Highlights
-
•
Microalgae are source of valuable compounds as lipids, proteins, carbohydrates, pigments among others.
-
•
Culture stress conditions increase biomass and high values compounds in microalgae.
-
•
Nitrogen and salt stress increase lipids in microalgae.
-
•
Two stages culture and electromagnetic fields enhancing microalgae biomass and pigments content.
Keywords: Culture strategies, Lipids, Pigments, Microalgae
Abstract
Microalgae are a major natural source for a vast array of valuable compounds as lipids, proteins, carbohydrates, pigments among others. Despite many applications, only a few species of microalgae are cultured commercially because of poorly developed of cultivation process. Nowadays some strategies of culture have been used for enhancing biomass and value compounds yield. The most strategies applied to microalgae are classified into two groups: nutrimental and physical. The nutrimental are considered as change in media composition as nitrogen and phosphorous limitation and changes in carbon source, while physical are described as manipulation in operational conditions and external factors such as application of high-light intensities, medium salinity and electromagnetic fields. The exposition to electromagnetic field is a promising technique that can improve the pigments and biomass yield in microalgae culture. Therefore, is important to describe the advantages and applications of the overall process. The aim of this review was to describe the main culture strategies used to improve the photosynthetic and lipids content in chlorophyceae species.
1. Introduction
Microalgae are biological resource for a vast array of high valuable compounds, are rich source of protein, carbohydrates, lipids (especially essential fatty acids) and diversity of pigments (such as chlorophylls, carotenoids and phycobiliproteins). The cultivation of these photosynthetic microorganisms represents an attractive process for obtaining biochemical components with high potential applications in different industries [21], [24], [58], [76]. Microalgae combine properties typical of higher plants (efficient oxygenic photosynthesis and simplicity of nutritional requirements) with biotechnological attributes properties of microbial cells (fast growth in liquid culture and ability to accumulate or secret some metabolites). This particular combination represents the basis of microalgal biotechnology for the use of these microorganisms on high-valued metabolites production [24].
In recent years the commercial applications of microalgae biomass have drastically increased. In alimentary industry, microalgae has been used to enhance the nutritional and organoleptic properties of foods (color, flavor and texture) such as pastas [31], bread [29], mayonnaises [70] and gelled desserts [9]. Microalgal biomass also plays a crucial role in aquaculture and cosmetic industries, especially in the astaxanthin production [65]. For this reason is necessary enhancing the biomass and value compounds yield in the culture of microalgae. The cultivation of microalgae in photobioreactors is recognized as the best way to achieve high production speed, while maintaining monocultures without contamination. This form of culture requires strict control of operating conditions for the development of microorganism such as nutrients, pH, temperature, aeration rate, CO2 concentration and light regime, inoculum stage and size [78].
Actually, some strategies have been studied for enhancing biomass and high value compounds yields. Stress strategies have been used as culture strategies. These conditions are defined as a significant deviation from the optimal conditions for the normal development and growth of microalgae and cause changes in all functional levels of the organism. Among the stress factors applied to microalgae cultures can be classified into two groups: nutrimental and physical. The nutrimental factors are considered as manipulation of culture media composition (carbon source, nitrogen, phosphorus and iron deficiency), while physical are described as manipulation in operation conditions and external factors that affect the microalgae growth (high light intensities, temperature, pH, salinity and electromagnetic fields) [68], [59], [85], [89], [63].
In this review, we present a general perspective of several culture conditions (N and P depravation, salt-stress, high light intensities, electromagnetic fields and two stage cultivation) used as strategies to increases the biomass yield, pigments and lipids content in chlorophyceae species.
2. Microalgae cultures conditions
The growth characteristics and composition of microalgae are known to significantly depend on the cultivation conditions. There are four major types of cultivation conditions for microalgae: photoautotrophic, heterotrophic, mixotrophic and photoheterotrophic cultivation [22]. Table 1 shows the principal differences between each cultivation.
Table 1.
Characteristics of the different culture conditions [16].
| Culture | Energy source | Carbon source |
|---|---|---|
| Photoautotrophy | Light | Inorganic carbón |
| Heterotrophy | Organic carbon | Organic carbón |
| Photoheterotrophy | Light | Organic carbón |
| Mixotrophy | Light and organic carbon | Inorganic and organic carbon |
Phoautototrophic cultivation occurs when the microalgae uses light, such as sunlight, as energy source, and inorganic carbon (e.g., carbon dioxide) as the carbon source to produce chemical energy through photosynthesis [42]. This is the most commonly cultivation condition used for microalgae growth [33], [32], [44], [91]. In contrast, heterotrophic cultivation is when microalgae undergo photosynthesis and uses organic carbons such as sugars and organic acids as carbon and energy sources in absence of light [17]. In contrast to photoautotrophic culture, heterotrophic culture can be performed in conventional microbial bioreactors (CSTR). The mixotrophic culture regime is a variant of the heterotrophic culture, where CO2 and organic carbons are simultaneously assimilated and both respiratory and photosynthetic metabolism operates concurrently Cheirsilp and Torpee, 2012. Photoheterotrophic cultivation is when the microalgae require light when using organic compounds as the carbon source. The main difference between mixotrophic and photoheterotrophic cultivation is that the latter requires light as the energy source, while mixotrophic cultivation can use organic compounds to serve this purpose.
3. Culture strategies
Changes in culture conditions, nutrients deficiency, physical modifies and regimen growth are developed as strategies in microalgae cultures to increase compounds of interest to different process [68], [59], [89], [63], [11]. This section describes some of the most important strategies to increase microalgae biomass yields with high content of photosynthetic pigments and lipids.
3.1. Nitrogen starvation
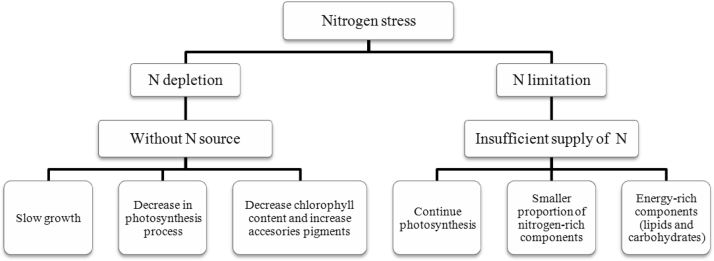
Nitrogen is a very important element for life, as a main constituent of protein and genetic material and is the most abundant element in microalgae (after carbon, oxygen and hydrogen) without mineralized walls [83]. Therefore, as cells grow and divide they require a supply of nitrogen. In microalgae culture, N starvation is carried out either by N depletion or N limitation. Under N depletion, microalgae grow in a medium lacking an N source, while under N limitation there is a constant but insufficient supply of N [13]. If nitrogen supply is limited in proportion to other elements, photosynthesis may continue but the resultant compounds will include a smaller proportion of nitrogen-rich components, accessories pigments as carotenoids and more energy-rich components such as lipids and carbohydrates (Fig. 1). In addition, nitrogen-depleted cells may slowly convert nitrogen-containing non-lipid, cell components to lipids, thereby liberating nitrogen [77].
Fig. 1.
Overview of nitrogen stress in microalgae cultures.
In marine phytoplankton, nitrogen limitation affects photosynthesis by reducing the efficiency of energy collection due to loss of chlorophyll (chl) and increases in non-photochemically active carotenoid pigments. It also directly affects photochemical energy conversion because of a decrement in protein synthesis that appears to affect chloroplastic proteins (and thus the proteins of PSI and PSII reaction centers) more strongly than cytoplasmic proteins [12].
