We find here that Ccr4, Pop2, and Dhh1 modulate the levels of mRNAs for specific Rho1 regulators, Rom2 and Lrg1. In budding yeast, Rho1 activity is tightly regulated both temporally and spatially. It is anticipated that Ccr4, Pop2, and Dhh1 may contribute to the precise spatiotemporal control of Rho1 activity by regulating expression of its regulators temporally and spatially. Our finding on the roles of the components of the Ccr4-Not complex in yeast would give important information for understanding the roles of the evolutionary conserved Ccr4-Not complex.
KEYWORDS: Ccr4-Not complex, Rho1, cell wall, mRNA stability, yeasts
ABSTRACT
Ccr4, a component of the Ccr4-Not cytoplasmic deadenylase complex, is known to be required for the cell wall integrity (CWI) pathway in the budding yeast Saccharomyces cerevisiae. However, it is not fully understood how Ccr4 and other components of the Ccr4-Not complex regulate the CWI pathway. Previously, we showed that Ccr4 functions in the CWI pathway together with Khd1 RNA binding protein. Ccr4 and Khd1 modulate a signal from Rho1 small GTPase in the CWI pathway by regulating the expression of ROM2 mRNA and LRG1 mRNA, encoding a guanine nucleotide exchange factor (GEF) and a GTPase-activating protein (GAP) for Rho1, respectively. Here we examined the possible involvement of the POP2 gene encoding a subunit of the Ccr4-Not complex and the DHH1 gene encoding a DEAD box RNA helicase that associates with the Ccr4-Not complex in the regulation of ROM2 and LRG1 expression. Neither ROM2 mRNA level nor Rom2 function was impaired by pop2Δ or dhh1Δ mutation. The LRG1 mRNA level was increased in pop2Δ and dhh1Δ mutants, as well as the ccr4Δ mutant, and the growth defects caused by pop2Δ and dhh1Δ mutations were suppressed by lrg1Δ mutation. Our results suggest that LRG1 expression is regulated by Ccr4 together with Pop2 and Dhh1 and that ROM2 expression is regulated by Khd1 and Ccr4, but not by Pop2 and Dhh1. Thus, Rho1 activity in the CWI pathway is precisely controlled by modulation of the mRNA levels for Rho1-GEF Rom2 and Rho1-GAP Lrg1.
IMPORTANCE We find here that Ccr4, Pop2, and Dhh1 modulate the levels of mRNAs for specific Rho1 regulators, Rom2 and Lrg1. In budding yeast, Rho1 activity is tightly regulated both temporally and spatially. It is anticipated that Ccr4, Pop2, and Dhh1 may contribute to the precise spatiotemporal control of Rho1 activity by regulating expression of its regulators temporally and spatially. Our finding on the roles of the components of the Ccr4-Not complex in yeast would give important information for understanding the roles of the evolutionary conserved Ccr4-Not complex.
INTRODUCTION
Gene expression can be regulated at many of the steps in the pathway from DNA to protein. In these regulations, posttranscriptional regulation includes the control of mRNA degradation and translation. Both 5′-cap and 3′ poly(A) tail structures of mRNAs have important roles in the control of mRNA degradation and translation. In eukaryotes, there are two general mechanisms of cytoplasmic degradation of mRNAs, 5′-to-3′ degradation and 3′-to-5′ degradation (1). Both degradations are initiated by shortening of the 3′ poly(A) tail in a process referred to as deadenylation. This deadenylation is carried out by the Pan2-Pan3 complex as well as by the Ccr4-Not complex. In the 5′-to-3′ degradation pathway, the deadenylated mRNAs are decapped by the Dcp1/Dcp2 decapping enzyme and then subjected to 5′-to-3′ degradation by Xrn1 exonuclease. Several decapping activators, such as Dhh1, Pat1, Edc3, and Scd6, stimulate the activity of decapping enzyme. In the 3′-to-5′ degradation pathway, the deadenylated mRNAs are subjected to 3′-to-5′ degradation by the exosome complex. Translation initiation is promoted by binding of the translation initiation complex eIF4F (eukaryotic initiation factor 4F) to the 5′-cap structure. This eIF4F complex contains eIF4E that directly binds to the 5′-cap structure, eIF4A that acts as an RNA helicase, and eIF4G that serves as a scaffold for the complex. Binding of the eIF4F complex to the 5′-cap structure recruits the 43S preinitiation complex, which includes the small ribosomal subunit, the initiator tRNA, and additional initiation factors (2). Translation initiation is also enhanced by the 3′ poly(A) tail and the poly(A) binding protein that interacts with eIF4G. In most cases, control of mRNA degradation and translational initiation is mediated by the 3′ untranslated regions (3′ UTR) of the regulated mRNAs where RNA binding proteins such as Puf family RNA binding proteins bind (3, 4).
The Ccr4-Not complex consists of nine core subunits, Ccr4, Pop2/Caf1, Not1, Not2, Not3, Not4, Not5, Caf40, and Caf130, in the budding yeast Saccharomyces cerevisiae (5, 6). In this complex, Ccr4 and Pop2 are catalytic subunits of deadenylase, and Not4 acts as a ubiquitin ligase. The ccr4Δ mutant shows pleiotropic phenotypes, including weak cell lysis, abnormal morphology, and defects in checkpoint control and cell cycle progression (7–11). The pop2Δ mutant also shows similar pleiotropic phenotypes, including weak cell lysis (7). Ccr4 and Pop2 physically and genetically interact with Dhh1, a DExD/H box protein known as decapping activator (1, 7). Overexpression of Dhh1 suppresses the phenotypes associated with ccr4Δ and pop2Δ mutant cells, and the dhh1Δ mutant shows a weak cell lysis phenotype, similar to ccr4Δ and pop2Δ mutants (7).
The cell wall of the budding yeast is required to maintain cell shape and integrity (12). Yeast cells must remodel the rigid structure of the cell wall during vegetative growth and during pheromone-induced morphogenesis. The cell wall remodeling is monitored and regulated by the cell wall integrity (CWI) signaling pathway (12). In the CWI signaling pathway, signals are initiated at the plasma membrane through the cell surface sensors, Wsc1, Wsc2, Wsc3, Mid2, and Mtl1. Together with phosphatidylinositol 4,5-bisphosphate (PI4,5P2), which recruits Rom1/2 guanine nucleotide exchange factors (GEFs) to the plasma membrane, the cell wall sensors stimulate nucleotide exchange on a small GTPase Rho1 through the activation of Rom1/2. The activated Rho1, Rho1-GTP, then activates several effectors, including protein kinase C (Pkc1), β1,3-glucan synthase, Bni1 formin protein, exocyst component Sec3, and Skn7 transcription factor. Pkc1 activates downstream mitogen-activated protein (MAP) kinase cascade, which is comprised of Bck1, Mkk1/2, and Mpk1. Mpk1 phosphorylates and activates two transcription factors, Rlm1 and the SBF complex (Swi4/Swi6), which induce gene expression. Rho1-GTP is inactivated by GTPase-activating proteins (GAPs), including Bem2, Sac7, Bag7, and Lrg1.
We have previously found that Ccr4 negatively regulates expression of the LRG1 mRNA encoding one of the Rho1-GAPs in the CWI pathway (11). Loss of LRG1 suppressed the cell lysis of the ccr4Δ mutant. Ccr4, together with RNA binding protein Khd1, also positively regulates expression of ROM2 mRNA encoding Rho1-GEF (11). The ccr4Δ khd1Δ double mutant shows more severe cell lysis.
In this study, we examined the roles of Pop2 and Dhh1 in the CWI signaling pathway. The LRG1 mRNA level was increased in pop2Δ and dhh1Δ mutants as well as ccr4Δ mutant and the increased LRG1 mRNA level contributes to the growth defect of pop2Δ and dhh1Δ mutants. On the other hand, ROM2 expression or Rom2 function was not impaired in pop2Δ and dhh1Δ mutants. Our results indicate that, in addition to the involvement of Ccr4 in the CWI signaling pathway, Dhh1 and Pop2 take a part in the regulation of Rho1 activity through the Rho1-GAP Lrg1.
RESULTS
The ccr4Δ and pop2Δ mutants, but not the dhh1Δ mutant, display a synthetic growth defect with the khd1Δ mutation.
We have shown that ccr4Δ and pop2Δ mutants displayed a synthetic growth defect with the khd1Δ mutation (11). Tetrad analysis revealed that ccr4Δ and pop2Δ mutant cells grew slower than wild-type cells, while khd1Δ ccr4Δ and khd1Δ pop2Δ double mutant cells grew much more slowly than either khd1Δ, ccr4Δ, or pop2Δ single mutant cells (Fig. 1A and B). To examine whether the dhh1Δ mutant shows a synthetic growth defect with the khd1Δ mutation, we performed tetrad analysis using a diploid strain that was heterozygous for khd1Δ and dhh1Δ alleles. The dhh1Δ mutant cells grew slower than wild-type cells, and khd1Δ dhh1Δ double mutant cells and dhh1Δ single mutant cells grew similarly (Fig. 1C). Therefore, unlike ccr4Δ and pop2Δ mutants, the dhh1Δ mutant does not display a synthetic growth defect with the khd1Δ mutation.
FIG 1 .
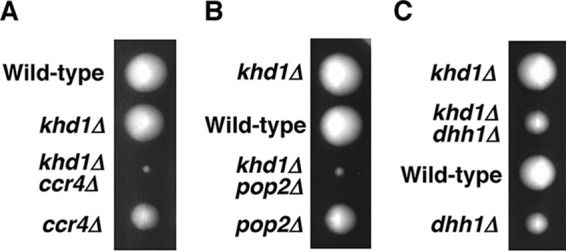
Growth of the khd1Δ ccr4Δ, khd1Δ pop2Δ, and khd1Δ dhh1Δ mutant strains. (A) Strain 10BD-c163 that was heterozygous for khd1Δ and ccr4Δ alleles was sporulated, and tetrads were dissected onto yeast extract-peptone-dextrose (YPD) plates. Growth after 4 days at 25°C is shown. The genotypes are indicated to the left of the image. More than 50 tetrads were dissected, and representative data are shown. (B) Strain 10BD-p163 that was heterozygous for khd1Δ and pop2Δ alleles was sporulated, and tetrads were dissected onto YPD plates. The genotypes are indicated to the left of the image. (C) Strain 10BD-d163 that was heterozygous for khd1Δ and dhh1Δ alleles was sporulated, and tetrads were dissected onto YPD plates. The genotypes are indicated to the left of the image.
ROM2 mRNA level was not decreased in the pop2Δ and dhh1Δ mutants.
