Abstract
Objectives: The incidence of lymphoproliferative disorders (LPDs) is increasing in sub-Saharan Africa (SSA) due to population growth, aging, and human immunodeficiency virus (HIV). Despite significant burden, resources for diagnosis and treatment of LPDs are limited, with little infrastructure to deliver modern pathology services. Diagnostic and therapeutic decisions are therefore frequently made without tissue confirmation, leading to high rates of misdiagnosis and inappropriate treatment.
Methods: We have established a laboratory in Malawi to support clinical and research efforts at a national teaching hospital. Consensus real-time diagnoses are rendered by local pathologists after weekly clinicopathologic teleconferences involving clinicians and pathologists from the United States and Malawi. Additional ancillary studies are then performed in the United States prior to final diagnosis.
Results: We report our first 2 years' experience and demonstrate high concordance between real-time diagnoses in Malawi and final diagnoses in the United States (5% major discordance rate for formalin-fixed, paraffin-embedded samples). In addition, we describe characteristics of pathologically confirmed LPDs in Malawi, highlighting differences by HIV status.
Conclusions: Our multidisciplinary approach can be a model for strong pathology services that provide direct, real-time support to clinical care and research in SSA.
Keywords: Sub-Saharan Africa, Lymphoproliferative disorders, Telepathology, Resource-limited setting
Lymphoproliferative disorder (LPD) incidence is increasing in sub-Saharan Africa (SSA). In Uganda, the age-standardized incidence of non-Hodgkin lymphoma (NHL) increased nearly 10% per year from 1991 to 2006,1 and similar trends have been reported elsewhere in the region.2 Although population growth and aging contribute, human immunodeficiency virus (HIV) plays a major role.3,4
Despite growing cancer burden, resources for diagnosis and care remain limited in SSA.5 Pathology services are in especially short supply, with fewer than one pathologist per 1 million residents in SSA, compared with more than 60 pathologists per 1 million residents in the United States.6‐8 Scarcity of diagnostic pathology results in frequent misdiagnosis with ensuing undertreatment and overtreatment. Inappropriate chemotherapy for nonmalignant conditions may have particularly severe consequences in this setting, where infectious complications during cytotoxic treatment are frequent and supportive care resources limited.
Lack of pathologists hinders not only patient care but also research in SSA. While occasional retrospective series with pathologic characterization have been published,9 few prospective studies in SSA have been supported by accurate real-time pathologic diagnoses. Instead, much of what is known about cancer in Africa comes from tumor registries, which suffer from low rates of pathologic confirmation.5 In the registry from the southern African nation of Malawi, for instance, only 18% of cancer cases were pathologically confirmed in the most recent report.10
Pathology services were not available in Malawi’s capital city, Lilongwe, until 2011, when a joint effort between Kamuzu Central Hospital (KCH) and the University of North Carolina, Chapel Hill (UNC) led to the opening of a new pathology laboratory, staffed during the study period by two pathologists, including a senior Malawian pathologist, and Malawian technicians.11,12 The laboratory offers basic cytology and histology services, as well as a limited panel of immunohistochemical (IHC) stains.
After opening the laboratory, we established a weekly clinicopathologic teleconference, in which new cases are discussed by clinicians and pathologists based in Malawi, along with pathologists in the United States, who review scanned slides using an online virtual microscopy system. After the multidisciplinary conference, a consensus real-time diagnosis is rendered by the Malawian pathologists to direct initial therapy. Cases are then shipped to the United States, where additional studies are performed before a final diagnosis is rendered.
Herein, we report pathology results from the first 2 years of this study, with specific focus on LPDs from patients enrolled in this ongoing prospective cohort. We provide descriptive statistics for LPDs among children and adults and demonstrate high concordance between real-time diagnoses in Malawi and final diagnoses in the United States. Our successful multidisciplinary model can inform efforts to increase access to pathology services in SSA.
Materials and Methods
Patient Enrollment
KCH is a national teaching hospital located in Lilongwe, Malawi, and serves as a referral center for approximately half of the nation’s 16 million residents. The prospective KCH Lymphoma Study was initiated in June 2013 and continues to actively enroll patients. The cohort described in this article is limited to patients enrolled between June 1, 2013, and May 31, 2015, with final US pathology review completed by November 15, 2015. Patients evaluated for suspected lymphoma at KCH or one of its referring clinics are invited to participate. Informed consent is obtained from patients or guardians prior to enrollment.