As chlorophyll is a nitrogen-rich compound and is easily accessible, it is utilized as an intracellular nitrogen pool to support further cell growth and biomass production as the nitrogen in the media becomes depleted [55]. For example, Chlamydomonas reinhardtii and Scenedesmus subspicatus only entered the stationary growth phase once nitrogen levels in the media were below 0.05 mg/L [23]. In a study with the freshwater microalga Chlorella minutissima, Ördög et al. [63] reported that when nitrogen was added to the nitrogen starved C. minutissima inoculum, an increase in chlorophyll (a and b) content was observed, being this an indicative of nitrogen is available, and accumulates in the chlorophyll molecules while carotenoid concentrations did not increase in this microalga in response to a nitrogen spike. The restoration of chlorophyll would account for the faster growth rates measured in the higher nitrogen treatments. However, as growth depleted the nitrogen in the media, chlorophyll was degraded to reutilize the nitrogen for growth with chlorophyll a and b levels decreasing. Similar results were reported in Neochloris oleoabundus by Li et al. [55], where a rapid decrease in chlorophyll a was observed after 2 days of culture in low-nitrogen treatments. The chl a content started to decrease by day 4 in intermediate nitrogen treatment and did not decrease in higher nitrogen treatments. This trend followed the inorganic nitrogen in the media with nitrogen depleted by day 2 in the low nitrogen treatments, by day 3 in the intermediate treatment and in the high treatment; nitrogen was not depleted [55].
The accumulation of the carotenoid astaxanthin also can be improving by nitrogen stress. In culture of Haematococcus pluvialis, Fábregas et al. [27] reported the highest values of astaxanthin cellular content with a N deficiency for high and low light intensities (40 and 230 μmol/m2 s), indicating that N deficiency has a greater effect than light intensity on astaxanthin synthesis. This behavior proposes the use of N limitation as economic strategy for carotenoids production rather than application of high light intensities.
Neochloris oleoabundans is a green microalga well known for the ability to increase its fatty acid (FA) content when it is cultured under nitrogen depletion. Li et al. [55] reported that during the culture of N. oleoabundans, as long as nitrogen was available, the rapid chlorophyll accumulation and cell division was observed. However when nitrogen was consumed, cell division stopped, even though biomass accumulation continued for several days. The new biomass was composed mostly of lipids and storage oils. In the same study, the consumption of nitrogen by the cells resulted in a fast decrease in chlorophyll a levels in the cultures, suggesting that cells metabolized chlorophyll during of nitrogen stress. There was also an increase in the ratio of carotenoid to chlorophyll. These observations indicated that the photo-physiology (chlorophyll content) the cells was adversely affected.
Also different reports have been applied N limitation to increase lipids in microalgae. Ho et al. [40] studied the N starvation time, obtaining the highest lipid content of 22.40% at 5 day of N starvation. In other study, the accumulation of FA and their composition were compared by cultivating N. oleoabundans in N limitation and N depletion conditions [13], using a semi-continuous cultivation mode as a strategy to optimize lipid accumulation. N limitation culture attained higher values of algal productivity and total FA concentration (0.42 mg/L d and 91.2 mg/L) in comparison with N depletion culture (0.15 mg/L d and 53.2 mg/L), where N limitation led to a significant increase of polyunsaturated triacylglycerols (PUFA), while N depletion led to the highest level of monounsaturated triacylglycerols. Similarly study was done by Chen et al. [18], these authors evaluated the effect of each medium component in lipid accumulation, founded that medium lacking the maximal accumulation in the third day of culture with lipid content 6 times higher than basal media.
Based on the above, the limitation of essential nutrients such as N in the culture medium increases the content of metabolites of interest like lipids and pigments, being this strategy a scalable technology in mass cultures, providing the same effect as the use of external factors such as light intensity.
3.2. Phosphorous limitation
Phosphorous (P) is an important rate limiting nutrient in many ecosystems [26]. It is considered as one of the nutrients most likely to limit the rate of phytoplankton production [67], being also associated with various other facets of phytoplankton growth. Phosporous is one of the major elements in the cell, and plays a critical role in producing ATP for energy metabolism, forming ribotides for nucleic acid metabolism, biosynthesis of phospholipids for membrane biogenesis, and production of other cell components [16]. Phosphorus is the component of several intermediate and final biological compounds; participating in energy conversion process and in the transfer of genetic information. The inorganic orthophosphate in the cell regulates enzyme activity and metabolic pathways as well as the transport processes. It affects various aspects of photosynthesis [61], [25]. Therefore, this element is an essential ingredient in microalgal culture media [87].
Kozlowska-Szerenos et al. [51] evaluated the effect of phosphorus limitation on Chlorella vulgaris growth, reporting a lower microalgae growth by 30–40% in cultures with P deficiency in comparison with controls. However, pigment content was no affected by P deficiency, showing no significant difference in total chlorophyll (a and b) content.
The photosynthetic oxygen evolution of C. vulgaris cells taken from phosphate-deficient (−P) cultures during 8 days of culture growth was also evaluated [52]. In this study, the phosphorus deficiency did not markedly affect the total content of total chlorophyll (30.12 ± 0.52 × 10–8 μg cell – 1) in the cells and only slightly increment in Chl a/b ratio was observed. However the −P cells showed a significantly decrement in Chls/carotenoids mass comparing with controls. These results indicated alterations in photosynthetic apparatus of C. vulgaris −P cells.
Qu et al. [67] reported the phosphate assimilation by Chlorella pyrenoidosa in biomass production under heterotrophic, mixotrophic and autotrophic culture conditions. During heterotrophic cultures the final biomass production under phosphate deficiency was lower, whereas under mixotrophic and autotrophic conditions maximal phosphate assimilation was observed. These findings suggested that illumination and autotrophic growth might significantly increase the metabolic requirement for phosphorous by C. pyrenoidosa.
Phosphorous-starvation was also used in biomass production by Scenedesmus sp. [87]. In this study, two cultivation modes were evaluated referred as phosphorous-starvation (high initial nitrogen-to-phosphorous N/P = 46:1) and luxury-nutrient. Under phosphorous starvation the algal biomass exhausted the phosphorous in the earlier stage of growth achieving that the cells are exposed to phosphorous starvation for some time. The results showed that continuous nitrogen and phosphorous feeding in luxury nutrient mode had no stimulating effect on biomass productivity (less than 40 g biomass/g −P), in contrast, the sustained growth of biomass after the exhaust of phosphate in phosphorous-starvation mode led to significant increase in the biomass yield (160 g biomass/g −P). This study proposed a phosphorous-starvation cultivation mode to minimize phosphorous resource consumption during the production of algal biomass.
Lipid accumulation can be improved by manipulating the levels of phosphorus in culture media. Liang et al. [57] reported the effect of phosphorus concentration at different stages of culture in the lipid accumulation by Chlorella sp. These authors reported that lipid accumulation in cells was increased when incubation was carried out at low phosphorus concentration (K2HPO4, 32 μM), indicating that Chlorella sp. could accumulate lipids under phosphorus-deprived conditions. Similar study was carried out by Chia et al. [20] with C. vulgaris varying phosphate (PO43−) concentrations plus Cd addition, obtaining an increment in neutral lipids proportion, showing an interactive effect between Cd added and PO43−. This effect was also observed in Scenedesmus sp., where an increment in lipid content by 35% was observed when −P starvation was used [86]. In base of these reports, it can be observed that during microalgal cell exposition to phosphorus deprivation, the biosynthetic pathways are altered, thus the synthesis of lipids is increased as a response to this stress. Hu et al. [41] mentioned that under stress conditions, the formation of lipids is a response to consume 24 NADPH derived from the excess of electron transport chain (formation of reactive oxygen species), which is twice that demanded for the synthesis of a carbohydrate or protein molecule of the same mass.