We have previously shown that the level of ROM2 mRNA (encodes Rho1 GEF) was slightly decreased in the ccr4Δ mutant, and this reduction was enhanced by the khd1Δ mutation (11) (Fig. 2A). Rom2 and Rom1 comprise a redundant pair of GEF for Rho1 (13). Loss of ROM2 function results in temperature-sensitive growth, whereas loss of both ROM2 and ROM1 is lethal. Using a mutation of ROM1, we have obtained the genetic evidence indicating that Rom2 function was indeed impaired in ccr4Δ mutant and khd1Δ ccr4Δ double mutant cells (11) (Fig. 3A). If ROM2 function were impaired in a strain harboring a given mutation, the mutant would show a synthetic growth defect with the rom1Δ mutation. Consistent with the fact that the ROM2 mRNA level is decreased in ccr4Δ mutant and khd1Δ ccr4Δ double mutant cells, ccr4Δ rom1Δ double mutant cells showed much slower growth than ccr4Δ single mutant cells, and khd1Δ ccr4Δ rom1Δ triple mutant cells showed much slower growth than khd1Δ ccr4Δ double mutant cells (Fig. 3A). This is also consistent with the observation that overexpression of ROM2 from a multicopy plasmid can suppress the growth defects of khd1Δ ccr4Δ double mutant, ccr4Δ rom1Δ double mutant, and khd1Δ ccr4Δ rom1Δ triple mutant cells (11) (see Fig. 12A) (data not shown).
FIG 2 .
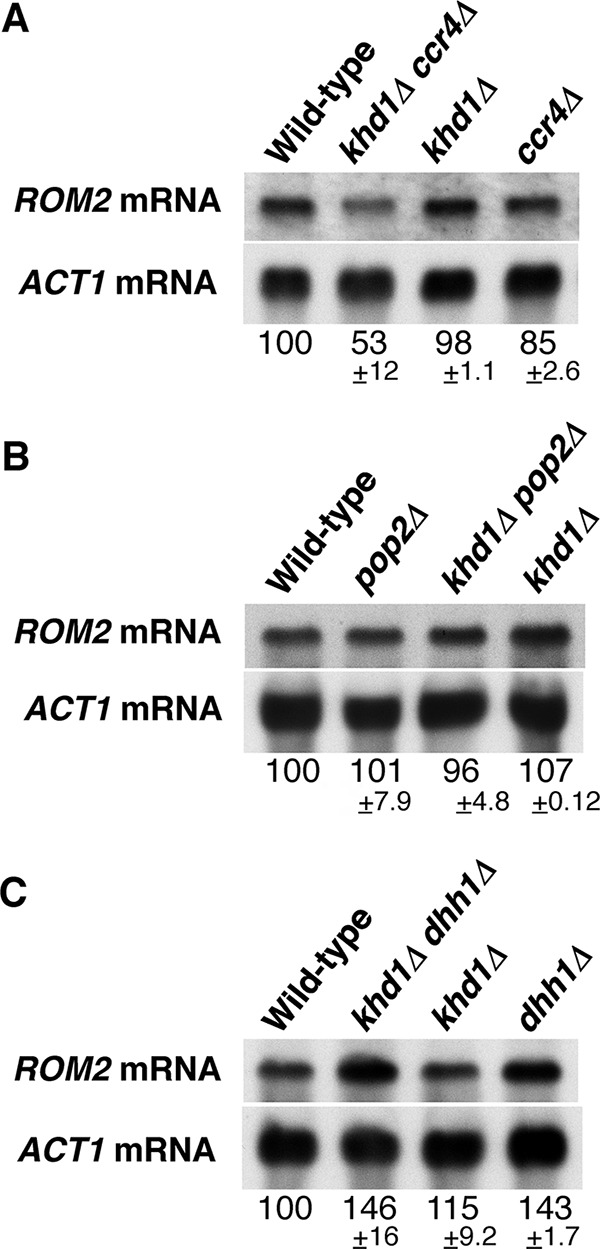
ROM2 mRNA levels in the khd1Δ ccr4Δ, khd1Δ pop2Δ, and khd1Δ dhh1Δ mutant strains. (A) ROM2 mRNA levels in wild-type, khd1Δ ccr4Δ, khd1Δ, and ccr4Δ cells. Wild-type (c1H-1A), khd1Δ ccr4Δ (c1H-1B), khd1Δ (c1H-1C), and ccr4Δ (c1H-1D) cells were cultured to mid-logarithmic phase in YPD medium and collected, and total RNA was prepared. The ROM2 transcripts were quantified by Northern blotting as described in Materials and Methods. ACT1 mRNA was included as a quantity control. mRNA levels are shown below the lanes. The mRNA levels are shown as percentages of the wild-type levels and represent the means ± standard deviations from three independent experiments. (B) ROM2 mRNA levels in wild-type, pop2Δ, khd1Δ pop2Δ, and khd1Δ cells. Wild-type (p1H-2A), pop2Δ (p1H-2B), khd1Δ pop2Δ (p1H-2C), and khd1Δ (p1H-2D) cells were cultured to mid-logarithmic phase in YPD medium and collected, and total RNA was prepared. (C) ROM2 mRNA levels in wild-type, khd1Δ dhh1Δ, khd1Δ, and dhh1Δ cells. Wild-type (d1H-1A), khd1Δ dhh1Δ (d1H-1B), khd1Δ (d1H-1C), and dhh1Δ (d1H-1D) cells were cultured to mid-logarithmic phase in YPD medium and collected, and total RNA was prepared.
FIG 3 .
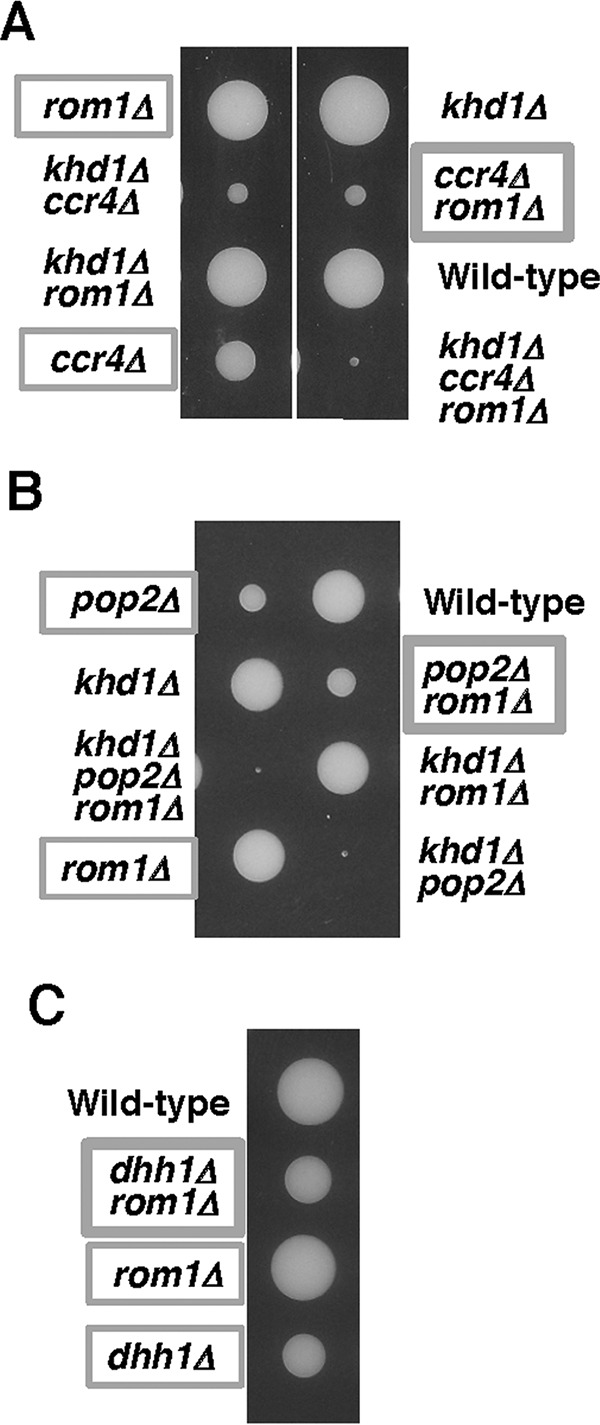
Growth of the ccr4Δ rom1Δ, pop2Δ rom1Δ, and dhh1Δ rom1Δ mutant strains. (A) Strain 10BD-c163r1 that was heterozygous for khd1Δ, ccr4Δ, and rom1Δ alleles was sporulated, and tetrads were dissected onto YPD containing 10% sorbitol. Growth after 6 days at 25°C is shown. Genotypes are indicated on both sides of the blots. More than 20 tetrads were dissected, and representative data are shown. (B) Strain 10BD-p163r1 that was heterozygous for khd1Δ, pop2Δ, and rom1Δ alleles was sporulated, and tetrads were dissected onto YPD containing 10% sorbitol. Growth after 6 days at 25°C is shown. Genotypes are indicated on both sides of the blots. More than 20 tetrads were dissected, and representative data are shown. (C) Strain 10BD-d1r1 that was heterozygous for dhh1Δ and rom1Δ alleles was sporulated, and tetrads were dissected onto YPD containing 10% sorbitol. Growth after 6 days at 25°C is shown. Genotypes are indicated to the left of the image. More than 20 tetrads were dissected, and representative data are shown.
FIG 12 .
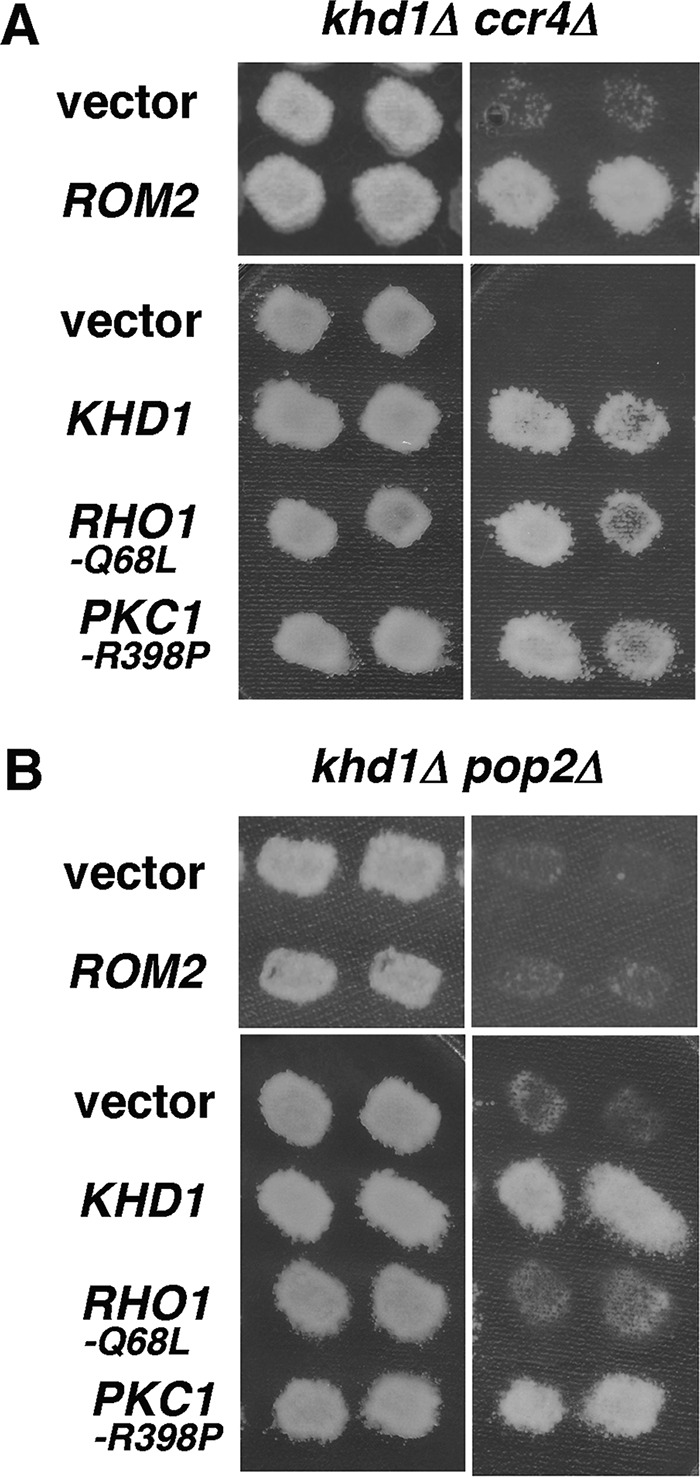
Different roles of Ccr4 and Pop2 in the CWI pathway. (A) Overexpression of ROM2 and expression of activated Rho1 and Pkc1 alleles suppress the growth defect of khd1Δ ccr4Δ double mutants. The plasmids were the ROM2 plasmid and plasmid harboring activated Rho1 and Pkc1 alleles in khd1Δ ccr4Δ. Transformants of the khd1Δ ccr4Δ strain (c1H-1B) carrying the indicated plasmids were streaked onto YPD medium and incubated at 25°C (left) or 37°C (right). Each patch represents an independent transformant. Plasmids were YEplac195 (vector), YEplac195-ROM2 (ROM2), YCplac33 (vector), YCplac-RHO1-Q68L (RHO1-Q68L), and YCplac33-PKC1-R398P (PKC1-R398P). (B) Overexpression of ROM2 and expression of activated Rho1 and Pkc1 alleles suppress the growth defect of khd1Δ pop2Δ double mutants. The plasmids were the ROM2 plasmid and plasmid harboring activated Rho1 and Pkc1 alleles in khd1Δ pop2Δ. Transformants of the khd1Δ pop2Δ strain (p1H-2C) carrying the indicated plasmids were streaked onto YPD medium and incubated at 25 °C (left) or 37°C (right).
