Abstract
Background
The ‘Asia-Pacific Expert Panel (APEX) for donepezil 23 mg’ met in November 2015 to review evidence for the recently approved high dose of donepezil and to provide recommendations to help physicians in Asia make informed clinical decisions about using donepezil 23 mg in patients with moderate-to-severe Alzheimer's disease (AD).
Summary
In a global phase III study (study 326) in patients with moderate-to-severe AD, donepezil 23 mg/day demonstrated significantly greater cognitive benefits versus donepezil 10 mg/day, with a between-treatment difference in mean change in the Severe Impairment Battery score of 2.2 points (p < 0.001) in the overall population and 3.1 points (p < 0.001) in patients with advanced AD. A subanalysis of study 326 demonstrated that the benefits and risks associated with donepezil 23 mg/day versus donepezil 10 mg/day in Asian patients with moderate-to-severe AD were comparable to those in the global study population.
Key Message
Donepezil 23 mg is a valuable treatment for patients with AD, particularly those with advanced disease. The APEX emphasized the importance of patient selection (AD severity, tolerability of lower doses of donepezil, and absence of contraindications), a stepwise titration strategy for dose escalation, and appropriate monitoring and counseling of patients and caregivers in the management of patients with AD.
Key Words: Donepezil, Alzheimer's disease, Acetylcholinesterase inhibitors, Dementia, Aging, Disease progression, Dose-response relationship
Introduction
Alzheimer's disease (AD), a progressive neurodegenerative disorder, is a common cause of dementia worldwide. The rising prevalence and socioeconomic burden associated with AD poses significant challenges to patients, caregivers, and healthcare systems. In 2015, the estimated worldwide prevalence of dementia was 47 million; East Asia had the highest prevalence of dementia (9.8 million), and four Asian countries - China, India, Japan, and Indonesia - had more than a million people living with dementia [1]. The prevalence of dementia is projected to double every 20 years to 131 million people living with dementia in 2050 [1]. China, India, and countries in South and Western Pacific Asia are set to experience a rapid growth in the number of people with dementia, driven by population growth and aging, exerting a mounting pressure on the healthcare systems [1,2].
Disease progression in AD places a significant burden not only on the person with AD and the family and caregivers, but also on society at large through the cost of care, with higher costs and burden associated with greater impairments [3,4]. Factors such as a limited awareness of dementia and inadequate understanding of and resources for the management of dementia contribute to the burden in several regions in the Asia-Pacific; the costs associated with dementia in the Asia-Pacific are estimated to be USD 185 billion [4]. Of the total cost related to the management of AD, about three-fourths is incurred in the severe stages of AD, mainly due to institutionalization [5]. A recent study in Singapore demonstrated that the median annual cost of care for each community-dwelling patient with late-onset dementia was USD 8,396; this cost was significantly higher in young-onset dementia patients living in the community [6]. Thus, there is a need for more aggressive medical treatment of dementia to delay and reduce progression to more advanced stages of dementia.
Persistent symptomatic treatment at the moderate-to-severe stage of AD may improve the quality of life of the patient, ease caregiver stress, and alleviate the care costs [7]. Currently, approved pharmacological therapies for AD include the acetylcholinesterase inhibitors (AChEIs) rivastigmine, galantamine, and donepezil, and the N-methyl-D-aspartate receptor antagonist, memantine. Only donepezil (oral tablets) and rivastigmine (transdermal patch) are approved for use in all stages - mild, moderate and severe - of AD [8,9]. This indicates a need for further treatment options which are effective through all stages of AD.
Recently, a higher-dose formulation of donepezil - a once-daily 23 mg tablet - was approved based on the results of a large, 24-week, randomized, double-blind study (study 326; ClinicalTrials.gov: NCT00478205) and is licensed for use in the United States, Hong Kong, South Korea, the Philippines, India, Singapore and Thailand [10].
Methodology
Although donepezil 23 mg/day is licensed in six Asian countries, there are no available guidelines or recommendations for its use in Asian patients with moderate-to-severe AD. The objective of this article is to review the available evidence for donepezil 23 mg and to provide clinical recommendations to help treating physicians in the Asia-Pacific region in making informed decisions about using the higher dose of donepezil in patients with AD. The ‘Asia-Pacific Expert Panel (APEX) for donepezil 23 mg’ includes 14 participants from seven Asian countries (India, Indonesia, the Philippines, Singapore, South Korea, Taiwan, and Thailand) and the United States; all participants are recognized experts in their respective countries. The APEX convened in November 2015 to assess the clinical evidence for donepezil 23 mg in conjunction with the clinical experience with its use in moderate-to-severe AD, and to provide recommendations to assist clinicians in the Asia-Pacific region in making treatment decisions. These recommendations were based on published literature, clinical trial experience, or practical experience in routine clinical practice; consensus was reached after discussions within the group.
Scientific Rationale for Donepezil 23 mg
Functional imaging studies have shown that doses of 5-10 mg of donepezil inhibit cortical AChE activity in vivo by only 20-40% [11,12]. This finding supported the idea that the current dosing of AChEIs might not achieve the maximum inhibition of cortical AChE, suggesting that higher doses of donepezil would increase AChE inhibition, potentially resulting in improved efficacy. Further, larger cholinergic deficits have been demonstrated in brain tissue samples of patients with AD with more advanced symptoms [11,13], indicating that higher AChE inhibition may be required in these patients. In clinical trials, a dose-response relationship between benefits in cognition and a higher dose of donepezil has been demonstrated in patients with AD randomized to treatment with donepezil 5 or 10 mg/day, or placebo [14,15]. The benefits of the higher dose of donepezil were most apparent in studies which included patients with more advanced AD [16]. In another study, once-daily donepezil 20 mg was found to be safe and well tolerated in subjects with mild-to-moderate AD who were stabilized on donepezil 10 mg/day [17]. These findings supported the development of donepezil 23 mg for patients with moderate-to-severe AD.
Efficacy and Safety of Donepezil 23 mg in Study 326
Clinical evidence for the efficacy and safety of donepezil 23 mg/day came primarily from study 326, a 24-week randomized, double-blind trial which evaluated the efficacy and safety of donepezil 23 mg/day compared with donepezil 10 mg/day in 1,467 patients with moderate-to-severe AD [Mini Mental State Examination (MMSE) 0-20] from the Asia-Pacific region, Europe, North and South Americas, and South Africa [10]. Patients who were on a stable dose of donepezil 10 mg/day were randomized 2:1 to either increase their dosage to donepezil 23 mg/day or to continue on the 10 mg/day dose. Primary outcome measures were the Severe Impairment Battery (SIB; cognitive function) and the Clinician's Interview-Based Impression of Change plus Caregiver Input Scale (CIBIC-plus; global function); secondary outcome measures included Alzheimer's disease Cooperative Study - Activities of Daily Living - severe version (ADCS-ADL-sev) and MMSE.
