Highlights
-
•
Crocus is a medicinally important plant and it is costliest spice of the world.
-
•
An efficient microprapogation protocol of five Turkish Crocus species was developed.
-
•
Crocus species: C. specious ssp. Specious, C. oliveri spp. Oliveri, C. pestalozzae, C. abantensis, and C. paschei..
-
•
Different combinations and concentrations of auxins and cytokinins were used.
-
•
Plant regeneration was developed via somatic embryogenesis.
Abbreviations: ABA, abscisic acid; BAP, 6-benzylaminopurine; CHE, Chee and Pool medium; GB5, Gamborg’s B-5 medium; IAA, indole-3-acetic acid; IBA, indole-3-butyric acid; LS, Linsmaier and Skoog medium; MS, Murashige and Skoog medium; NAA, α-naphthalene acetic acid; TDZ, 1-phenyl-3-(1,2,3-thiadiazol-5-yl)-urea
Keywords: Crocus species, Organogeneis, Somatic embryogenesis, Plant regeneration
Abstract
Callus induction, somatic embryogenesis and plant regeneration were initiated in selected five species of Turkish crocus using three diffrent explants (leaf, stem and corm) cultured on four different media (MS, GB5, LS and CHE). The highest frequencies of callus induction (100%) and shoot regeneration (70%, with 7.2 shoots/callus) were found in the crocus species Crocus oliveri ssp. Oliveri, using the MS medium containing 5% (w/v) sucrose supplemented with (4 mg/L NAA + 4 mg/L TDZ) and (2 mg/L IAA + 2 mg/L TDZ + 2 mg/L BAP). When the embryogenic calli were transferred into the four nutrient media containing (2 mg/L IAA + 2 mg/L TDZ) and 100 mg/L ABA, these further developed into cotyledonary embryos. Maximum number of somatic embryos (2.9 embryos per leaf explant, with a frequency 46.6%) was obtained in C. oliveri ssp. Oliveri. During subculture using the half strength media, cotyledonary embryos gradually developed into plantlets.
1. Introduction
The genus Crocus belongs to the large Iridaceae family that includes more than 80 species, of which approximately 30 are cultivated worldwide. The name saffron applies indistinctly to Crocus sativus L., an herbaceous plant with corms, which grows widespread throughout the tropical and subtropical regions of the northern hemisphere. However, saffron (the dried orange-red trifid stigma of C. sativus and the costliest culinary spice of the world) is an autumn-flowering, triploid (2n = 3× = 24) male-sterile plant, propagated vegetatively by means of corms. Saffrons have been used for their smell, color and healing qualities and are cultivated for their spice value for more than 4000 years [1]. Spain and Iran are the largest producers of the spice, accounting together for more than 80% of the world production. In the Mediterranean region, in Italy, Greece, and Turkey, saffron is also cultivated, though on a smaller scale. However, Turkey is among the richest country in producing the Crocus species. There are almost 70 Crocus taxa and 31 of them are endemic to Turkey [2]. When the diversity of the taxa is taken into consideration, Turkey is regarded as the gene center of this particular species. Currently, the production of saffron in the Turkish region has decreased owing to the drift of agricultural practices.
Saffron is a spice of great economic value; one kilogram of good quality saffron produced from C. sativus L. can cost more than 2000 US dollars [3]. Approximately 150,000 flowers are needed to produce one kilogram of dried saffron and in order to grow this amount, one would need about 2000 m2 of land under cultivation [4]. According to Sobolev et al. [5], biologically active compounds such as crocetin, picrocrocin, and safranal are found in saffron. These compounds make saffron a promising candidate for imparting flavor and nutritional ingredients into the prepared food. The highly water soluble crocins (or, crocetins) are widely used as a natural food colourant and also act as antioxidants, thus protecting cells and tissues against oxidation [6]. Saffron is also used extensively in the Indian medicinal system (Ayurveda) for healing a variety of diseases including arthritis, cold, asthma, acne, skin disorders, impotence and infertility [7]. Saffron finds its applications as a textile dye and in perfume preparations [8]. It has traditionally been considered as an anodyne, antidepressant, respiratory decongestant, antispasmodic, aphrodisiac, diaphoretic, emmenagogue, expectorant and sedative. Saffron is also used in folk medicine as a remedy against scarlet fever, smallpox, cold, asthma, eye and heart disease [9]. Apart from these, often saffron is used to help clear up sores and to reduce the discomfort of teething infants [10]. Recently, there has been a growing interest in its anti-carcinogenic compounds which can be used in the prevention of tumors [11].
Of late, there is an increasing interest in the exploitation of tissue culture and genetic improvement of C. sativus L., but the other members of genus Crocus have received very little attention. Saffron is a triploid plant conventionally propagated by corm, which makes its continued cultivation a bit problematic. Yield and quality improvement of saffron through breeding have not been possible because of the sterility problem [12]. In vitro culture is proved to be greatly advantageous for mass propagation and development of diseases-resistant cultivars, mainly for vegetatively propagated crops such as saffron [13]. Due to its ecological and economic importance, there is a need for germplasm preservation of the Crocus species. This could in turn contribute to the maintenance of genetic material of C. sativus allies, which is one of the major topics of the current saffron research [14].
Several reports have appeared on in vitro propagation of Crocus species [13], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. An efficient in vitro tissue culture system is the need of the hour since the Crocus species are threatened in many countries where they are cultivated [25]. Therefore, improvement of an in vitro tissue culture protocol for the species is of great importance for germplasm conservation as well as for commercial propagation. However, there are no reports concerning in vitro regeneration of the selected five Turkish Crocus species. In this paper, for the first time, we describe a simple and very effective protocol for indirect somatic embryogenesis, organogenesis and plant regeneration of the five Crocus species [Crocus specious ssp. Specious, Crocus oliveri ssp. Oliveri, Crocus pestalozzae (endemic), Crocus abantensis (endemic) and Crocus paschei (C. abantensis × C. oliveri ssp. Oliveri) (endemic)]. The current protocol can easily be extended to other Crocus species for their propagation and conservation.
With respect to the commercial exploitation of the five crocus species under consideration in this article, all of these are sold in the local Turkish markets for their culinary use and also for use in folk medicines. Since the middle ages, the Persian, Mesopotamian and Arabian world have been well-known for their exotic food preparations in royal kitchens, where saffron was used as an integral ingredient. While data on individual species are not available, there are growing demands of the five crocus species among spice-loving consumers in the Middle East and in South East Asia.
2. Materials and methods
2.1. Plant materials
Crocus species were collected from natural populations of Bolu province [C. specious ssp. Specious: Abant Izeet baysal Campus (altitude 850 m, Coordinates: N 40° 42.864′, E 31° 31.166′); C. oliveri ssp. Oliveri, C. abantensis, and C. paschei: Abant lake, on the road of Mudurnu (altitude 1480 m, Coordinates: N 40° 35.549′, E 32° 16.956′)] and Istanbul [C. pestalozzae (altitude 130–155 m, N 40° 57′, E 29° 47.51′)] in Turkey. Identification of the collected species was done according to Davis et al. [2], and specimens were deposited at the Herbarium, Abant Izzet Baysal University, Turkey.