We next applied this approach to examine whether the Rom2 function is impaired in pop2Δ and dhh1Δ mutants. Tetrad analysis using the diploid strain that was heterozygous for pop2Δ, rom1Δ, and khd1Δ alleles showed that pop2Δ rom1Δ double mutant cells and pop2Δ single mutant cells grew similarly (Fig. 3B). The khd1Δ pop2Δ rom1Δ triple mutant cells and khd1Δ pop2Δ double mutant cells also grew similarly (Fig. 3B). Tetrad analysis using the diploid strain that was heterozygous for dhh1Δ and rom1Δ alleles showed that dhh1Δ rom1Δ double mutant cells and dhh1Δ single mutant cells also grew similarly (Fig. 3C). These results suggest that Rom2 normally operates in pop2Δ and dhh1Δ mutant cells. The ROM2 mRNA level was consistently not altered in pop2Δ and khd1Δ pop2Δ mutant cells compared to wild-type cells (Fig. 2B). Rather, ROM2 mRNA level was marginally increased in dhh1Δ single mutant and khd1Δ dhh1Δ double mutant cells (Fig. 2C). Thus, Rom2 function and ROM2 expression were impaired in ccr4Δ mutant and khd1Δ ccr4Δ double mutant cells, but not in pop2Δ and dhh1Δ mutant cells. These results indicate that only Ccr4 functions in regulation of the expression level of ROM2 mRNA.
Rom2 protein level was decreased in ccr4Δ and khd1Δ ccr4Δ mutants.
To address how Ccr4 functions in ROM2 expression, we quantified the level of Rom2 protein in ccr4Δ single mutant and khd1Δ ccr4Δ double mutant cells using the myc-tagged ROM2 construct. As shown in Fig. 4A, myc-tagged Rom2 (Rom2myc) protein levels were decreased in ccr4Δ single mutant and khd1Δ ccr4Δ double mutant cells compared to wild-type cells. Decreased protein levels in ccr4Δ and khd1Δ ccr4Δ mutant cells (61% in ccr4Δ mutant and 25% in khd1Δ ccr4Δ mutant cells in Fig. 4A) were more evident than the decreased mRNA levels (85% in ccr4Δ mutant and 53% in khd1Δ ccr4Δ mutant cells in Fig. 2A), implying that Rom2 expression is regulated at both mRNA and protein levels. The myc-tagged ROM2 construct used here had the ADH1 3′ UTR instead of endogenous ROM2 3′ UTR (Fig. 4A), implying that the ROM2 3′ UTR is not essential for the regulation of ROM2 expression. To investigate the protein level regulation, we utilized the pGAL-HA-ROM2 construct harboring the ROM2 3′ UTR (Fig. 4B and C). While the HA-ROM2 mRNA levels from the GAL1 promoter were not altered in ccr4Δ and khd1Δ ccr4Δ mutant cells compared to wild-type cells (Fig. 4B), the hemagglutinin-tagged Rom2 (HA-Rom2) protein levels were clearly decreased in ccr4Δ and khd1Δ ccr4Δ mutant cells compared to wild-type cells (Fig. 4C). Together with the observation that ROM2 mRNA levels from the endogenous ROM2 promoter were slightly decreased in ccr4Δ mutant and khd1Δ ccr4Δ double mutant cells (Fig. 2A), Rom2 expression is likely to be regulated at the both mRNA and protein levels.
FIG 4 .

Rom2 protein levels in the ccr4Δ and khd1Δ ccr4Δ mutant strains. (A, top) Schematic representation of the regulatory elements in the ROM2myc construct. (Bottom) Rom2myc protein levels in wild-type, khd1Δ ccr4Δ, khd1Δ, and ccr4Δ cells. Wild-type (180-3B-4A), khd1Δ ccr4Δ (180-3B-1A), ccr4Δ (180-3B-7C), and khd1Δ (180-3B-7D) cells harboring the ROM2myc construct were cultured to mid-logarithmic phase in YPD medium and collected, and total protein was prepared. The Rom2myc proteins were quantified by Western blotting as described in Materials and Methods. Mcm2 protein was included as a quantity control. The protein levels shown below the lanes are indicated as percentages of the wild-type levels and represent the means ± standard deviations from three independent experiments. (B, top) Schematic representation of the regulatory elements in the pGAL-HA-ROM2 construct. (Bottom) HA-ROM2 mRNA levels in wild-type, khd1Δ ccr4Δ, khd1Δ, and ccr4Δ cells. Wild-type (c1H-1A), khd1Δ ccr4Δ (c1H-1B), khd1Δ (c1H-1C), and ccr4Δ (c1H-1D) cells harboring pGAL-HA-ROM2 plasmid were cultured to mid-logarithmic phase in SG−Ura medium and collected, and total RNA was prepared. The HA-ROM2 transcripts were quantified by Northern blotting as described in Materials and Methods. ACT1 mRNA was included as a quantity control. The mRNA levels are indicated as percentages of wild-type levels and represent the means ± standard deviations from three independent experiments. (C) HA-Rom2 protein levels in wild-type, khd1Δ ccr4Δ, khd1Δ, and ccr4Δ cells. Wild-type (c1H-1A), khd1Δ ccr4Δ (c1H-1B), khd1Δ (c1H-1C), and ccr4Δ (c1H-1D) cells harboring pGAL-HA-ROM2 plasmid were cultured to mid-logarithmic phase in SG−Ura medium and collected, and total protein was prepared. The HA-Rom2 proteins were quantified by Western blotting as described in Materials and Methods. Mcm2 protein was included as a quantity control. The protein levels are indicated as percentages of wild-type levels and represent the means ± standard deviations from three independent experiments.
We have previously shown that the ccr4Δ single mutant shows weak cell lysis and that the khd1Δ ccr4Δ double mutant shows more severe cell lysis (11). Due to the cell lysis, Mpk1 is constitutively activated in ccr4Δ and khd1Δ ccr4Δ mutants (data not shown). Since it has been reported that Mpk1 downregulates Rom2 (14), we speculated that Mpk1 might be involved in ROM2 expression. We found that the decreased Rom2myc protein levels in ccr4Δ and khd1Δ ccr4Δ mutants were partially suppressed by the mpk1Δ mutation (Fig. 5). Thus, the decreased Rom2myc protein levels in ccr4Δ and khd1Δ ccr4Δ mutants are partly due to the constitutive activation of Mpk1.
FIG 5 .
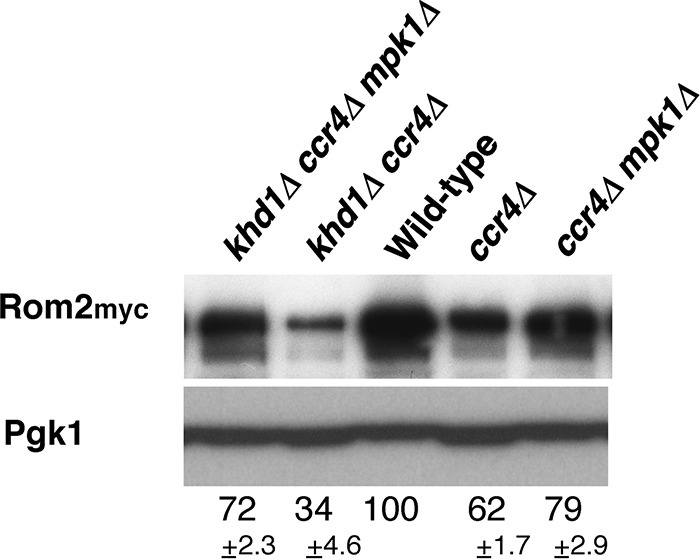
Rom2 protein levels in the ccr4Δ, khd1Δ ccr4Δ, ccr4Δ mpk1Δ, and khd1Δ ccr4Δ mpk1Δ mutant strains. The Rom2myc protein levels in wild-type, ccr4Δ, khd1Δ ccr4Δ, ccr4Δ mpk1Δ, and ccr4Δ khd1Δ mpk1Δ cells are shown. Wild-type (180-m-1D4A), khd1Δ ccr4Δ (180-m-3D), khd1Δ ccr4Δ mpk1Δ (180-m-7A), ccr4Δ (180-m-6B), and ccr4Δ mpk1Δ (180-m-4C) cells harboring the ROM2myc construct were cultured to mid-logarithmic phase in YPD containing 10% sorbitol medium and collected, and total protein was prepared. The Rom2myc proteins were quantified by Western blotting as described in Materials and Methods. Pgk1 protein was included as a quantity control. The protein levels are indicated as percentages of wild-type levels and represent the means ± standard deviations from three independent experiments.
LRG1 expression is negatively regulated by Pop2 and Dhh1.