The use of media composition changes as phosphorous deprivation reduces process costs in large-scale culture and it is applicable in open and closed systems.
3.3. Salt-stress
Salt-stress causes a multitude of bioenergetics and biochemical changes in photosynthetic organisms. In plants, salt-stress is caused due to the excess of ions Na+ and Cl− in the environment, decreasing the osmotic potential of soil and hence water uptake by the plant root. During osmotic stress, photosynthetic organism accumulate low molecular mass compounds known as compatible solutes or osmolytes such as proline, protein, mannitol, sorbitol, glycine, etc. [2]. Among the important non-specific changes of microalgal cells to salt stress are: (i) increased rates of biopolymers and lipid catabolism; (ii) changes in the rates of energy yielding processes; (iii) change of membrane permeability with interruption of ion homeostasis [3].
The effect of salt-stress, as increase of NaCl concentration, on biomass and pigments production, specifically carotenoids, has been studied in freshwater microalgae species suggest that salt-stress can replace light stress to induce carotenoid production, however, in several cases the microalgae growth decreased as NaCl concentration increased [6].
Pelah et al. [64] found that this phenomenon affect Chlorella zofingiensis growth under low light irradiance and subjected to salt and nitrogen stress, accumulating higher amounts of total secondary carotenoids. Furthermore, C. zofingiensis growing under salt-stress conditions and low light accumulated higher amounts of canthaxanthin than astaxanthin, suggesting that for canthaxanthin accumulation under salt stress light is not a limiting factor, but for astaxanthin accumulation high light irradiance is mandatory [54]. These results may be applied in a commercial production of canthaxanthin by C. zofingiensis in systems where light availability is poor. The above was observed in study with C. vulgaris [11] where different salt concentrations (0.17, 0.34 and 0.51 M) were evaluated under different light regimes (24:0 h, 12:12 h and 0:24 h light:dark regimens). At 0.017 M, biomass yield was higher in comparison with cultures without salt. Pigment content changed under salt treatments in C. vulgaris, the higher pigment yield was observed at 0.34 M of NaCl using a photoperiod of 12:12 h light/dark cycles. At these conditions, the total chlorophyll content was 49.92 mg/g (68% chl a and 32% chl b) and 5.96 mg/g for carotenoids. For treatment with 0.34 and 0.51 M under dark conditions the total chlorophyll reduces 54.6 and 78.2% respectively, while carotenoids production was higher by 88.9 and 6.9% respectively in comparison with control samples.
Tam et al. [96] in a recent study with H. pluvialis reported the effect of high NaCl concentrations (0.13, 0.25 and 0.43 M NaCl) on astaxanthin accumulation. The obtained results have indicated that the high NaCl concentrations caused an increase in carotenoid content per cell and a decrease in the algal growth. The best carotenogenic condition by addition of salt was obtained at 0.43 M NaCl under high temperature. Astaxanthin content per cell increased 4.8 folds in comparison to the initial value after 15 days of salt stress. It was also observed that accumulation of total lipid content was correlated with an increasing in astaxanthin content. The total lipid content of control was 15% of dry cell weight whereas for cells exposed to salt-stress was 19% and 26% at 0.25 and 0.43 M NaCl concentrations, respectively. This salt tolerance has been reported in some freshwater microalgaes where concentration ranged between 0.1 and 0.2 M promoted the growth [50], [48].
The tolerance of Scenedesmus almeriensis to medium salt concentrations has been well documented, where it was found higher biomass productivities at 0.1 M NaCl in comparison with fresh media [75]. However in some species of Chlorella, specifically C. vulgaris, the observed growth was lower at 0.17 M of NaCl in comparison with the media without salt, and a total inhibition of growth was observed when NaCl concentration ranged from 0.34 to 0.51 M [6]. The inhibitory effect of salinity on microalgae growth can be attributed by imbalance in ion homeostasis, osmotic change and by the accumulation of reactive oxygen species (ROS), and consequently, a programmed cell death [1].
The decrease in microalga growth rate with high levels of salinity has frequently been reported in the literature [69]. It is accompanied by a decrease in photosynthetic efficiency, probably by that salinity stress affects light utilization and metabolism (particularly carbohydrates involved in osmoregulation) to counteract ionic and osmotic stresses [73].
It is noted that the increase in salinity can increase the lipid content of microalgae, but can decrease growth rate of a species. Salt stress is a major abiotic environmental factor that limits plant growth and productivity. The salinity stress and unfavorable light conditions are the main limiting factors of plant productivity both in aquatic and terrestrial, natural and anthropically modified environments [30]. Microalgae differ in their adaptability to salinity and other stress conditions. The ability of cells to survive and flourish in saline environment under the influence of osmotic stress has received considerable attention. Under favorable and unlimited growth conditions microalgae produces primarily polar lipids (e.g., glycolipids and phospholipids), which enrich chloroplast and cellular membranes. However, under unfavorable growth conditions microalgae accumulate neutral lipids in lipid droplets located in the cytoplasm. Asulabh et al. [5] studied the effect of salt-stress in Chlorococcum sp., Microcystis sp. (fresh water algae) and Chaetoceros sp. (marine alga) for 7 days under light conditions. The halotolerance of all the three algae were determined by growing them in three different salinity concentrations (0.013, 0.014 and 0.034 mM for freshwater algae and 0.6, 3 and 6 mM for marine alga). The cell growth of all the three algae did not show a definite pattern. The total lipid content was found to be higher on the 5th day of culture experiment in case of Chaetoceros sp. (8.06 mg/L at 3 mM) and Microcystis sp. (8.4 mg/L at 0.013 mM) whereas, it was higher on 6th day for Chlorococcum sp. (6.6 mg/L at 0.0133 mM). The increase in lipid content at higher NaCl concentration may be due to adaptation under stress conditions, which help in accumulation of lipid content in cells.
Talukdar et al. [80] documented a study with oleaginous microalga Ankistrodesmus falcatus in batch culture under light and dark diurnal cycles. An improved growth and total lipid contents were observed with the culture salinity reached up to 160 mM. Total lipid content increase to 55.3% under salinity compared to control medium (lipid 38.3%). It was found that the major fatty acids synthetized where C16:0, C18:1 and C18:3.
Salt stress not only occurs in freshwater microalgal species. This behavior has been reported in Botryococcus braunii cells when grown in 0.5 M NaCl and halophile strains of Dunaliella salina and Dunaliella tertioletca when grown in 4.0 M NaCl found low contents of proteins, carbohydrates and pigments except lipids [10], [81]. In similar study, the accumulation of glycerol in Dunaliella cells determines the cell osmotic status and protects the enzyme functions at low activity of intracellular water caused by salinity [92].