2.2. Sterilization
The corm, leaf, and stem were thoroughly washed under running tap water for 1 h and then cleaned with detergent Tween-20 with the help of a sable hair brush. These corm, leaf, and stem were then surface-disinfected with a savlon antiseptic (0.6 ml/100 ml; v/v) solution for 30 min. After rinsing with distilled water, the surface was sterilized with 70% (v/v) ethanol for 30–45 s and 0.1% (w/v) freshly prepared aqueous mercuric chloride (HgCl2) for 10–20 min in a laminar flow cabinet, followed by washing with sterile distilled water. Small segments of the corm, leaf and stem were cultured in media for further experimentation.
2.3. Media preparation and culture conditions
The culture media used were MS (Murashige and Skoog [26]), GB5 (Gamborg’s B-5, 1968) [27], LS (Linsmaier and Skoog [28]) and CHE’ (Chee and Pool [29]) with 2% or 5% (w/v) sucrose and 0.8% agar. The pH of the medium was adjusted to 5.8 with 0.1 N HCl or 0.1 N KOH prior to autoclaving (121 °C and 1.06 kg cm−2 pressure for 15 min). The cultures were incubated at 23 ± 1 °C for the first two days in dark and then transferred to 16 h light: 8 h dark photoperiod (provided by cool-white fluorescent light, irradiance 50 μ mol−2 S−1) and a relative humidity level of 50–60% were maintained.
2.4. Induction of callus and somatic embryogenesis
Three different types of explants (leaf, stem and corm) were used as explants for callus induction in MS, GB5, LS and CHE’ media, each supplemented with 4 mg/L TDZ + 4 mg/L NAA as shown in Table 1. Callus and embryogenic callus induction frequencies were calculated as the percentage of cultured explants (leaf, stem and corm) producing callus and embryogenic callus respectively. After 2 months of culturing, calli were produced from all the explants. Nonembryogenic calli were compact and granular in shape. Embryogenic nature of the cultures was maintained by visual identification and selection of embryogenic sectors and removal of soft and translucent nonembryogenic portions were done at the time of subculturing. For the maintenance of embryogenic potential, embryogenic calli together with globular embryos were transferred to the media (MS, GB5, LS and CHE’) containing 2 mg/L IAA + 2 mg/L TDZ + 100 mg/L ABA + 5% sucrose (w/v). The cultures were incubated at 23 ± 1 °C in 16 h light: 8 h dark photoperiod, for maturation of somatic embryos (cotyledonary stages). The embryos were then subcultured after 4 weeks in half strength media for further conversion into plantlets. All media formulations and plant growth regulators (PGR) were purchased from Duchefa Biochemie (Netherlands). Each experiment was repeated three times, each using 20 replicates (i.e., a total of 60 explants per treatment). Both the frequency (%) of callus developing somatic embryos and a mean number of somatic embryos per explant derived callus were recorded after 8 or 12 weeks of culture, respectively.
Table 1.
Callus induction from the different explants (leaf, stem and corm) of the five crocus species cultured in the presence of the four different media. Each medium is supplemented with (4 mg/L NAA + 4 mg/L TDZ) along with either 2% (w/v) or 5% (w/v) sucrose. Data were collected after three weeks of culturing.
| Crocus species | Explants | Callus induction frequency (%)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MS medium |
GB5 medium |
LS medium |
CHE’ medium |
||||||
| 2% sucrose | 5% sucrose | 2% sucrose | 5% sucrose | 2% sucrose | 5% sucrose | 2% sucrose | 5% sucrose | ||
| Crocus specious ssp. Specious | Leaf | 66.6c | 73.3d | 23.3f | 33.3e | 40.0e | 46.6f | 63.3d | 73.3e |
| Stem | 43.3g | 56.6g | 26.6e | 36.6d | 36.6f | 46.6f | 56.6f | 66.6f | |
| Corm | 76.6b | 86.6c | 36.6b | 43.3c | 53.3c | 66.6c | 73.3b | 83.3b | |
| Crocus oliveri ssp. Oliveri | Leaf | 76.6b | 90.0b | 26.6e | 36.6d | 46.6d | 60.0e | 70.0c | 80.0c |
| Stem | 56.6e | 66.6e | 33.3c | 43.3c | 53.3c | 63.3d | 63.3d | 76.6d | |
| Corm | 83.3a | 100.0a | 40.0a | 50.0a | 66.6a | 73.3a | 76.6a | 90.0a | |
| Crocus pestalozzae | Leaf | 50.0f | 56.6g | 20.0g | 33.3e | 36.6f | 43.3g | 56.6f | 63.3g |
| Stem | 36.6h | 43.3i | 26.6e | 33.3e | 33.3g | 36.6i | 50.0g | 56.6h | |
| Corm | 63.3d | 73.3d | 33.3c | 46.6b | 60.0b | 70.0b | 63.3d | 73.3e | |
| Crocus abantensis | Leaf | 43.3g | 53.3h | 20.0g | 26.6g | 33.3g | 36.6i | 50.0g | 56.6h |
| Stem | 30.0i | 33.3j | 23.3f | 26.6g | 26.6h | 30.0j | 40.0h | 46.6j | |
| Corm | 56.6e | 63.3f | 30.0d | 36.6d | 53.3c | 60.0e | 60.0e | 73.3e | |
|
Crocus paschei (C. abantensis × C. oliveri ssp. Oliveri) |
Leaf | 30.0i | 33.3j | 16.6h | 23.3h | 23.3i | 26.6k | 36.6i | 40.0k |
| Stem | 23.3j | 30.0k | 16.6h | 20.0i | 16.6j | 23.3l | 26.6j | 30.0l | |
| Corm | 43.3g | 53.3h | 23.3f | 30.0f | 33.3g | 40.0h | 40.0h | 53.3i | |
Mean number of callus formed per explant with the same letter within columns are not significantly different according to Duncan’s multiple range test at p < 0.05.
2.5. Shoot regeneration
Selected nonembryogenic calli (Table 1) were further subcultured on media supplemented with 2 mg/L IAA + 2 mg/L TDZ + 2 mg/L BAP for shoot regeneration. The shoots obtained from calli were maintained through regular subculturing at 4 weeks intervals on fresh medium with the same composition in order to obtain more shoots. After sufficient growth, shoots (6–7 cm length) were excised from the parent culture and transferred to the MS rooting media containing IAA or IBA (0–2 mg/L). Root did not differentiate from the regenerated shoots.
2.6. Hardening and acclimatization
The plantlets regenerated through somatic embryos were removed from magenta and planted into 2.5 cm deep plastic pots containing a mixture of sterile soil: manure: moss: sand (1:2:2:1, w/w/w/w) and were kept in the greenhouse for acclimatization. The plants were watered at 2-days intervals and maintained at 70–75% relative humidity. Their survival rate was observed for a period of 4 weeks in greenhouse conditions.