We have previously shown that the level of LRG1 mRNA encoding Rho1 GAP was increased in the ccr4Δ single mutant and khd1Δ ccr4Δ double mutant cells (11) (Fig. 6A). Therefore, we quantified LRG1 mRNA levels in pop2Δ single mutant and khd1Δ pop2Δ double mutant cells. As shown in Fig. 6B, LRG1 mRNA levels were increased in pop2Δ and khd1Δ pop2Δ mutant cells than in wild-type cells. In addition, we found that LRG1 mRNA levels were increased in dhh1Δ and khd1Δ dhh1Δ mutant cells than in wild-type cells (Fig. 6C). Therefore, LRG1 expression is downregulated by Pop2 and Dhh1.
FIG 6 .
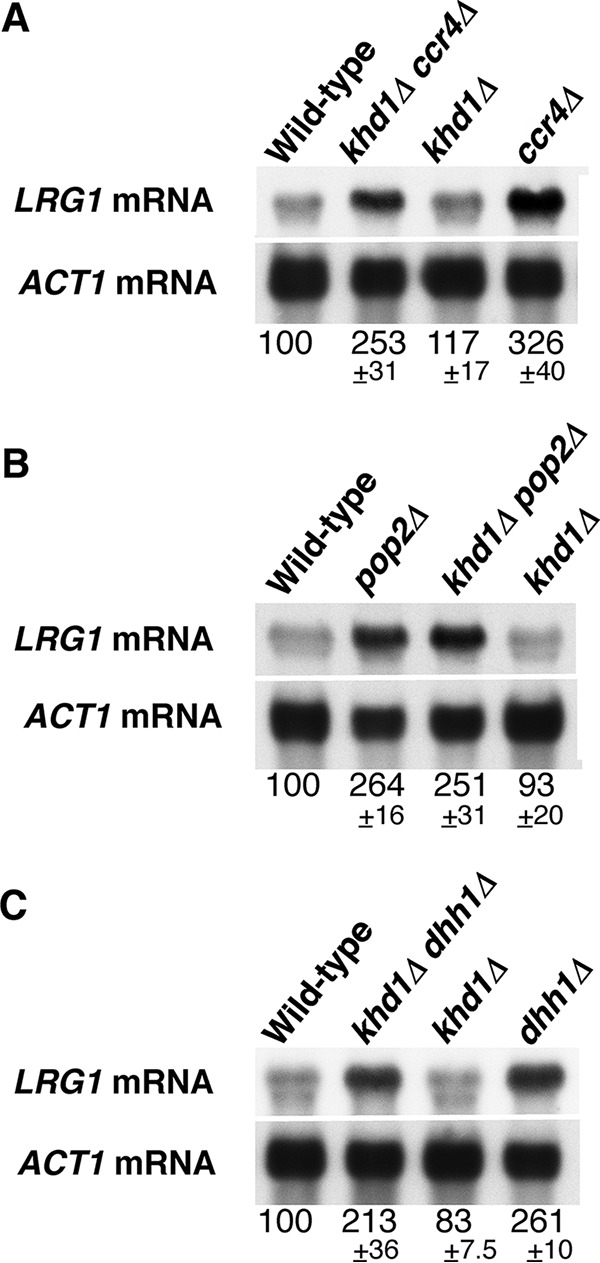
LRG1 mRNA levels in the khd1Δ ccr4Δ, khd1Δ pop2Δ, and khd1Δ dhh1Δ mutant strains. (A) LRG1 mRNA levels in wild-type, khd1Δ ccr4Δ, khd1Δ, and ccr4Δ cells. Wild-type (c1H-1A), khd1Δ ccr4Δ (c1H-1B), khd1Δ (c1H-1C), and ccr4Δ (c1H-1D) cells were cultured to mid-logarithmic phase in YPD medium and collected, and total RNA was prepared. The LRG1 transcripts were quantified by Northern blotting as described in Materials and Methods. ACT1 mRNA was included as a quantity control. The mRNA levels are indicated as percentages of wild-type levels and represent the means ± standard deviations from three independent experiments. The bands smaller than the LRG1 mRNA bands show cross hybridization to rRNA. (B) LRG1 mRNA levels in wild-type, pop2Δ, khd1Δ pop2Δ, and khd1Δ cells. Wild-type (p1H-2A), pop2Δ (p1H-2B), khd1Δ pop2Δ (p1H-2C), and khd1Δ (p1H-2D) cells were cultured to mid-logarithmic phase in YPD medium and collected, and total RNA was prepared. (C) LRG1 mRNA levels in wild-type, khd1Δ dhh1Δ, khd1Δ, and dhh1Δ cells. Wild-type (d1H-1A), khd1Δ dhh1Δ (d1H-1B), khd1Δ (d1H-1C), and dhh1Δ (d1H-1D) cells were cultured to mid-logarithmic phase in YPD medium and collected, and total RNA was prepared.
Pop2 and Dhh1 encode a cytoplasmic deadenylase and a DExD/H box RNA helicase known as mRNA decapping activator, respectively, and they are important factors acting in mRNA degradation (1). Therefore, we speculate that Pop2 and Dhh1 are involved in the degradation of LRG1 mRNA. To analyze the decay rates of LRG1 mRNA, we employed the controllable GAL1 promoter to express LRG1 mRNA. As shown in Fig. 7A and B, LRG1 mRNA were stabilized in pop2Δ and dhh1Δ mutant cells. Notably, in pop2Δ and dhh1Δ mutant cells, LRG1 mRNA has a twofold-longer half-life than in wild-type cells. These results indicate that Pop2 and Dhh1 are involved in the degradation of LRG1 mRNA.
FIG 7 .
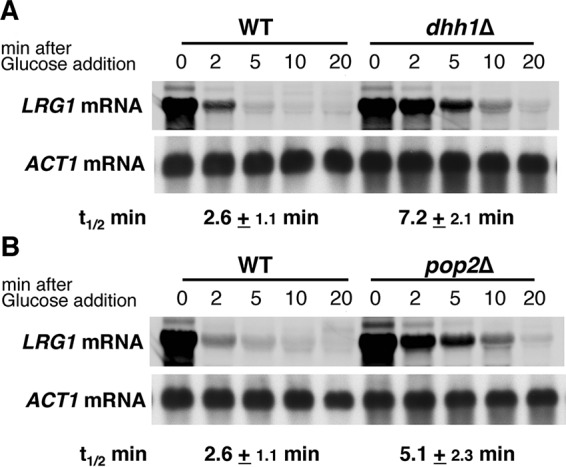
Degradation of the LRG1 mRNA in the pop2Δ and dhh1Δ mutant strains. (A) Wild-type (WT) d1H-1A cells carrying the pGAL-LRG1 plasmid and dhh1Δ cells (d1H-1D cells) carrying pGAL-LRG1 plasmid. (B) WT cells (p1H-2A) carrying pGAL-LRG1 plasmid and pop2Δ cells (p1H-2B) carrying pGAL-LRG1 plasmid. Cells harboring the pGAL-LRG1 plasmid were grown in SG−Ura, and the medium was changed to SC−Ura to inhibit transcription from the GAL1 promoter. Cells were harvested at the times indicated above the lanes, and total RNA was isolated. Samples were analyzed by Northern blotting with specific probes, and the half-lives (t1/2) (in minutes) were determined as the means from three independent experiments. ACT1 mRNA was used as a reference for quantification.
Loss of LRG1 suppresses the growth defect of the pop2Δ and dhh1Δ mutations.
We have shown that LRG1 mRNA expression is increased in the khd1Δ ccr4Δ mutant and that deletion of LRG1 suppressed the growth defect of the khd1Δ ccr4Δ mutant (11). At high temperature, the severe growth defect was observed even in the ccr4Δ single mutant (Fig. 8A). The defect associated with the ccr4Δ single mutation was effectively suppressed by deletion of LRG1 (Fig. 8A), indicating that the increased level of LRG1 contributes to the growth defect of ccr4Δ mutant cells. Since LRG1 mRNA levels were also increased in pop2Δ and dhh1Δ mutant cells, we examined whether deletion of LRG1 can also suppress the growth defect caused by pop2Δ and dhh1Δ mutations. The pop2Δ and dhh1Δ mutant cells failed to grow at elevated temperature (37°C) (Fig. 8B and C). Their growth defects are due to cell lysis, since addition of osmotic stabilizer sorbitol to medium improved their growth at 37°C (data not shown). The pop2Δ lrg1Δ and dhh1Δ lrg1Δ double mutant cells could grow at 37°C, although their growth was slightly slower than that of wild-type cells (Fig. 8B and C). These results indicate that the increased LRG1 mRNA level contributes to the growth defect of pop2Δ and dhh1Δ mutant cells.
FIG 8 .
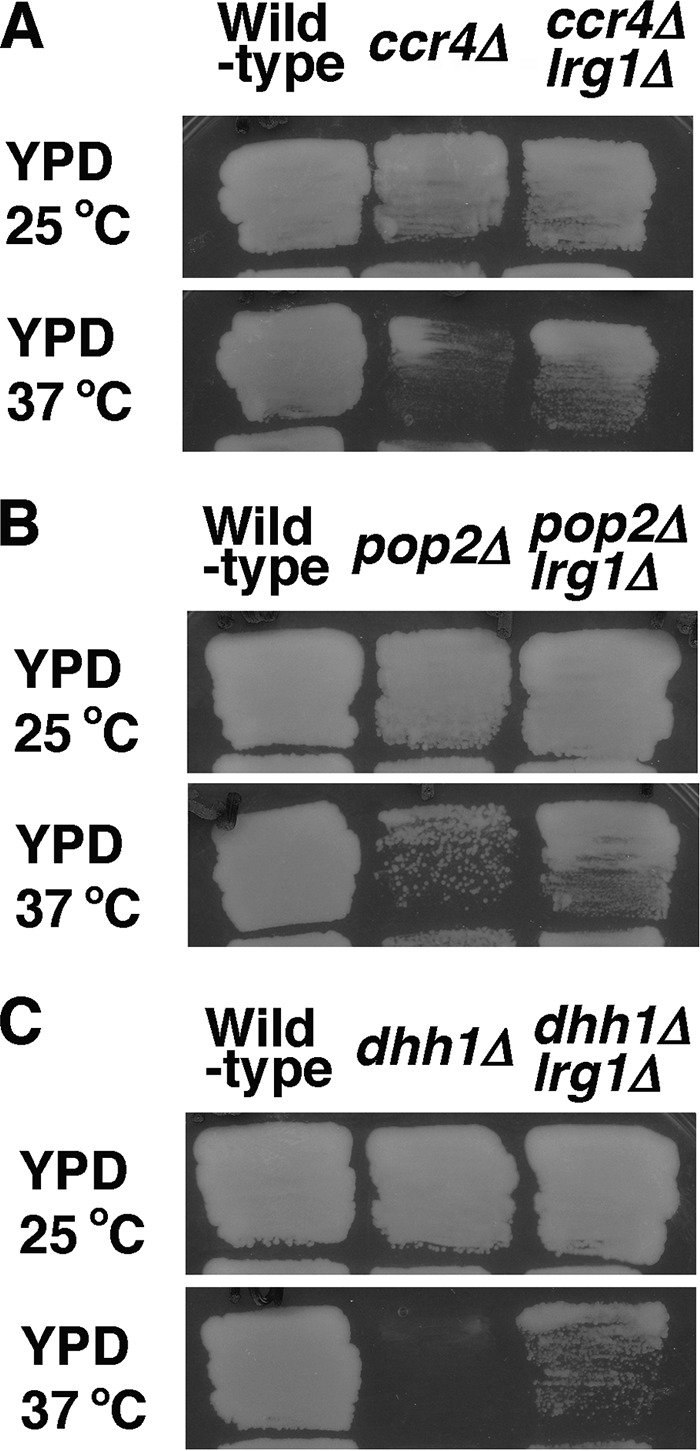
Loss of LRG1 suppresses cell lysis of the ccr4Δ, pop2Δ, and dhh1Δ mutants. (A) Wild-type (c1H-1A), ccr4Δ (c1H-1D), and ccr4Δ lrg1Δ (cl4-1B) cells were plated on YPD medium plates and grown at either 25°C or 37°C for 3 days. (B) Wild-type (p1H-2A), pop2Δ (p1H-2B), and pop2Δ lrg1Δ (pl4-1B) cells were plated on YPD medium plates and grown at either 25°C or 37°C for 3 days. (C) Wild-type (d1H-1A), dhh1Δ (d1H-1D), and dhh1Δ lrg1Δ (dl4-1B) cells were plated on YPD medium plates and grown at either 25°C or 37°C for 3 days.