Salt-stress was also used for maintaining the culture under axenic conditions. Open cultivation systems are more susceptible to contamination for others microorganism. Increasing lipid production in these systems while minimizing the invasion of non-target algae (competitors) and grazers (predators) will improve the economic viability of microalgal biomass. Bartley et al. [8] manipulated a basic environmental parameter, salinity, to promote algal growth and limit invading organisms, monitoring the growth of marine microalga Nannochloropsis salina and invasion of algal competitors and predators (ciliates and rotifers) in open cultures grown at different salinities ranging from brackish to hypersaline. Algal growth rate and biomass yield were higher at salinities of 0.61 and 0.94 M, whereas the density of invading organisms was lowest at 0.61 M. To determine if lipid accumulation could be maximized by salinity stress, N. salina was growth at 0.61 M until the population was at stationary phase and then evaluated different salinities up to 1.60. The results showed that lipid content increased significantly at higher salinities, and was highest at 0.94 M (36% dry tissue mass), where high triglycerides content was observed, meanwhile membrane lipids decrease as salinity increased. These findings represent an advantage to massive culture of chlorophycae species using seawater as a growth media to reduce freshwater utilization and to decrease the risk to bacterial contamination.
3.4. High-light intensities
Light conditions are the main factors affecting microalgal physiology and the most important factor affecting the photosynthesis. Light regime determinates the quantity and quality of the amount of light energy available to photosynthetic organisms to conduct their metabolic activities [53]. Numerous studies analyzing the light effect on microalgae production of pigments, unsaturated fatty acids, carbohydrates and proteins content have been carried out [76], [49]. Also from those reports it has observed that changes in production yields responds to the increments or decrements in light intensity. In this topic, several studies describes that microalgae growth is a function of the total amount of light per day and this can be controlled by the photoperiod [28], [72], [46]. In this section the description of the use of light intensity to stimulate the growth, accumulation of pigments, fatty acids and proteins in different species of microalgae is addressed.
In outdoor photobioreactors the light intensity is determinate by light harvesting process that plays a crucial role in the overall growth response to such light fluctuations. Ma et al. [60] evaluated the growth of C. zofingiensis in semi-continuous culture in an outdoor enclosed tubular photobioreactor with special emphasis on N and C metabolism. They found that of carbon and nitrogen content in the biomass showed a linear correlation with incident (photosynthetically active radiance, PAR), suggesting that the cultures were light limited. On sunny days, a rapid increase in the C/N ratio of the biomass was attributed to a slow response of protein synthesis to a high increment in PAR, compared to carbohydrate synthesis suggesting that carbon from carbohydrate storage polymers can be transferred to protein during night.
Seyfabadi et al. [76] studied the effects of irradiance and photoperiod on growth rate, chlorophyll a, β-carotene, total protein and fatty acid content of C. vulgaris. They evaluated the phototrophic growth under different irradiances and cycles (light/dark). In this study, the maximal growth rate (1.13 d−1), protein content (46%), saturated fatty acids and β-carotene were obtained at irradiance of 100 μmol photons/m2 s and 16:8-h light/dark photoperiod, while chlorophyll a, monounsaturated and polyunsaturated fatty acids decreased with increasing irradiance and light duration.
Similar finding were observed during the culture of H. pluvialis where high light levels accelerated the growth process, increasing the rate of nutrient depletion and providing more energy for astaxanthin biosynthesis [27]. The same effect was observed in β-carotene and phyocyanin production by Dunalliella salina and Spirulina platensis, respectively; where increasing light exposure the synthesis of these pigments increased [38], [84].
Khoeyi et al. [49] reported a maximum biomass yield (2.05 g L−1) when C. vulgaris was cultured at 62.5 μmol photons/m2 s and 16:8 h light/dark photoperiod. Fatty acid composition changed in different light regimes, the maximum percentage of total saturated fatty acids (SFA) (33.38%) was recorded at 100 μmol photons/m2 s and 16:8 h photoperiod, while maximal production of monounsaturated (MUFA) (15.93%) and polyunsaturated (PUFA) (27.40%) was recorded at 37.5 μmol photons m−2 s−1 and 8:16 h photoperiod.
The growth and biochemical composition of H. pluvialis is controlled or regulated by light. Imamaglu et al. [45] found that maximal growth rate in batch culture for this strain (0.195 1/d) was obtained the light intensity of 40 μmol photons/m2 s, in contrast, in similar study with this microalgae where evaluated different light intensities H. pluvialis showed a maximal growth rate 0.243 1/d at a light intensity of 170 μmol photons/m2 s used acetate as carbon source [47].
From a practical point of view, these results emphasizes that a high light irradiation had improved the content of high values compounds, where pigments and lipids production are dependent and controlled by the irradiance and photoperiod in microalgal cultures. This technology is widely used for laboratory scales studies; however, results are very expensive in massive cultivation due to the use of artificial light and its application will depend on natural irradiance of sunlight.
3.5. Electromagnetic fields
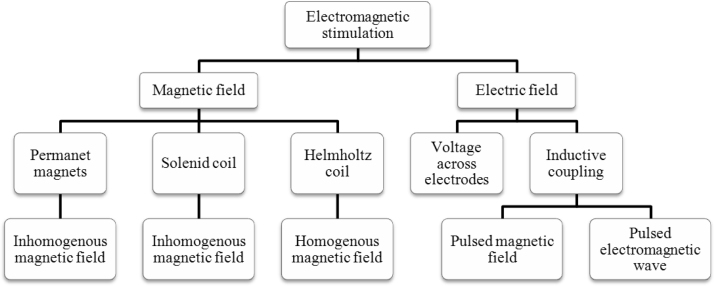
In recent years, the application of electromagnetic fields (EMF) to improve or accelerated processes has been used successfully in many areas such as chemical engineering, biomedical engineering, food technology and agriculture. However, few studies regarding the application of EMF in biotechnological processes have been reported [4], [7]. A number of bioprocesses could be successfully integrated with electromagnetic or electrochemical stimulation if the cultivation conditions are properly engineered using specialized reactors as electrolytic bioreactors, electro-bioreactors and bioelectro-reactors [82]. Fig. 2 shows the different forms for apply electromagnetic stimulation in biological systems.
Fig. 2.
Overview of various electromagnetic stimulation modalities from fields in biological systems [43].
Electromagnetic fields treatments cause a variety of effects in microorganisms and plants such as changes in gene expression, growth rate, lag phase duration and biomass yields during fermentative process in bacteria and yeast [15], [66], increased seed germination, growth, and pigments content in plants such as wheat and been [14], and increased oxidative stress in plant cells [74]. Photosynthetic cells have been used as models of electric fields induced oxidative stress [85]. Electromagnetic stimulation can alter the energy levels and spin orientation of electrons to increase the activity, concentration, and lifetime of free radicals [71], [74]. It also affects enzyme activity, gene expression, and release of calcium from intracellular storage sites, which in turn influences the membrane structure, cell growth and cell death [85]. Living organisms have developed metabolic pathways leading to the accumulation of antioxidants including carotenoids and enzyme systems to protect cells against oxidation under conditions of stress [34].
Several studies were carried out to investigate the effect of electromagnetic stimulation in microalgae for enhancing growth and metabolites production. Hader [37] evaluated the effect of external electric field in Phorrnidium uncinatum and found that low voltages (<2.5 V) did not affect the photophobic reactions in this blue-green algae, but at electric field between 3 and 7 V the photophobic reactions are replaced and at high voltage (>7 V) induced cell death.