3. Results
3.1. Callus induction
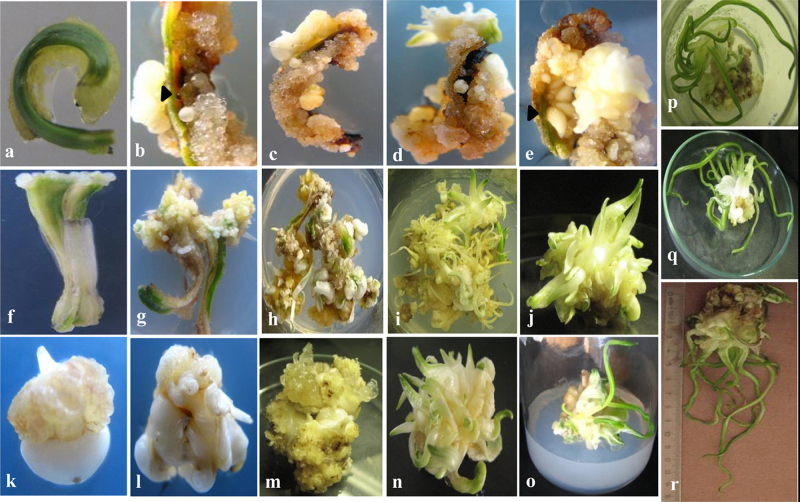
As shown in Table 1, there are significant differences in the callus induction data arising from the variations of the plant growth media and explants used with respect to callus initiation and growth. It is seen that all explants (leaf, stem and corm) of the five crocus species cultured on the four nutrient media (MS, GB5, LS and CHE’) without any plant growth regulator did not show any formation of callus, but the explants remained viable for up to 2 months. The explants successfully formed the callus on the four media upon the fortification of 4 mg/L TDZ + 4 mg/L NAA (Table 1, Fig. 1, Fig. 2). Calluses at the base of the leaf (Fig. 1a), at the cut end of the stem (Fig. 1f) and corm (Fig. 1k) had developed after 4 weeks of culturing in all the media supplemented with 4 mg/L TDZ + 4 mg/L NAA (Fig. 1a). However, other parts of the explants remained unchanged for another 2 weeks but covered with callus later (Fig. 1b, g, and i). Well-organized, cream-colored, callus appeared at the surface of explants after 8 weeks of culturing in media supplemented with 4 mg/L TDZ + 4 mg/L NAA (Table 1, Fig. 1c, h, and m).
Fig. 1.
Induction of crocus calli from five selected Turkish crocus species (Crocus specious ssp. Specious, Crocus oliveri ssp. Oliveri, Crocus pestalozzae, Crocus abantensis, and Crocus paschei (C. abantensis × C. oliveri ssp. Oliveri) and plant regeneration from leaf, stem and corm explants (a-r). Callus induction on the bottom surface of leaf (a-e) as well as well-developed globular somatic embryos at different stages of development (arrow heads) from leaf explants within three months of culture on MS medium supplemented with 4 mg/L α-naphthaleneacetic acid (NAA) and 4 mg/L thidiazuron (TDZ) (b, e); stem (f-i) and corm (k-o) explants after three months of culture on MS medium with 4 mg/L α-naphthaleneacetic acid (NAA) and 4 mg/L thidiazuron (TDZ). A plantlet regenerated from well-developed callus after six months of culture on MS medium supplemented with 2 mg/L indole-3-acetic acid (IAA) plus 2 mg/L thidiazuron (TDZ) and 6-benzylaminopurine (BAP) (o-r).
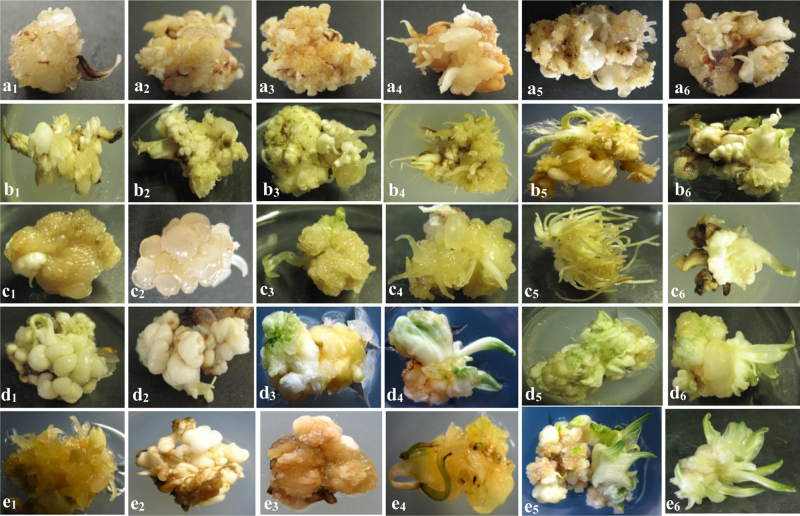
Fig. 2.
Callus induction and organogenesis from the corms of different crocus species, Crocus oliveri ssp. Oliveri (a1-a6), Crocus specious ssp. Specious (b1-b6), Crocus pestalozzae (c1-c6), Crocus abantensis (d1-d6), Crocus paschei (C. abantensis × C. oliveri ssp. Oliveri) (e1-e6), on MS medium supplemented with 2 mg/L α-naphthaleneacetic acid (NAA) and 2 mg/L thidiazuron (TDZ) within six months of culturing.
With respect to the media variations, we found that the callus induction frequencies are comparatively much higher in the case of MS medium, using both 2% and 5% sucrose (w/v).
Among the three explants, corms showed highest callus induction response with frequencies 83.3% (in MS medium containing 2% sucrose) and 100% (in MS medium containing 5% sucrose) in the C. oliveri ssp. Oliveri species (Fig. 2a1-a6) followed by the C. specious ssp. Specious species (Fig. 2b1-b6) in the same MS medium, with response frequencies 76.6% (in 2% sucrose) and 86.6% (in 5% sucrose) (Table 1). Using corms as explants, we found a minimum response in callus induction in C. paschei (C. abantensis × C. oliveri ssp. Oliveri) in the presence of MS medium, with response frequencies 43.3% (in 2% sucrose) and 53.3% (in 5% sucrose) (Table 1, Fig. 2 e1-e2).
The leaf explants showed second highest callus induction with frequencies 76.6% (in MS medium with 2% sucrose) and 90.0% (in MS medium with 5% sucrose) in the C. oliveri ssp. Oliveri species (Fig. 1a-e) followed by the C. specious ssp. Specious species, with frequencies 66.6% (in MS medium with 2% sucrose) and 73.3% (in MS medium with 5% sucrose). A closely similar callus induction was achieved in CHE’ medium, using leaf explants of C. specious ssp. Specious species [frequencies 63.3% (in MS medium with 2% sucrose) and 73.3% (in MS medium with 5% sucrose)]. Using leaf explants, the species C. paschei (C. abantensis × C. oliveri ssp. Oliveri) had shown minimum response with callus induction frequencies 30.0% (in 2% sucrose) and 33.3% (in 5% sucrose) in the MS medium.