Real-Time Diagnosis in Malawi
For most patients in this study, real-time diagnoses were made by pathologists at KCH in Lilongwe. For these cases, Diff-Quik–stained smears from fine-needle aspirates (FNAs) and H&E-stained slides from formalin-fixed, paraffin-embedded tissues (FFPE) were first reviewed by the local pathologists (N.G.L. and B.M.D.). Using an Aperio virtual microscopy system (Leica Biosystems, Buffalo Grove, IL), slides were then scanned (histology objective magnification ×20, cytology objective magnification ×40), loaded onto a secure server, and reviewed by US pathologists (Y.F. and N.D.M.) over a virtual private network. Weekly, new cases were discussed as part of a clinicopathologic teleconference involving clinicians and pathologists in the United States and Malawi. When indicated, diagnoses were supported by manual IHC using a small panel of antibodies available in Lilongwe (Supplementary Table 1; all supplemental materials can be found at American Journal of Clinical Pathology online). After review, a consensus real-time diagnosis was rendered by the Malawian pathologists based on conference discussion.
In a minority of cases, patients were enrolled in the KCH Lymphoma Study after diagnosis at the University of Malawi College of Medicine in Blantyre. Pathologists in Blantyre (S.K. and T.T.) did not participate in weekly clinicopathologic teleconferences during the study period, nor did they have reliable access to the IHC stains offered at KCH. As such, diagnoses were rendered by Malawian pathologists based on morphologic impression alone.
US Review
After real-time consensus diagnosis, specimens were batched and shipped to the United States on a quarterly basis for additional characterization. Automated IHC stains were performed on a Leica Bond platform (Leica Biosystems) according to the manufacturer’s instructions using antibodies and in situ hybridization (ISH) probes listed in Supplementary Table 2. In a subset of cases, fluorescence in situ hybridization (FISH) was performed using MYC (Vysis LSI MYC Dual Color Break Apart Probe; Abbott Laboratories, Abbott Park, IL) and/or BCL2 (Vysis LSI BCL2 Break Apart Probe; Abbott Laboratories) probes. Once all special studies were complete, a final diagnosis was rendered by US pathologists (Y.F. and N.D.M.).
Statistical Analysis
Differences for categorical data were assessed with Fisher’s exact test, and continuous variables were evaluated using a two-tailed Student t test. All statistical analyses were performed using Prism 6 (GraphPad Software, La Jolla, CA), and a two-sided α value less than .05 was considered significant.
Results
Clinical Characteristics
Between June 1, 2013, and May 31, 2015, a total of 85 pediatric and 82 adult patients received real-time LPD diagnoses in Malawi followed by final diagnoses in the United States. Clinical characteristics of pediatric and adult patients are shown in Table 1. Fifty-two percent of adults were HIV infected, and demographic characteristics were similar between HIV-positive and HIV-negative adult patients.
Table 1.
Clinical Characteristics and Diagnostic Modality in Pediatric and Adult Casesa
| Characteristic | HIV+ | HIV– | Total | P Value (HIV+ vs HIV–) |
|---|---|---|---|---|
| Pediatric cases | ||||
| No. of cases | 3 | 82 | 85 | |
| Age, mean ± SD, y | 12.7 ± 5.2 | 9.5 ± 3.2 | 9.8 ± 3.3 | .10 |
| Male-to-female ratio | 2:1 | 1.9:1 | 1.9:1 | .97 |
| Cases with FFPE tissue, No. (%) | 2 (67) | 20 (24) | 22 (25) | .16 |
| Adult cases | ||||
| No. of cases | 43 | 39 | 82 | |
| Age, mean ± SD, y | 43.6 ± 9.5 | 45.4 ±18.5 | 44.0 ± 14.6 | .58 |
| Male-to-female ratio | 1.5:1 | 2.5:1 | 1.9:1 | .35 |
| Cases with FFPE tissue, No. (%) | 39 (91) | 37 (95) | 76 (93) | .68 |
FFPE, formalin-fixed, paraffin-embedded; HIV, human immunodeficiency virus; HIV+, human immunodeficiency virus positive; HIV–, human immunodeficiency virus negative.
aClinical characteristics of enrolled pediatric and adult patients, stratified by HIV status.
Diagnostic Modality
Data summarizing the diagnostic modality (FNA cytology vs FFPE histology) used to reach real-time diagnoses are also shown in Table 1. While pediatric cases in our cohort were more often diagnosed by cytology alone (75%), FFPE tissue was available in almost all adult cases (93%). Differences between children and adults were largely driven by high frequency of Burkitt lymphoma in pediatric patients. This typically presents in Malawi as jaw or abdominal masses that may be difficult to biopsy in young children with limited facilities for sedation and few pediatric surgeons. For typical clinical presentations, characteristic Burkitt cytologic features, including intermediate cell size, high nuclear-to-cytoplasmic ratio, and vacuolated cytoplasm Image 1, can be diagnostic with a high degree of accuracy. Clinicopathologic teams did not insist on tissue in these situations to avoid placing children at unnecessary risk for adverse iatrogenic events, unless the cytologic diagnosis remained uncertain after consensus telepathology review.