We have previously shown that ccr4Δ rom2Δ double mutants and khd1Δ ccr4Δ rom2Δ triple mutants were inviable (11) (Fig. 9A). This raised the possibility that the lethality of the ccr4Δ rom2Δ mutant was attributed to the increased LRG1 mRNA level. To test this, we examined whether the lrg1Δ mutation suppresses the growth defect of the ccr4Δ rom2Δ mutant. Indeed, the lrg1Δ mutation suppressed the growth defect of the ccr4Δ rom2Δ mutant (Fig. 9A). We then examined growth of pop2Δ rom2Δ and dhh1Δ rom2Δ double mutant cells and found that both mutants were also inviable (Fig. 9B and C). The lrg1Δ mutation also suppressed the growth defect of pop2Δ rom2Δ and dhh1Δ rom2Δ mutants (Fig. 9B and C), indicating that the increased LRG1 mRNA level causes the lethality in the pop2Δ rom2Δ and dhh1Δ rom2Δ mutants. These results suggest that LRG1 mRNA is a target mRNA for Ccr4, Pop2, and Dhh1 and that regulation of the LRG1 mRNA stability mediated by Ccr4, Pop2, and Dhh1 is important for yeast cells to grow at high temperature.
FIG 9 .
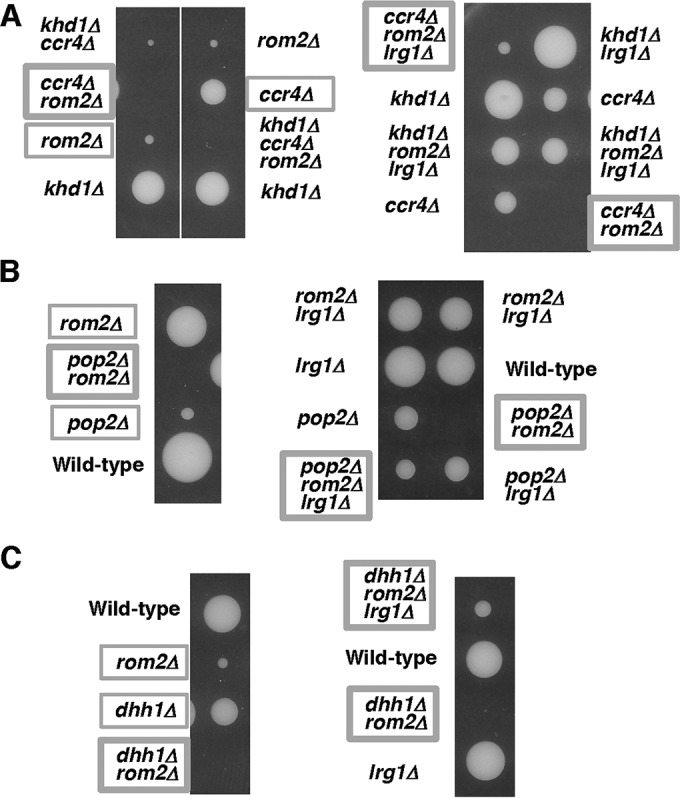
Loss of LRG1 suppresses the lethality of the ccr4Δ rom2Δ, pop2Δ rom2Δ, and dhh1Δ rom2Δ mutants. (A) Strain 10BD-c163r2l1 that was heterozygous for khd1Δ, ccr4Δ, rom2Δ, and lrg1Δ alleles was sporulated, and tetrads were dissected onto YPD containing 10% sorbitol. Growth after 6 days at 25°C is shown. Genotypes are indicated on both sides of the blots. More than 20 tetrads were dissected, and representative data are shown. (B) Strain 10BD-pr1l1 that was heterozygous for pop2Δ, rom2Δ, and lrg1Δ alleles was sporulated, and tetrads were dissected onto YPD containing 10% sorbitol. Growth after 6 days at 25°C is shown. Genotypes are indicated on both sides. More than 20 tetrads were dissected, and representative data are shown. (C) Strain 10BD-d1r1l1 that was heterozygous for dhh1Δ, rom2Δ, and lrg1Δ alleles was sporulated, and tetrads were dissected onto YPD containing 10% sorbitol. Growth after 6 days at 25°C is shown. Genotypes are indicated to the left of the blots. More than 20 tetrads were dissected, and representative data are shown.
Overexpression of Dhh1 suppressed the growth defect of the khd1Δ ccr4Δ mutant.
A previous study showed not only that Dhh1 interacts physically with Ccr4 and Pop2 but also that overexpression of Dhh1 suppressed the phenotypes associated with the pop2Δ and ccr4Δ mutations (7). These results raised the possibility that Dhh1 overexpression could suppress the growth defect of the khd1Δ ccr4Δ mutant. To test this, we transformed multicopy plasmids carrying either the DHH1, CCR4, or POP2 gene into khd1Δ ccr4Δ mutant cells. As shown in Fig. 10A, overexpression of Dhh1 suppressed the growth defect of the khd1Δ ccr4Δ mutant at 37°C, but overexpression of Pop2 did not. In the khd1Δ ccr4Δ mutant, the expression levels of ROM2 and LRG1 mRNAs are decreased and increased, respectively (11) (Fig. 2A and 6A). We hypothesized that DHH1 overexpression suppresses the growth defect of the khd1Δ ccr4Δ mutant by reducing LRG1 expression, since the dhh1Δ mutation affects LRG1 expression, but not ROM2 expression (Fig. 2C and 6C). Indeed, as shown in Fig. 10B, the LRG1 mRNA level in the khd1Δ ccr4Δ mutant was reduced by Dhh1 overexpression. This result supports the model in which Dhh1 negatively regulates LRG1 expression.
FIG 10 .
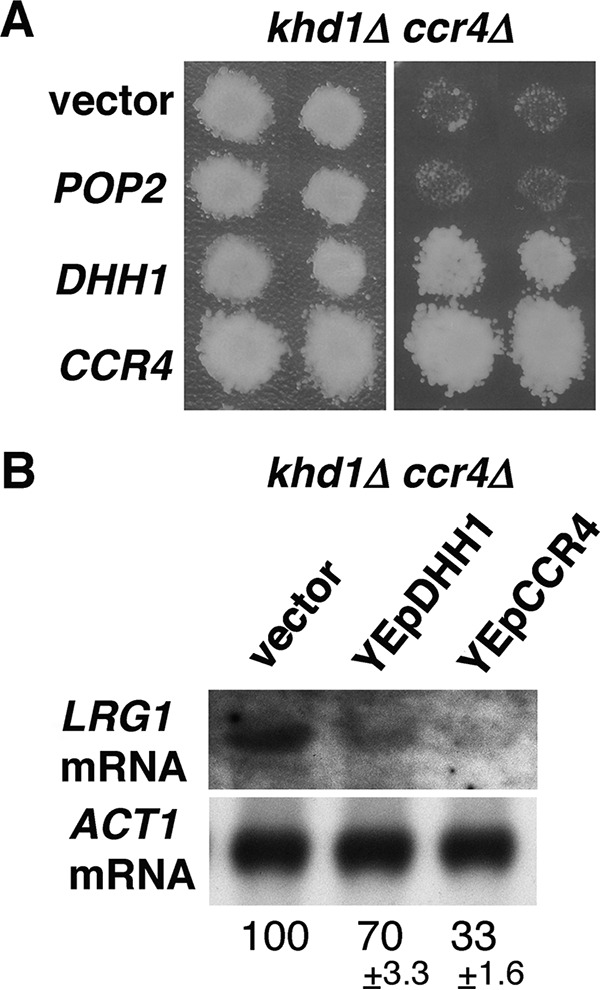
Overexpression of DHH1 suppresses the growth defect of khd1Δ ccr4Δ double mutants. (A) Multicopy suppressors of khd1Δ ccr4Δ. Transformants of the khd1Δ ccr4Δ strain (c1H-1B) carrying the plasmid indicated to the left of the blot were streaked onto YPD medium and incubated at 25°C (left) or 37°C (right). Each patch represents an independent transformant. The plasmids were YEplac195 (vector), YEplac195-POP2 (POP2), YEplac195-DHH1 (DHH1), and YEplac195-CCR4 (CCR4). (B) LRG1 mRNA levels in khd1Δ ccr4Δ cells harboring plasmid. The khd1Δ ccr4Δ (c1H-1B) cells harboring vector, YEplac195-DHH1, or YEplac195-CCR4 were cultured to mid-logarithmic phase in SC−Ura medium and collected, and total RNA was prepared. The LRG1 transcripts were quantified by Northern blotting as described in Materials and Methods. ACT1 mRNA was included as a quantity control. The mRNA levels are indicated as percentages of the cells harboring vector and represent the means ± standard deviations from two independent experiments.
To clarify whether Ccr4 and Dhh1 function in the linear pathway, we examined the growth of ccr4Δ dhh1Δ double mutant cells. Surprisingly, ccr4Δ dhh1Δ double mutant cells are inviable (Fig. 11A). This result was inconsistent with a previous observation of Hata et al. (7), in which ccr4Δ and dhh1Δ mutations do not have any additive phenotypes. Deletion of LRG1 failed to suppress the growth defect of ccr4Δ dhh1Δ double mutant cells (Fig. 11). Furthermore, the addition of sorbitol, an active allele of RHO1 (Q-to-L change at position 68 encoded by RHO1 [RHO1-Q68L]), or an active allele of PKC1 (PKC1-R398P) failed to suppress the growth defect of the ccr4Δ dhh1Δ double mutant (Fig. 11B and data not shown). These results suggest that, in addition to the CWI pathway, Ccr4 and Dhh1 cooperatively regulate another biological process.
FIG 11 .