The stem explants showed the third highest callus inductions. The highest callus induction in stems was obtained in the species C. oliveri ssp. Oliveri, with frequencies 56.6% (in 2% sucrose) and 66.6% (in 5% sucrose) in the presence of MS medium, followed by C. specious ssp. Specious species (frequencies 43.3% and 56.6%, respectively, for the 2% and 5% sucrose concentrations). Two crocus species, namely, C. abantensis (Fig. 2d1-d2) and C. paschei (C. abantensis × C. oliveri ssp. Oliveri) (Fig. 2e1-e2) displayed successful callus induction (Table 1) but failed to develop into embryos (Table 2), in the presence of the four nutrient media.
Table 2.
Development of cotyledonary somatic embryos from the different explants (leaf, stem and corm) of the five crocus species cultured in the presence of the four different media. Each medium is supplemented with (2 mg/L NAA + 2 mg/L TDZ) and 100 mg/L ABA. Data were collected after five weeks of culturing.
| Crocus species | Explants | Callus response for cotyledonary somatic embryos |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MS medium |
GB5 medium |
LS medium |
CHE’ medium |
||||||
| Average number of embryos formeda | Percent frequency of embryos formed |
Average number of embryos formeda | Percent frequency of embryos formed | Average number of embryos formeda | Percent frequency of embryos formed | Average number of embryos formeda | Percent frequency of embryos formed | ||
| Crocus specious ssp. Specious | Leaf | 2.5 ± 0.2b | 36.6b | 1.2 ± 0.4b | 13.3c | 1.4 ± 0.3c | 36.6a | 1.3 ± 0.6dc | 43.3a |
| Stem | 1.1 ± 0.4d | 23.3e | 1.0 ± 0.3c | 10.0d | 1.5 ± 0.7c | 10.0e | 1.0 ± 0.4de | 16.6g | |
| Corm | 1.5 ± 0.2c | 26.6d | 1.0 ± 0.3c | 13.3c | 1.3 ± 0.6c | 16.6d | 1.1 ± 0.4dc | 30.0d | |
| Crocus oliveri ssp. Oliveri | Leaf | 2.9 ± 0.4a | 46.6a | 1.5 ± 0.2a | 16.6b | 1.3 ± 0.6c | 33.3b | 1.8 ±0.4a | 40.0b |
| Stem | 1.5 ± 0.2c | 30.0c | 1.1 ± 0.1cb | 20.0a | 1.8 ± 0.4b | 16.6d | 1.4 ± 0.3bc | 23.3e | |
| Corm | 1.8 ± 0.4c | 36.6b | 1.1 ± 0.1cb | 16.6b | 2.0 ± 0.3a | 26.6c | 1.7 ± 0.7ab | 33.3c | |
| Crocus pestalozzae | Leaf | 2.0 ± 0.3b | 26.6d | 0.6 ± 0.3d | 13.3c | 0.7 ± 0.3d | 16.6d | 1.0 ± 0.3dc | 23.3e |
| Stem | 0.7 ± 0.3d | 6.6g | 0.2 ± 0.3e | 3.3f | 0.2 ± 0.3e | 3.3g | 0.4 ± 0.2e | 10.0h | |
| Corm | 0.9 ± 0.6d | 13.3f | 0.3 ± 0.3e | 6.6e | 0.4 ± 0.2e | 6.6f | 0.6 ± 0.3e | 20.0f | |
| Crocus abantensis | Leaf | – | – | – | – | – | – | – | – |
| Stem | – | – | – | – | – | – | – | – | |
| Corm | – | – | – | – | – | – | – | – | |
|
Crocus paschei (C. abantensis × C. oliveri ssp. Oliveri) |
Leaf | – | – | – | – | – | – | – | – |
| Stem | – | – | – | – | – | – | – | – | |
| Corm | – | – | – | – | – | – | – | – | |
Mean number of somatic embryos formed per callus explant ± SE (standard error) with the same letter within columns are not significantly different according to Duncan’s multiple range test at p < 0.05.
3.2. Effect of sucrose on callus induction
Of the two different concentrations of sucrose (2% and 5%, w/v) used in the presence of different media (MS, GB5, LS and CHE’), the medium with 5% sucrose was proved to be better for the formation of callus, which was friable and creamy in color. The combination of 4 mg/L TDZ + 4 mg/L NAA with 5% sucrose was superior, as friable callus was initiated on the crocus species, within 20 days. Although callus also formed in the presence of 2% sucrose using the same medium, the texture was relatively hard. The media containing 5% sucrose showed about 10% increased callus formation frequencies than those of 2% sucrose containing media (Table 1).
3.3. Somatic embryogenesis, maturation and conversion of embryos
As shown in Table 2, medium supplemented with 2 mg/L NAA + 2 mg/L TDZ + 5% sucrose (w/v), induced somatic embryogenesis on the leaf explants within 4 weeks, and the other explants (stem and corm) had shown a later response, showing the somatic embryogenesis induction after 8 weeks of culture. It was observed that callus initiated from a leaf explant in the presence of 2 mg/L NAA + 2 mg/L TDZ formed somatic embryos earlier than other explants (stem and corm) in the MS medium. Among the four media tested, the MS medium was the best (frequency of embryo formation is 46.6%) for somatic embryo regeneration, followed by the CHE’ medium (with frequency 43.3%). The crocus species had shown positive and early somatic embryogenesis in terms of mean frequencies of leaf explants developing into embryos (46.6%) followed by the corm explants (36.6%) in the presence of the MS medium. The average number of somatic embryos formed per leaf explant was 2.9 embryos while per corm explant only 1.8 embryos were formed. Somatic embryos were tightly packed and inseparable (Fig. 1b and e). Such somatic embryos were separated from initial explants and sub-cultured into a fresh medium (2 mg/L IAA + 2 mg/LTDZ + 100 mg/L ABA) for proliferation (Fig. 3b-e). In the present study, somatic embryos further developed and enlarged after the addition of an auxin into the medium. Successive cultures increased the number of embryos and the embryos further developed into cotyledonary stages (Fig. 3a-e). Interestingly, globular embryos (Figs. 1 b and 3 a) were found to be predominant. Embryos were white or pale yellow in color, small and globular in shape appearing individually or in the form of clusters. The heart and torpedo developmental stages were not observed in the present study. Globular somatic embryos with the soft and shining surface (Fig. 3a) grew further to form somatic embryos with a notch that differentiated to form scutellum, coleoptiles, root and shoot pole (i.e., cotyledonary stage of embryos) (Fig. 3a-e). We observed that the embryos grew up to the cotyledonary stage (maturation) in the same medium. Cotyledons, which initially appeared white in color, turned green and underwent germination (Fig. 3a, f). These regenerated embryos gradually formed shoots and roots, after further four weeks culture in the same media. Later, plantlet formation was achieved after culturing in half-strength MS medium containing no plant growth regulators, for six weeks (Fig. 3f).