Image 1.
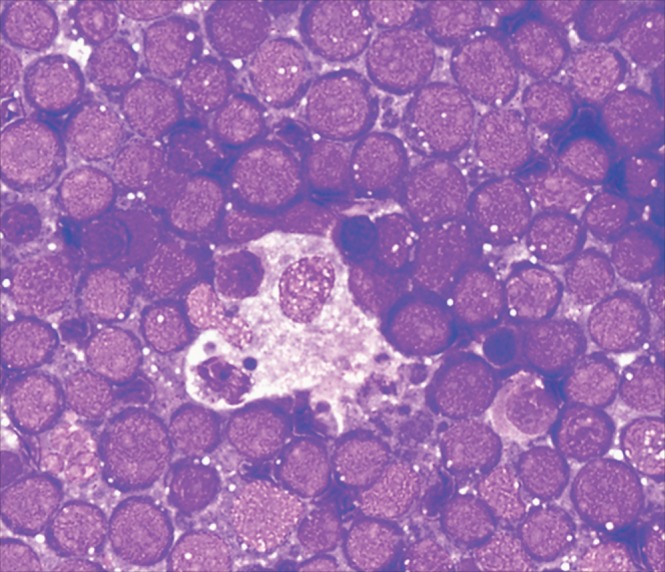
Burkitt lymphoma cytology. Diff-Quik-stained fine-needle aspirates from Malawi are frequently abundantly cellular due to standard use of large-bore needles. With this approach, some degree of tissue architecture is often retained in cytologic preparations. Characteristic cytologic features of Burkitt lymphoma are shown, including intermediate to large cell size, high nuclear-to-cytoplasmic ratio, and vacuolated cytoplasm. In addition, a tingible body macrophage is present in the center of the image, imparting a starry sky appearance to the smear. In the appropriate clinical context (eg, a child with a jaw mass or an adult who is human immunodeficiency virus positive with diffuse lymphadenopathy and a high serum lactate dehydrogenase level), these findings are considered diagnostic of Burkitt lymphoma.
Concordance Between Real-Time and Final Diagnoses at KCH
To quantify concordance between real-time diagnoses rendered at KCH after weekly clinicopathologic teleconferences and final diagnoses in the United States, we developed a four-tier system categorizing levels of agreement and incorporating the clinical implications of any discordance. For cytologic preparations, the highest degree of concordance, level 1 cytology concordance, was reserved for cases in which the final diagnosis exactly matched the original, real-time diagnosis. Level 2 cytology concordance denoted cases in which real-time and final diagnoses were similar but one or the other was less specific. In most cases, level 2 cytology concordance was seen in cases called Burkitt lymphoma in real time but given a less specific diagnosis of “intermediate- to high-grade lymphoma” on US review. Level 3 cytology concordance was reserved for a small number of cases in which a definitive diagnosis was rendered in real time but a qualifying term, such as suspicious for, was added on US review, indicating a change in certainty between real-time and final diagnosis. Finally, cases were classified as “major cytology discordance” when differences in real-time and final diagnosis would have resulted in changes in treatment in Malawi.
In 74% of cytology cases, real-time and final cytology diagnoses showed complete concordance Table 2. Another 17% of cases showed level 2 or 3 cytology concordance, with nearly equal numbers of less specific (level 2) or less certain (level 3) diagnoses on US review. Level 2 and 3 discrepancies would typically not lead to change in management in Malawi based on current KCH treatment guidelines, where many different lymphoma subtypes are treated similarly with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).13 Finally, 9% of review diagnoses were discordant with the real-time diagnosis. These included six cases diagnosed as lymphoma initially but considered nondiagnostic on US review and one case initially diagnosed as lymphoma and later revised to a small round blue cell tumor.
Table 2.
