Abstract
Background
The aim of this study was to explore temporal trends in incidence and case fatality rates of intracerebral hemorrhage (ICH) over the last two decades in a Norwegian municipality.
Methods
Incident cases of primary ICH were registered in the period from 1995 through 2012 in 32,530 participants of the longitudinal population-based Tromsø Study. Poisson regression models were used to obtain incidence rates over time in age- and sex-adjusted and age- and sex-specific models. Case fatality rates were calculated and age- and sex-adjusted trends over time were estimated using logistic regression.
Results
A total of 226 ICHs were registered. The age- and sex-adjusted incidence rate [95% confidence interval (CI)] in the overall population was 0.42 (0.37-0.48) per 1,000 person-years. Age-adjusted incidence rates were 0.53 (0.43-0.62) in men and 0.33 (0.26-0.39) in women. In individuals aged <75 years, the age- and sex-adjusted incidence rate was 0.27 (0.22-0.32) and in individuals aged ≥75 years, it was 2.42 (1.95-2.89) per 1,000 person-years. There was no significant change in incidence rates over time. The incidence rate ratio (95% CI) in the overall population was 0.73 (0.47-1.12) in 2012 compared with 1995. The overall 30-day case fatality (95% CI) was 23.9% (18.3-29.5) and did not change substantially over time [odds ratio in 2012 vs. 1995 = 0.83 (95% CI 0.27-2.52)].
Conclusion
No significant changes in incidence and case fatality rates of ICH were observed during the last two decades.
Key Words: Intracerebral hemorrhage, Stroke incidence, Cohort study, Epidemiology
Introduction
Stroke is the second leading cause of death worldwide and the third leading cause of death in Norway [1,2]. Intracerebral hemorrhage (ICH) accounts for 10-15% of all strokes in Western countries, with an incidence rate of 0.1-0.3/1,000/year [3]. Morbidity and case fatality are high: only 12-39% of patients live independently after an ICH and case fatality rates at 1 month range between 13 and 61% (median 40%) [4]. Treatment possibilities for ICH are limited [5]. However, recent studies show that early, intensive lowering of blood pressure may improve outcome [6].
Studies of trends in incidence and 1-month case fatality rates of ICH over the last three decades have shown divergent results. While some studies have reported stable incidence rates, others have found decreasing or increasing rates [7,8,9,10,11,12,13,14,15,16]. Studies of trends in case fatality rates have reported stable as well as decreasing rates [7,9,11,12,13,14,16,17,18]. Reviews based on studies published between 1970 and 2008 showed no significant change in incidence and case fatality rates [4,19], while a recent review reported a decrease in incidence of intracerebral and subarachnoidal hemorrhage in high-income countries and a significant increase in low- to middle-income countries between 1990 and 2010 [20]. The aim of our study was to explore temporal trends in incidence and case fatality rates of ICH over the last two decades in a Norwegian municipality.
Methods
Study Population
The Tromsø Study is an ongoing, longitudinal population-based study started in 1974. The municipality of Tromsø is located in the northern part of Norway. The population has increased; from approximately 42,200 in 1974 to the current population of approximately 73,000 inhabitants [21,22]. The vast majority of the population is of Caucasian origin.
Details of the study have been described earlier [23,24]. Based on the official population registry, full birth cohorts and random samples of residents in the municipality of Tromsø have been invited to attend the surveys. To the first survey (Tromsø 1), only men were invited. Of the 53,731 individuals who were invited, 40,051 attended at least 1 of the 6 surveys (table 1) [24]. Participants are being followed up with regard to incident stroke and cardiovascular events. The Tromsø Study has been approved by the Regional Committee for Medical and Health Research Ethics and the Data Inspectorate of Norway.
Table 1.