Fig. 3.
Plant regeneration through indirect somatic embryogenesis from the leaf explants of crocus species Crocus oliveri ssp. Oliveri. Well-developed globular somatic embryos (a); developing stages of cotyledonary stages of somatic embryos (b-d); on the callus from leaf explants after three months of culture on MS medium supplemented with 2 mg/L indole-3-acetic acid (IAA) plus 2 mg/L thidiazuron (TDZ) and 100 mg/L abscisic acid (ABA). (e) Well-developed cotyledonary stage somatic embryos. (f) A plantlet regenerated from a somatic embryo. (h): Regenerated plants in the potted soil.
3.4. Shoot regeneration
Shoot regeneration from the well-developed calli from different explants of the crocus species cultured on four different media has been summarized in Table 3. Specific ratios of NAA and TDZ supported callus induction (Table 1). A decrease in TDZ concentration with the addition of IAA into the media progressively increased the percent response and enhanced shoot formation in the crocus species (Fig. 1o-r). The majority of the responsive cultures producing shoots developed these from the basal portion of the callus (as shown in Fig. 1n) and very rarely from all over the surface (as shown in Fig. 1j). A maximum number of shoots per callus was observed for the (2 mg/L IAA + 2 mg/L TDZ + 2 mg/L BAP) combination in MS medium. Therefore, the reduction in TDZ concentration (from 4 mg/L to 2 mg/L) and the addition of (2 mg/L IAA + 2 mg/L BAP) proved to be more suitable for the production of distinct shoots (Table 3).
Table 3.
Shoot regeneration from the well-developed calluses of different explants (leaf, stem and corm) of the five crocus species cultured in the presence of four different media supplemented with (2 mg/L IAA + 2 mg/L TDZ) and 2 mg/L BAP. Data were collected after 6 months of culturing.
| Crocus species | Explants | Callus response for shoot regeneration |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MS medium |
GB5 medium |
LS medium |
CHE’ medium |
||||||
| Average number of shoots formeda | Percent frequency of shoots formed |
Average number of shoots formeda | Percent frequency of shoots formed | Average number of shoots formed* | Percent frequency of shoots formed | Average number of shoots formeda | Percent frequency of shoots formed | ||
| Crocus specious ssp. Specious | Leaf | 3.0 ± 0.2cde | 46.6e | 1.5 ± 0.4dfe | 16.6c | 1.7 ± 0.7cbd | 33.3f | 4.3 ± 0.4bc | 50.0d |
| Stem | 2.5 ± 0.7fde | 26.6h | 1.8 ± 0.2de | 13.3d | 1.5 ± 0.4cd | 20.0h | 2.1 ± 0.2dfe | 43.3f | |
| Corm | 6.3 ± 0.4a | 63.3b | 3.9 ± 0.3ab | 20.0b | 4.7 ± 0.7a | 50.0b | 5.2 ± 0.8ba | 56.6b | |
| Crocus oliveri ssp. Oliveri | Leaf | 4.0 ± 0.5cb | 60.0c | 2.0 ± 0.3dce | 20.0b | 2.0 ± 0.3cb | 40.0d | 5.2 ± 0.5ba | 53.3c |
| Stem | 3.0 ± 0.4cde | 50.0d | 2.0 ± 0.3dce | 16.6c | 1.8 ± 0.6cdb | 36.6e | 3.9 ± 0.3dce | 46.6e | |
| Corm | 7.2 ± 0.5a | 70.0a | 4.7 ± 0.7a | 23.3a | 5.2 ± 0.5a | 56.6a | 6.3 ± 0.4a | 66.6a | |
| Crocus pestalozzae | Leaf | 2.5 ± 0.7fde | 33.3f | 1.2 ± 0.7def | 10.0e | 1.4 ± 0.5ced | 30.0g | 2.9 ± 0.4dce | 36.6g |
| Stem | 2.0 ± 0.3fge | 30.0g | 1.1 ± 0.4dfe | 20.0b | 1.2 ± 0.7ced | 16.6i | 1.8 ± 0.4dce | 33.3h | |
| Corm | 4.7 ± 0.7b | 46.6e | 3.0 ± 0.2bc | 16.6c | 2.7 ± 0.1b | 43.3c | 3.9 ± 0.1dce | 43.3f | |
| Crocus abantensis | Leaf | 2.0 ± 0.9fge | 26.6h | 1.1 ± 0.4dfe | 10.0e | 1.3 ± 0.6ced | 16.6i | 2.3 ± 0.4dfe | 33.3h |
| Stem | 1.5 ± 0.2fg | 13.4i | 1.0 ± 0.3fe | 6.6f | 1.0 ± 0.4ced | 20.0h | 1.5 ± 0.2dfe | 23.3i | |
| Corm | 3.4 ± 0.3cd | 50.0d | 2.3 ± 0.4dc | 13.3d | 2.0 ± 0.9cd | 36.6e | 3.0 ± 0.2dc | 43.3f | |
|
Crocus paschei (C. abantensis × C. oliveri ssp. Oliveri) |
Leaf | 1.4 ± 0.3g | 13.3i | 0.9 ± 0.2fe | 6.6f | 0.7 ± 0.3ed | 6.6j | 1.1 ± 0.4fe | 20.0j |
| Stem | 1.1 ± 0.4g | 6.6j | 0.4 ± 0.2f | 3.3g | 0.4 ± 0.2e | 3.3k | 0.9 ± 0.2f | 10.0k | |
| Corm | 2.5 ± 0.2fde | 26.6h | 1.2 ± 0.7dfe | 13.3d | 1.7 ± 0.7cbd | 16.6l | 2.0 ± 0.9dfe | 23.3l | |
Mean number of shoots formed per callus explant ± SE (standard error) with the same letter within columns are not significantly different according to Duncan’s multiple range test at p < 0.05.
Of the four media tested, the best growth occurred in the MS medium containing 5% sucrose (w/v), which support a maximum frequency of callus formation, the maximum frequency of somatic embryo formation and the maximum frequency of shoot regeneration (Data in Tables 1–3 and Figs. 1–3). However, none of the media combined with the auxins (IAA and IBA) could support the formation of roots in the crocus species from the regenerated shoots. The whole plant regeneration was achieved from the mature somatic embryos (Fig. 3a-g).
3.5. Transplantation
The crocus plantlets were transferred to a mixture of soil:manure:moss:sand (1:2:2:1, w/w/w/w). The plantlets continued growing well and getting acclimatized in the greenhouse conditions with 40% survival (Fig. 3h).
