chest computerized tomography (CT) scanning,4,5,6 sputum cytology analysis,7,8 biomarker analysis,9,10 circulating tumor cell (CTC) detection, 11 and circulating cell-free tumor DNA (ctDNA) detection.12,13 An average solid tumor may release an estimated million cells per day into the bloodstream and although most dispersed cancer cells do not survive, the cells that do survive clearly pose an existential threat to the host organism.14
Detection, monitoring, and molecular investigation of CTCs and ctDNA offer a meaningful and noninvasive way for the detection of the early phase of the disease, prognosis prediction and assessing therapeutic response in patients with lung cancer.15,16 As a so-called 'liquid biopsy', single tumor cell analysis and ctDNA analysis hold significant potential in providing understanding into lung cancer biology and the cancer spreading process.17 Until now CTC characterization and enumeration has been extensively studied in several cancers such as colorectal carcinoma,18,19 prostate cancer,20,21 breast cancer,22,23 ovarian cancer,24,25 head and neck carcinoma,26,27 brain cancer,28,29,30 gastric cancer,31,32 hepatocellular carcinoma,33,34,35 renal cell carcinoma,36 mesothelioma,37,38 and lung cancer.39,40 In general, the objectives of studies on CTCs in solid tumor include (a) risk prediction for metastatic progression and relapse , (b) staging determination and real-time observation of response to therapies, (c) determination of therapeutic targets and mechanisms of resistance, and (d) understanding metastasis process in solid tumor patients.41
Circulating ctDNA can be identified in lung cancer patients with the polymerase chain reaction (PCR) assay and high plasma DNA levels could also identify high-risk persons for lung cancer screening.42 Moreover, ctDNA in patients with lung cancer displays genetic and epigenetic differences common for tumors including oncogene activation, chromosome loss, and tumor-suppressor gene silencing by methylation.43 Studies have shown that ctDNA levels were associated with tumor grade, tumor stage, lymph node spreading, the number of metastatic sites, tumor response to treatment and, finally, survival in patients with non-small cell lung cancer (NSCLC).44
METHODS
The purpose of this review is to discuss the basic concept and methods of detection of CTCs and ctDNA in solid tumors generally. We also aimed to see how these advancements of knowledge have been applied in assessing patients with lung cancer, both small NSCLC as well as small cell lung cancer, based on accumulated evidence. A review of the published peer-reviewed reports in the Medline database up to October 2015 was performed. The terms used to gather relevant original articles, reports, and reviews were: circulating tumor cells, circulating cell-free tumor DNA, lung cancer, and epithelial-mesenchymal transition. A narrative review format was used to present the information collected.
DEFINITIONS
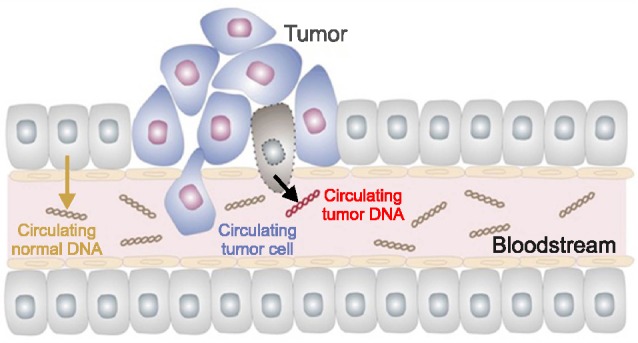
CTCs, with a spectrum from one cell to aggregates consisting of 2-5 cells, are tumor cells that detach from either a primary lesion or a metastatic site and spread in the peripheral blood as the cellular seed of metastasis (Fig. 1).45,46 1 gram of tumor tissue, about 1 million tumor cells, can disseminate daily into the blood stream.47 CTCs in blood circulation derive from solid tumors. They are involved in the metastatic spread to distant organs that leads to the formation of secondary sites of the disease.48 Meanwhile, circulating tumor microemboli (CTM) is a tumor cell aggregate that circulates in the blood.49 Another term is disseminating tumor cells (DTCs), which are defined as a settlement of CTCs in secondary organs and may stay in a quiescence state or may cause an observable metastasis.50 CTCs and DTCs in blood and bone marrow are considered as potential metastases-inducing cells.51 At least one CTC per 3 ml of blood was reported in 17 (65%) and one CTM per 3 ml of blood in 15 (58%) of 26 NSCLC cases.52
FIG. 1. Circulating tumor cells (CTCs) and cell-free circulating tumor (ctDNA) originated from the primary tumor or metastatic lesion circulates in the blood. Reprinted with permission from Sysmex Inostics Corp.