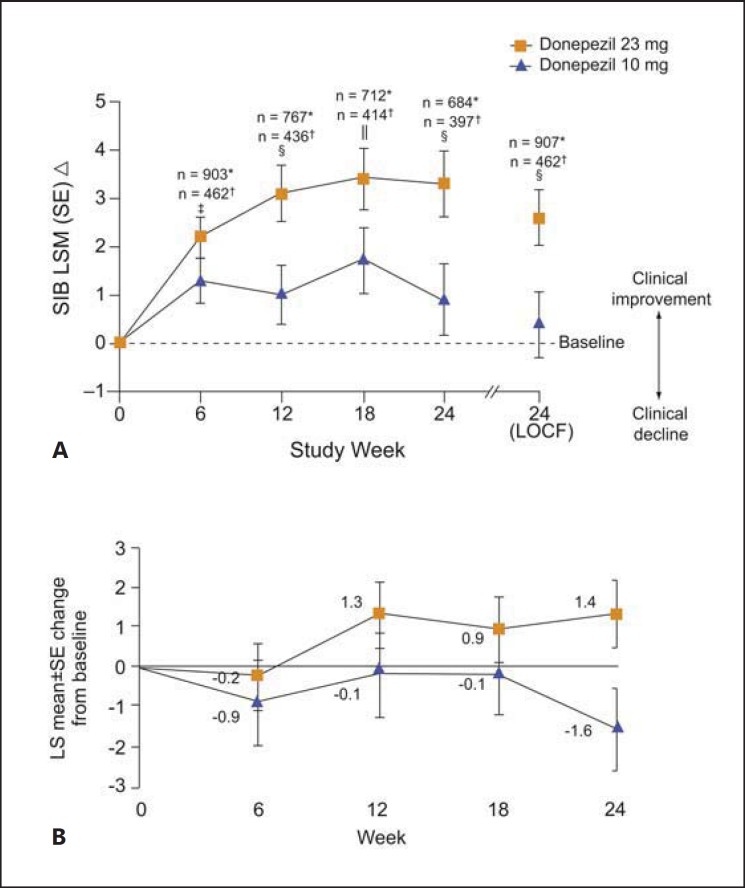
After 24 weeks, patients in the donepezil 23 mg/day treatment group demonstrated a statistically significant improvement in cognition on the SIB score compared with those on donepezil 10 mg/day, with a least-square mean difference between treatments for the change from baseline to week 24 of 2.2 points (p < 0.001; fig. 1A). In patients with more advanced AD, the between-treatment difference in change in SIB score was 3.1 (p < 0.001), which was greater than the difference seen in the overall population. No incremental benefits were seen with donepezil 23 mg/day over donepezil 10 mg/day on CIBIC-plus or the two pre-specified secondary outcome measures, ADCS-ADL-sev and MMSE. In an analysis of SIB scores in patients taking and not taking concomitant memantine, significant benefits in cognition were reported with donepezil 23 mg compared with donepezil 10 mg [18].
Fig. 1.
Changes from baseline in SIB total score in study 326 (A) and subanalysis of study 326 (B) in the Asian population. * Donepezil 23 mg; † donepezil 10 mg; ‡ p < 0.05 between treatment groups; § p < 0.001 between treatment groups; ‖ p < 0.01 between treatment groups. LOCF = Last observation carried forward; LS = least square; LSM = least square mean; SE = standard error; SIB = Severe Impairment Battery. A Reprinted from Farlow et al. [10] with permission from Elsevier. B Reprinted from Han et al. [23] (Copyright© 2016 John Wiley & Sons A/S).
Treatment emergent adverse events (TEAEs) were reported in more patients in the donepezil 23 mg/day treatment group compared with the donepezil 10 mg/day treatment group (74 vs. 64%; table 1); the majority of these events were mild-to-moderate in severity [10]. Cholinergic gastrointestinal (GI) events, such as nausea, vomiting, and diarrhea, were the most common TEAEs and also contributed most to early discontinuations; these AEs as well as discontinuations occurred most frequently in the first month of treatment in patients randomized to increase their dose of donepezil. Post hoc analyses revealed a higher incidence of TEAEs in patients in the lowest weight group (≤55 kg) [19]. Patients who completed study 326 were enrolled into an open-label extension study which evaluated the long-term safety and tolerability of donepezil 23 mg/day; all patients received donepezil 23 mg/day irrespective of the dose received in the double-blind phase [20]. No new safety signals were observed; patients in the donepezil 10-23 mg subgroup had a higher incidence of cholinergic TEAEs compared with those in the donepezil 23-23 mg subgroup; the majority of the TEAEs were mild-to-moderate in severity and were seen in the initial weeks of the study when the dose of donepezil was increased from 10 to 23 mg/day (table 2).
Table 1.
| Parameter | Study 326 [10] |
Asian subanalysis [23] |
||
|---|---|---|---|---|
| Donepezil 23 mg/day (n = 963) | Donepezil 10 mg/day (n = 471) | Donepezil 23 mg/day (n = 145) | Donepezil 10 mg/day (n = 78) | |
| Patients with at least 1 TEAE | 73.7 | 63.7 | 82.1 | 71.8 |
| TEAEs | ||||
| Nausea | 11.8 | 3.4 | 15.9 | 6.4 |
| Vomiting | 9.2 | 2.5 | 15.2 | 3.8 |
| Diarrhea | 8.3 | 5.3 | 6.9 | 3.8 |
| Anorexia | 5.3 | 1.7 | 10.3 | 2.6 |
| Dizziness | 4.9 | 3.4 | 8.3 | 6.4 |
| Weight decrease | 4.7 | 2.5 | 5.5 | 3.8 |
| Headache | 4.3 | 3.2 | 6.9 | 3.8 |
| Patients who discontinued due to TEAEs | 18.6 | 7.9 | 21.4 | 10.3 |
| Most common TEAEs leading to discontinuation | ||||
| Vomiting | 2.9 | 0.4 | 4.1 | 1.3 |
| Nausea | 1.9 | 0.4 | 2.1 | 0 |
Values are percentages.