Wang et al. [85] in a study with C. vulgaris, proposed three mechanisms by which EMF interact with living organisms. The first one is so called electromagnetic induction that is EMF exerts forces on moving ions in solution (e.g., electrolytes) which gives rise to induced currents. The second is the electromechanical effect that is uniform EMF produce torques on certain molecules and any ferromagnetic materials in the body. The third mechanism, which is often used to explain the variation of biological reaction in cells, is electronic interactions that is static EMF can alter energy levels and spin orientation of electrons. EMP alters the spin states of the free radicals, which in turn changes the relative probabilities of recombination and other interactions, possibly with biological consequences [71].
In a study with Chlorella kessleri, the exposition to electromagnetic field (10 mT) resulted an increased in biomass, pigments, proteins, Ca and Zn contents [79]. In similar study with C. vulgaris, Benavente-Valdés [13] reported maximal production of pigments (15.02 mg/L for total chlorophyll and 3.33 mg/L for carotenoids) when the microalga was exposure in the exponential phase of culture (96 h) with an EMF of 2 V and 4 h of duration under photoheterotrophic conditions.
Li et al. [56] observed similar behavior in the cyanobacterium Spirulina platensis when was exposed at static magnetic field intensities stimulated growth rate, uptake of carbon and light energy utilization. They observed that the levels of micro and trace elements (Ni, Sr, Cu, Mg, Fe, Mn, Ca, Co and V) and essential amino acids such as histidine improved at 250 mT magnetic field treatments. Also, chlorophyll a content of the magnetically treated sample was higher than the control, suggesting better light harvesting for photosynthesis. In this concept, Hirano et al. [39] observed that S. platensis was stimulated by geomagnetic field increasing the phycocyanin content which plays an important role in the activation of photosystem II to help the activation of electron transfer reactions during photosynthesis.
In D. salina, β-carotene content was increased when it was exposed to static magnetic field (10 mT) and addition of Fe-EDTA. It also showed higher accumulation of the heavy metals (Co, Cd, Cu and Ni) indicating its potential for bioremediation of heavy metals [88].
The application of EMF fields in microalgae cultures represents an alternative for production of microalgal biomass with high contents of pigments, protein and minerals. Also it is important to evaluate the operation cost at large scale by studying the impact on yields in open and closed systems in comparison with other strategies.
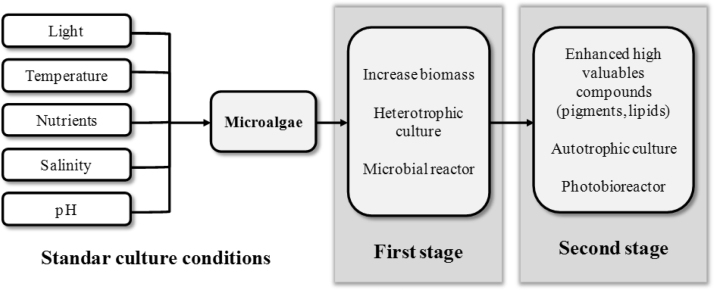
3.6. Two-stage culture
In commercial production of microalgae biomass, high cell density culture is desirable in order to reduce the cost for down-stream processing. The two-stage culture technique refers to microalgae growth under two different culture conditions (generally changing carbon source or type of reactor) in order to increase biomass productivity and metabolites of interest (Fig. 3). Although most of the microalgae are autotrophic organisms, some species are able to use organic carbon as source of nutrients for cell growth and development. The use of substances containing organic carbon and energy source in addition of supplementation with CO2 creates an additive or synergistic effect of the two processes which are reflected in increased productivity.
Fig. 3.
Two stage strategy for microalgae culture.
Two-stage culture strategy has been used for enhancing accumulation of high-valuable compounds in microalgae cells. In a study with Schizochytrium limacinum [19] for production of docosahexaenoic acid (DHA) the cultivation was divided into two stages: (1) a cell-number-increasing stage in which cell reproduction and cell number increase with little increase in the size and weight of each cell; and (2) a cell-size-increasing stage in which cells stop reproduction but cell size enlarges due to lipids accumulation, where the production of algae biomass and DHA was improved to levels of 37.9 g/L and 6.56 g/L.
Sequential heterotrophic and autotrophic cultivation methods were investigated for production of high concentration of C. pyrenoidosa biomass with high cellular protein and chlorophyll contents [62]. In this study, the cultivation system was composed of the conventional mini-jar reactor for the heterotrophic phase and a tubular photobioreactor for the autotrophic phase. During of steady state cultivation, 27% of CO2 emitted in heterotrophic phase was used in the autotrophic phase. In this system was possible to produce high biomass concentration (14 g/L) containing 60.1% of protein and 3.6% of chlorophyll continuously for more than 640 h.
Yen and Cheng [90] developed a two-stage strategy for the cultivation of C. vulgaris using an autotrophic growth followed by a mixotrophic process. The experimentation was carried out in glass flask bubbled with supplemented air (2% CO2) illuminated with LED lights (1300 μmol photon/m2 s) for autotrophic phase. In the mixotrophic stage, carbon sources (glucose or glycogen) were added diary to the medium. The results indicated that a two-stage cultivation process achieved 7.4 g/L of biomass, which was about a 64% increase over simple autotrophic cultivation; however it was observed lower levels of lipids. In contrast, the lipid content increased when the two-stage culture process started with heterotrophic phase using organic waste and municipal wastewater followed by phototrophic conditions was used with Chlorella sorokiniana [93]. These results indicated this two-stage algae culture system could also benefit waste treatment.
Two-stage cultivation for nutrient removal is often adopted in wastewater treatment. Zhou et al. [94] developed a sequential two-stage mix-photoautotrophic culture strategy for swine wastewater treatment for the production of biofuels and animal feeds with the advantage of remove total phosphorus (100%) and ammonia (89.46%) after second-stage cultivation. They used facultative heterotrophic microalgal strains isolated from local waste. Also a hetero-photoautotrophic algal growth culture was studied to improve wastewater treatment and to produce a low-cost algal biofuel feedstock using Auxenochlorella protothecoides [95]. Therefore, two-stage cultivation represents an economic and feasible technology to enhance the industrial viability of microalgae pigments and lipids.
4. Conclusions
Limitation of N and P, and increment of salinity in microalgae cultures represents a feasible strategy to increase biomass, pigments and lipids in chlorophyceae species. Also, the control irradiance and photoperiod in cultures are crucial to the development of metabolic activities of the microalgae for the production of these high-value compounds. The application of physical stress-strategies such electromagnetic or electric fields to increase microalgae yields it is at the early development stage, and more research is needed to quantify their impact at commercial scale production systems. Two-stage cultivation processes are promising strategies to explore at commercial levels in a near future to maximize yields and decreasing costs. Finally it is important to fully understand the operation of the overall process, making more efficient and economically viable the application of these technologies to enhance the commercial viability of microalgae industry.
Acknowledgments
J.R. Benavente-Valdes expresses his gratitude to the Mexican National Council for Science and Technology (CONACyT) for the grant received to pursue his postgraduate studies.