Cell-free circulating tumor DNA (ctDNA) may come from primary or metastatic tumor deposits and is enriched for detection of metastatic precursors.53 ctDNA genotyping has some advantages over CTCs because (1) CTCs must be separated from the much more abundant hematologic cells in the blood requiring comprehensive laboratory facilities to obtain a viable population of CTCs for study; (2) CTCs in circulation encounter substantial apoptosis and fragility leading to variability between different CTC assays; (3) most of the ctDNA genotyping methods require a minimum of special handling and do not depend on special equipment, and lastly; (4) ctDNA could be processed at the same time with plasma DNA from normal cells, which always exists in the bloodstream.54
FORMATION OF CTC AND CTDNA
CTC takes part in the long process of tumor metastasis, it is believed that metastasis is started by a sub-group of CTC seen in the blood of patients.55 Metastasis consists of the two steps: The first steps is the displacement of a cancer cell to a distant site, while the second step is related to the capacity of the cancer cell to generate a metastatic foci at that distant organ.56 To detach from the primary tumor, cancer cells needs to undergo a cellular process known as the epithelial-mesenchymal transition (EMT).57 EMT allows the tumor cells to gain motility and migratory capacity which results in penetration into bloodstream and circulation as CTC.58
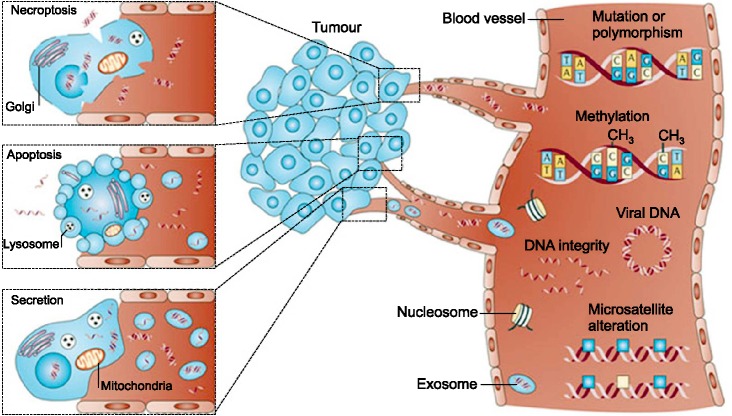
The release of DNA into circulation is a common phenomenon in cancer patients due to apoptotic and necrotic processes characteristic of tumor cells.59 Methylation, mutations, microsatellite alterations, DNA integrity, and viral DNA can be studied in the ctDNA, as well as contributing factors of tumor DNA released into circulation which are tumor burden and tumor cell proliferation (Fig. 2).60 Cell-free DNA found in lung cancer patients originates primarily from tumor development and necrotic malignant cells that have been enveloped by macrophages rather than the chronic inflammatory response.61,62
FIG. 2. Circulating cell-free tumor DNA exist in the blood in various forms, and could provide information such as mutations, DNA integrity, methylation, viral DNA, and microsatellite alterations. Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Cancer 11: 426-437, copyright (2011).
METHODS OF DETECTION
CTC identification, enumeration, and molecular analysis are very difficult because CTCs occur typically with a frequency 1 per 106,7 leukocytes and the amount of available sample is very limited.63,64 Many methods have been tested to detect and characterize CTCs in lung cancer patients.65 Most of the current strategies to enumerate CTC are generally based on CTC markers and size. In the surface marker-based strategy, CTC detection is based on epithelial cell adhesion molecule (EpCAM) and keratin markers.66 One of the methods that are commonly used to enumerate CTC is CellSearch® CTC Test which has been shown to consistently capture clinically important CTCs.67 So far, only the CellSearch® system has been approved by the FDA for CTCs detection of epithelial origin in whole blood and to assess prognosis.68 CellSearch® isolates CTCs by using the EpCAM-based enrichment technique.69 The nanotechnology approach in CTCs detection has also been studied. Gazouli and colleagues demonstrated a sensitive assay that combines magnetic beads, coupled with EpCAM and CK19 antibodies, and quantum dots (QDs) fluorescence detection for the detection of as little as 10 CTCs per ml blood samples.70 However, because CTCs of lung cancer often present non-epithelial characteristics and lose their epithelial markers during the EMT process, this detection method can become ineffective and result in poor sensitivity.51,71 In breast cancer, loss of EpCAM is accompanied by the upregulation of caveolin1 (CAV1) in CTC. CTC detection devices using CAV1-EpCAM conjugated beads give significantly improved throughput compared to EpCAM only.72 However, there has been no reporting about the use of CAV1-EpCAM-based CTC detection in lung cancer.
To address the limitation of EpCAM-based strategies, efforts to classify CTCs based on their EMT markers and to elucidate the heterogeneity of CTC populations have been made.73 Recently, the CanPatrol CTC enrichment method was used to detect CTCs using EMT markers in various types of malignancies.74 This technique might offer a solution because EpCAM-based enrichment methods have led to missed CTC detection that has lost their EpCAM expression due to EMT.75
As for the size-based CTC detection, most commonly, microfluidic chips are used, which involves filtration of micro-channels to isolate the larger CTCs from the other blood components.76 One study reported that an ultra-high-throughput spiral microfluidic biochip device could enrich CTC with a fast processing time (7.5 mL blood within 10 min), 100% detection rate (10/10) of blood samples collected from patients with advanced-stage metastatic lung and breast cancers, and was able to capture 20 to 135 CTCs/mL with high purity of 1 CTC out of every 30-100 white blood cells.77 Another sized-based CTC detection, microcavity array (MCA) system connected with a small device for CTC detection independent of EpCAM expression, has been reported to be successful in isolating CTC populations from patients who had been determined as CTC negative by the CellSearch system.78 The MCA system is a microfabricated nickel filter with a rectangular MCA (10(4) cavities/filter) and the shape and porosity of the MCA were customized to efficiently identify small tumor cells on the microcavities under low flow resistance while at the same time allowing other blood cells to pass through.79
Ligand-targeted PCR (LT-PCR) is a system where CTCs, that were enriched from erythrocytes and leukocytes, were marked with a conjugate of a tumor-specific ligand and a synthesized oligonucleotide followed by quantitative PCR analysis.80 LT-PCR provide quantification of CTC in NSCLC patients with greater sensitivity, about 80% sensitivity of stage I/II, 67% sensitivity of stage III, and 93% sensitivity of stage IV NSCLC.81 An assay that identified live cells using an adenoviral probe that detected elevated telomerase activity present in almost all cancer cells, but not in normal cells, could detect CTC in 65% of lung cancer patients.82 ISET (RareCell Diagnostics, Paris, France) is a technology that does not rely on the tumor marker expression, in which CTCs are captured by filtration independent of the tumor-related markers, because of their large size compared to circulating white blood cells.83 Filtration using the ISET system could detect larger numbers of CTCs, including epithelial marker negative cancer cells and isolate CTM and allow molecular analysis.84 The challenge is not limited to detection only but also culturing. If we could culture and expand the number CTCs ex vivo, it would allow us to examine genotype and phenotype of the tumor cells. In this regard, the Thermoresponsive NanoVelcro CTC purification system has been investigated for culture expansion of CTC from NSCLC.85 The Thermoresponsive NanoVelcro is the third generation of NanoVelcro in which agent-coated nanostructured substrates immobilized CTC while maintaining cells viability and molecular integrity.45