4. Discussion
Crocus is a difficult geophytic species and recalcitrant towards adventitious shoot and root induction under in vitro conditions. After preliminary experiments with several plant growth regulators [NAA plus TDZ; IAA plus TDZ and BAP; IAA plus TDZ and ABA (data not shown)], we have successfully identified optimum concentrations and combinations for: callus induction [4 mg/L TDZ + 4 mg/L NAA, in 2% sucrose and 5% sucrose (w/v)], somatic embryogenesis: [2 mg/L IAA + 2 mg/L TDZ + 100 mg/L ABA], shoot regeneration: [2 mg/L IAA + 2 mg/L TDZ + 2 mg/L BAP] and maturation of the crocus species in half strength MS medium. Comparisons were made with four different media formulation (MS, GB5, LS and CHE’) that were supplemented with the above plant growth regulators (Tables 1–3).
The present study confirms that the leaf explants of the crocus species were faster to induce callus formation in all the crocus species. This is in contrast with Demeter et al.’s [20] study, who investigated the response of different Crocus heuffelians explants to different auxin/cytokinin combinations. These authors could not achieve callus induction from leaf explants. In the in vitro recalcitrat plants, usually, the juvenile parts are responsive to somatic embryogenesis and plant regeneration [30]. In order to effect somatic embryogenesis in various saffron varieties, different authors had used different explants, including vegetative apex [18], apical buds [31], shoot meristem along with the pair of leaf primordia [32], meristematic tissues of saffron corms [33], rectangular section from the central meristematic region [13], and cataphylls [34]. Several combinations of auxins (NAA or IAA) and cytokinins (BAP or TDZ) efficiently induced caulogenesis. In the present work, the [4 mg/L NAA + 4 mg/L TDZ] combination was the best for callus induction. These results are in agreement with the findings of Vatankhah et al. [15]. It has been established that the phytohormone auxin (IAA) plays a critical role in influencing all the aspects of plant growth and development. Abscisic acid (ABA) is known to exert influence in exogenous applications of the hormone initiation during rapid cell proliferation, cell expansion and differentiation [35]. Auxins and cytokinins are the key factors to determine the embryogenic response due to their pervasive participation in the cell cycle regulation and cell division [36]. It was postulated that the concentration of endogenous phytohormones affected the TDZ-induced morphogenesis [37]. TDZ increased the supply of purines for cellular development and accumulation of purine metabolites [38] as well as the conversion of adenine to adenosin [39]. These are required during the cell division process and protein synthesis, both are fast processes that take place during the somatic embryo development [40]. At concentrations higher than 1 μM, TDZ can stimulate the formation of calluses, adventitious shoots and somatic embryos [41]. TDZ also affects the embryogenic callus formation and somatic embryogenesis [37], [38], [41] in C. sativus in which 2.27 μM TDZ has been found to be the most effective concentration. In the present work, the used TDZ concentration was 4 mg/L, in combination with 4 mg/L of NAA. We observed that the application of (TDZ + NAA) combination successfully induced callus and somatic embryogenesis in the five Turkish crocus species. For the sake of comparison, Sharifi et al. [13] observed that the application of TDZ alone at concentrations below 10 μM induced shoot, instead of somatic embryos in C. sativus. Additionally, the present study showed that ABA might have an important role in the somatic embryogenesis in Crocus species (Table 2 and Fig. 3a-e). This result is consistent with several earlier studies that suggested ABA’s participation in somatic embryogenesis in various plants [42], [43], [44]. Further studies are needed to ascertain the detailed mechanism of the function of ABA as well as the role of auxin + ABA combination in influencing embryogenesis. The morphology and structure of the embryogenic callus and somatic embryos developed in this work agreed with those previously reported in C. sativus [33] and Crocus cancelatus [19]. In the case of somatic embryogenesis through callus, the maximum number of embryos per callus was 2.9 with a frequency 46.6% (Table 2) for C. oliveri ssp. Oliveri. Thus, it is evident that the auxin (NAA), cytokinin (TDZ) and the hormone (ABA) are required for the development of somatic embryos (Table 2), while for shoot regeneration in the crocus species only auxin (IAA) and cytokinins (TDZ + BAP) are needed (Table 3).
Increasing the concentration of sucrose in the media used for the callus formation in the five Turkish crocus species caused a favorable response. The presence of sucrose causes an increase in the biomass and influences organogenesis in crocus species. In the contrary, a low sucrose concentration requirement for shoot regeneration from the leaf explants of Solanum melogens was reported [45]. Sucrose may act as a source of carbon, energy and osmotic agent [46]. Callus formation, somatic embryogenesis and shoot regeneration were highest in the media containing 5% sucrose (w/v) (Tables 1–3). Earlier reports have established that the somatic embryogenesis in saffron can be induced by TDZ in the presence of the MS medium with 6.0% sucrose (w/v) [47]. Similarly, 4.5% sucrose (w/v) + 2.38 μM jasmonic acid were successful in effecting somatic embryogenesis in saffron [31]. Sucrose probably substituted the starch reserves in crocus, whereas the decrease in percent germination response at high sucrose concentrations has been attributed to osmotic shock [48].
In the case of shoot regeneration through organogenesis, the maximum number of crocus shoots per explant was 12, as reported by Ilahi et al. [49]. This modestly differs from our experimental finding (7.2 shoots per corm explant, Table 3). Whereas 85.7% of the calluses had been reported to regenerate shoots after transfer to a (4.44 μM NAA + 0.53 μM BAP) [50], we found only 70% of the corm explants formed shoots, when the MS medium was supplemented with (2 mg/L IAA + 2 mg/L TDZ + 2 mg/L BAP). We also obtained the different percent of shoot regeneration in all media using same plant hormones containing 5% sucrose (w/v). The shoot regeneration order is as follows: MS > CHE’ > LS > GB5. In the present study, shoot regeneration was also achieved from embryogenic calli that was already cultured for somatic embryos (Data not shown; Table 2). However, shoot development on the callus was mediated via organogenic-like shoot meristems previously reported by Verma et al. [51]. Therefore, it can not be ruled out that, next to somatic embryogenesis, adventitious shoot formation was also involved in the plant propagation [51].
With a view to effect root induction in crocus species under in vitro conditions, IAA and IBA were chosen to supplement the medium containing 5% sucrose (w/v) for adventitious root formation. The auxins (IAA and IBA) could not induce any root formation on the regenerated shoots. Limited number of studies are available in the literature focusing on the in vitro root induction in the crocus species. One such study by Ilahi et al. [49] described the morphogenesis of saffron using tissue culture. These authors found that shoot formation and development were enhanced with low levels of 2,4-D and root formation was increased with the addition of NAA. Similar results were obtained on Indian saffrons with corm explants, where NAA induced the formation of in vitro root formation and kinetin induced shoot generation [52]. In addition to IBA, sucrose concentration was increased to 5% (w/v) in the growth media to induce root formation in C. sativus [53].