Concordance Between Malawi Diagnoses and US Reviewa
|
Concordance, No. (%) |
||||
|---|---|---|---|---|
| Characteristic | Level 1 | Level 2 | Level 3 | Major Discordance, No. (%) |
| Cytology cases | 57 (74) | 7 (9) | 6 (8) | 7 (9) |
| FFPE cases | ||||
| Lilongwe | 63 (76) | 7 (8) | 9 (11) | 4 (5) |
| Blantyre | 3 (21) | 10 (71) | 0 (0) | 1 (8) |
aConcordance between real-time and final diagnoses from fine-needle aspiration cytology specimens and formalin-fixed, paraffin-embedded (FFPE) tissues. For both cytology and FFPE cases, level 1 concordance indicates cases in which the final diagnosis exactly matched the original, real-time diagnosis, and level 2 concordance denotes cases in which the real-time and final diagnoses were similar but one or the other was less granular/specific (eg, Burkitt lymphoma vs intermediate to large lymphoma for cytology cases or non-Hodgkin lymphoma vs diffuse large B-cell lymphoma for FFPE cases). For cytology cases, level 3 concordance refers to cases in which a definitive diagnosis, such as Burkitt lymphoma, was rendered in real time, but a qualifying term such as suspicious for was added on re-review. For FFPE cases, level 3 concordance indicates a minor discordance that would result in change in the World Health Organization classification but no change in treatment in Malawi (eg, diffuse large B-cell lymphoma vs Burkitt lymphoma). Finally, for both cytology and FFPE specimens, cases were classified as “major discordance” when differences in real time and final diagnosis would have resulted in changes in treatment in Malawi. FFPE cases were further stratified based on site of diagnosis. Cases initially reviewed in Lilongwe were included in the weekly telepathology conference, whereas cases initially reviewed in Blantyre were not. Using Fisher’s exact test, there is a statistically significant difference in distribution of concordance categories between FFPE cases initially reviewed in Lilongwe vs those initially reviewed in Blantyre (P < .00001).
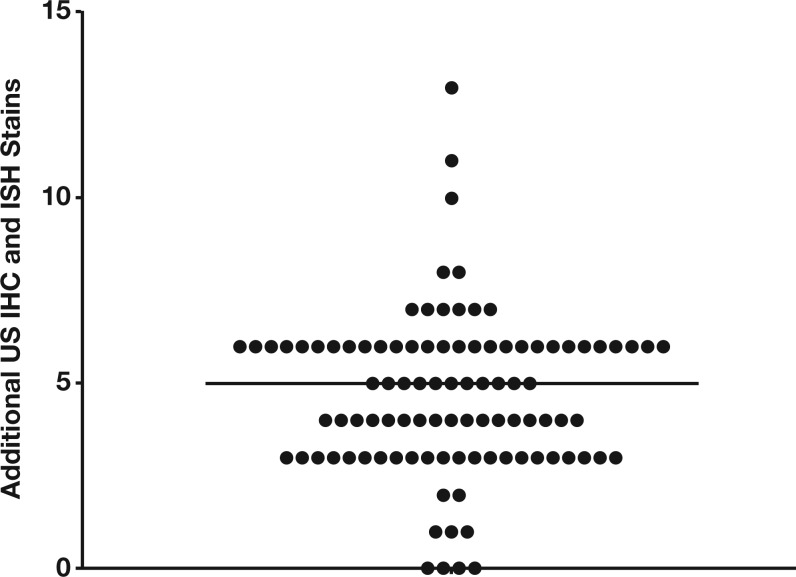
For FFPE cases, a number of additional IHC and ISH stains were performed during the US review process, in some cases supplemented by FISH. The median number of additional stains performed during US review beyond those available in Malawi was five (range, 0-13) Figure 1. As with cytology specimens, a four-tier concordance system was developed to assess agreement between real-time and final diagnoses. Comparable to the cytology classification, level 1 FFPE concordance indicated complete agreement, and level 2 FFPE concordance was reserved for cases in which the real-time and final diagnoses were similar but one or the other was less specific. Level 3 FFPE concordance indicated a minor discordance that would change World Health Organization (WHO) classification but not treatment in Malawi based on current guidelines. Finally, major discordance was reserved for those cases in which the revised final diagnosis would have changed therapy in Malawi.
Figure 1.
Additional stains performed on US review. The number of additional immunohistochemical (IHC) and/or in situ hybridization (ISH) stains performed during US review beyond those available in Malawi is shown for each case. The median number of additional stains performed was five (range, 0-13).
In 95% of FFPE cases, there was level 1, 2, or 3 concordance between the real-time and final diagnosis (Table 2), meaning that the diagnosis rendered in Lilongwe led to patient management that was appropriate for the setting. More specifically, 76% of all cases showed complete (ie, level 1) concordance, demonstrating the accuracy of diagnoses in Lilongwe using the real-time telepathology system. An additional 8% and 11% of cases showed level 2 and 3 concordance, respectively.