Calculation of ctDNA could assist in objective response assessments, identification of subclinical residual disease and provide a noninvasive approach for tumor genotyping.86 The quantity of plasma ctDNA can be determined using quantitative Real-Time PCR.87 It has been shown to reach the level of 12.8 ng/mL in lung cancer.12 Novel methods called the 'cancer personalized profiling by deep sequencing' (CAPP-Seq) for quantifying ctDNA can detect ctDNA in all the patients with stage II-IV and in half of the patients with stage I NSCLC.88
CLINICAL APPLICATIONS
In the future, CTC applications will not be limited to enumeration. Daily characterization using a high-content approach that studies protein expression, morphometrics, genotype profiling and eventually prediction of the metastasis of the disease from the primary tumors to distant organs will become possible.89 Circulating cancer cells may be reliable markers in the diagnostic procedures of lung lesions.90 Even if without obvious tumor nodules, the CTCs can be captured in patients with COPD and non-detectable lung malignancy.91 Generally, the rate of CTC detection is positively correlated with NSCLC patients' overall stage and metastatic status.92
It has been demonstrated that CTC investigation through serial blood sampling could help provide personalized medicine for SCLC.93 Circulating tumor cells, prior to the initiation of chemotherapy, are valuable predictors of specific progression-free survival (PFS) in Stage III patients with SCLC.94 CTC enumerations could also be used for overall survival (OS) and PFS prediction. It has been shown that patients with no CTCs detected before treatment have a prolonged PFS and OS and show a greater percentage of stable disease compared to patients with 1-3 and >3 CTCs which impose a higher risk of having progressive disease.95 In terms of evaluation after treatment, patients with positive detection of CTCs after therapy show a poorer response to therapy.96
BIOMARKER ANALYSIS OF CTC
Real-time PCR and melting curve analysis have been used to find sensitizing EGFR mutations in blood cells enriched in CTC.97 Analysis of EGFR can also be performed in captured CTCs by immunofluorescence staining.98 EGFR mutation analysis on DNA recovered from CTCs showed high sensitivity, including identification of the T790M secondary mutation which confers drug resistance.99 Using ctDNA specimens, it was found that follows up to the EGFR mutations in the circulation allow for identification of the T790M mutation for up to 344 days before patients suffered from disease progression.100 Consistent with this finding, studies that quantitatively detected activating EGFR mutations and the resistant T790 secondary mutation showed a 72.7% detection rate of EGFR mutation in all lung cancer patient enrolled in the study. The detection rate of the T790M mutation among patients who experienced progressive disease after EGFR-TKI treatment was 43.5%. The BEAMing (beads, emulsion, amplification, and magnetics) method was used for detection.101
As for KRAS mutation, it was reported that the colorimetric membrane array analysis technique could identify an activated KRAS mutation from CTC in various cancers.102,103 This finding was then followed by the establishment of the platform-weighted chemiluminescent membrane array (WCHMA) which is able to detect the KRAS mutation from minimally 3 CTCs/ml blood with a sensitivity of 93% and a specificity of 94%.104 In the WCHMA method, the best intensity value for each gene, for example, KRAS, is calculated by the linear value of the chemiluminescent emission and is more accurate than the color concentration readings in colorimetric membrane array method.105 However, recent evidence suggests that ctDNA was superior and preferable as type of specimen for KRAS mutation analysis in lung malignancies compared to CTC DNA because it could lead to greater mutation detection than CTCs.40
CTCs have also been used clinically to diagnose echinoderm microtubule associated proteins like 4-anaplastic lymphoma kinase (EML4-ALK) gene rearrangement in NSCLC to select patients who are eligible to receive ALK inhibitor.106 ALK rearrangement could be determined in CTCs of ALK-positive NSCLC patients by applying a filtration technique and filter adapted fluorescence in situ hybridization (FA-FISH).107 FA-FISH comprises a filter for detecting CTCs that have been previously enriched by blood filtration, followed by preparation of a filter spot for cells fixation and the cells analysis using the FISH assay.108 Moreover, ALK protein expression could also be observed in CTCs isolated from lung cancer patients by immunocytochemistry.109 FISH analysis provided sufficient positivity of EML4-ALK gene rearrangement detection from 10-1535 CTCs/mL and only required 7.5 mL of patient blood samples.110
Taken together, these advancements show that genetic modifications in solid cancers can be characterized by massively parallel sequencing of ctDNA shed from cancer cells into plasma. Repeated biopsies to investigate genomic dynamic changes as a consequence of therapy are not easy, invasive and may be confounded by the heterogeneity nature within the tumor.111 The potential of ctDNA as a replacement of metastasized tumor biopsy becomes more evident because, in some patients, mutation expression analysis from metastasis lesion biopsies failed because of insufficient biopsy sizes, but was successful in all plasma ctDNA samples.112 However, despite the promising application and their applicability for employing CTCs to diagnose genomic changes and monitor responses to therapies (ie, as a liquid biopsy) in lung cancer, these technologies have been limited by significant hurdles, such as complex systems that requires high-level laboratory capacity, contaminated blood cells, and undefined gold-standard method, and have not compiled momentum to add tissue-based diagnostics.113
CONCLUSIONS
CTCs are tumor cells that can be captured through a liquid biopsy from blood and can be genetically and phenotypically studied to provide important data for guiding cancer therapy. The clinical values of CTCs as a biomarker for early-phase cancer screening, diagnosis, the prediction of treatment response, prognosis, and stratification have been widely explored in recent years.114 It is expected that an understanding of CTC biology and its implications in their clinical application will help clinicians in the treatment of lung cancer. Despite high sensitivity and specificity, technological issues have limited the broad clinical utility of the method. It may need several years for CTC detection to become applicable in the clinic for the routine diagnosis of cancer. Obviously, further investigation is required to establish standardized techniques for sample collection, processing, and analysis.