Table 2.
Cumulative incidence of TEAEs in the safety population by study week in the extension, open-label, long-term safety study [20]
| Donepezil 23/23 mg (n = 570) |
Donepezil 10/23 mg (n = 332) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 week | 2 weeks | 4 weeks | 52 weeks | 1 week | 2 weeks | 4 weeks | 52 weeks | |
| Patients with at least 1 TEAE | 15.8 | 18.6 | 23.2 | 72.8 | 20.8 | 25.9 | 31.9 | 78 |
| Patients who discontinued due to TEAEs Patients with TEAEs | 0.5 | 1.6 | 2.3 | 11.4 | 2.4 | 3.9 | 4.8 | 17.5 |
| Diarrhea | 0.4 | 0.4 | 0.9 | 3.5 | 1.5 | 2.1 | 2.7 | 5.7 |
| Nausea | 0.2 | 0.2 | 0.2 | 2.1 | 3.9 | 4.2 | 4.2 | 6 |
| Vomiting | 0 | 0 | 0 | 1.6 | 2.4 | 2.4 | 2.7 | 4.5 |
| Dizziness | 0.4 | 0.4 | 0.5 | 1.1 | 0.9 | 1.2 | 1.5 | 3.6 |
Values are percentages. Reprinted from Tariot et al. [20] (Copyright© Tariot et al.; licensee BioMed Central Ltd., 2012).
Overall, the results from study 326 provided clinical evidence to support the use of a higher dose of donepezil in patients with moderate-to-severe AD to improve treatment outcomes. Further research to evaluate the effects of donepezil 23 mg on activities of daily living (ADL) as well as behavioral and psychological symptoms of dementia is warranted.
Efficacy and Safety of Donepezil 23 mg in an Asian Population
It is believed that interethnic differences are responsible for variations in pharmacokinetics and pharmacodynamics of drugs, resulting in differences in drug response [21]. Asia comprises several ethnic populations who may have different disease patterns [22]; these heterogeneous populations may have physical and biological differences that could potentially influence drug response with respect to efficacy and safety. A subanalysis of study 326 was undertaken to ascertain the efficacy and safety of donepezil 23 mg/day in Asian patients with moderate-to-severe AD [23].
A total of 223 Asian patients with AD from India, South Korea, Taiwan, Singapore, and Hong Kong from the global study were included in the subanalysis [23]. Baseline demographics and clinical characteristics of the group of patients receiving donepezil 10 mg/day and those receiving 23 mg/day were similar (table 3). It is noteworthy that 50% of the subjects in both treatment groups had a low body weight (<55 kg). Donepezil 23 mg provided significantly greater cognitive benefits, as measured by the SIB score, compared with donepezil 10 mg in Asian patients with moderate-to-severe AD, with a similar between-group difference in the SIB score at 24 weeks (2.92; p = 0.028) as seen in the global study (fig. 1B). There was no difference between the groups in global function measured by the CIBIC-plus scale.
Table 3.
Baseline demographic and clinical characteristics of patients in study 326 and the Asian subanalysis [10,23]
| Characteristic | Study 326 [10] |
Asian subanalysis [23] |
||
|---|---|---|---|---|
| Donepezil 23 mg/day (n = 963) | Donepezil 10 mg/day (n = 471) | Donepezil 23 mg/day (n = 145) | Donepezil 10 mg/day (n = 78) | |
| Age, years | 73.9±8.53 | 73.8±8.56 | 70.7±9.32 | 69.2±9.04 |
| Female sex | 607 (63.0) | 294 (62.4) | 74 (51.0) | 39 (50.0) |
| Weight group | ||||
| <55 kg | 218 (22.6) | 111 (23.6) | 75 (51.7) | 37 (47.4) |
| 55–<65 kg | 245 (25.4) | 129 (27.4) | 42 (29.0) | 27 (34.6) |
| 65–<75 kg | 240 (24.9) | 110 (23.4) | 22 (15.2) | 12 (15.4) |
| ≥75 kg | 259 (26.9) | 121 (25.7) | 6 (4.1) | 2 (2.6) |
| Concurrent memantine use | 352 (36.6) | 168 (35.7) | 19 (13.1) | 16 (20.5) |
| SIB score | 74.2±17.58 | 75.6±16.28 | 72.6±17.32 | 71.5±19.04 |
| MMSE score | 13.1±4.99 | 13.1±4.72 | 12.7±4.69 | 12.2±4.37 |
Values are means ± standard deviations or n (%).
In the Asian population, TEAEs associated with donepezil 23 mg were typically cholinergic and included nausea, vomiting, diarrhea, anorexia, headache, and dizziness (table 1). More patients receiving donepezil 23 mg/day discontinued the study medication compared with those receiving donepezil 10 mg (42 vs. 19%); of these patients, 21% receiving donepezil 23 mg/day and 10% receiving donepezil 10 mg discontinued due to TEAEs. Most TEAEs leading to the discontinuation of the study medication occurred during the first month of the treatment. In the donepezil 23 mg group, the incidence of TEAEs was higher among patients with a lower body weight (<55 kg) at baseline.
The results of the subanalysis demonstrate that the benefits and risks associated with donepezil 23 mg/day versus donepezil 10 mg/day in Asian patients are comparable to those of the global study population. This subanalysis has some limitations: this was a post hoc analysis, and the study population included a small number of patients; the results are subject to careful interpretation. Large-scale, well-designed clinical studies in Asian populations will potentially confirm this result. Further, the impact of donepezil 23 mg on other aspects of dementia, such as the ADL and the behavioral and psychological symptoms of dementia, must also be evaluated in future studies.
Translation of Benefits Seen in Clinical Trials to Routine Clinical Practice
The progressive nature of AD means that the moderate-to-severe stages are characterized by progressively greater impairments in cognition and in the ability to engage in normal ADL and an increasing probability of behavioral disturbances [24,25]. Improvement or even preservation of any aspect of cognition and ADL may be clinically meaningful to the patient and the caregivers and may postpone institutionalization. The APEX believes that this is extremely relevant in the Asian scenario, where the availability of professional nursing care varies between countries and most patients with dementia are cared for within the family and community [4,26,27].