Real-time and final FFPE diagnoses were discordant in only four cases. Two of these were originally called classical Hodgkin lymphoma in Malawi, with diagnoses later revised to ALK-negative anaplastic large cell lymphoma and Epstein-Barr virus (EBV)–positive diffuse large B-cell lymphoma (DLBCL) of the elderly, respectively. In both cases, CD3 and CD20 stains performed in Malawi were negative, and the final diagnosis required additional T- and B-cell stains unavailable in Lilongwe. In a third case, an original diagnosis of plasmacytoma was revised to plasmablastic lymphoma, based on detection of EBV in the United States, as well as greater appreciation of plasmablastic morphologic features that were initially difficult to discern using digital slides. Finally, the fourth discordant case was originally called HIV lymphadenitis but later recognized as multicentric Castleman disease (MCD).14,15 Interestingly, three of four discordant diagnoses were during the first 6 months of teleconferences, suggesting improved agreement as collaborating clinicians and pathologists learned to work more effectively together.
Comparison to Real-Time Diagnoses From a Remote Site in Malawi
While most cases enrolled in the KCH Lymphoma Study were diagnosed after FNA or biopsy in Lilongwe, occasionally patients were enrolled after diagnosis at the University of Malawi College of Medicine in Blantyre. Pathologists in Blantyre do not participate in the weekly clinicopathologic teleconferences, nor do they have reliable access to the IHC stains offered at KCH. To determine whether agreement with the final diagnosis differed between diagnoses rendered in Blantyre vs Lilongwe, we compared concordance between both locations with final US review.
Despite limited resources, 92% of diagnoses in Blantyre showed level 1 to 3 concordance, similar to what was seen in Lilongwe (Table 2). Overall, however, the distribution of concordance levels differed between Lilongwe and Malawi (P < .00001). This difference was largely driven by a tendency for Blantyre diagnoses to be less specific without IHC capabilities, leading to a lower percentage of cases with level 1 concordance (76% in Lilongwe vs 21% in Blantyre) and a higher percentage of cases with level 2 concordance (8% in Lilongwe vs 71% in Blantyre). Overall, these results suggest excellent performance by Blantyre pathologists working without IHC, while also demonstrating the utility of IHC toward improving diagnostic specificity in Lilongwe.
Pathologic Characteristics of Pediatric Cases
Final diagnoses of pediatric patients were evaluated to assess the relative frequency of LPDs in this cohort. As has been reported in previous publications from the region,3,10,16 Burkitt lymphoma was the most common diagnosis in pediatric patients, representing at least 74% of all LPDs Table 3. Another 10 (12%) cases, all diagnosed by cytology, received a nonspecific diagnosis of “intermediate- to high-grade lymphoma” on US review. Seven of these cases were originally called Burkitt lymphoma in Malawi, and these children were subsequently treated as such. Despite small numbers, survival appeared similar between cases in which a Malawi FNA diagnosis of Burkitt lymphoma was confirmed in the United States and cases in which the diagnosis was revised to intermediate- to high-grade lymphoma (Supplementary Figure 1). Children with Burkitt lymphoma diagnosed by FFPE tissue also exhibited similar survival (Supplementary Figure 1), suggesting that many pediatric cases called Burkitt lymphoma in Malawi and later revised to intermediate- to high-grade lymphoma may represent bona fide cases of Burkitt lymphoma with similar outcomes. Other pediatric diagnoses in this cohort included CHL (7%), DLBCL (2%), and peripheral T-cell lymphoma not otherwise specified (4%). Overall, this distribution is similar to that reported previously in a cohort of pediatric patients from Tanzania and Kenya.3
Table 3.