ACKNOWLEDGEMENTS
We would like to acknowledge Katrin Tomson for her support in proofreading and editing of this manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Kanodra NM, Silvestri GA, Tanner NT. Screening and early detection efforts in lung cancer. Cancer. 2015;121:1347–1356. doi: 10.1002/cncr.29222. [DOI] [PubMed] [Google Scholar]
- 5.Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. 2015;191:1166–1175. doi: 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 6.Shlomi D, Ben-Avi R, Balmor GR, Onn A, Peled N. Screening for lung cancer: time for large-scale screening by chest computed tomography. Eur Respir J. 2014;44:217–238. doi: 10.1183/09031936.00164513. [DOI] [PubMed] [Google Scholar]
- 7.Sagawa M, Kobayashi T, Uotani C, Kibe Y, Tanaka M, Machida Y, et al. A survey about further work-up for cases with positive sputum cytology during lung cancer mass screening in Ishikawa Prefecture, Japan: a retrospective analysis about quality assurance of lung cancer screening. Jpn J Clin Oncol. 2015;45:297–302. doi: 10.1093/jjco/hyu214. [DOI] [PubMed] [Google Scholar]
- 8.Yu L, Shen J, Mannoor K, Guarnera M, Jiang F. Identification of ENO1 as a potential sputum biomarker for early-stage lung cancer by shotgun proteomics. Clin Lung Cancer. 2014;15:372–378.e1. doi: 10.1016/j.cllc.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, Kim DH. CpG island hypermethylation as a biomarker for the early detection of lung cancer. Methods Mol Biol. 2015;1238:141–171. doi: 10.1007/978-1-4939-1804-1_8. [DOI] [PubMed] [Google Scholar]
- 10.Hirales Casillas CE, Flores Fernández JM, Padilla Camberos E, Herrera López EJ, Leal Pacheco G, Martínez Velázquez M. Current status of circulating protein biomarkers to aid the early detection of lung cancer. Future Oncol. 2014;10:1501–1513. doi: 10.2217/fon.14.21. [DOI] [PubMed] [Google Scholar]
- 11.Yu N, Zhou J, Cui F, Tang X. Circulating tumor cells in lung cancer: detection methods and clinical applications. Lung. 2015;193:157–171. doi: 10.1007/s00408-015-9697-7. [DOI] [PubMed] [Google Scholar]
- 12.Paci M, Maramotti S, Bellesia E, Formisano D, Albertazzi L, Ricchetti T, et al. Circulating plasma DNA as diagnostic biomarker in non-small cell lung cancer. Lung Cancer. 2009;64:92–97. doi: 10.1016/j.lungcan.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Sirera R, Bremnes RM, Cabrera A, Jantus-Lewintre E, Sanmartín E, Blasco A, et al. Circulating DNA is a useful prognostic factor in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:286–290. doi: 10.1097/JTO.0b013e31820189a5. [DOI] [PubMed] [Google Scholar]
- 14.Dong Y, Skelley AM, Merdek KD, Sprott KM, Jiang C, Pierceall WE, et al. Microfluidics and circulating tumor cells. J Mol Diagn. 2013;15:149–157. doi: 10.1016/j.jmoldx.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Han Y, Su C, Liu Z. Methods for detection of circulating cells in non-small cell lung cancer. Front Biosci (Landmark Ed) 2014;19:896–903. doi: 10.2741/4255. [DOI] [PubMed] [Google Scholar]
- 16.Karachaliou N, Mayo-de-Las-Casas C, Molina-Vila MA, Rosell R. Real-time liquid biopsies become a reality in cancer treatment. Ann Transl Med. 2015;3:36. doi: 10.3978/j.issn.2305-5839.2015.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tognela A, Spring KJ, Becker T, Caixeiro NJ, Bray VJ, Yip PY, et al. Predictive and prognostic value of circulating tumor cell detection in lung cancer: a clinician's perspective. Crit Rev Oncol Hematol. 2015;93:90–102. doi: 10.1016/j.critrevonc.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Bork U, Rahbari NN, Schölch S, Reissfelder C, Kahlert C, Büchler MW, et al. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer. 2015;112:1306–1313. doi: 10.1038/bjc.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbazán J, Muinelo-Romay L, Vieito M, Candamio S, Díaz-López A, Cano A, et al. A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer. Int J Cancer. 2014;135:2633–2643. doi: 10.1002/ijc.28910. [DOI] [PubMed] [Google Scholar]
- 20.Goldkorn A, Ely B, Tangen CM, Tai YC, Xu T, Li H, et al. Circulating tumor cell telomerase activity as a prognostic marker for overall survival in SWOG 0421: a phase III metastatic castration resistant prostate cancer trial. Int J Cancer. 2015;136:1856–1862. doi: 10.1002/ijc.29212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thalgott M, Heck MM, Eiber M, Souvatzoglou M, Hatzichristodoulou G, Kehl V, et al. Circulating tumor cells versus objective response assessment predicting survival in metastatic castration-resistant prostate cancer patients treated with docetaxel chemotherapy. J Cancer Res Clin Oncol. 2015;141:1457–1464. doi: 10.1007/s00432-015-1936-z. [DOI] [PubMed] [Google Scholar]