Treatment Response in Severe Cognitive Impairment
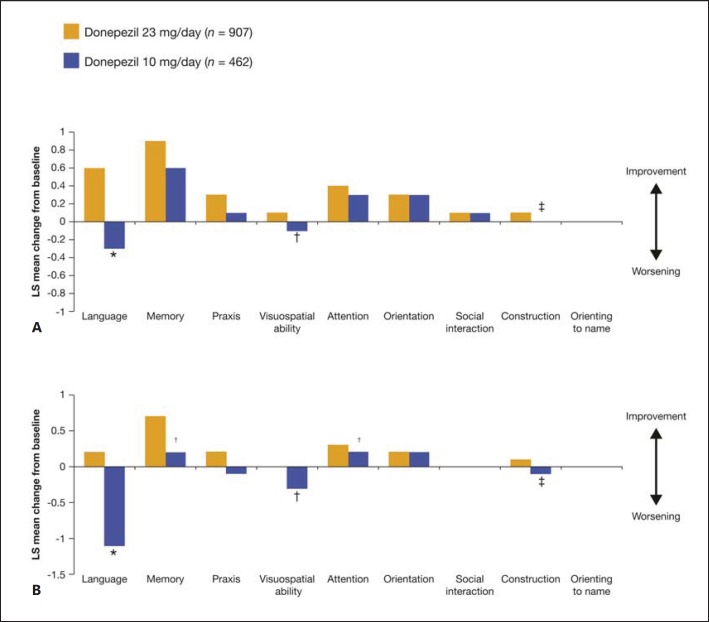
Several post hoc analyses of study 326 have been performed to further characterize the efficacy of donepezil 23 mg. A subgroup analysis assessing the impact of baseline severity on the effect of donepezil 23 mg/day on SIB domains in patients with moderate-to-severe AD reported significant treatment differences in favor of donepezil 23 mg/day over donepezil 10 mg/day across a range of domains, with the magnitude of the benefit and the domains impacted varying with the stage of AD (fig. 2) [28]. In the analysis, most incremental benefits of treatment with donepezil 23 mg/day over donepezil 10 mg/day were observed in patients with more advanced disease at baseline (MMSE 0-16; fig. 2B).
Fig. 2.
Change from baseline in SIB subdomain scores at week 24. A Overall population (MMSE 0-20). B MMSE 0-16. * p < 0.001; † p < 0.05; ‡ p < 0.01. LS = Least square; MMSE = Mini Mental State Examination; SIB = Severe Impairment Battery. A, B Reprinted from Ferris et al. [28] (Copyright© Ferris et al.; licensee BioMed Central Ltd., 2013).
Another post hoc analysis investigated the relationship between baseline demographics and cognitive response in patients treated with either donepezil 23 or 10 mg/day [29]. The cognitive benefits of donepezil 23 mg/day over donepezil 10 mg/day were observed irrespective of the patients’ age, gender, weight, duration of prior donepezil 10 mg/day, and functional severity. However, baseline cognitive severity influenced treatment response depending on the level of impairment - cognitive benefits of donepezil 23 mg/day over donepezil 10 mg/day were most apparent in patients with more severe impairment.
Impact on Language Function
With progressing disease severity, patients with AD often lose the ability to communicate, to express their needs for medical attention, and respond to caregivers, and this impacts their emotional and physical well-being; the loss of the ability to communicate is one of the most distressing factors in AD contributing to the declining quality of life of the patients [30,31]. Preservation or improvement of language abilities is an important focus of treatment in AD.
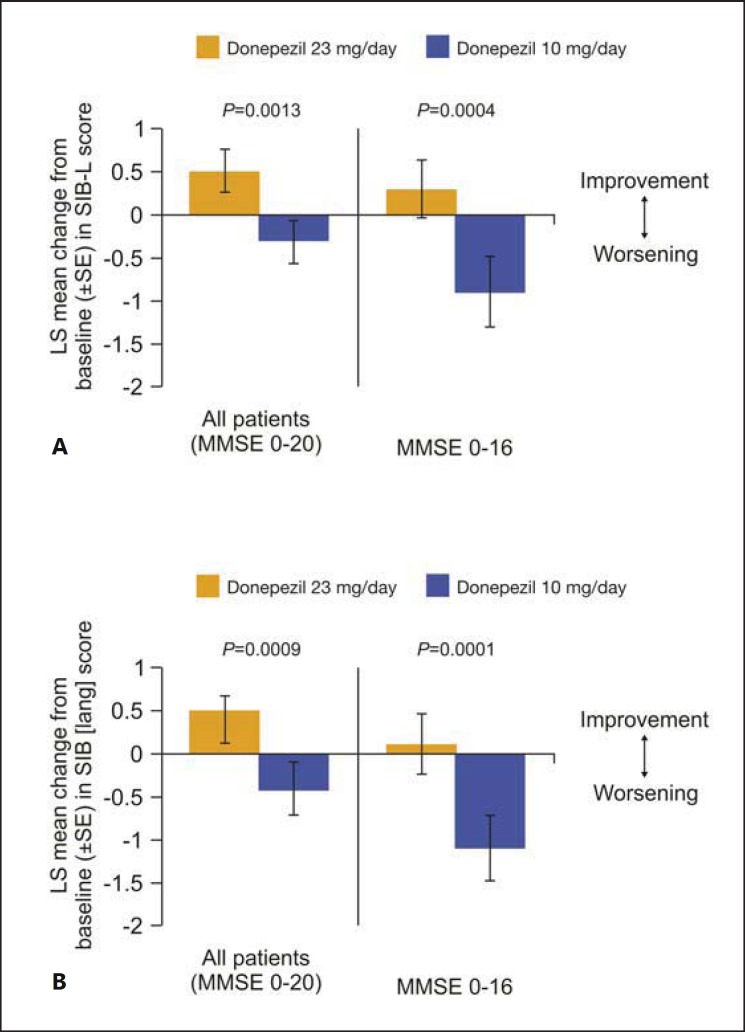
In a post hoc subgroup analysis of study 326, SIB-language scale (SIB-L) and a new SIB-derived language scale (SIB [lang]) were used to explore differences in the language function between the groups receiving donepezil 23 mg/day and those receiving 10 mg/day [32]. Patients with moderate-to-severe AD on donepezil 23 mg/day showed greater language benefits than those receiving donepezil 10 mg/day as measured by language assessment scores (fig. 3). This improvement in the language domain may be clinically meaningful to patients with AD, particularly those in the advanced stages of the disease, and their caregivers in terms of the improvement in or preservation of the communication potentially translating to an improvement in the quality of life of patients and easing the caregiver burden [30]. The reason behind the greater impact of donepezil 23 mg/day on the language function compared with the lower doses was not explored in the post hoc analysis. However, several direct and indirect reasons can be hypothesized, including the higher dose of donepezil leading to a greater enhancement of cholinergic function in regions of the brain specifically controlling speech and language or in regions controlling other cognitive processes, such as attention and memory (which could potentially impact a patient's language ability) [32].