Pathologic Characteristics of Pediatric and Adult Casesa
| Characteristic | HIV+, No. (%) | HIV–, No. (%) | Total, No. (%) |
|---|---|---|---|
| Pediatric cases | n = 3 | n = 82 | n = 85 |
| Burkitt lymphoma | 1 (33) | 62 (76) | 63 (74) |
| Intermediate to large lymphoma | 0 (0) | 10 (12) | 12 (12) |
| Classic Hodgkin lymphoma | 1 (33) | 5 (6) | 6 (7) |
| Peripheral T-cell lymphoma, NOS | 0 (0) | 3 (4) | 3 (4) |
| Diffuse large B-cell lymphoma | 0 (0) | 2 (2) | 2 (2) |
| High-grade B-cell lymphoma | 1 (33) | 0 (0) | 1 (1) |
| Adult cases | n = 43 | n = 39 | n = 82 |
| Diffuse large B-cell lymphoma | 23 (53) | 14 (36) | 37 (45)b |
| Classic Hodgkin lymphoma | 2 (5) | 10 (26) | 12 (15)c |
| Multicentric Castleman disease | 6 (14) | 0 (0) | 6 (7)d |
| Burkitt lymphoma | 4 (9) | 1 (3) | 4 (5)b |
| High-grade B-cell lymphoma, NOS | 3 (7) | 1 (3) | 4 (5) |
| Mature T/NK-cell lymphoma | 0 (0) | 4 (10) | 4 (5)e |
| Extranodal NK/T-cell lymphoma | — | 3 (8) | 3 (4) |
| ALK-negative ALCL | — | 1 (3) | 1 (1) |
| Low-grade B-cell lymphoma | 1 (2) | 3 (8) | 4 (5) |
| CLL/SLL | 1 (2) | 2 (5) | 3 (4) |
| Marginal zone lymphoma | — | 1 (3) | 1 (1) |
| Plasmablastic lymphoma | 2 (5) | 1 (3) | 3 (4)b |
| Intermediate- to high-grade lymphoma | 2 (5) | 1 (3) | 3 (4) |
| Mantle cell lymphoma | 0 (0) | 1 (3) | 1 (1) |
| Plasmacytoma | 0 (0) | 1 (3) | 1 (1) |
| T-lymphoblastic lymphoma | 0 (0) | 1 (3) | 1 (1) |
| Indolent EBV-positive NK-cell LPD | 0 (0) | 1 (3) | 1 (1) |
ALCL, anaplastic large cell lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; HIV+, human immunodeficiency virus positive; HIV–, human immunodeficiency virus negative; LPD, lymphoproliferative disorder; NK, natural killer; NOS, not otherwise specified.
aPathologic classification of enrolled pediatric and adult patients, stratified by HIV status. The number of patients diagnosed with various lymphoproliferative disorders is shown. For rare diagnoses in our cohort, similar entities are listed as a group (eg, mature NK/T-cell lymphomas and low-grade B-cell lymphomas), with specific diagnoses listed within those groups.
bIndicates high-grade B-lineage lymphomas, which represented a higher proportion of adult HIV+ cases (32/43) than adult HIV– cases (17/39) (P = .004, Fisher’s exact test).
cIndicates a statistically significant difference in the proportion of classic Hodgkin lymphoma cases between adult HIV+ and adult HIV– patients (P = .007, Fisher’s exact test).
dIndicates a statistically significant difference in the proportion of multicentric Castleman disease cases in adult HIV+ and adult HIV– patients (P = .03, Fisher’s exact test).
eIndicates a statistically significant difference in the proportion of mature T- and NK-cell lymphomas between adult HIV+ and adult HIV– patients (P = .03).
Pathologic Characteristics of Adult Cases
LPDs in adult patients, stratified by HIV status, are shown in Table 3. As reported previously,17,18 LPDs in HIV-positive patients were more frequently intermediate- to high-grade B-lineage neoplasms, such as DLBCL, Burkitt lymphoma, or plasmablastic lymphoma (74% in HIV-positive patients vs 44% in HIV-negative patients, P = .004). Cases of MCD were also common among HIV-positive patients, as we have previously reported.14,15 In contrast, CHL and mature T-cell and natural killer (NK)–cell lymphomas represented a higher proportion of cases in HIV-negative patients (P = .007 and P = .03, respectively).
Discussion
We describe a program to accurately diagnose LPDs in real time in Malawi using a limited panel of IHC stains, along with weekly clinicopathologic teleconferences. On review, major discordance for cytology specimens was rare (9% of all cases), although there was a tendency for US pathologists to be less definitive and specific than their Malawi counterparts when FNAs were the only available diagnostic material. Among cases with FFPE tissue, major discordances that would have changed care in Malawi were seen in only 5% of cases, which is similar to interobserver disagreement between pathologists in resource-rich settings.19 While minor discordances resulting in change of WHO classification were observed in another 11% of cases, these discrepancies did not affect care in Malawi, meaning that real-time diagnoses resulted in management appropriate to the setting.
Our work demonstrates that a small, carefully chosen panel of IHC stains can both ensure diagnostic accuracy and provide added granularity to LPD diagnoses in resource-limited settings. Limited chemotherapy formularies and logistical constraints result in many NHL subtypes being treated similarly in Malawi. Nevertheless, added diagnostic granularity has informed clinical management, for instance, providing a basis for observation or omission of anthracyclines among indolent NHL subtypes where appropriate. In addition, it has facilitated recognition of MCD, which was recurrently being misdiagnosed as reactive lymphadenopathy, and reduced instances where solid tumors or benign conditions were inappropriately treated as lymphoma.