- 22.Helissey C, Berger F, Cottu P, Diéras V, Mignot L, Servois V, et al. Circulating tumor cell thresholds and survival scores in advanced metastatic breast cancer: the observational step of the CirCe01 phase III trial. Cancer Lett. 2015;360:213–218. doi: 10.1016/j.canlet.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Beije N, Jager A, Sleijfer S. Circulating tumor cell enumeration by the CellSearch system: the clinician's guide to breast cancer treatment? Cancer Treat Rev. 2015;41:144–150. doi: 10.1016/j.ctrv.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Pearl ML, Dong H, Tulley S, Zhao Q, Golightly M, Zucker S, et al. Treatment monitoring of patients with epithelial ovarian cancer using invasive circulating tumor cells (iCTCs) Gynecol Oncol. 2015;137:229–238. doi: 10.1016/j.ygyno.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolostova K, Spicka J, Matkowski R, Bobek V. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am J Transl Res. 2015;7:1203–1213. [PMC free article] [PubMed] [Google Scholar]
- 26.Weller P, Nel I, Hassenkamp P, Gauler T, Schlueter A, Lang S, et al. Detection of circulating tumor cell subpopulations in patients with head and neck squamous cell carcinoma (HNSCC) PLoS One. 2014;9:e113706. doi: 10.1371/journal.pone.0113706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulasinghe A, Perry C, Jovanovic L, Nelson C, Punyadeera C. Circulating tumour cells in metastatic head and neck cancers. Int J Cancer. 2015;136:2515–2523. doi: 10.1002/ijc.29108. [DOI] [PubMed] [Google Scholar]
- 28.Adamczyk LA, Williams H, Frankow A, Ellis HP, Haynes HR, Perks C, et al. Current understanding of circulating tumor cells - potential value in malignancies of the central nervous system. Front Neurol. 2015;6:174. doi: 10.3389/fneur.2015.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lombard A, Goffart N, Rogister B. Glioblastoma circulating cells: reality, trap or illusion? Stem Cells Int. 2015;2015:182985. doi: 10.1155/2015/182985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan JP, Nahed BV, Madden MW, Oliveira SM, Springer S, Bhere D, et al. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov. 2014;4:1299–1309. doi: 10.1158/2159-8290.CD-14-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ, Lee J, Kim ST, Park SH, Park JO, Park YS, et al. Circulating tumor cells are predictive of poor response to chemotherapy in metastatic gastric cancer. Int J Biol Markers. 2015;30:e382–e386. doi: 10.5301/jbm.5000151. [DOI] [PubMed] [Google Scholar]
- 32.Kolostova K, Matkowski R, Gürlich R, Grabowski K, Soter K, Lischke R, et al. Detection and cultivation of circulating tumor cells in gastric cancer. Cytotechnology. 2016;68:1095–1102. doi: 10.1007/s10616-015-9866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu HY, Qian HH, Zhang XF, Li J, Yang X, Sun B, et al. Improved method increases sensitivity for circulating hepatocellular carcinoma cells. World J Gastroenterol. 2015;21:2918–2925. doi: 10.3748/wjg.v21.i10.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan JL, Yang YF, Yuan CH, Chen H, Wang FB. Circulating tumor cells for predicting the pognostic of patients with hepatocellular varcinoma: a meta analysis. Cell Physiol Biochem. 2015;37:629–640. doi: 10.1159/000430382. [DOI] [PubMed] [Google Scholar]
- 35.Nel I, David P, Gerken GG, Schlaak JF, Hoffmann AC. Role of circulating tumor cells and cancer stem cells in hepatocellular carcinoma. Hepatol Int. 2014;8:321–329. doi: 10.1007/s12072-014-9539-3. [DOI] [PubMed] [Google Scholar]
- 36.Gradilone A, Iacovelli R, Cortesi E, Raimondi C, Gianni W, Nicolazzo C, et al. Circulating tumor cells and "suspicious objects" evaluated through CellSearch® in metastatic renal cell carcinoma. Anticancer Res. 2011;31:4219–4221. [PubMed] [Google Scholar]
- 37.Raphael J, Massard C, Gong IY, Farace F, Margery J, Billiot F, et al. Detection of circulating tumour cells in peripheral blood of patients with malignant pleural mesothelioma. Cancer Biomark. 2015;15:151–156. doi: 10.3233/CBM-140448. [DOI] [PubMed] [Google Scholar]
- 38.Bobek V, Kacprzak G, Rzechonek A, Kolostova K. Detection and cultivation of circulating tumor cells in malignant pleural mesothelioma. Anticancer Res. 2014;34:2565–2569. [PubMed] [Google Scholar]
- 39.Myung JH, Roengvoraphoj M, Tam KA, Ma T, Memoli VA, Dmitrovsky E, et al. Effective capture of circulating tumor cells from a transgenic mouse lung cancer model using dendrimer surfaces immobilized with anti-EGFR. Anal Chem. 2015;87:10096–10102. doi: 10.1021/acs.analchem.5b02766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freidin MB, Freydina DV, Leung M, Montero Fernandez A, Nicholson AG, Lim E. Circulating tumor DNA outperforms circulating tumor cells for KRAS mutation detection in thoracic malignancies. Clin Chem. 2015;61:1299–1304. doi: 10.1373/clinchem.2015.242453. [DOI] [PubMed] [Google Scholar]
- 41.Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110–118. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 42.Sozzi G, Conte D, Leon M, Ciricione R, Roz L, Ratcliffe C, et al. Quantification of free circulating DNA as a diagnostic marker in lung cancer. J Clin Oncol. 2003;21:3902–3908. doi: 10.1200/JCO.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Xue X, Zhu YM, Woll PJ. Circulating DNA and lung cancer. Ann N Y Acad Sci. 2006;1075:154–164. doi: 10.1196/annals.1368.021. [DOI] [PubMed] [Google Scholar]
- 44.Nie K, Jia Y, Zhang X. Cell-free circulating tumor DNA in plasma/serum of non-small cell lung cancer. Tumour Biol. 2015;36:7–19. doi: 10.1007/s13277-014-2758-3. [DOI] [PubMed] [Google Scholar]