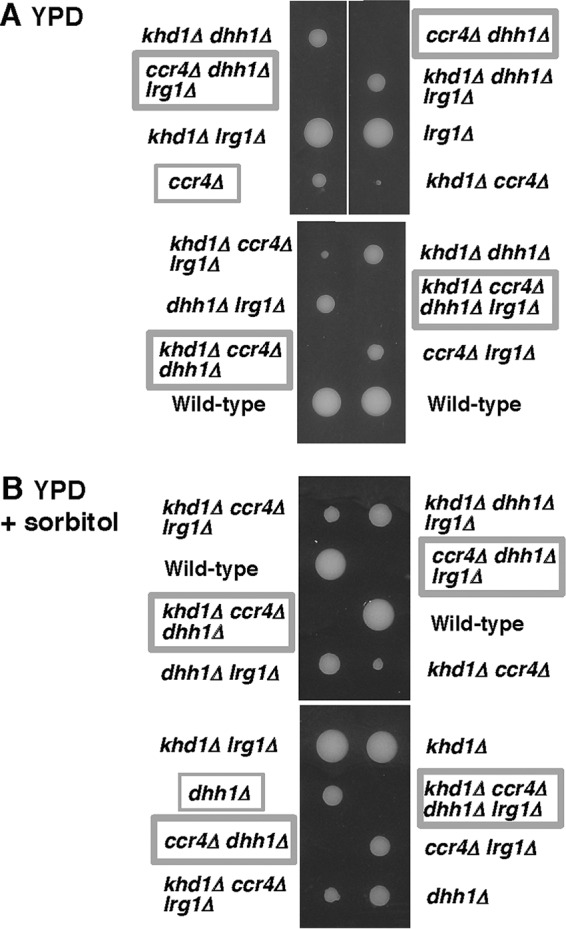
Growth of the ccr4Δ dhh1Δ and ccr4Δ dhh1Δ lrg1Δ mutant strains. Strain 10BD-c163d1l1 that was heterozygous for khd1Δ, ccr4Δ, dhh1Δ, and lrg1Δ alleles was sporulated, and tetrads were dissected onto YPD (A) and YPD containing 10% sorbitol (B). Growth after 6 days at 25°C is shown. Genotypes are indicated on both sides of the blots. More than 20 tetrads were dissected, and representative data are shown.
Different roles of Ccr4 and Pop2 in the CWI pathway.
Both ccr4Δ and pop2Δ mutants displayed a synthetic growth defect with the khd1Δ mutation (11). In the khd1Δ ccr4Δ double mutant, Rom2 function is decreased and Lrg1 function is increased. These results suggest that, in the khd1Δ ccr4Δ double mutant, Rho1 activity is severely decreased, which results in its growth defect. This idea is supported by the findings that the growth defect of the khd1Δ ccr4Δ double mutant could be suppressed by ROM2 overexpression and expression of RHO1-Q68L (11) (Fig. 12A). On the other hand, in the khd1Δ pop2Δ double mutant, Lrg1 function is increased, but Rom2 function is normal. Therefore, it is anticipated that a reduction of Rho1 activity is less severe in khd1Δ pop2Δ double mutant cells than in khd1Δ ccr4Δ double mutant cells. ROM2 overexpression and RHO1-Q68L failed to suppress the growth defect of the khd1Δ pop2Δ double mutant (Fig. 12B), indicating that decreased Rho1 activity caused by the increased Lrg1 level cannot account for the growth defect of the khd1Δ pop2Δ double mutant.
Rho1 acts as an activator of five effectors, including Pkc1, Fks1, Bni1, Sec3, and Skn7 (12). The growth defect of the khd1Δ ccr4Δ double mutant can be suppressed by PKC1-R398P (11) (Fig. 12A), suggesting that reduction of Pkc1 activity is responsible for the growth defect of the khd1Δ ccr4Δ double mutant. We unexpectedly found that PKC1-R398P also suppressed the growth defect of the khd1Δ pop2Δ double mutant (Fig. 12B). This result suggests that the signaling from Rho1 to Pkc1 requires a cooperative function of Pop2 and Khd1. Taken together, these results suggest that Pop2 and Ccr4 not only destabilize a common target, LRG1 mRNA, but also function upstream of Pkc1 in the CWI pathway in a manner independent of each other.
DISCUSSION
In this study, we found that the LRG1 mRNA level was increased in pop2Δ and dhh1Δ mutants and the ccr4Δ mutant than in the wild type. The growth defect of pop2Δ and dhh1Δ mutants at high temperature and the lethality of pop2Δ rom2Δ and dhh1Δ rom2 double mutants are suppressed by the lrg1Δ mutation. Thus, the increased LRG1 mRNA level does contribute to the growth defect of pop2Δ and dhh1Δ mutants. The ccr4Δ, pop2Δ, and dhh1Δ mutants show more severe growth defects at high temperature, suggesting that the negative regulation of LRG1 expression is more important at high temperature. Since it is well-known that the CWI pathway is activated at high temperature (12, 15), the negative regulation of LRG1 expression is more important at high temperature to ensure the proper activation of Rho1 at high temperature. Besides Lrg1, there are three other Rho1-GAPs, Bem2, Sac7, and Bag7 (12). The level of expression of BEM2, SAC7, or BAG7 mRNA was not altered significantly in ccr4Δ and pop2Δ mutants (data not shown). In these GAPs, Lrg1 has been reported to participate in the regulation of β-1,3-glucan synthase (16). Bem2 and Sac7 are involved in the downregulation of the Pkc1-activated mitogen-activated protein kinase (MAPK) pathway (17, 18). Thus, it is possible that the negative regulation of LRG1 expression by Ccr4, Pop2, and Dhh1 is important for Rho1 to activate β-1,3-glucan synthase properly. Data from Candida albicans also support this idea, as ccr4 and pop2 mutants showed relatively lower glucan in the cell wall (19).
How is LRG1 mRNA specifically recognized by Ccr4, Pop2, and Dhh1 as a target mRNA? Stewart et al. (20) have reported that an RNA binding protein Puf5/Mpt5 negatively regulates the LRG1 mRNA level and that the lrg1Δ mutation suppresses the growth defect of the puf5Δ mutant. Puf5 was originally isolated as a multicopy suppressor of the pop2 mutation (7). Puf5 directly binds to the 3′ UTR of LRG1 mRNA (21, 22) and physically interacts with Ccr4, Pop2, and Dhh1 (4, 7). Previous studies showed that Puf5 does not bind to the 3′ UTR of BEM2, SAC7, or BAG7 mRNA encoding other Rho1-GAPs (21, 22). Thus, Ccr4, Pop2, and Dhh1 may specifically regulate LRG1 mRNA via the ability of Puf5 to recruit them to the 3′ UTR of LRG1 mRNA.
Ccr4 and Pop2 shorten the poly(A) tail of LRG1 mRNA, and Dhh1 stimulates decapping by Dcp1/2.
The ccr4Δ, pop2Δ, and dhh1Δ mutants show severe growth defect at high temperature, and their growth defects are suppressed by lrg1Δ mutation, suggesting that rapid degradation of LRG1 mRNA is important for cell growth, especially at high temperature. We have shown here that overexpression of Dhh1 suppressed the growth defect of the khd1Δ ccr4Δ mutant at 37°C and that the elevated LRG1 mRNA level in the khd1Δ ccr4Δ mutant was reduced by Dhh1 overexpression. Dhh1 overexpression could be inducing the deadenylation-independent decapping, and in that way confer a decrease of the LRG1 mRNA level. Thus, Dhh1 acts downstream of Ccr4 in the degradation pathway of LRG1 mRNA. While Ccr4, Pop2, and Dhh1 share LRG1 mRNA as a target, they may act independently on other targets. We found here that the combination of ccr4Δ dhh1Δ mutations was lethal and that deletion of LRG1 failed to suppress the lethality of the ccr4Δ dhh1Δ double mutant. Thus, the LRG1 mRNA is not the sole target mRNA for Ccr4 and Dhh1. Since Ccr4 and Dhh1 are global regulators acting on practically all mRNAs, the lethality of ccr4Δ dhh1Δ double mutant cells could be caused by more general changes in mRNA degradation and translational repression, rather than control of specific target mRNAs.
The level of ROM2 mRNA encoding Rho1 GEF was slightly decreased in the ccr4Δ mutant, and this reduction was enhanced by the khd1Δ mutation (11) (Fig. 2A). We also confirmed genetically that Rom2 function was indeed impaired in the ccr4Δ single mutant and khd1Δ ccr4Δ double mutant using a mutation of the ROM1 gene. Using this genetic approach, we found that Rom2 function was not impaired in pop2Δ and dhh1Δ mutants. Consistently, ROM2 mRNA level was not decreased in pop2Δ and dhh1Δ mutants. Thus, Ccr4 acts independently of Pop2 and Dhh1 in regulating ROM2 expression. Rom2 protein levels and ROM2 mRNA levels were decreased in ccr4Δ and khd1Δ ccr4Δ mutants than in wild-type cells, and the decreased protein levels were more evident than the decreased mRNA levels. Thus, Rom2 expression level is regulated at both the mRNA and protein levels. How do Khd1 and Ccr4 positively regulate the expression of ROM2? The myc-tagged ROM2 construct used in Fig. 4A and 5 had the ADH1 3′ UTR instead of the endogenous ROM2 3′ UTR, and the Rom2myc protein levels were decreased in ccr4Δ single mutant and khd1Δ ccr4Δ double mutant cells, implying that the ROM2 3′ UTR seems not to be essential for the regulation of ROM2 expression. In the case of the regulation of MTL1 mRNA stability by Khd1, MTL1 mRNA itself bears the multiple CNN repeats involved in destabilization by the decapping enzyme Dcp1/2 and the 5′-to-3′ exonuclease Xrn1, and Khd1 stabilizes MTL1 mRNA by binding to this element (23, 24). Since ROM2 mRNA contains three CNN repeats in the coding sequence and Khd1 associates with ROM2 mRNA (11, 23), Khd1, together with Ccr4, may stabilize ROM2 mRNA by binding to the CNN repeats of the ROM2 mRNA. Since Rom2 expression is regulated at both mRNA and protein levels, the binding to the CNN repeats by Khd1 may also be involved in translational control. Consistently, the HA-Rom2 protein levels expressed from the GAL1 promoter were decreased in ccr4Δ and khd1Δ ccr4Δ mutant cells compared to wild-type cells, while the HA-ROM2 mRNA levels were not altered. Additionally, Mpk1, which is activated in ccr4Δ and khd1Δ ccr4Δ mutants, may be involved in the decrease of the ROM2 mRNA. The decreased Rom2myc protein levels in ccr4Δ and khd1Δ ccr4Δ mutant cells were partially suppressed by the mpk1Δ mutation, implying the possibility that Mpk1 is also involved in ROM2 expression at the protein level.