Fig. 3.
Mean change from baseline to week 24 in SIB-L scores (A) and SIB [lang] scores (B). LS = Least square; SE = standard error; SIB-L = Severe Impairment Battery-Language scale. A, B Reprinted from Ferris et al. [32] (Copyright© Ferris et al.; licensee BioMed Central Ltd., 2011).
Assessment of Benefits in Clinical Practice
The APEX noted that from their experience in routine clinical practice, appreciable cognitive benefits were observed with donepezil 23 mg, particularly in patients with more advanced AD. However, the assessment of benefits in clinical practice can be challenging. The MMSE and Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) are frequently used for measuring cognition in clinical settings. The MMSE and ADAS-cog are known to have ‘floor’ effects in patients with advanced AD [33]. Also, factors such as education and cultural background may impact the sensitivity and validity of the assessment of treatment effects with the MMSE [33]. The cognitive benefit of pharmacologic treatment may not be clearly seen if these tests are used in the evaluation of patients in the clinics. The SIB, commonly used to evaluate the therapeutic efficacy in cognition in clinical studies, was used to assess cognition in study 326 [10]. The SIB was created to allow assessment of cognition in later AD stages; it relies on measuring the preserved abilities in various cognitive domains, such as social interactions, memory, orientation, and language, and may be a useful instrument for use in routine clinical practice [34]. The SIB-L, a version of the SIB based on the language domain, can help clinicians in analyzing language and communication skills in patients with moderate-to-severe dementia [35]. The SIB, however, is not routinely used in clinical practice. Additionally, the importance of caregivers' reports on perceived or actual benefits should not be underestimated; a detailed caregiver interview can also assist in assessing cognition in patients with moderate-to-severe dementia.
Donepezil 23 mg in Clinical Practice
Patient Selection
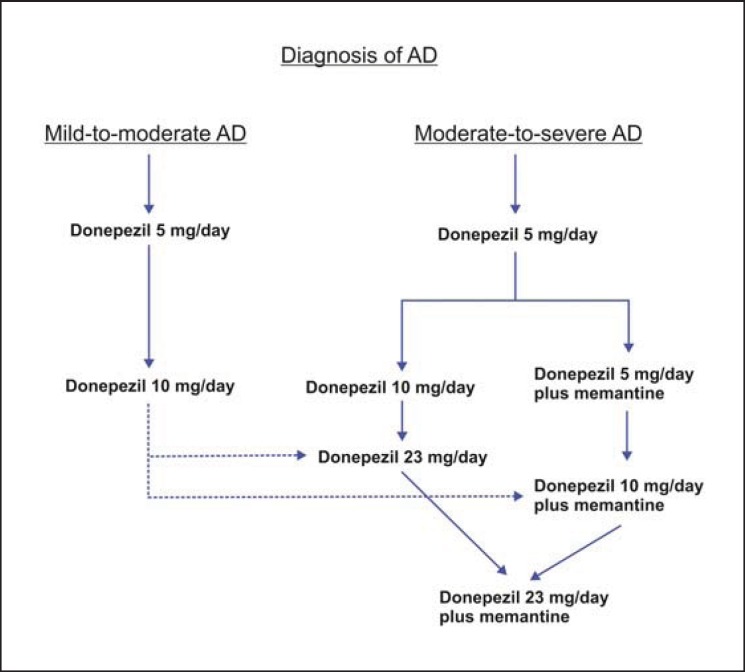
In Asia, donepezil 23 mg/day is licensed in Hong Kong, South Korea, the Philippines, India, Singapore, and Thailand for use in patients with a diagnosis of moderate-to-severe AD who have been on a stable dose of donepezil 10 mg/day for at least 3 months. Given the evidence and the clinical experience of the panel members, patients with more severe cognitive impairment are expected to experience the most clinical benefit with the higher dose of donepezil. It can be administered concomitantly with memantine; in patients taking memantine, donepezil can be added and then up-titrated to 23 mg, or memantine can be added to donepezil 10 mg before up-titrating the dose to 23 mg (fig. 4). Caution is advocated when using donepezil 23 mg in patients with a low body weight (<55 kg), a history of GI bleeding, and atrioventricular block. In patients with AD with a pacemaker and in patients on beta blockers, management in consultation with a cardiologist may be advisable. Clinicians should be aware that patients who have experienced side effects while making the transition from donepezil 5 to 10 mg/day may be expected to experience similar difficulty when up-titrating to 23 mg/day.
Fig. 4.
Donepezil for the treatment of AD.
Mitigating Side Effects
Patients enrolled in study 326 had been on lower donepezil doses for an average time of 2 years, i.e. they were not de novo patients. A common observation in several Asian countries is that many patients first present to the clinicians when dementia is already in the moderate-to-severe stage; in Korea, a study reported that the mean duration from the onset of first symptoms to the time of diagnosis was 2.8 years [36]. Donepezil dose escalation in these de novo patients could be challenging given the increased incidence of GI side effects observed when increasing the daily dose of donepezil from 10 to 23 mg/day in study 326 [10]. However, in the experience of the members of the APEX and as seen in study 326, these side effects seldom persist beyond a 1-month period. Using stepwise titration strategies for escalating donepezil doses may address the GI side effects during the transition, thereby driving adherence to treatment. These titration strategies could potentially involve increasing the dose of donepezil from 10 to 23 mg over a 1- to 2-month period by taking one 10-mg tablet plus one 5-mg tablet once daily for 1 month followed by 23 mg once daily or a 10-mg tablet and a 23-mg tablet on alternating days [37]. A study in South Korea has been designed to determine the optimal dose escalation strategy for successfully up-titrating to 23 mg donepezil (ODESA; ClinicalTrials.gov: NCT02550665) [38]; results from this study will provide further insights into up-titrating with minimal side effects.