The present study records the development of complete plantlets with root system via somatic embryos cultured in half-strength MS medium supplemented with (2 mg/L IAA + 2 mg/L TDZ + 100 mg/L ABA) (Table 2 and Fig. 3). This is consistent with a previous study [54], where germinated embryos were transferred to half-strength MS medium supplemented with BAP and NAA. Regeneration via somatic embryogenesis has been derived from corm-derived callus cultures [18], [32], [55].
5. Conclusions
For the first time, this study reports a high-frequency somatic embryogenesis and in vitro plant regeneration protocol for the five selected Turkish crocus species (C. specious ssp. Specious, C. oliveri ssp. Oliveri, C. pestalozzae, C. abantensis and C. paschei (C. abantensis × C. oliveri ssp. Oliveri). We have developed an efficient tissue culture system that originated from a natural population of the five selected crocus species. As three of the species are known to be endemic [C. pestalozzae, C. abantensis and C. paschei (C. abantensis × C. oliveri ssp. Oliveri)], the developed protocol is immensely important in terms of germplasm preservation. The protocol can efficiently contribute to the maintenance of genetic material of C. sativus L. and its allies, which is one of the major topics of the global saffron research. The efficient shoot regeneration and somatic embryogenesis described here will serve as a powerful tool for the biochemical and cytological studies of the developmental processes in the crocus genus. The taxon includes C. sativus, an important species for culinary and medicinal purposes. Such embryological and regeneration studies are of great importance to the plant biotechnologists as well as agricultural scientists.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank TUBITAK for partial financial support. We are grateful to the Department of Biology, University of AIBU, for extending the lab facilities.
References
- 1.Escribano J., Diaz-Guerra M., Riese H.H., Alvarez A., Proenza R., Fernández J.A. The cytolytic effect of a glycoconjugate extracted from corms of saffron plant (Crocus sativus) on human cell lines in culture. Planta Med. 2000;66:157–162. doi: 10.1055/s-2000-11127. [DOI] [PubMed] [Google Scholar]
- 2.Davis P.H., Mill R., Tan K. vol. 10. Edinburgh University Press; Edinburgh: 1988. (Flora of Turkey and the East Aegean Islands (Supplement I)). 278pp. [Google Scholar]
- 3.Negbi M. Sterility and improvement of saffron crocus. Proceedings of the International Conference on Saffron (Crocus Sativus L.); L’Àquilla, Italy, 27–29 October; 1989. pp. 183–207. [Google Scholar]
- 4.Aytekin A., Acikgoz A.O. Hormone and microorganism treatments in the cultivation of saffron (Crocus sativus L.) plants. Molecules. 2008;13:1135–1147. doi: 10.3390/molecules13051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobolev A.P., Carradori S., Capitani D., Vista S., Trella A. Saffron samples of different origin: an NMR study of microwave-assisted extracts. Food. 2014;3:403–419. doi: 10.3390/foods3030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melnyk J.P., Wang S., Marcone M.F. Chemical and biological properties of the world’s most expensive spice: saffron. Food Res. Int. 2010;43:1981–1989. [Google Scholar]
- 7.Peter K.V. Woodhead Publishing Ltd.; Cambridge, UK and CRC USA: 2006. Handbook of Herbs and Spices. 500p. [Google Scholar]
- 8.Estilai A. Variability in saffron (Crocus sativus L.) Experientia. 1978;34:725. [Google Scholar]
- 9.Abdullaev F.I. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp. Biol. Med. 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 10.Abdullaev F., Espinosa-Aguirre J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detec. Prevent. 2002;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Nair S., Pannikar B., Panikkar K. Antitumour activity of saffron (Crocus sativus) Cancer Lett. 1991;57:109–114. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- 12.Dhar A., Sapru R., Rekha K. Studies on saffron in Kashmir 1: variation in natural population and its cytological behaviour. Crop Improv. (India) 1988;15:48–52. [Google Scholar]
- 13.Sharifi G., Ebrahimzadeh H., Ghareyazie B., Karimi M. Globular embryo-like structures and highly efficient thidiazuron-induced multiple shoot formation in saffron (Crocus sativus L.) In Vitro Cell Dev. Biol. Plant. 2010;46:274–280. [Google Scholar]
- 14.Kafi M., Koocheki A., Rashed M., Nassiri M. Science Publishers; Enfield, NH, USA: 2006. Saffron (Crocus Sativus) Production and Processing; p. 244. [Google Scholar]
- 15.Vatankhah E., Niknam V., Ebrahimzadeh H. Activity of antioxidant enzyme during in vitro organogenesis in Crocus sativus. Biol. Plant. 2010;54:509–514. [Google Scholar]
- 16.Sarma K.S., Maesato K., Hara T., Sonoda Y. In vitro production of stigma-like structures from stigma explants of Crocus sativus L. J. Exp. Bot. 1990;41:745–748. [Google Scholar]
- 17.Plessner O., Ziv M., Negbi M. In vitro corm production in the saffron (Crocus sativus L.) Plant Cell Tissue Organ Cult. 1990;20:89–94. [Google Scholar]
- 18.George P., Visvanath S., Ravishankar G., Venkataraman L. Tissue culture of saffron (Crocus sativus L.): somatic embryogenesis and shoot regeneration. Food Biotechnol. 1992;6:217–223. [Google Scholar]
- 19.Karamian R., Ebrahimzadeh H. Plantlet regeneration from protoplast-derived embryogenic calli of Crocus cancellatus. Plant Cell Tissue Organ Cult. 2001;65:115–121. [Google Scholar]
- 20.Demeter Z., Surányi G., Molnár V.A., Sramkó G., Beyer D., Kónya Z., Vasas G., Márta M., Máthé C. Somatic embryogenesis and regeneration from shoot primordia of Crocus heuffelianus. Plant Cell Tissue Organ Cult. 2010;100:349–353. [Google Scholar]
- 21.Ahouran M., Hosseini R., Zarghami R. Corms as a source of explants for the successful clonal propagation of Crocus cancellatus. J. Crop Sci. Biotechnol. 2012;15:47–51. [Google Scholar]
- 22.Mir J.I., Ahmed N., Wani S.H., Rashid R., Mir H., Sheikh M.A. In vitro development of microcorms and stigma like structures in saffron (Crocus sativus L.) Phys. Mol. Biol. Plant. 2010;16:369–373. doi: 10.1007/s12298-010-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devi K., Sharma M., Ahuja P. Direct somatic embryogenesis with high frequency plantlet regeneration and successive cormlet production in saffron (Crocus sativus L.) S. Afr. J. Bot. 2014;93:207–216. [Google Scholar]