- 45.Lin M, Chen JF, Lu YT, Zhang Y, Song J, Hou S, et al. Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Acc Chem Res. 2014;47:2941–2950. doi: 10.1021/ar5001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King MR, Phillips KG, Mitrugno A, Lee TR, de Guillebon AM, Chandrasekaran S, et al. A physical sciences network characterization of circulating tumor cell aggregate transport. Am J Physiol Cell Physiol. 2015;308:C792–C802. doi: 10.1152/ajpcell.00346.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97:14608–14613. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res. 2014;20:2553–2568. doi: 10.1158/1078-0432.CCR-13-2664. [DOI] [PubMed] [Google Scholar]
- 49.Carlsson A, Nair VS, Luttgen MS, Keu KV, Horng G, Vasanawala M, et al. Circulating tumor microemboli diagnostics for patients with non-small-cell lung cancer. J Thorac Oncol. 2014;9:1111–1119. doi: 10.1097/JTO.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med. 2014;7:1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gires O, Stoecklein NH. Dynamic EpCAM expression on circulating and disseminating tumor cells: causes and consequences. Cell Mol Life Sci. 2014;71:4393–4402. doi: 10.1007/s00018-014-1693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mascalchi M, Falchini M, Maddau C, Salvianti F, Nistri M, Bertelli E, et al. Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC. J Cancer Res Clin Oncol. 2016;142:195–200. doi: 10.1007/s00432-015-2021-3. [DOI] [PubMed] [Google Scholar]
- 53.Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4:650–661. doi: 10.1158/2159-8290.CD-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang WL, Wei F, Wong DT, Lin CC, Su WC. The emergent landscape of detecting EGFR mutations using circulating tumor DNA in lung cancer. Biomed Res Int. 2015;2015:340732. doi: 10.1155/2015/340732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 56.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 57.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogden A, Rida PC, Aneja R. Heading off with the herd: how cancer cells might maneuver supernumerary centrosomes for directional migration. Cancer Metastasis Rev. 2013;32:269–287. doi: 10.1007/s10555-012-9413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulivi P, Silvestrini R. Role of quantitative and qualitative characteristics of free circulating DNA in the management of patients with non-small cell lung cancer. Cell Oncol (Dordr) 2013;36:439–448. doi: 10.1007/s13402-013-0155-3. [DOI] [PubMed] [Google Scholar]
- 60.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 61.Szpechcinski A, Chorostowska-Wynimko J, Struniawski R, Kupis W, Rudzinski P, Langfort R, et al. Cell-free DNA levels in plasma of patients with non-small-cell lung cancer and inflammatory lung disease. Br J Cancer. 2015;113:476–483. doi: 10.1038/bjc.2015.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112–123. doi: 10.1373/clinchem.2014.222679. [DOI] [PubMed] [Google Scholar]
- 63.Lianidou ES, Strati A, Markou A. Circulating tumor cells as promising novel biomarkers in solid cancers. Crit Rev Clin Lab Sci. 2014;51:160–171. doi: 10.3109/10408363.2014.896316. [DOI] [PubMed] [Google Scholar]
- 64.McInnes LM, Jacobson N, Redfern A, Dowling A, Thompson EW, Saunders CM. Clinical implications of circulating tumor cells of breast cancer patients: role of epithelial-mesenchymal plasticity. Front Oncol. 2015;5:42. doi: 10.3389/fonc.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hofman V, Ilie M, Long E, Guibert N, Selva E, Washetine K, et al. Detection of circulating tumor cells from lung cancer patients in the era of targeted therapy: promises, drawbacks and pitfalls. Curr Mol Med. 2014;14:440–456. doi: 10.2174/1566524014666140414205455. [DOI] [PubMed] [Google Scholar]
- 66.Joosse SA, Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer Res. 2013;73:8–11. doi: 10.1158/0008-5472.CAN-12-3422. [DOI] [PubMed] [Google Scholar]
- 67.Adams DL, Stefansson S, Haudenschild C, Martin SS, Charpentier M, Chumsri S, et al. Cytometric characterization of circulating tumor cells captured by microfiltration and their correlation to the CellSearch(®) CTC test. Cytometry A. 2015;87:137–144. doi: 10.1002/cyto.a.22613. [DOI] [PubMed] [Google Scholar]
- 68.Truini A, Alama A, Dal Bello MG, Coco S, Vanni I, Rijavec E, et al. Clinical applications of circulating tumor cells in lung cancer patients by cellsearch system. Front Oncol. 2014;4:242. doi: 10.3389/fonc.2014.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer. 2013;133:2165–2171. doi: 10.1002/ijc.28230. [DOI] [PubMed] [Google Scholar]
- 70.Gazouli M, Lyberopoulou A, Pericleous P, Rizos S, Aravantinos G, Nikiteas N, et al. Development of a quantum-dot-labelled magnetic immunoassay method for circulating colorectal cancer cell detection. World J Gastroenterol. 2012;18:4419–4426. doi: 10.3748/wjg.v18.i32.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young R, Pailler E, Billiot F, Drusch F, Barthelemy A, Oulhen M, et al. Circulating tumor cells in lung cancer. Acta Cytol. 2012;56:655–660. doi: 10.1159/000345182. [DOI] [PubMed] [Google Scholar]