Managing Caregiver Expectations
In patients with moderate-to-severe AD, the quality of life is paramount to the family and the caregivers. Improvement in the language domain of cognition, as demonstrated by study 326 [10], can translate into an everyday benefit for the patient and the caregiver by easing caregiver burden and improving the patient's quality of life. Open and consistent communication with patients and caregivers, focusing on the expected side effects upon dose escalation, the transient nature of these side effects and ways to counter them, and the benefits of persisting with therapy, will help in managing patient and caregiver expectations. Caregivers are central to managing compliance with therapy; clinicians must help the patients and caregivers understand the importance of persisting with the strategy adopted for stepwise dose escalation and reaching an optimal therapeutic dose of donepezil [39]. Apart from pharmacological therapy, diet and exercise, cognitive stimulation and training, management of behavioral disturbances, and monitoring the health of caregivers are also important for the successful management of patients.
In Asia, managing caregiver expectations is particularly important, given the central role they play in the management of patients with AD, even those with severe impairments. In the experience of the expert panel, although AD is a progressive disease, the importance of preserving cognitive abilities for as long as possible cannot be underestimated. Family and caregiver expectations should be managed by properly communicating attainable outcomes that are easily understood. Additionally, outcome parameters conveyed to caregivers should be real-life benefits that they can experience, such as the patient's ability to do basic household chores, participate in family activities, and communicate with family members. Such observable outcomes will be more meaningful to the caregivers.
Patients with Severe AD - Stop Treatment or Continue?
There is a lack of clear guidelines for discontinuation of medication in patients with advanced AD. In these patients, the cognitive benefits of therapy may be perceived as slight, influencing the decision to discontinue treatment. However, even slight benefits in patients with moderate-to-severe AD could potentially translate into a positive impact on the lives of the patients and caregivers. There is evidence to suggest a long-term benefit from patients with AD staying on medication, as indicated by a delay in nursing home admission [40,41,42]. DOMINO-AD, a randomized, double-blind study in patients with moderate-to-severe AD, demonstrated that withdrawal of donepezil increased the risk of nursing home placement in 12 months [40]. Institutionalization is often considered a negative determinant of quality of life; the decrease in the quality of life after nursing home placement, in conjunction with the cost associated with institutionalization, should be an incentive to continue treatment in patients with moderate-to-severe AD, even when the perceived benefits are small.
In the experience of the expert panel, it is important to make individualized decisions about treatment discontinuation, taking into account patients', caregivers', and societal perspectives. The expert panel concluded that in the context of the benefits seen with donepezil 23 mg in patients with moderate-to-severe AD, it may be worthwhile to continue medication in patients with severe AD to keep them within the community.
Conclusion
In most countries in the Asia-Pacific region, patients with AD are typically cared for within the family. The cultural practice of the family being the primary caregiving resource combined with the disparity in the availability of professional nursing care/institutions places a significant burden on caregivers [4,26,28]. In this context, maintenance or preservation of cognitive functions and ADL in patients with AD for as long as possible is essential.
Donepezil 23 mg/day has demonstrated significantly greater cognitive benefits than donepezil 10 mg/day in a global clinical study, with typically GI AEs which occurred most frequently in the first month after starting therapy. Similar efficacy and safety results have been reported in a subanalysis of this global study conducted in Asian patients with moderate-to-severe AD.
Table 4 summarizes the recommendations of the APEX for the use of donepezil 23 mg in patients with moderate-to-severe AD. Overall, donepezil 23 mg is a valuable treatment option for patients with AD, particularly those with advanced disease who are expected to experience the maximum benefit.
Table 4.
APEX recommendations for the use of donepezil 23 mg/day in the treatment of moderate-to-severe AD
| Ensure an accurate diagnosis of moderate-to-severe AD – evaluate cognition, function, and behavior. |
| Assess for contraindications and cautions to donepezil – low body weight ( 55 kg), history of GI bleeding, and atrioventricular block. |
| Review medical history – manage comorbid conditions, provide education, support, and referrals to support groups or social workers for patient and caregivers. |
| Nonpharmacological therapies – offer diet and exercise counseling, cognitive stimulation, and training exercises. |
| History of prior donepezil use – ensure that the patient has been on donepezil 10 mg/day for at least 3 months. Assess history of side effects with donepezil – nausea, vomiting, weight loss, and anorexia. |
| Consider stepwise escalation of donepezil to 23 mg/day. Monitor side effects. |
| At all stages, maintain open communication channels with the patient and caregivers. Set realistic expectations from treatment, in a language that is meaningful, emphasizing real-life benefits and observable outcomes in the everyday life of the patient, the family, and the caregiver. |
Disclosure Statement
Marwan Sabbagh has received grants/clinical trial support from Biogen, Lilly, vTv therapeutics, Merck, Roche, Avid, Axovant, and Lundbeck; has served as a consultant or in an advisory capacity for vTv therapeutics, Forum, Lilly, Biogen, FujiRebio, and Axovant; has received royalties from TenSpeed and Wiley; has stock ownership interests in Versanum, Muses Labs, and Brain Health Inc., and is a non-CME speaker for Eisai.
SangYun Kim has received clinical trial support from PeopleBio, Merck, Lundbeck, AstraZeneca, Daewoong Pharmaceuticals, Ildong Pharmaceuticals, Eisai, Roche, and Novartis.
Nagaendran Kandiah has received honoraria and CME sponsorship from Eisai, Lundbeck, Schwabbe, and Novartis and has received research funding from the Biomedical Research Council of Singapore, Media Development Authority of Singapore, National Medical Research Council of Singapore, and the Singhealth Foundation.
Kammant Phanthumchinda has been a speaker for Eisai in scientific meetings.
Vorapun Senanarong has received honoraria as a speaker from Eisai, Novartis, Lundbeck, and Otsuka (Thailand).
Ming-Chyi Pai has received grants/clinical trial support from AB Science, Lundbeck, Biogen, BAC, Lilly, and Axovant and has served as a consultant and/or has received lecture fees from Janssen, Lilly, Novartis, Lotus, and Eisai.
Diatri Narilastri and Ajit M. Sowani have served as advisors to Eisai Co. Ltd.
Encarnita Ampil is on the Advisory Board of Lundbeck Philippines and, previously, Novartis Healthcare Philippines, Inc., and Hi Eisai Pharmaceutical, Inc. She has been speaker for and has received honoraria from Lundbeck Philippines, Hi Eisai Pharmaceutical, Inc., Menarini, Novartis Healthcare Philippines, Inc., Janssen Pharmaceuticals, Otsuka Philippines, Astra Zeneca, Pharmalink, Inc., and Torrent Pharma Philippines. She is currently involved in clinical trials with E Chimes and Amylex Pharmaceuticals, Inc.
Amitabh Dash is an employee of Eisai Pharmaceuticals India Pvt. Ltd., Mumbai, India.