Year of survey, age, number and attendance rate of eligible participants (the Tromsø Study)
| Survey year | Men |
Women |
||||
|---|---|---|---|---|---|---|
| age group, years | participants, n | Attendance rate, % | age group, years | participants, n | Attendance rate, % | |
| 1974 | 20–49 | 6,595 | 74.4 | – | – | – |
| 1979–80 | 20–54 | 8,477 | 73.8 | 20–49 | 8,143 | 81.8 |
| 1986–87 | 12–64 | 10,963 | 71.8 | 12–67 | 10,863 | 79.0 |
| 1994–95 | 25–97 | 12,865 | 69.6 | 25–97 | 14,293 | 74.9 |
| 2001–02 | 30–89 | 3,511 | 75.7 | 30–89 | 4,619 | 80.8 |
| 2007–08 | 30–87 | 6,054 | 62.9 | 30–87 | 6,930 | 68.4 |
Individuals who were not officially registered as inhabitants of the Tromsø municipality at the date of enrolment (n = 162), individuals who were younger than 20 years at enrolment and did not attend later studies (n = 785), those who did not have valid written consent to medical research (n = 225), and individuals who had prevalent ICH (n = 18) or unspecified stroke (n = 45) were excluded. Because older birth cohorts were not enrolled in the earliest surveys, and individuals <30 years were not enrolled in the two latest surveys (table 1), analyses were limited to individuals aged ≥30 years in the period January 1, 1995 to December 31, 2012. Individuals who emigrated out of the municipality (n = 5,145), died (n = 788) or suffered an ICH (n = 24) before 1995 or did not reach 30 years of age during follow-up (n = 329) were censored, leaving 32,530 individuals (16,771 women and 15,759 men) to be included. Individuals were followed up with registration of incident stroke from the date of first attendance. For individuals who were younger than 30 years when first attending a survey, the start of follow-up was assigned from the date they turned 30 years. Participants were followed up until the first-ever ICH event, emigration out of the municipality, death or end of study (December 31, 2012).
Case Ascertainment
Cases were retrieved by linking the participation list to the discharge and outpatient diagnosis registers at the University Hospital of North Norway, and to the National Causes of Death Registry. The University Hospital is the only hospital serving the Tromsø region (the nearest hospital in the county being located 300 km away by road, 134 km by air). Cases of stroke were retrieved by searching for International Classification of Disease (ICD) versions 8 and 9 diagnosis codes 430-438, and ICD-10 diagnosis codes I60-I69 (cerebrovascular disease). In 2006 through 2007, ICD-10 codes G45 (transitory ischemic attack), G46 (vascular syndromes of brain in cerebrovascular diseases) and G81 (hemiplegia) were added to the search. In addition, systematic text searches were made for the words ‘stroke’, ‘ischemic stroke’ and ‘intracerebral hemorrhage’ in the medical records of all participants with ICD-8 to ICD-10 diagnosis codes 410-414 and I20-I25 (ischemic heart disease), 798/R96 (sudden death, cause unknown), R98 (unattended death) and 799/R99 (other ill-defined and unknown causes of morbidity and mortality).
Each case was reviewed separately by an independent endpoint committee by use of medical records from the hospital (including autopsy reports). Cases retrieved from the National Causes of Death registry were additionally validated by medical records from nursing homes, general practitioners, emergency services and/or death certificates. Stroke was defined according to the WHO criteria: ‘rapidly developed clinical signs of focal (or global) disturbance of cerebral function, lasting more than 24 h or leading to death, with no apparent cause other than of vascular origin’ [25]. Strokes were defined as an ICH where a parenchymal hemorrhage was identified on computed tomography (CT) and/or magnetic resonance imaging (MRI) and/or autopsy. ICHs caused by hemorrhagic transformation of ischemic stroke, trauma, brain surgery, hematologic disease or brain tumor were excluded. Cases where neither imaging nor autopsy was performed in the acute phase were categorized as unspecified stroke.
Dates for death and emigration out of the municipality were obtained from the Population Registry of Norway. Linkage to registers was performed using the Norwegian, unique 11-digit personal identification numbers.