While the ccr4Δ mutant displays a synthetic growth defect with the khd1Δ mutation, the dhh1Δ mutant does not. The simple explanation is that in the ccr4Δ mutant, where Rom2 function is decreased and Lrg1 function is increased, Rho1 activity is severely decreased. Consistently, a constitutively active RHO1 allele is able to suppress the growth defect of the khd1Δ ccr4Δ double mutant (11) (Fig. 12A). In the dhh1Δ mutant, where Rom2 function is normal and Lrg1 function is increased, the decrease in Rho1 activity is lower than that in the ccr4Δ mutant. This raises the possibility that khd1Δ mutation would affect cell growth only when Rho1 activity is more severely impaired. However, this explanation is not consistent with the pop2Δ case. The pop2Δ mutant displays a synthetic growth defect with the khd1Δ mutation, but Rom2 function is normal in the pop2Δ mutant. In the pop2Δ mutant, where Rom2 function is normal and Lrg1 function is increased, the decrease in Rho1 activity is lower than that in the ccr4Δ mutant. Since a constitutively active RHO1 allele cannot suppress the growth defect of the khd1Δ pop2Δ double mutant (Fig. 12B), the khd1Δ pop2Δ double mutant might have an additional defect in the CWI signaling pathway. Intriguingly, the growth defect of the khd1Δ pop2Δ double mutant as well as the khd1Δ ccr4Δ double mutant could be suppressed by the constitutively active PKC1 allele. These results suggest that Pop2 and Ccr4 not only destabilize a common target, LRG1 mRNA, but also regulate the CWI pathway at different points, and that Ccr4 and Pop2 act at a point upstream of Pkc1 in the CWI pathway. Although Rho1 activity is severely decreased in the khd1Δ ccr4Δ double mutant, where Rom2 function is decreased and Lrg1 function is increased, Mpk1 seems to be activated in the khd1Δ ccr4Δ double mutant. Since Lrg1 participates in the regulation of β-1,3-glucan synthase (16), one possibility is that the decreased Rho1 activity could not activate β-1,3-glucan synthase due to the increased Lrg1 but could still activate the Pkc1-Mpk1 branch in the khd1Δ ccr4Δ double mutant. The levels of expression of BEM2 and SAC7, which are involved in the downregulation of the Pkc1-activated MAPK pathway (17, 18), were not altered significantly in the khd1Δ ccr4Δ double mutant (data not shown). Regulation of the levels of different Rho1-GAPs by modulation of mRNAs might ensure Rho1 activation in a target-specific manner.
Previously, we revealed that Ccr4, a component of the Ccr4-Not cytoplasmic deadenylase complex, functions in the CWI pathway (11). In this study, we further identified Pop2 deadenylase and Dhh1 DExD/H box protein as the regulator of the CWI pathway. Ccr4, Pop2, and Dhh1 modulate the levels of mRNAs for specific Rho1 regulators, Rom2 and Lrg1. In budding yeast, Rho1 activity is tightly regulated both temporally and spatially (12). It is anticipated that Ccr4, Pop2, and Dhh1 may contribute to the precise spatiotemporal control of Rho1 activity by regulating expression of its regulators temporally and spatially. Therefore, to further elucidate how Ccr4, Pop2, and Dhh1 regulate ROM2 and LRG1 mRNAs will undoubtedly provide valuable insights into the precise spatiotemporal regulation of this signaling pathway.
MATERIALS AND METHODS
Strains and general methods.
Escherichia coli DH5α was used for DNA manipulations. S. cerevisiae strains used in this study are described in Table 1. Standard procedures were followed for yeast manipulations (25). The media used in this study included rich medium, synthetic complete medium (SC), and synthetic minimal medium (SD) (25). SC lacking amino acids or other nutrients (e.g., SC−Ura is SC lacking uracil) were used to select transformants. Recombinant DNA procedures were carried out as described previously (26).
TABLE 1 .
S. cerevisiae strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| 10B | MATα ade2 trp1 can1 leu2 his3 ura3 GAL psi+ HOp-ADE2-HO 3′ UTR | 33 |
| 10BD | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 | 33 |
| 10BD-c163 | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 KHD1/khd1Δ::CgHIS3 CCR4/ccr4Δ::CgLEU2 | 11 |
| 10BD-p163 | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 KHD1/khd1Δ::CgHIS3 POP2/pop2Δ::CgLEU2 | 11 |
| 10BD-d163 | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 KHD1/khd1Δ::CgHIS3 DHH1/dhh1Δ::CgLEU2 | 11 |
| c1H-1A | MATα ade2 trp1 can1 leu2 his3 ura3 | 11 |
| c1H-1B | MATα ade2 trp1 can1 leu2 his3 ura3 khd1Δ::CgHIS3 ccr4Δ::CgLEU2 | 11 |
| c1H-1C | MATa ade2 trp1 can1 leu2 his3 ura3 khd1Δ::CgHIS3 | 11 |
| c1H-1D | MATa ade2 trp1 can1 leu2 his3 ura3 ccr4Δ::CgLEU2 | 11 |
| p1H-2A | MATα ade2 trp1 can1 leu2 his3 ura3 | This study |
| p1H-2B | MATα ade2 trp1 can1 leu2 his3 ura3 pop2Δ::CgLEU2 | This study |
| p1H-2C | MATa ade2 trp1 can1 leu2 his3 ura3 khd1Δ::CgHIS3 pop2Δ::CgLEU2 | This study |
| p1H-2D | MATa ade2 trp1 can1 leu2 his3 ura3 khd1Δ::CgHIS3 | This study |
| d1H-1A | MATα ade2 trp1 can1 leu2 his3 ura3 | This study |
| d1H-1B | MATα ade2 trp1 can1 leu2 his3 ura3 khd1Δ::CgHIS3 dhh1Δ::CgLEU2 | This study |
| d1H-1C | MATa ade2 trp1 can1 leu2 his3 ura3 khd1Δ::CgHIS3 | This study |
| d1H-1D | MATa ade2 trp1 can1 leu2 his3 ura3 dhh1Δ::CgLEU2 | This study |
| 10BD-c163-r1 | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 KHD1/khd1Δ::CgTRP1 CCR4/ccr4Δ::CgLEU2 ROM1/rom1Δ::CgHIS3 | 11 |
| 10BD-p163-r1 | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 KHD1/khd1Δ::CgTRP1 POP2/pop2Δ::CgLEU2 ROM1/rom1Δ::CgHIS3 | This study |
| 10BD-d1-r1 | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 DHH1/dhh1Δ::CgLEU2 ROM1/rom1Δ::CgHIS3 | This study |
| 180-3B-4A | MATα ade2 trp1 can1 leu2 his3 ura3 ROM2myc-kan | This study |
| 180-3B-1B | MATα ade2 trp1 can1 leu2 his3 ura3 khd1Δ::CgHIS3 ccr4Δ::CgLEU2 ROM2myc-kan | This study |
| 180-3B-7D | MATa ade2 trp1 can1 leu2 his3 ura3 ccr4Δ::CgLEU2 ROM2myc-kan | This study |
| 180-3B-7C | MATa ade2 trp1 can1 leu2 his3 ura3 khd1Δ::CgHIS3 ROM2myc-kan | This study |
| 180-m-1D | MATα ade2 trp1 can1 leu2 his3 ura3 ROM2myc-kan | This study |
| 180-m-3D | MATα ade2 trp1 can1 leu2 his3 ura3 khd1Δ::CgHIS3 ccr4Δ::CgLEU2 ROM2myc-kan | This study |
| 180-m-7A | MATa ade2 trp1 can1 leu2 his3 ura3 khd1Δ::CgHIS3 ccr4Δ::CgLEU2 mpk1Δ::CgHIS3 ROM2myc-kan | This study |
| 180-m-6B | MATa ade2 trp1 can1 leu2 his3 ura3 ccr4Δ::CgLEU2 ROM2myc-kan | This study |
| 180-m-6C | MATa ade2 trp1 can1 leu2 his3 ura3 ccr4Δ::CgLEU2 mpk1Δ::CgHIS3 ROM2myc-kan | This study |
| cl4-1B | MATa ade2 trp1 can1 leu2 his3 ura3 ccr4Δ::CgLEU2 lrg1Δ::CgHIS3 | This study |
| pl4-1B | MATa ade2 trp1 can1 leu2 his3 ura3 pop2Δ::CgLEU2 lrg1Δ::CgHIS3 | This study |
| dl4-1B | MATa ade2 trp1 can1 leu2 his3 ura3 dhh1Δ::CgLEU2 lrg1Δ::CgHIS3 | |
| 10BD-c163-r2l1 | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 KHD1/khd1Δ::CgTRP1 CCR4/ccr4Δ::CgLEU2 ROM2/rom2Δ::CgHIS3 LRG1/lrg1Δ::KlURA3 | This study |
| 10BD-p-r2l1 | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 POP2/pop2Δ::CgLEU2 ROM2/rom2Δ::CgHIS3 LRG1/lrg1Δ::KlURA3 | This study |
| 10BD-p-r2l1 | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 DHH1/dhh1Δ::CgLEU2ROM2/rom2Δ::CgHIS3 LRG1/lrg1Δ::KlURA3 | This study |
| 10BD-c163-d1l1 | MATa/MATα ade2/ade2 trp1/trp1 can1/can1 leu2/leu2 his3/his3 ura3/ura3 KHD1/khd1Δ::CgTRP1 CCR4/ccr4Δ::CgLEU2 DHH1/dhh1Δ::CgHIS3 LRG1/lrg1Δ::KlURA3 | This study |
Plasmids.
Plasmids used in this study are described in Table 2. Plasmids pCgLEU2, pCgHIS3, and pCgTRP1 are pUC19 carrying the Candida glabrata LEU2, HIS3, and TRP1 genes, respectively (27). Plasmid pKlURA3 is pUC19 carrying the Kluyveromyces lactis URA3. Plasmid pGAL-HA-LRG1 expressing HA-LRG1 from the GAL1 promoter was used for the experiment for LRG1 mRNA degradation. Plasmid YCplac33-ROM2myc expressing ROM2myc from the endogenous promoter and plasmid pGAL-HA-ROM2 expressing HA-ROM2 from the GAL1 promoter were used for Western blotting of Rom2 protein.
TABLE 2 .
Plasmids used in this study
| Plasmid | Relevant marker(s) | Reference |
|---|---|---|
| YCplac33 | URA3 CEN-ARS | 34 |
| pRS316-GAL-LRG1 | URA3 CEN-ARS pGAL-LRG1-LRG1 3′ UTR | This study |
| YCplac33-ROM2myc | URA3 CEN-ARS pROM2-ROM2myc-ADH1 3′ UTR | This study |
| pRS316-GAL-HA-ROM2 | URA3 CEN-ARS pGAL-HA-ROM2-ROM2 3′ UTR | This study |
| YCplac33-RHO1-Q86L | URA3 CEN-ARS RHO1-Q86L | 35 |
| pRS316-PKC1-R398P | URA3 CEN-ARS PKC1-R398P | 36 |
| YEplac195 | URA3 2μ | 34 |
| YEplac195-POP2 | URA3 2μ POP2 | 7 |
| YEplac195-DHH1 | URA3 2μ DHH1 | 7 |
| YEplac195-CCR4 | URA3 2μ CCR4 | 7 |
| YEplac195-ROM2 | URA3 2μ ROM2 | 11 |
| pCgLEU2 | C. glabrata LEU2 in pUC19 | 27 |
| pCgHIS3 | C. glabrata HIS3 in pUC19 | 27 |
| pCgTRP1 | C. glabrata TRP1 in pUC19 | 27 |
| pKlURA3 | K. lactis URA3 in pUC19 | 11 |
| pKlURA3 | K. lactis URA3 in pUC19 | 11 |
| pFA6a-13myc-kanMX6 | myc | 30 |
Gene deletion and protein tagging.