A key feature of our program has been closely linking the parallel development of clinical and pathology services. While it is critical in SSA to distinguish infection from malignancy, hematopoietic from nonhematopoietic neoplasms, and even some lymphomas from others, it may be less critical to distinguish one high-grade B-cell lymphoma from another. The panel of IHC stains offered in our laboratory has largely met local needs, while being mindful of limited laboratory human capacity and the need to perform many assays manually. Importantly, Malawian pathologists, technicians, and clinicians have been integrally involved in developing the laboratory, providing input regarding the importance and feasibility of new assays. Consistent close collaboration among the group has also been important. Processes have improved over time, as colleagues have better understood how to work most effectively together. The value of such continuity is emphasized by the observation that three of four major discordances on FFPE cases occurred in the first 6 months.
The spectrum of LPDs in our cohort is generally similar to what has been reported previously in the region.3,10,16 Even using relatively strict cytologic criteria preferred by US-based pathologists, Burkitt lymphoma represented nearly three-fourths of all pediatric lymphomas. Among adults, there was a distinct distribution of LPDs between HIV-positive and HIV-negative patients. As reported previously, aggressive B-cell lymphomas predominated in HIV-positive patients,17,18 and consistent with our own earlier observations, MCD was also frequently observed in this population.14,15 On the other hand, classic Hodgkin lymphoma and mature T- and NK-cell lymphomas represented a higher proportion of cases in HIV-negative patients.
This study and the model for pathology services described herein have several notable strengths. Most notably, high concordance on US review demonstrates that local pathology infrastructure supported by IHC and a novel real-time clinicopathologic teleconference is able to produce timely and accurate real-time diagnoses in this resource-limited setting. At an institution that lacked pathology services just 5 years ago, tissue diagnosis is now a central part of clinical decision making, thus reducing the number of patients treated unnecessarily and inappropriately. Moreover, a prospective study design with active case finding across all hospital departments resulted in a representative case distribution for a national teaching hospital in SSA in the current HIV treatment era. As such, this cohort adds to a small number of published series describing characteristics of pathologically confirmed LPDs in SSA.
Conversely, there are several limitations to this work. First, despite 2 years of accrual at a national teaching hospital, the sample size is relatively small. Second, referral bias influences the spectrum of cases seen in our cohort. At KCH, patients often have aggressive, high-stage disease, and individuals with lower grade or earlier stage disease may be less likely to be referred. Conversely, patients with an especially aggressive course may die at home, before they can travel to Lilongwe for specialty services. Finally, given that most ancillary studies cannot be performed on routine cytology preparations, we were unable to support cytology diagnoses with additional studies in the United States. However, the impression of US pathologists generally affirmed the consensus diagnosis. The laboratory in Malawi has begun to generate cell blocks from FNA specimens, which will facilitate ancillary studies on cytology specimens in the future.
In conclusion, the relative lack of pathology services and personnel in SSA has received increasing international attention, and many organizations have initiated programs to expand access to pathology resources in the region.20‐22 Our experience in Malawi can inform these efforts. Our results demonstrate that accurate diagnostic services can be implemented in SSA with relatively modest investments. The initial cost to establish our laboratory included approximately $200,000 in equipment, donated space, and funds for training and supplementary salary support of Malawian staff. This investment has led to dramatically improved diagnostic services and a collaborative academic environment for Malawian pathologists. Enhanced collaboration between clinicians and pathologists has allowed appropriate tailoring of services to the environment. Together, these efforts provide a strong foundation for continued growth of clinical and laboratory programs for care and research over many years to come.
Supplementary Material
Acknowledgments
Funding: This work was supported by multiple grants to S.G. from the National Institutes of Health (K01TW009488, R21CA180815, and U54CA190152), Lineberger Comprehensive Cancer Center (P30CA016086), and AIDS Malignancy Consortium (U01CA121947).
Acknowledgments: We thank the leadership of Kamuzu Central Hospital (Jonathan Ngoma), UNC Project-Malawi (Irving Hoffman, Innocent Mofolo, Mina Hosseinipour, Francis Martinson), and Lineberger Comprehensive Cancer Center (Shelley Earp, Ned Sharpless, Lisa Carey, Blossom Damania, Dirk Dittmer) for their support of this study. Additional IHC and ISH stains were performed at the University of North Carolina Translational Pathology Laboratory (UNC TPL), with extensive technical contribution from Michelle Mathews. The UNC TPL is supported in part by a National Cancer Institute Core Support Grant (CA16086) to the UNC Lineberger Comprehensive Cancer Center. Finally, we are grateful to the staff in the UNC Hospitals Cytogenetics Laboratory, especially the late Kathleen Rao, PhD, and Kathy Kaiser-Rogers, PhD, for assistance with FISH studies.