Statistical Analyses
Statistical analyses were conducted using STATA version 13.0 (StataCorp LP, College Station, Tex., USA). Analyses of the overall study population, stratified by age (predefined age groups: <75 and ≥75 years) and sex were conducted. The stsplit function in STATA was used to produce a new record in the data file for each year a participant was under follow-up, with updated calendar time and attained age variables. Crude incidence rates for incident primary ICH per 1,000 person-years from January 1, 1995 through December 31, 2012 were calculated with the number of events registered during the study period as numerator and person-years at risk as denominator (table 2). Calendar year-specific incidence rates were estimated. In addition, crude incidence rates in 10-year age bands were calculated.
Table 2.
Incidence rates of primary ICH per 1,000 person-years (the Tromsø Study 1995–2012)
| ICH, n | Person-years at risk, n | Crude incidence rate (95% CI) | Adjusted incidence ratea (95% CI) | |
|---|---|---|---|---|
| Men | 122 | 216,279 | 0.56 (0.47–0.67) | 0.53 (0.43–0.62) |
| Women | 104 | 236,873 | 0.44 (0.36–0.53) | 0.33 (0.26–0.39) |
| Age <75 | 121 | 410,607 | 0.29 (0.25–0.35) | 0.27 (0.22–0.32) |
| Age ≥75 | 105 | 42,545 | 2.47 (2.04–2.99) | 2.42 (1.95–2.89) |
| Overall | 226 | 453,152 | 0.50 (0.44–0.57) | 0.42 (0.37–0.48) |
Incidence rates adjusted to age/age and sex by the direct method using the European standard population of 1976 as reference.
Incidence rates adjusted for age and sex were calculated by the direct method using the European standard population of 1976 as reference. Incidence rate ratios (IRRs) between men and women, with women as reference, adjusted for age, were estimated using Poisson regression.
Trends in incidence rates over time, adjusted for age or age and sex (fig. 1, 2), were obtained from a Poisson regression model. In the overall population, trend was estimated with age set at 64 years, while trends were estimated at 62 years in men, 65 in women, 58 in individuals <75 years of age and 82 in individuals aged ≥75 years, respectively. In sex-adjusted models, the mean value of sex was used. To assess a possible nonlinear trend over time, the models were fitted with fractional polynomials, with time as covariate [26]. Powers were chosen from the set: φ = (−2, −1, −0.5, 0, 0.5, 1, 2, 3). Model selection was performed by comparing a Poisson regression model with a linear time variable with the best fitting first- and second-degree models using the Akaike Information Criteria (AIC). In the overall population and in all subgroups, the best AIC was observed in the models with a linear time term. Tests of interaction between age and time and sex and time were performed by including two-way interaction terms (age × time and sex × time) in the regression models. IRRs between 2012 and 1995 were estimated from each regression model.
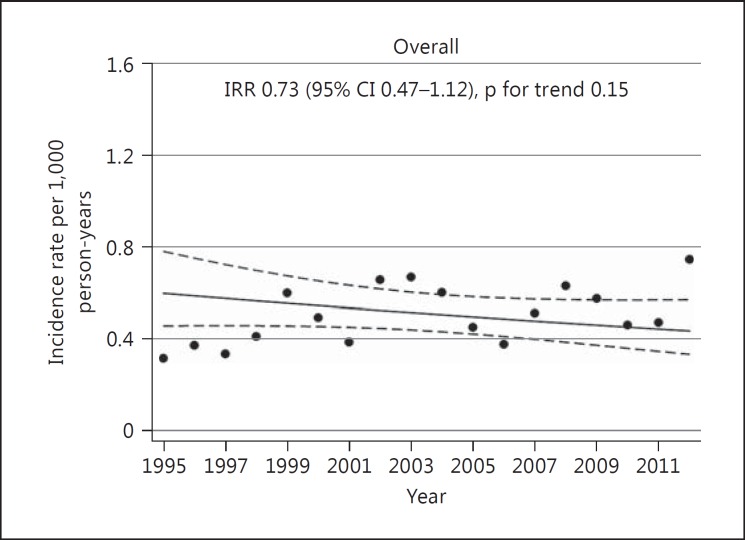
Fig. 1.