- 72.Kim YJ, Koo GB, Lee JY, Moon HS, Kim DG, Lee DG, et al. A microchip filter device incorporating slit arrays and 3-D flow for detection of circulating tumor cells using CAV1-EpCAM conjugated microbeads. Biomaterials. 2014;35:7501–7510. doi: 10.1016/j.biomaterials.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 73.Mohamadi RM, Besant JD, Mepham A, Green B, Mahmoudian L, Gibbs T, et al. Nanoparticle-mediated binning and profiling of heterogeneous circulating tumor cell subpopulations. Angew Chem Int Ed Engl. 2015;54:139–143. doi: 10.1002/anie.201409376. [DOI] [PubMed] [Google Scholar]
- 74.Wu S, Liu S, Liu Z, Huang J, Pu X, Li J, et al. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One. 2015;10:e0123976. doi: 10.1371/journal.pone.0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T, et al. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang T, Jia CP, Jun-Yang, Sun WJ, Wang WT, Zhang HL, et al. Highly sensitive enumeration of circulating tumor cells in lung cancer patients using a size-based filtration microfluidic chip. Biosens Bioelectron. 2014;51:213–218. doi: 10.1016/j.bios.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 77.Warkiani ME, Khoo BL, Tan DS, Bhagat AA, Lim WT, Yap YS, et al. An ultra-high-throughput spiral microfluidic biochip for the enrichment of circulating tumor cells. Analyst. 2014;139:3245–3255. doi: 10.1039/c4an00355a. [DOI] [PubMed] [Google Scholar]
- 78.Hosokawa M, Kenmotsu H, Koh Y, Yoshino T, Yoshikawa T, Naito T, et al. Size-based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. PLoS One. 2013;8:e67466. doi: 10.1371/journal.pone.0067466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hosokawa M, Yoshikawa T, Negishi R, Yoshino T, Koh Y, Kenmotsu H, et al. Microcavity array system for size-based enrichment of circulating tumor cells from the blood of patients with small-cell lung cancer. Anal Chem. 2013;85:5692–5698. doi: 10.1021/ac400167x. [DOI] [PubMed] [Google Scholar]
- 80.Chen X, Zhou F, Li X, Yang G, Zhang L, Ren S, et al. Folate receptor-positive circulating tumor cell detected by LT- PCR-based method as a diagnostic biomarker for non-small-cell lung cancer. J Thorac Oncol. 2015;10:1163–1171. doi: 10.1097/JTO.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 81.Lou J, Ben S, Yang G, Liang X, Wang X, Ni S, et al. Quantification of rare circulating tumor cells in non-small cell lung cancer by ligand-targeted PCR. PLoS One. 2013;8:e80458. doi: 10.1371/journal.pone.0080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dorsey JF, Kao GD, MacArthur KM, Ju M, Steinmetz D, Wileyto EP, et al. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: pilot study results. Cancer. 2015;121:139–149. doi: 10.1002/cncr.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma YC, Wang L, Yu FL. Recent advances and prospects in the isolation by size of epithelial tumor cells (ISET) methodology. Technol Cancer Res Treat. 2013;12:295–309. doi: 10.7785/tcrt.2012.500328. [DOI] [PubMed] [Google Scholar]
- 84.Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, Nonaka D, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7:306–315. doi: 10.1097/JTO.0b013e31823c5c16. [DOI] [PubMed] [Google Scholar]
- 85.Ke Z, Lin M, Chen JF, Choi JS, Zhang Y, Fong A, et al. Programming thermoresponsiveness of NanoVelcro substrates enables effective purification of circulating tumor cells in lung cancer patients. ACS Nano. 2015;9:62–70. doi: 10.1021/nn5056282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bratman SV, Newman AM, Alizadeh AA, Diehn M. Potential clinical utility of ultrasensitive circulating tumor DNA detection with CAPP-Seq. Expert Rev Mol Diagn. 2015;15:715–719. doi: 10.1586/14737159.2015.1019476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodriguez-Lee M, Kuhn P, Webb DR. Advancing cancer patient care by integrating circulating tumor cell technology to understand the spatial and temporal dynamics of cancer. Drug Dev Res. 2014;75:384–392. doi: 10.1002/ddr.21225. [DOI] [PubMed] [Google Scholar]
- 90.Fiorelli A, Accardo M, Carelli E, Angioletti D, Santini M, Di Domenico M. Circulating tumor cells in diagnosing lung cancer: clinical and morphologic analysis. Ann Thorac Surg. 2015;99:1899–1905. doi: 10.1016/j.athoracsur.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 91.Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud JM, Padovani B, et al. "Sentinel" circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9:e111597. doi: 10.1371/journal.pone.0111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheu CC, Yu YP, Tsai JR, Chang MY, Lin SR, Hwang JJ, et al. Development of a membrane array-based multimarker assay for detection of circulating cancer cells in patients with non-small cell lung cancer. Int J Cancer. 2006;119:1419–1426. doi: 10.1002/ijc.21999. [DOI] [PubMed] [Google Scholar]