- 24.Renau-Morata B., Moyá L., Nebauer S., Seguí-Simarro J., Parra-Vega V., Gómez M., Molina R. The use of corms produced under storage at low temperatures as a source of explants for the in vitro propagation of saffron reduces contamination levels and increases multiplication rates. Ind. Crop Prod. 2013;46:97–104. [Google Scholar]
- 25.Fernández J.A., Pandalai S. Biology, biotechnology and biomedicine of saffron. Recent Res. Dev. Plant Sci. 2004;2:127–159. [Google Scholar]
- 26.Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- 27.Gamborg O., Miller R., Ojima K. Nutrient requirement of suspensions cultures of soybean root cells. Exp. Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- 28.Linsmaier E., Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 1965;18:100–127. [Google Scholar]
- 29.Chee R., Pool R. Improved inorganic media constitutents for in vitro shoot multiplication of Vitis. Sci. Horti. Amst. 1987;32:85–95. [Google Scholar]
- 30.Schulze J. Improvements in cereal tissue culture by thidiazuron: a review. Fruit Veg. Cereal Sci. Biotechnol. 2007;1:64–79. [Google Scholar]
- 31.Blazquez S., Piqueras A., Serna M.D., Casas J.L., Fernández J.A. Somatic embryogenesis in saffron: optimisation through temporary immersion and polyamine metabolism. Acta Horti. 2004:269–276. [Google Scholar]
- 32.Ahuja A., Koul S., Ram G., Kaul B. Somatic embryogenesis and regeneration of plantlets in saffron, Crocus sativus L. Indian J. Exp. Biol. 1994;32 135–135. [Google Scholar]
- 33.Blazquez S., Olmos E., Hernández J.A., Fernández-García N., Fernández J.A., Piqueras A. Somatic embryogenesis in saffron (Crocus sativus L.). Histological differentiation and implication of some components of the antioxidant enzymatic system. Plant Cell Tissue Organ Cult. 2009;97:49–57. [Google Scholar]
- 34.Devi K., Sharma M., Ahuja P. Direct somatic embryogenesis with high frequency plantlet regeneration and successive cormlet production in saffron (Crocus sativus L.) S. Afr. J. Bot. 2014;93:207–216. [Google Scholar]
- 35.Ogata Y., Iizuka M., Nakayama D., Ikeda M., Kamada I.I., Koshiba T. Possible involvement of abscisic acid in the induction of secondary somatic embryogenesis on seed-coat-derived carrot somatic embryos. Planta. 2005;221:417–423. doi: 10.1007/s00425-004-1449-5. [DOI] [PubMed] [Google Scholar]
- 36.Francis D., Sorrell D.A. The interface between the cell cycle and plant growth regulators: a mini review. Plant Growth Regul. 2001;33:1–12. [Google Scholar]
- 37.Guo B., Abbasi B.H., Zeb A., Xu L., Wei Y. Thidiazuron: a multi-dimensional plant growth regulator. Afr. J. Biot. 2013;10:8984–9000. [Google Scholar]
- 38.Victor J., Murch S., KrishnaRaj S., Saxena P. Somatic embryogenesis and organogenesis in peanut: the role of thidiazuron and N6-benzylaminopurine in the induction of plant morphogenesis. Plant Growth Regul. 1999;28:9–15. [Google Scholar]
- 39.Capelle S.C., Mok D.W., Kirchner S.C., Mok M.C. Effects of thidiazuron on cytokinin autonomy and the metabolism of N6-(Δ2-isopentenyl)[8–14C] adenosine in callus tissues of Phaseolus lunatus L. Plant Phys. 1983;73:796–802. doi: 10.1104/pp.73.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujimura T., Komamine A. Mode of action of 2,4-D and zeatin on somatic embryogenesis in a carrot cell suspension culture. Zeitsc für Pflanz. 1980;99:1–8. [Google Scholar]
- 41.Huetteman C.A., Preece J.E. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 1993;33:105–119. [Google Scholar]
- 42.Nolan K., Rose R. Plant regeneration from cultured Medicago truncatula with particular reference to abscisic acid and light treatments. Aust. J. Bot. 1998;46:151–160. [Google Scholar]
- 43.Fernando S., Gamage C. Abscisic acid induced somatic embryogenesis in immature embryo explants of coconut (Cocosnucifera L.) Plant Sci. 2000;151:193–198. doi: 10.1016/s0168-9452(99)00218-6. [DOI] [PubMed] [Google Scholar]
- 44.Vahedi M., Kalantari S., Salami S.A. Effects of osmolytic agents on somatic embryogenesis of saffron (Crocus sativus L.) Notulae Sci. Biol. 2015;7:57–61. [Google Scholar]
- 45.Mukherjee S.K., Rathinasabapathi B., Gupta N. Low sugar and osmotic requirements for shoot regeneration from leaf pieces of Solanum melongena L. Plant Cell Tissue Organ Cult. 1991;25:13–16. [Google Scholar]
- 46.Carrier D., Cunningham J., Taylor D., Dunstan D. Sucrose requirements and lipid utilization during germination of interior spruce (Picea glauca engelmannii complex) somatic embryos. Plant Cell Rep. 1997;16:550–554. doi: 10.1007/BF01142322. [DOI] [PubMed] [Google Scholar]
- 47.Sheibani M., Nemati S., Davarinejad G., Azghandi A., Habashi A. Induction of somatic embryogenesis in saffron using thidiazuron (TDZ) II International Symposium on Saffron Biology and Technology 739. 2006:259–267. [Google Scholar]
- 48.Mondal T.K., Bhattacharya A., Sood A., Ahuja P.S. Factors affecting germination and conversion frequency of somatic embryos of tea [Camellia sinensis (L.) O. Kuntze] J. Plant Phys. 2002;159:1317–1321. [Google Scholar]
- 49.Ilahi I., Jabeen M., Firdous N. Morphogenesis with saffron tissue culture. J. Plant Phys. 1987;128:227–232. [Google Scholar]
- 50.Isa T., Ogasawara T. Efficient regeneration from the callus of saffron (Crocus sativus) Jpn. J. Breed. 1988;38:371–374. [Google Scholar]
- 51.Verma S.K., Yucesan B., Sahin G., Gurel E. Embryogenesis, plant regeneration and cardiac glycoside determination in Digitalis ferruginea subsp ferruginea L. Plant Cell Tissue Organ Cult. 2014;119:625–634. [Google Scholar]
- 52.Dhar A., Sapru R. Studies on saffron in Kashmir. III. In vitro production of corm and shoot like structures. Indian J. Genet. Plant Breed. 1993;53:193–196. [Google Scholar]
- 53.Zeybek E., Önde S., Kaya Z. Improved in vitro micropropagation method with adventitious corms and roots for endangered saffron. Open Life Sci. 2012;7:138–145. [Google Scholar]
- 54.Bhagyalakshmi N. Factors influencing direct shoot regeneration from ovary explants of saffron. Plant Cell Tissue Organ Cult. 1999;58:205–211. [Google Scholar]
- 55.Karamian R., Ranjbar M. Efficient somatic embryogenesis and plantlet regeneration from protoplast culture of Crocus sativus L. III International Symposium on Saffron: Forthcoming Challe Culti Rese. Econo. 850. 2009:113–116. [Google Scholar]