Temporal trend in incidence rates of ICH, overall population. The Tromsø Study 1995-2012.
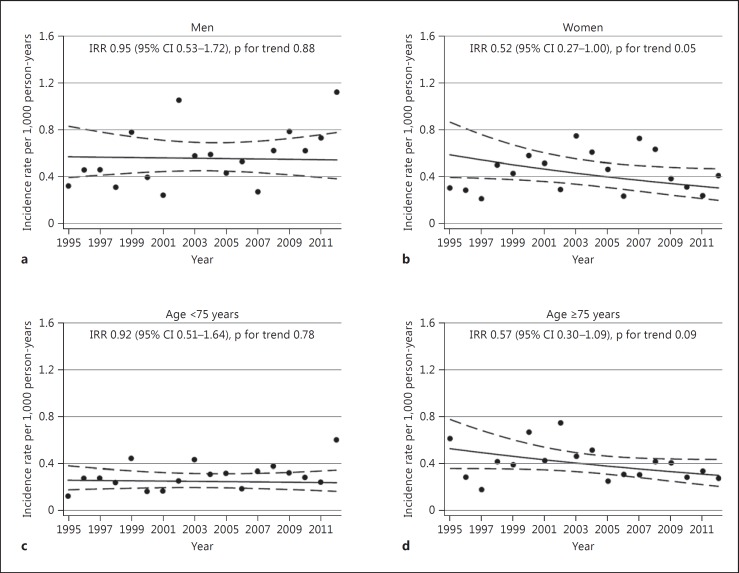
Fig. 2.
Temporal trends in incidence rates of ICH, stratified by sex (a, b) or age (c, d). The Tromsø Study 1995-2012.
Case fatality rates were calculated with the number of deaths occurring within 30 days after the event as numerator and the total number of ICH cases as denominator (table 3). Analysis of temporal trend was performed using a logistic regression model, adjusted for age and sex (fig. 3). The adjusted time trend was presented using the mean values of age and sex. The model was fitted with fractional polynomials and model selection performed using AIC as described earlier. Based on the model selection criteria, time was included as a linear term in the logistic regression model. Odds ratio (OR) was calculated for the year 2012 versus 1995. Tests of interaction between age and time and sex and time were performed by including two-way interaction terms (age × time and sex × time) in the model. Additional analyses of trends in case fatality were performed by calculating ORs between time periods (1995-2000, 2001-2006 and 2007-2012), unadjusted and adjusted for age and sex (table 3).
Table 3.
ORs for 30-day case fatality rates of ICH according to time period (the Tromsø Study 1995–2012)
| Year of ICH |
|||
|---|---|---|---|
| 1995–2000 | 2001–2006 | 2007–2012 | |
| ICH, n | 67 | 79 | 80 |
| 30-day CFR, % (n) | 25.37 (17) | 24.05 (19) | 22.50 (18) |
| OR (95% CI)a | 1 (reference) | 0.93 (0.44–1.98) | 0.85 (0.40–1.83) |
| OR (95% CI)b | 1 (reference) | 0.83 (0.38–1.81) | 0.85 (0.39–1.88) |
CFR = Case fatality rate.
Unadjusted.
Adjusted for age and sex.
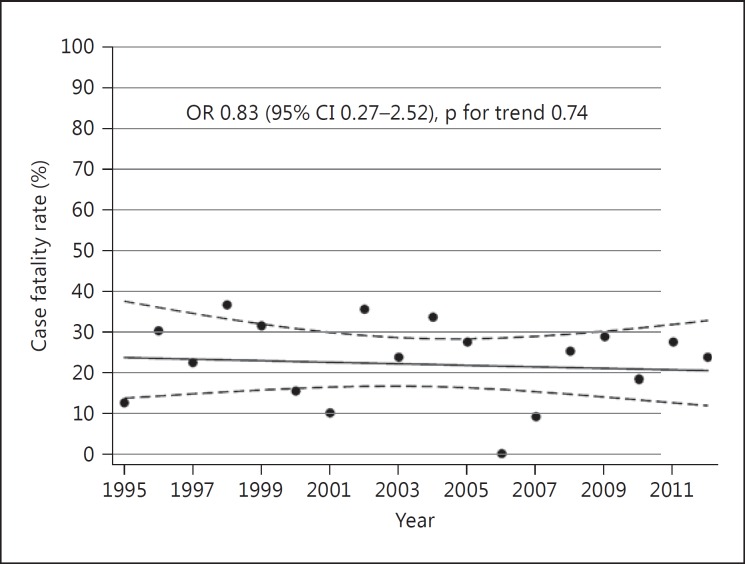
Fig. 3.