SeolHeui Han, Hae-Ri Na, Jae-Hong Lee, and Chuthamanee Suthisisang declare no conflicts of interest.
Acknowledgements
The APEX meeting was sponsored by Eisai Co. Ltd. All authors participated in the discussions at the meeting and are responsible for the content of this article, which reflects their views and opinions. Dr. Shilpa Mudgal from In Vivo Communications (Asia) Pte. Ltd. provided medical writing assistance in the development of the manuscript.
References
- 1.Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015 - The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer's Disease International; 2015. [Google Scholar]
- 2.Catindig JA, Venketasubramanian N, Ikram MK, Chen C. Epidemiology of dementia in Asia: insights on prevalence, trends and novel risk factors. J Neurol Sci. 2012;321:11–16. doi: 10.1016/j.jns.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Dauphinot V, Delphin-Combe F, Mouchoux C, Dorey A, Bathsavanis A, Makaroff Z, Rouch I, Krolak-Salmon P. Risk factors of caregiver burden among patients with Alzheimer's disease or related disorders: a cross-sectional Study. J Alzheimers Dis. 2015;44:907–916. doi: 10.3233/JAD-142337. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer's Disease International and Alzheimer's Australia . Dementia in the Asia Pacific Region. London: Alzheimer's Disease International; 2014. [Google Scholar]
- 5.Wimo A, Winblad B, Stöffler A, Wirth Y, Möbius HJ. Resource utilization and cost analysis of memantine in patients with moderate to severe Alzheimer's disease. Pharmacoeconomics. 2003;21:327–340. doi: 10.2165/00019053-200321050-00004. [DOI] [PubMed] [Google Scholar]
- 6.Kandiah N, Wang V, Lin X, Nyu MM, Lim L, Ng A, Hameed S, Wee HL. Cost related to dementia in the young and the impact of etiological subtype on cost. J Alzheimers Dis. 2015;49:277–285. doi: 10.3233/JAD-150471. [DOI] [PubMed] [Google Scholar]
- 7.Deardorff WJ, Feen E, Grossberg GT. The use of cholinesterase inhibitors across all stages of Alzheimer's disease. Drugs Aging. 2015;32:537–547. doi: 10.1007/s40266-015-0273-x. [DOI] [PubMed] [Google Scholar]
- 8.Eisai Co. Ltd. Aricept (donepezil hydrochloride): highlights of prescribing information. 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020690s035,021720s008,022568s005lbl.pdf
- 9.Novartis Pharmaceutical Corporation Exelon (rivastigmine): highlights of prescribing information. 2015. https://www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf
- 10.Farlow MR, Salloway S, Tariot PN, Yardley J, Moline ML, Wang Q, Brand-Schieber E, Zou H, Hsu T, Satlin A. Effectiveness and tolerability of high-dose (23 mg/d) versus standard dose (10 mg/d) donepezil in moderate to severe Alzheimer's disease: a 24-week, randomized, double-blind study. Clin Ther. 2010;32:1234–1251. doi: 10.1016/j.clinthera.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabbagh M, Cummings J. Progressive cholinergic decline in Alzheimer's disease: consideration for treatment with donepezil 23 mg in patients with moderate to severe symptomatology. BMC Neurol. 2011;11:21. doi: 10.1186/1471-2377-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boehnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Koeppe RA, Meltzer CC, Constantine G, Davis JG, Mathis CA, Dekosky ST, Moore RY. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:315–319. doi: 10.1136/jnnp.2004.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis KL, Yesavage JA. Brain acetylcholine and disorders of memory. In: Davis KL, Berger PA, editors. Brain Acetylcholine and Neuropsychiatric Disease. New York: Springer US; 1979. pp. 205–213. [Google Scholar]
- 14.Homma A, Imai Y, Tago H, Asada T, Shigeta M, Iwamoto T, Takita M, Arimoto I, Koma H, Ohbayashi T. Donepezil treatment of patients with severe Alzheimer's disease in a Japanese population: results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord. 2008;25:399–407. doi: 10.1159/000122961. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead A, Perdomo C, Pratt RD, Birks J, Wilcock GK, Evans JG. Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer's disease: a meta-analysis of individual patient data from randomised controlled trials. Int J Geriatr Psychiatry. 2004;19:624–633. doi: 10.1002/gps.1133. [DOI] [PubMed] [Google Scholar]
- 16.Nozawa M, Ichmiya Y, Nozawa E, Utumi Y, Sugiyama H, Murayama N, Iseki E, Arai H. Clinical effects of high oral dose of donepezil for patients with Alzheimer's disease in Japan. Psychogeriatrics. 2009;9:50–55. doi: 10.1111/j.1479-8301.2009.00291.x. [DOI] [PubMed] [Google Scholar]
- 17.Doody RS, Corey-Bloom J, Zhang R, Li H, Leni J, Schindler R. Safety and tolerability of donepezil 20 mg/day: results from a pilot study in patients with Alzheimer's disease. Drugs Aging. 2008;25:163–174. doi: 10.2165/00002512-200825020-00008. [DOI] [PubMed] [Google Scholar]
- 18.Doody RS, Geldmacher DS, Farlow MR, Sun Y, Moline M, Mackell J. Efficacy and safety of donepezil 23 mg versus donepezil 10 mg for moderate-to-severe Alzheimer's disease: a subgroup analysis in patients already taking or not taking concomitant memantine. Dement Geriatr Cogn Disord. 2012;33:164–173. doi: 10.1159/000338236. [DOI] [PubMed] [Google Scholar]
- 19.Farlow MR, Veloso F, Moline M, Yardley J, Brand-Schieber E, Bibbiani F, Zou H, Hsu T, Satlin A. Safety and tolerability of donepezil 23 mg in moderate to severe Alzheimer's disease. BMC Neurol. 2011;11:57. doi: 10.1186/1471-2377-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tariot P, Salloway S, Yardley J, Mackell J, Moline M. Long-term safety and tolerability of donepezil 23 mg in patients with moderate to severe Alzheimer's disease. BMC Res Notes. 2012;5:283. doi: 10.1186/1756-0500-5-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417–423. doi: 10.1038/clpt.2008.141. [DOI] [PubMed] [Google Scholar]