Deletions of KHD1, CCR4, POP2, DHH1, ROM1, ROM2, and LRG1 were constructed by PCR-based gene deletion method (27–29). Primer sets were designed such that 46 bases at the 5′ ends of the primers were complementary to those at the corresponding region of the target gene and 20 bases at their 3′ ends were complementary to the pUC19 sequence outside the polylinker region in the plasmid pCgLEU2, pCgHIS3, pCgTRP1, or pKlURA3. Primer sets for PCR were designed to delete the open reading frame (ORF) completely. The PCR products were transformed into the wild-type strain and selected for Leu+, His+, Trp+, or Ura+. The ROM2myc strains were prepared by the method of Longtine et al. (30) using pFA6a-13myc-kanMX6.
Northern blot analysis.
Total RNA was prepared from cells using Isogen reagent (Nippon Gene) and RNeasy minikit (Qiagen). RNA samples were separated by 1.5% denatured agarose gel electrophoresis and transferred to a nylon membrane. Then, RNA was hybridized using digoxigenin (DIG)-labeled antisense probe. The primer pair j298 (TGACGATATGATGAGCTCCTCCTTACGTCA) and j297 (TTAACCCCAGAAATCTAACGACG) and primer pair j259 (ATGATTCAAAATTCTGCTGGTTA) and j260 (GCCAATATTTATGAATTCCATAAC) were used to detect transcript containing ROM2 and LRG1, respectively. After washing and blocking, the membrane was incubated with alkaline phosphatase-conjugated anti-DIG antibody, and the signal was detected by enhanced chemiluminescence.
mRNA degradation was determined from Northern blots as described previously (31, 32). Cells were grown in SG−Ura, and the medium was changed to SC−Ura to inhibit transcription from the GAL1 promoter. Cells were harvested at the times indicated in the figures, and total RNA was isolated. Samples were analyzed by Northern blotting with specific probes, and half-lives (t1/2) (in minutes) were determined as the means from three independent experiments.
Western blot analysis.
Extracts were prepared as described previously (23, 24, 33). Extracts were subjected to SDS-PAGE on 8% acrylamide gels followed by electroblotting onto an Immobilon membrane (Millipore). To detect myc-tagged and hemagglutinin (HA)-tagged proteins, the membrane was incubated with anti-myc antibody (9E10; Santa Cruz Biotechnology) (1:2,000) and anti-HA antibody (HA11; Santa Cruz Biotechnology) (1:2,000), respectively, and then with HRP-labeled secondary antibody (Calbiochem) (1:4,000). To control for equal loading of the lanes, the blots were probed with anti-Mcm2 antibody (Santa Cruz Biotechnology) (1:1,000) or anti-Pgk1 antibody (Invitrogen) (1:1,000) and peroxidase-conjugated secondary antibody (Calbiochem) (1:3,000).
ACKNOWLEDGMENTS
We thank the members of the Molecular Cell Biology Laboratory for valuable discussions.
Kenji Irie is supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture, and Technology, Japan (2012 to 2015) (grant 24570192). Xia Li is supported by the Japan Society for Promotion of Science (JSPS) Postdoctoral Fellowship for Foreign Researchers.
REFERENCES
- 1.Parker R. 2012. RNA degradation in Saccharomyces cerevisiae. Genetics 191:671–702. doi: 10.1534/genetics.111.137265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonenberg N, Hinnebusch AG. 2009. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quenault T, Lithgow T, Traven A. 2011. PUF proteins: repression, activation and mRNA localization. Trends Cell Biol 21:104–112. doi: 10.1016/j.tcb.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Goldstrohm AC, Hook BA, Seay DJ, Wickens M. 2006. PUF proteins bind Pop2p to regulate messenger RNAs. Nat Struct Mol Biol 13:533–539. doi: 10.1038/nsmb1100. [DOI] [PubMed] [Google Scholar]
- 5.Collart MA. 2003. Global control of gene expression in yeast by the Ccr4-Not complex. Gene 313:1–16. doi: 10.1016/S0378-1119(03)00672-3. [DOI] [PubMed] [Google Scholar]
- 6.Collart MA, Panasenko OO. 2012. The Ccr4–Not complex. Gene 492:42–53. doi: 10.1016/j.gene.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Hata H, Mitsui H, Liu H, Bai Y, Denis CL, Shimizu Y, Sakai A. 1998. Dhh1p, a putative RNA helicase, associates with the general transcription factors Pop2p and Ccr4p from Saccharomyces cerevisiae. Genetics 148:571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolstencroft RN, Beilharz TH, Cook MA, Preiss T, Durocher D, Tyers M. 2006. Ccr4 contributes to tolerance of replication stress through control of CRT1 mRNA poly(A) tail length. J Cell Sci 119:5178–5192. doi: 10.1242/jcs.03221. [DOI] [PubMed] [Google Scholar]
- 9.Manukyan A, Zhang J, Thippeswamy U, Yang J, Zavala N, Mudannayake MP, Asmussen M, Schneider C, Schneider BL. 2008. Ccr4 alters cell size in yeast by modulating the timing of CLN1 and CLN2 expression. Genetics 179:345–357. doi: 10.1534/genetics.108.086744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traven A, Beilharz TH, Lo TL, Lueder F, Preiss T, Heierhorst J. 2009. The Ccr4-Pop2-NOT mRNA deadenylase contributes to septin organization in Saccharomyces cerevisiae. Genetics 182:955–966. doi: 10.1534/genetics.109.104414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito W, Li X, Irie K, Mizuno T, Irie K. 2011. RNA-binding protein Khd1 and Ccr4 deadenylase play overlapping roles in the cell wall integrity pathway in Saccharomyces cerevisiae. Eukaryot Cell 10:1340–1347. doi: 10.1128/EC.05181-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin DE. 2011. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozaki K, Tanaka K, Imamura H, Hihara T, Kameyama T, Nonaka H, Hirano H, Matsuura Y, Takai Y. 1996. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J 15:2196–2207. [PMC free article] [PubMed] [Google Scholar]
- 14.Guo S, Shen X, Yan G, Ma D, Bai X, Li S, Jiang Y. 2009. A MAP kinase dependent feedback mechanism controls Rho1 GTPase and actin distribution in yeast. PLoS One 4:e6089. doi: 10.1371/journal.pone.0006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamada Y, Jung US, Piotrowski J, Levin DE. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev 9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe D, Abe M, Ohya Y. 2001. Yeast Lrg1p acts as a specialized RhoGAP regulating 1,3-beta-glucan synthesis. Yeast 18:943–951. doi: 10.1002/yea.742. [DOI] [PubMed] [Google Scholar]
- 17.Martín H, Rodríguez-Pachón JM, Ruiz C, Nombela C, Molina M. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J Biol Chem 275:1511–1519. doi: 10.1074/jbc.275.2.1511. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt A, Schmelzle T, Hall MN. 2002. The RHO1-GAPs SAC7, BEM2, and BAG7 control distinct RHO1 functions in Saccharomyces cerevisiae. Mol Microbiol 45:1433–1441. doi: 10.1046/j.1365-2958.2002.03110.x. [DOI] [PubMed] [Google Scholar]
- 19.Dagley MJ, Gentle IE, Beilharz TH, Pettolino FA, Djordjevic JT, Lo TL, Uwamahoro N, Rupasinghe T, Tull DL, McConville M, Beaurepaire C, Nantel A, Lithgow T, Mitchell AP, Traven A. 2011. Cell wall integrity is linked to mitochondria and phospholipid homeostasis in Candida albicans through the activity of the post-transcriptional regulator Ccr4-Pop2. Mol Microbiol 79:968–989. doi: 10.1111/j.1365-2958.2010.07503.x. [DOI] [PubMed] [Google Scholar]
- 20.Stewart MS, Krause SA, McGhie J, Gray JV. 2007. Mpt5p, a stress tolerance- and lifespan-promoting PUF protein in Saccharomyces cerevisiae, acts upstream of the cell wall integrity pathway. Eukaryot Cell 6:262–270. doi: 10.1128/EC.00188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerber AP, Herschlag D, Brown PO. 2004. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol 2:E79. doi: 10.1371/journal.pbio.0020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilinski D, Qiu C, Lapointe CP, Nevil M, Campbell ZT, Tanaka Hall TM, Wickens M. 2015. RNA regulatory networks diversified through curvature of the PUF protein scaffold. Nat Commun 6:8213. doi: 10.1038/ncomms9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa Y, Irie K, Gerber AP. 2008. Distinct roles for Khd1p in the localization and expression of bud-localized mRNAs in yeast. RNA 14:2333–2346. doi: 10.1261/rna.1016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauchi N, Ohtake Y, Irie K. 2010. Stability control of MTL1 mRNA by the RNA-binding protein Khd1p in yeast. Cell Struct Funct 35:95–105. doi: 10.1247/csf.10011. [DOI] [PubMed] [Google Scholar]
- 25.Adams A, Gottschling DE, Kaiser CA. 1997. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 26.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning; a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 27.Sakumoto N, Mukai Y, Uchida K, Kouchi T, Kuwajima J, Nakagawa Y, Sugioka S, Yamamoto E, Furuyama T, Mizubuchi H, Ohsugi N, Sakuno T, Kikuchi K, Matsuoka I, Ogawa N, Kaneko Y, Harashima S. 1999. A series of protein phosphatase gene disruptants in Saccharomyces cerevisiae. Yeast 15:1669–1679. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. 1993. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res 21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider BL, Steiner B, Seufert W, Futcher AB. 1996. pMPY-zap: a reusable polymerase chain reaction-directed gene disruption cassette for Saccharomyces cerevisiae. Yeast 12:129–134. [DOI] [PubMed] [Google Scholar]
- 30.Longtine MS, McKenzie AR, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953–961. doi:. [DOI] [PubMed] [Google Scholar]
- 31.Tadauchi T, Matsumoto K, Herskowitz I, Irie K. 2001. Post-transcriptional regulation through the HO 3′-UTR by Mpt5, a yeast homolog of Pumilio and FBF. EMBO J 20:552–561. doi: 10.1093/emboj/20.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inada T, Aiba H. 2005. Translation of aberrant mRNAs lacking a termination codon or with a shortened 3'-UTR is repressed after initiation in yeast. EMBO J 24:1584–1595. doi: 10.1038/sj.emboj.7600636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irie K, Tadauchi T, Takizawa PA, Vale RD, Matsumoto K, Herskowitz I. 2002. The Khd1 protein, which has three KH RNA-binding motifs, is required for proper localization of ASH1 mRNA in yeast. EMBO J 21:1158–1167. doi: 10.1093/emboj/21.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gietz RD, Sugino A. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 35.Sekiya-Kawasaki M, Abe M, Saka A, Watanabe D, Kono K, Minemura-Asakawa M, Ishihara S, Watanabe T, Ohya Y. 2002. Dissection of upstream regulatory components of the Rho1p effector, 1,3-beta-glucan synthase, in Saccharomyces cerevisiae. Genetics 162:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nonaka H, Tanaka K, Hirano H, Fujiwara T, Kohno H, Umikawa M, Mino A, Takai Y. 1995. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J 14:5931–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]