- 93.Hodgkinson CL, Morrow CJ, Li Y, Metcalf RL, Rothwell DG, Trapani F, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014;20:897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 94.Fu L, Liu F, Fu H, Liu L, Yuan S, Gao Y, et al. Circulating tumor cells correlate with recurrence in stage III small-cell lung cancer after systemic chemoradiotherapy and prophylactic cranial irradiation. Jpn J Clin Oncol. 2014;44:948–955. doi: 10.1093/jjco/hyu109. [DOI] [PubMed] [Google Scholar]
- 95.Bao H, Burke PA, Huang J, Chen X, Brohawn PZ, Yao Y, et al. Circulating tumor cells: application as a biomarker for molecular characterization and predictor of survival in an all-comer solid tumor phase I clinical study. PLoS One. 2013;8:e58557. doi: 10.1371/journal.pone.0058557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sher YP, Shih JY, Yang PC, Roffler SR, Chu YW, Wu CW, et al. Prognosis of non-small cell lung cancer patients by detecting circulating cancer cells in the peripheral blood with multiple marker genes. Clin Cancer Res. 2005;11:173–179. [PubMed] [Google Scholar]
- 97.Breitenbuecher F, Hoffarth S, Worm K, Cortes-Incio D, Gauler TC, Köhler J, et al. Development of a highly sensitive and specific method for detection of circulating tumor cells harboring somatic mutations in non-small-cell lung cancer patients. PLoS One. 2014;9:e85350. doi: 10.1371/journal.pone.0085350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh RF, et al. Molecular biomarker analyses using circulating tumor cells. PLoS One. 2010;5:e12517. doi: 10.1371/journal.pone.0012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sorensen BS, Wu L, Wei W, Tsai J, Weber B, Nexo E, et al. Monitoring of epidermal growth factor receptor tyrosine kinase inhibitor-sensitizing and resistance mutations in the plasma DNA of patients with advanced non-small cell lung cancer during treatment with erlotinib. Cancer. 2014;120:3896–3901. doi: 10.1002/cncr.28964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taniguchi K, Uchida J, Nishino K, Kumagai T, Okuyama T, Okami J, et al. Quantitative detection of EGFR mutations in circulating tumor DNA derived from lung adenocarcinomas. Clin Cancer Res. 2011;17:7808–7815. doi: 10.1158/1078-0432.CCR-11-1712. [DOI] [PubMed] [Google Scholar]
- 102.Yang MJ, Chiu HH, Wang HM, Yen LC, Tsao DA, Hsiao CP, et al. Enhancing detection of circulating tumor cells with activating KRAS oncogene in patients with colorectal cancer by weighted chemiluminescent membrane array method. Ann Surg Oncol. 2010;17:624–633. doi: 10.1245/s10434-009-0831-8. [DOI] [PubMed] [Google Scholar]
- 103.Chen YF, Wang JY, Wu CH, Chen FM, Cheng TL, Lin SR. Detection of circulating cancer cells with K-ras oncogene using membrane array. Cancer Lett. 2005;229:115–122. doi: 10.1016/j.canlet.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 104.Tsao DA, Yang MJ, Chang HJ, Yen LC, Chiu HH, Hsueh EJ, et al. A fast and convenient new technique to detect the therapeutic target, K-ras mutant, from peripheral blood in non-small cell lung cancer patients. Lung Cancer. 2010;68:51–57. doi: 10.1016/j.lungcan.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 105.Lin SR, Huang MY, Chang MS. A high-performance gene chip platform for detecting genetic markers from circulating tumor cells. Biomark Genom Med. 2013;5:12–17. [Google Scholar]
- 106.Faugeroux V, Pailler E, Auger N, Taylor M, Farace F. Clinical utility of circulating tumor cells in ALK-positive non-small-cell lung cancer. Front Oncol. 2014;4:281. doi: 10.3389/fonc.2014.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pailler E, Adam J, Barthélémy A, Oulhen M, Auger N, Valent A, et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J Clin Oncol. 2013;31:2273–2281. doi: 10.1200/JCO.2012.44.5932. [DOI] [PubMed] [Google Scholar]
- 108.Cho WC. Emerging techniques in molecular detection of circulating tumor cells. Expert Rev Mol Diagn. 2014;14:131–134. doi: 10.1586/14737159.2014.868308. [DOI] [PubMed] [Google Scholar]
- 109.Ilie M, Long E, Butori C, Hofman V, Coelle C, Mauro V, et al. ALK-gene rearrangement: a comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann Oncol. 2012;23:2907–2913. doi: 10.1093/annonc/mds137. [DOI] [PubMed] [Google Scholar]
- 110.Khoo BL, Warkiani ME, Tan DS, Bhagat AA, Irwin D, Lau DP, et al. Clinical validation of an ultra high-throughput spiral microfluidics for the detection and enrichment of viable circulating tumor cells. PLoS One. 2014;9:e99409. doi: 10.1371/journal.pone.0099409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 112.Lebofsky R, Decraene C, Bernard V, Kamal M, Blin A, Leroy Q, et al. Circulating tumor DNA as a non-invasive substitute to metastasis biopsy for tumor genotyping and personalized medicine in a prospective trial across all tumor types. Mol Oncol. 2015;9:783–790. doi: 10.1016/j.molonc.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Costa DB. Identification of somatic genomic alterations in circulating tumors cells: another step forward in non-small-cell lung cancer? J Clin Oncol. 2013;31:2236–2239. doi: 10.1200/JCO.2013.48.9229. [DOI] [PubMed] [Google Scholar]
- 114.Hong B, Zu Y. Detecting circulating tumor cells: current challenges and new trends. Theranostics. 2013;3:377–394. doi: 10.7150/thno.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]