References
- 1.Parkin DM, Nambooze S, Wabwire-Mangen F, et al. Changing cancer incidence in Kampala, Uganda, 1991-2006. Int J Cancer. 2010;126:1187-1195. [DOI] [PubMed] [Google Scholar]
- 2.Otieno MW, Remick SC, Whalen C. Adult Burkitt's lymphoma in patients with and without human immunodeficiency virus infection in Kenya. Int J Cancer. 2001;92:687-691. [DOI] [PubMed] [Google Scholar]
- 3.Naresh KN, Raphael M, Ayers L, et al. Lymphomas in sub-Saharan Africa—what can we learn and how can we help in improving diagnosis, managing patients and fostering translational research? Br J Haematol. 2011;154:696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopal S, Wood WA, Lee SJ, et al. Meeting the challenge of hematologic malignancies in sub-Saharan Africa. Blood. 2012;119:5078-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morhason-Bello IO, Odedina F, Rebbeck TR, et al. Challenges and opportunities in cancer control in Africa: a perspective from the African organisation for research and training in cancer. Lancet Oncol. 2013;14:e142-e151. [DOI] [PubMed] [Google Scholar]
- 6.Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14:e152-e157. [DOI] [PubMed] [Google Scholar]
- 7.Benediktsson H, Whitelaw J, Roy I. Pathology services in developing countries: a challenge. Arch Pathol Lab Med. 2007;131:1636-1639. [DOI] [PubMed] [Google Scholar]
- 8.Physician Characteristics and Distribution in the US. 2011th ed Washington, DC: American Medical Association; 2011. [Google Scholar]
- 9.Mwakigonja AR, Kaaya EE, Heiden T, et al. Tanzanian malignant lymphomas: WHO classification, presentation, ploidy, proliferation and HIV/EBV association. BMC Cancer. 2010;10:344-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Msyamboza KP, Dzamalala C, Mdokwe C, et al. Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry. BMC Res Notes. 2012;5:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopal S, Krysiak R, Liomba NG, et al. Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PLoS One. 2013;8:e70361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopal S, Krysiak R, Liomba G. Building a pathology laboratory in Malawi. Lancet Oncol. 2013;14:291-292. [DOI] [PubMed] [Google Scholar]
- 13.Gopal S, Fedoriw Y, Kaimila B, et al. CHOP chemotherapy for aggressive non-Hodgkin lymphoma with an without HIV in the antiretroviral therapy era in Malawi. PLoS One. 2016;11:e0150445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopal S, Liomba NG, Montgomery ND, et al. Characteristics and survival for HIV-associated multicentric Castleman disease in Malawi. J Int AIDS Soc. 2015;18:20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopal S, Fedoriw Y, Montgomery ND, et al. Multicentric Castleman's disease in Malawi. Lancet. 2014;384:1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinfield RL, Molyneux EM, Banda K, et al. Spectrum and presentation of pediatric malignancies in the HIV era: experience from Blantyre, Malawi, 1998-2003. Pediatr Blood Cancer. 2007;48:515-520. [DOI] [PubMed] [Google Scholar]
- 17.Raphael M, Said J, Borisch B, et al. Lymphomas associated with HIV infection In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed Lyon, France: IARC; 2008:340-342. [Google Scholar]
- 18.Hoffmann C, Tiemann M, Schrader C, et al. AIDS-related B-cell lymphoma (ARL): correlation of prognosis with differentiation profiles assessed by immunophenotyping. Blood. 2005;106:1762-1769. [DOI] [PubMed] [Google Scholar]
- 19.Strobbe L, van der Schans SA, Heijker S, et al. Evaluation of a panel of expert pathologists: review of the diagnosis and histological classification of Hodgkin and non-Hodgkin lymphomas in a population-based cancer registry. Leuk Lymphoma. 2014;55:1018-1022. [DOI] [PubMed] [Google Scholar]
- 20.ASCP. White House announces global initiative to accelerate fight against cancer in Africa. http://labculture.ascp.org/community/news/2015/10/29/white-house-announces-global-initiative-to-accelerate-fight-against-cancer-in-africa. Accessed November 27, 2015.
- 21.Friends of Africa USCAP Initiative. http://www.foa-uscapinitiative.org. Accessed November 27, 2015.
- 22.Wilson ML, Fleming KA. Global cancer care: the role of pathology. Am J Clin Pathol. 2016;145:6-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.