References
- 1.Affenzeller M.J., Darehshouri A., Andosch A., Cornelius-Lutz C., Lutz-Meindl U. Salt stress-induced cell death in the unicellular green alga Micrasterias denticulate. J. Exp. Bot. 2009;60 doi: 10.1093/jxb/ern348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad N., Fazal H., Abbasi B.H., Rashid M., Mahmood T., Fatima N. Efficient regeneration and antioxidant potential in regenerated-tissues of Piper nigrum L. Plant Cell Tissue Organ Cult. 2010;102 [Google Scholar]
- 3.Alyabyev A., Andreyeva I., Rachimova G. Influence of pH shift and salting on the energetics of microalgae Chlorella vulgaris and Dunaliella maritime. J. Therm. Anal. Calorim. 2011;104:201–207. [Google Scholar]
- 4.Araújo O.Q.F., Coelho M.A.Z., Margarit I.C.P., Vaz C.A., Rocha-Leão M.H.M. Electrical stimulation of Saccharomyces cerevisiae cultures. Braz. J. Microbiol. 2004;35:97–103. [Google Scholar]
- 5.Asulabh K.S., Supriya G., Ramachandra T.V. Effect of salinity concentrations on growth rate and lipid concentration in Microcystis sp., Chlorococcum sp. and Chaetoceros sp. LAKE 2012. National Conference on Conservation and Management of Wetland Ecosystems. 2012 [Google Scholar]
- 6.Barghbani R., Rezaei K., Javanshir A. Investigating the effects of several parameters on the growth of Chlorella vulgaris using Taguchi’s experimental approach. Int. J. Biotechnol. Wellness Ind. 2012;1:128–133. [Google Scholar]
- 7.Bartlett P.N., Pletcher D., Zeng J. Approaches to the integration of electrochemistry and biotechnology. J. Electrochem. Soci. 1997;144:3705–3710. [Google Scholar]
- 8.Bartley M.L., Boeing W.J., Corcoran A., Holguin F.O., Schaub T. Effects of salinity on growth and lipid accumulation of biofuels microalga Nannochloropsis salina and invading organisms. Biomass Bioenergy. 2013;54:83–88. [Google Scholar]
- 9.Batista A.P., Nunes M.C., Gouveia L., Sousa I., Raymundo A., Cordobés F., Guerrero A., Franco J.M. Microalgae biomass interaction in biopolymer gelled systems. Food Hydrocoll. 2011;25:817–825. [Google Scholar]
- 10.Ben-Amotz A., Tornabene T.G., Thomas W.H. Chemical profile of selected species of microalgae with special emphasis on lipids. J. Phycol. 1985;21 [Google Scholar]
- 11.Benavente-Valdés J.R. Master Thesis. University of Coahuila; 2013. Análisis de factores de estrés sobre la producción de pigmentos por Chlorella vulgaris en un fotobiorreactor airlift tipo flat panel. [Google Scholar]
- 12.Berges J.A., Charwbois O.D., Mauzerall D.C., Falkowski P.G. Differential effects of nitrogen limitation on photosynthetic efficiency of photosystems I and II in microalgae. Plant Physiol. 1996;110 doi: 10.1104/pp.110.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bona F., Capuzzo A., Franchino M., Maffei M.E. Semicontinuous nitrogen limitation as convenient operation strategy to maximize fatty acid production in Neochloris oleoabundans. Algal Res. 2014;5:1–6. [Google Scholar]
- 14.Cakmak T., Dumlupinar R., Erdal S. Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagnetics. 2010;31:120–129. doi: 10.1002/bem.20537. [DOI] [PubMed] [Google Scholar]
- 15.Castro I., Oliveira C., Domingues L., Teixeira J.A., Vicente A. The effect of the electric field on lag phase, β-galactosidase production and plasmid stability of a recombinant Saccharomyces cerevisiae strain growing on lactose. Food Bioprocess Technol. 2012;5:3014–3020. [Google Scholar]
- 16.Chen G.Q., Chen F. Growing phototrophic cells without light. Biotechnol. Lett. 2006;28:607–616. doi: 10.1007/s10529-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 17.Chen C.Y., Yeh K.L., Aisyah R., Lee D.J., Chang J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour. Technol. 2011;102:71–81. doi: 10.1016/j.biortech.2010.06.159. [DOI] [PubMed] [Google Scholar]
- 18.Chen M., Tang H., Ma H., Holland T.C., Simon Ng K.Y., Salley S.O. Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 2010;102:1649–1655. doi: 10.1016/j.biortech.2010.09.062. [DOI] [PubMed] [Google Scholar]
- 19.Chi Z.Y., Liu Y., Frear C., Chen S.L. Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl. Microbiol. Biotechnol. 2009;81:1141–1148. doi: 10.1007/s00253-008-1740-7. [DOI] [PubMed] [Google Scholar]
- 20.Chia M.A., Lombardi A.T., Melão M.G., Parrish G.C. Lipid composition of Chlorella vulgaris (Trebouxiophyceae) as a function of different cadmium and phosphate concentrations. Aquat. Toxicol. 2013;128:171–182. doi: 10.1016/j.aquatox.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Chisti Y. Biodiesel from microalgae. Biotechnol. Adv. 2007;25:294–306. doi: 10.1016/j.biotechadv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Chojnacka K., Marquez-Rocha F.J. Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology. 2004;3:21–34. [Google Scholar]
- 23.Dean A.P., Estrada B., Sigee D.C., Pittman J.K. Using FTIR spec-troscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour. Technol. 2010;101:4499–4507. doi: 10.1016/j.biortech.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 24.Del Campo J.A., García-González M., Guerrero M.G. Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl. Microbiol. Biotechnol. 2007;74:1163–1174. doi: 10.1007/s00253-007-0844-9. [DOI] [PubMed] [Google Scholar]
- 25.Dietz K.J., Harris G.C. Photosynthesis under nutrient deficiency. In: Pessarakli M., editor. Handbook of Photosynthesis. Marcel Dekker Inc.; New York: 1997. pp. 951–975. [Google Scholar]
- 26.Eurgain H.J., Flynn K.J. Modelling phosphate transport and assimilation in microalgae; how much complexity is warranted? Ecol. Modell. 2000;125:145–157. [Google Scholar]
- 27.Fábregas J., Domínguez A., García-Álvarez D., Lamela T., Otero A. Induction of astaxanthin accumulation by nitrogen and magnesium deficiencies in Haematococcus pluvialis. Biotechnol. Lett. 1998;20:623–626. [Google Scholar]
- 28.Fabregas J., Maseda A., Domınguez A., Ferreira M., Otero A. Changes in the cell composition of the marine microalga, Nannochloropsis gaditana, during a light: dark cycle. Biotechnol. Lett. 2002;24 [Google Scholar]
- 29.Figueira F., Crizel T., Silva C.R., Salas-Mellado M. Elaboration of gluten-free bread enriched with the microalgae Spirulina platensis. Braz. J. Food Sci. 2011;14 [Google Scholar]
- 30.Fodorpataki L., Bartha C. Studia. Universitatis. Babes. Bolyat. Biologia.; 2004. Salt Stress Tolerance of a Freshwater Green Alga Under Different Photon Flux Densities. (XLX, 2) [Google Scholar]
- 31.Fradique M., Batista A.P., Nunes M.C., Gouveia L., Bandarrac N.M., Raymundo A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: preparation and evaluation. J. Sci. Food Agric. 2010;90:1656–1664. doi: 10.1002/jsfa.3999. [DOI] [PubMed] [Google Scholar]