- 22.Venketasubramanian N, Sahadevan S, Kua EH, Chen CPL, Ng TP. Interethnic differences in dementia epidemiology: global and Asia-Pacific perspectives. Dement Geriatr Cogn Disord. 2010;30:492–498. doi: 10.1159/000321675. [DOI] [PubMed] [Google Scholar]
- 23.Han SH, Lee JH, Kim SY, Park KW, Chen C, Tripathi M, Dash A, Kubota N. Donepezil 23 mg in Asian patients with moderate-to-severe Alzheimer's disease. Acta Neurol Scand. 2016 doi: 10.1111/ane.12571. DOI: 10.1111/ane.12571. [DOI] [PubMed] [Google Scholar]
- 24.Cummings JL, Cole G. Alzheimer's disease. JAMA. 2002;287:2335–2338. doi: 10.1001/jama.287.18.2335. [DOI] [PubMed] [Google Scholar]
- 25.Gillette-Guyonnet S, Andrieu S, Nourhashemi F, Gardette V, Coley N, Cantet C, Gauthier S, Ousset PJ, Vellas B, REAL.FR study group Long-term progression of Alzheimer's disease in patients under antidementia drugs. Alzheimers Dement. 2011;7:579–592. doi: 10.1016/j.jalz.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Chan SW. Family caregiving in dementia: the Asian perspective of a global problem. Dement Geriatr Cogn Disord. 2010;30:469–478. doi: 10.1159/000322086. [DOI] [PubMed] [Google Scholar]
- 27.Tew CW, Tan LF, Luo N, Ng WY, Yap P. Why family caregivers choose to institutionalize a loved one with dementia: a Singapore perspective. Dement Geriatr Cogn Disord. 2010;30:509–516. doi: 10.1159/000320260. [DOI] [PubMed] [Google Scholar]
- 28.Ferris S, Cummings J, Christensen D, Doody R, Farlow M, Sabbagh M, Liu L, Mackell J, Fain R. Effects of donepezil 23 mg on severe impairment battery domains in patients with moderate to severe Alzheimer's disease: evaluating the impact of baseline severity. Alzheimers Res Ther. 2013;5:12. doi: 10.1186/alzrt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabbagh M, Cummings J, Christensen D, Doody R, Farlow M, Liu L, Mackell J, Fair R. Evaluating the cognitive effects of donepezil 23 mg/d in moderate and severe Alzheimer's disease: analysis of effects of baseline features on treatment response. BMC Geriatr. 2013;13:56. doi: 10.1186/1471-2318-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferris S, Ihl R, Robert P, Winblad B, Gatz G, Tennigkeit F, Gauthier S. Severe Impairment Battery Language scale: a language-assessment tool for Alzheimer's disease patients. Alzheimers Dement. 2009;5:375–379. doi: 10.1016/j.jalz.2009.04.1236. [DOI] [PubMed] [Google Scholar]
- 31.Blair M, Marczinski CA, Davis-Faroque N, Kertesz A. A longitudinal study of language decline in Alzheimer's disease and frontotemporal dementia. J Int Neuropsychol Soc. 2007;13:237–245. doi: 10.1017/S1355617707070269. [DOI] [PubMed] [Google Scholar]
- 32.Ferris SH, Schmitt FA, Saxton J, Richardson S, Mackell J, Sun Y, Xu Y. Analyzing the impact of 23 mg/day donepezil on language dysfunction in moderate to severe Alzheimer's disease. Alzheimers Res Ther. 2011;3:22. doi: 10.1186/alzrt84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert P, Ferris S, Gauthier S, Ihl R, Winblad B, Tenningkeit F. Review of Alzheimer's disease scales: is there a need for a new multi-domain scale for therapy evaluation in medical practice? Alzheimer Res Ther. 2010;2:24. doi: 10.1186/alzrt48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saxton J, McGonigle-Gibson KL, Swihart AA, Miller VJ, Boller F. Assessment of the severely impaired patient: description and validation of a new neuropsychological test battery. Psychol Assess. 1990;2:298–303. [Google Scholar]
- 35.Saxton J, Kastango KB, Hugonot-Diener L, Boller F, Verny M, Sarles CE, Girgis RR, Devouche E, Mecocci P, Pollock BG, DeKosky ST. Development of a short form of the Severe Impairment Battery. Am J Geriatr Psychiatry. 2005;13:999–1005. doi: 10.1176/appi.ajgp.13.11.999. [DOI] [PubMed] [Google Scholar]
- 36.Go SM, Lee KS, Seo SW, Chin J, Kang SJ, Moon SY, Na DL, Cheong HK. Survival of Alzheimer's disease patients in Korea. Dement Geriatr Cogn Disord. 2013;35:219–228. doi: 10.1159/000347133. [DOI] [PubMed] [Google Scholar]
- 37.Sabbagh M, Jacobson S, Christine B, Obradov Z, Malek-Ahmadi M, Powell J, Syal A, Bhalla N, Kahlon M, Shah J, Liebsack C, Drumm-Gurnee D. A pilot phase IV open-label study investigating the safety and tolerability of donepezil 23 mg once daily in patients with moderate to severe AD switched from Exelon patch 9.5 mg daily. Ann Psychiatry Ment Health. 2015;3:1041. [Google Scholar]
- 38.Asan Medical Center optimal dose escalation strategy to successful achievement of high dose donepezil 23 mg (ODESA) ClinicalTrials.gov. 2000. https://clinicaltrials.gov/ct2/show/NCT02550665 (accessed January 20, 2016).
- 39.Small G, Bullock R. Defining optimal treatment with cholinesterase inhibitors in Alzheimer's disease. Alzheimers Dement. 2011;7:177–184. doi: 10.1016/j.jalz.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, Burns A, Dening T, Findlay D, Holmes C, Jones R, Jones R, McKeith I, Macharouthu A, O'Brien J, Sheehan B, Juszczak E, Katona C, Hills R, Knapp M, Ballard C, Brown RG, Banerjee S, Adams J, Johnson T, Bentham P, Phillips PP. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer's disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171–1181. doi: 10.1016/S1474-4422(15)00258-6. [DOI] [PubMed] [Google Scholar]
- 41.Lopez OL, Becker JT, Wahed AS, Saxton J, Sweet RA, Wolk DA, Klunk W, Dekosky ST. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80:600–607. doi: 10.1136/jnnp.2008.158964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wattmo C, Wallin AK, Londos E, Minthon L. Risk factors for nursing home placement in Alzheimer's disease: a longitudinal study of cognition, ADL, service utilization, and cholinesterase inhibitor treatment. Gerontologist. 2011;51:17–27. doi: 10.1093/geront/gnq050. [DOI] [PubMed] [Google Scholar]