- 32.Gouveia L., Oliveira A. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009;36:269–274. doi: 10.1007/s10295-008-0495-6. [DOI] [PubMed] [Google Scholar]
- 33.Gouveia L., Marques A.E., Da Silva T.L., Reis A. Neochloris oleabundans UTEX #1185: a suitable renewable lipid source for biofuel production. J. Ind. Microbiol. Biotechnol. 2009;36:821–826. doi: 10.1007/s10295-009-0559-2. [DOI] [PubMed] [Google Scholar]
- 34.Gouveia L., Veloso V., Reis A., Fernandes H., Novais J., Empis J. Evolution of pigment composition in Chlorella vulgaris. Bioresour. Technol. 1996;57:157–163. [Google Scholar]
- 37.Hader D.P. Influence of electric fields on photophobic reactions in blue-green algae. Arch. Microbiol. 1977;114:83–86. doi: 10.1007/BF00429635. [DOI] [PubMed] [Google Scholar]
- 38.Hejazi M.A., Holwerda E., Wijffels R.H. Milking microalga Dunaliella salina for beta-carotene production in two-phase bioreactors. Biotechnol. Bioeng. 2004;85 doi: 10.1002/bit.10914. [DOI] [PubMed] [Google Scholar]
- 39.Hirano M., Ohta A., Abe K. Magnetic field effects on photosynthesis and growth of the cyanobacterium Spirulina platensis. J. Ferment. Bioeng. 1998;87:313–316. [Google Scholar]
- 40.Ho S.H., Chen C.Y., Chang J.S. Effect of light intensity and nitrogen starvationon CO2 fixation and lipid/carbohydrate production of an indigenous microalgae Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012;113 doi: 10.1016/j.biortech.2011.11.133. [DOI] [PubMed] [Google Scholar]
- 41.Hu Q., Sommerfeld M., Jarvis E., Ghirardi M., Posewitz M., Seibert M., Darzins A. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 2008;54:621–639. doi: 10.1111/j.1365-313X.2008.03492.x. [DOI] [PubMed] [Google Scholar]
- 42.Huang G.H., Chen F., Wei D., Zhang X.W., Chen G. Biodiesel production by microalgal biotechnology. Appl. Energy. 2010;87:38–46. [Google Scholar]
- 43.Hunt R.W., Zavalin A., Bhatnagar A., Chinnasamy S., Das K.C. Electromagnetic biostimulation of living cultures for biotechnology, biofuel and bioenergy applications. Int. J. Mol. Sci. 2009;10:4515–4558. doi: 10.3390/ijms10104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Illman A.M., Scragg A.H., Shales S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym. Microbiol. Technol. 2000;27:631–635. doi: 10.1016/s0141-0229(00)00266-0. [DOI] [PubMed] [Google Scholar]
- 45.Imamaglu E., Vardar-Sukan F., Conk-Dalay M. Effect of different culture media and light intensities on growth of Haematococcus pluvialis. Int. J. Nat. Eng. Sci. 2007;1:5–9. [Google Scholar]
- 46.Jacob-Lopes E., Gimenes-Scoparo C.H., Ferreira-Lacerda L.M.C., Teixeira-Franco T. Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors. Chem. Eng. Process. 2009;48 [Google Scholar]
- 47.Jeon Y.C., Cho C.W., Yun Y.S. Combined effects of light intensity and acetate concentration on the growth of unicellular microalga Haematococcus pluvialis. Enzyme Microb. Technol. 2006;39:490–495. [Google Scholar]
- 48.Kaewkannetra P., Enmak P., Chiu T.Y. The effect of CO2 and salinity on the cultivation of Scenedesmus obliquus for biodiesel production. Biotechnol. Bioprocess Eng. 2012;17 [Google Scholar]
- 49.Khoeyi Z.A., Seyfabadi J., Ramezanpour Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae Chlorella vulgaris. Aquacult. Int. 2012;20:41–49. [Google Scholar]
- 50.Kobayashi M., Kurimura Y., Tsuji Y. Light-independent, astaxanthin production by the green microalga Haematococcus pluvialis under salt stress. Biotechnol. Lett. 1997;19 [Google Scholar]
- 51.Kozłowska-Szerenos B., Zielinski P., Maleszewski S. Involvement of glycolate metabolism in acclimation of Chlorella vulgaris cultures to low phosphate supply. Plant Physiol. Biochem. 2000;38:727–734. [Google Scholar]
- 52.Kozłowska-Szerenos B., Zielinski P., Maleszewski S. Enhancement of photosynthetic O2 evolution in Chlorella vulgaris under high light and increased CO2 concentration as a sign of acclimation to phosphate deficiency. Plant Physiol. Biochem. 2004;42:403–409. doi: 10.1016/j.plaphy.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Latasa M. Pigment composition of Heterocapsa sp. and Thalassiosira weissflogii growing in batch cultures under different irradiances. Sci. Mar. 1995;59:25–37. [Google Scholar]
- 54.Li Y., Huang J. High-light and sodium chloride stress differentially regulate the biosynthesis of astaxanthin in Chlorella zofingiensis. J. Phycol. 2009;40 doi: 10.1111/j.1529-8817.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- 55.Li Y., Horsman M., Wang B., Wu N., Lan C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008;81:629–636. doi: 10.1007/s00253-008-1681-1. [DOI] [PubMed] [Google Scholar]
- 56.Li Z.Y., Guo S.Y., Lin L., Cai M.Y. Effects of electromagnetic field on the batch cultivation and nutritional composition of Spirulina platensis in an air-lift photobioreactor. Bioresour. Technol. 2007;98:700–705. doi: 10.1016/j.biortech.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 57.Liang K., Zhang Q., Gu M., Cong W. Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J. Appl. Phycol. 2013;25:311–318. [Google Scholar]
- 58.Lim S., Chu W., Phang S. Use of Chlorella vulgaris for bioremediation of textile wastewater. Bioresour. Technol. 2010;101:7314–7322. doi: 10.1016/j.biortech.2010.04.092. [DOI] [PubMed] [Google Scholar]
- 59.Liu Z.Y., Wang G.C., Zhou B.C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008;99:4717–4722. doi: 10.1016/j.biortech.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 60.Ma X., Chen K.W., Lee Y.K. Growth of Chlorella outdoors in a changing light environment. J. Appl. Phycol. 1997;9 [Google Scholar]
- 61.Mimura T. Homeostasis and transport of inorganic phosphate in plants. Plant Cell Physiol. 1995;36:1–7. [Google Scholar]
- 62.Ogbonna J.C., Masui H., Tanaka H. Sequential heterotrophic/autotrophic cultivation—an efficient method of producing Chlorella biomass for health food and animal feed. J. Appl. Phycol. 1997;9 [Google Scholar]
- 63.Ördög V., Stirk W., Bálint P., Staden J., Lovász C. Changes in lipid, protein and pigment concentrations in nitrogen-stressed Chlorella minutissima cultures. J. Appl. Phycol. 2012;24:907–914. [Google Scholar]
- 64.Pelah D., Sintov A., Cohen E. The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World J. Microbiol. Biotechnol. 2004;20:483–486. [Google Scholar]
- 65.Ponce-Palafox J.T., Arredondo-Figueroa J.L., Vernon-Carter E.J. Carotenoids from plants used in diets for the culture of the pacific white shrimp (Litopenaeus vannamei) Rev. Mex. Ing. Chim. 2006;5 [Google Scholar]
- 66.Potenza L., Ubaldi L., De-Sanctis R., De-Bellis R., Cucchiarini L., Dacha M. Effects of a static magnetic field on cell growth and gene expression in Escherichia coli. Mutat. Res. 2004;561:53–62. doi: 10.1016/j.mrgentox.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 67.Qu C.B., Wu Z.F., Shi X.M. Phosphate assimilation by Chlorella and adjustment of phosphate concentration in basal medium for its cultivation. Biotechnol. Lett. 2008;30:1735–1740. doi: 10.1007/s10529-008-9758-6. [DOI] [PubMed] [Google Scholar]
- 68.Rao A.R., Dayananda C., Sarada R., Shamala T.R., Ravishankar G.A. Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour. Technol. 2007;98:560–564. doi: 10.1016/j.biortech.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Ravelonandro P.H., Ratianarivo D.H., Joannis-Cassand C., Isambertc A., Raherimandimby M. Improvement of the growth of Arthrospira (Spirulina) platensis from Toliara (Madagascar): effect of agitation, salinity and CO2 addition. Food Bioprod. Process. 2011;89 [Google Scholar]
- 70.Raymundo A., Gouveia L., Batista A.P., Empis J., Sousa I. Fat mimetic capacity of Chlorella vulgaris biomass in oil-in-water food emulsions stabilized by pea protein. Food Res. Int. 2005;38:961–965. [Google Scholar]
- 71.Repacholi M.H., Greenebaum B. Interaction of static and extremely low frequency electric and magnetic fields with living systems: health effects and research needs. Bioelectromagnetics. 1999;20:133–160. doi: 10.1002/(sici)1521-186x(1999)20:3<133::aid-bem1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 72.Richmond A. Biological principles of mass cultivation. In: Richmond A., editor. Handbook of Microalgal Mass Culture: Biotechnology and Applied Phycology. Blackwell Oxford; 2004. [Google Scholar]
- 73.Rosales N., Ortega J., Mora R., Morales E. Influence of salinity on the growth and biochemical composition of the cyanobacterium Synechococcus sp. Ciencias marínas. 2005;31:349–355. [Google Scholar]
- 74.Sahebjamei H., Abdolmaleki P., Ghanati F. Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnetics. 2007;28:42–47. doi: 10.1002/bem.20262. [DOI] [PubMed] [Google Scholar]
- 75.Sanchez F., Fernández J.M., Acien-Fernández G., Rueda A., Perez-Parra J., Molina E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008;43:398–405. [Google Scholar]
- 76.Seyfabadi J., Ramezanpou Z., Khoeyi Z.A. Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J. Appl. Phycol. 2011;23:721–726. [Google Scholar]
- 77.Sheehan J., Dunahay T., Benemann J. and Roessler P. (1998). A Look Back at the U.S. Department of Energy’s Aquatic Species Program −Biodiesel From Algae, Golden, CO, National Renewable Energy Institute, NREL/TP-580-24190, 328.
- 78.Singh R.N., Sharma S. Development of suitable photobioreactor for algae production—a review. Renew. Sustain. Energy Rev. 2012;16:2347–2353. [Google Scholar]
- 79.Small D.P., Huner N.P.A., Wan W. Effect of static magnetic fields on the growth, photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics. 2012;33:98–308. doi: 10.1002/bem.20706. [DOI] [PubMed] [Google Scholar]
- 80.Talukdar J., Mohan-Chandra-Kalita M.C., Goswami B.C. Effects of salinity on growth and total lipid content of the biofuel potential microalga Ankistrodesmus falcatus (Corda) Ralfs. Int. J. Sci. Eng. Res. 2012;3 [Google Scholar]
- 81.Tammam A.A., Fakhry E.M., El-Sheekh M. Effect of salt stress on antioxidant system and the metabolism of the reactive oxygen species in Dunaliella salina and Dunaliella tertiolecta. Afr. J. Biotechnol. 2011;10 [Google Scholar]
- 82.Velizarov S. Electric and magnetic fields in microbial biotechnology: possiblities, limitations, and perspectives. Electro- Magnetobiol. 1999;18:185–212. [Google Scholar]
- 83.Vymazal J. Lewis Publishers; Boca Raton, FL: 1995. Algae and Element Cycling in Wetlands. (689 pp.) [Google Scholar]
- 84.Walter A., Carvalho J.C., Soccol V.T., Bisinella De Faria A.B., Ghiggi F., Soccol C.R. Study of phycocyanin production from Spirulina platensis under different light spectra. Braz. Arch. Biol. Technol. 2011;54:675–682. [Google Scholar]
- 85.Wang H.Y., Zeng X.B., Guo S.Y., Li Z.T. Effects of magnetic field on the antioxidant defense system of recirculation-cultured Chlorella vulgaris. Bioelectromagnetics. 2008;29:39–46. doi: 10.1002/bem.20360. [DOI] [PubMed] [Google Scholar]
- 86.Wu Y.H., Yin Y., Hu H.Y. Microalgal growth with intracellular phosphorus for achieving high biomass growth rate and high lipid/triacylglycerol content simultaneously. Bioresour. Technol. 2015;192:374–381. doi: 10.1016/j.biortech.2015.05.057. [DOI] [PubMed] [Google Scholar]
- 87.Wu Y.H., Yu Y., Li X., Hu H.Y., Su Z.F. Biomass production of a Scenedesmus sp. under phosphorous-starvation cultivation condition. Bioresour. Technol. 2012;112:193–198. doi: 10.1016/j.biortech.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 88.Yamaoka Y., Takimura O., Fuse H., Kamimura K. Effect of magnetism on growth of Dunaliella salina. Photosynth. Res. 1992;3 [Google Scholar]
- 89.Yeesang C., Cheirsilp B. Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour. Technol. 2011;102:3034–3040. doi: 10.1016/j.biortech.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 90.Yen H.W., Chang J.T. A two-stage cultivation process for the growth enhancement of Chlorella vulgaris. Bioprocess Biosyst. Eng. 2013;36:1797–1801. doi: 10.1007/s00449-013-0922-6. [DOI] [PubMed] [Google Scholar]
- 91.Yoo C., Jun S.Y., Lee J.Y., Ahn C.Y., Oh H.M. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010;101:71–74. doi: 10.1016/j.biortech.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 92.Zakhozhii I.G., Matalin D.A., Popova L.G., Balnokin Y.V. Responses of photosynthetic apparatus of the halotolerant microalga Dunalliella maritima to hyperosmotic salt shock. Russ. J. Plant Physiol. 2012;59:42–49. [Google Scholar]
- 93.Zheng Y., Chi Z., Lucker B., Chen S. Two-stage heterotrophic and phototrophic culture strategy for algal biomass and lipid production. Bioresour. Technol. 2012;103:484–488. doi: 10.1016/j.biortech.2011.09.122. [DOI] [PubMed] [Google Scholar]
- 94.Zhou W.G., Hu B., Li Y., Min M., Mohr M. Mass cultivation of microalgae on animal wastewater: a sequential two-stage cultivation process for energy crop and omega-3-rich animal feed production. Appl. Biochem. Biotechnol. 2012;168:348–363. doi: 10.1007/s12010-012-9779-4. [DOI] [PubMed] [Google Scholar]
- 95.Zhou W.G., Min M., Li Y., Hu B., Ma X. A hetero-photoautotrophic two-stage cultivation process to improve wastewater nutrient removal and enhance algal lipid accumulation. Bioresour. Technol. 2012;110:448–455. doi: 10.1016/j.biortech.2012.01.063. [DOI] [PubMed] [Google Scholar]
- 96.Tam L.T., Hoang D.D., Ngoc Mai D.T., Hoai Thu N.T., Lan Anh H.T., Hong D.D. Study on the effect of salt concentration on growth and astaxanthin accumulation of microalgae Haematococcus pluvialis as the initial basis for two phase culture of astaxanthin production. Tap Chi Sinh Hoc. 2012;34:213–223. [Google Scholar]