Temporal trend in 30-day case fatality rates. The Tromsø Study 1995-2012.
For all analyses, a two-sided p value <0.05 was considered significant. Power calculations based on the observed person-years at risk, the age-adjusted baseline incidence rate, and a 5% significance level showed that the smallest population effect size that would give us 80% power to detect a significantly decreasing incidence trend was IRR = 0.51. In subgroup analyses, the population effect size was IRR = 0.52 in men and IRR = 0.35 in women, and IRR = 0.48 in those <75 years of age and IRR = 0.17 in those ≥75 years of age. Power calculations based on a baseline case fatality rate of 26.4% and the number of ICH being 226 showed that we would have 80% power to detect a significant linear trend in case fatality rates if the population trend over 17 years was 0.24 (OR = 0.92 per year).
Results
We registered 226 incident primary ICHs during a total of 453,152 person-years (table 2). The age- and sex-adjusted incidence rate in the overall population was 0.42 (95% CI 0.37-0.48) per 1,000 person-years, 0.53 (95% CI 0.43-0.62) in men and 0.33 (95% CI 0.26-0.39) in women (table 2). Women were on average 5 years older than men at the time of ICH. Adjusted incidence rates were 0.27 (95% CI 0.22-0.32) per 1,000 person-years in individuals aged <75 years and 2.42 (95% CI 1.95-2.89) in individuals aged ≥75 years. The incidence rates increased steeply with age: compared with the age group 45-54 years, individuals in age groups 65-74 years and ≥85 years had a 9-fold and 30-fold higher risk of ICH, respectively [crude incidence rates 0.12 (95% CI 0.07-0.20), 1.08 (95% CI 0.85-1.39) and 3.65 (95% CI 2.61-5.11) per 1,000 person-years]. In the overall population, the incidence rate of ICH was significantly higher in men compared with women [IRR 1.63 (95% CI 1.25-2.13)]. In individuals aged <75 years, this difference remained significant [IRR 1.72 (95% CI 1.19-2.48)], while the difference between men and women in individuals aged ≥75 years was nonsignificant [IRR 1.43 (95% CI 0.97-2.11)].
Figures 1 and 2 show trends in incidence rates over time. In the overall population, the estimated incidence rate in 2012 was 27% lower than in 1995 [IRR 0.73 (95% CI 0.47-1.12)]. In women, there was a decrease by 48% [IRR 0.52 (95% CI 0.27-1.00)] and in individuals aged ≥75 years the rates decreased by 43% [IRR 0.57 (95% CI 0.30-1.09)] during the study period. However, none of these changes were statistically significant (p value for trend: 0.15, 0.05 and 0.09, respectively). Incidence rates in men and in individuals aged <75 years remained stable [IRR 0.95 (95% CI 0.53-1.72) and 0.92 (95% CI 0.51-1.64), respectively]. There were no significant interactions between age and time (p values for the overall population 0.06, others ranging between 0.21 and 0.76) or sex and time (p values for the overall population 0.21, p values for individuals <75 and ≥75 years of age 0.56 and 0.39, respectively).
Among the 226 individuals suffering an ICH, 54 died within the first 30 days after the ICH event, resulting in a 30-day case fatality rate of 23.9% (95% CI 18.3-29.5). Of the individuals who died within the first 30 days, 48.2% died within the first 2 days and 74.1% died within the first 7 days after the event. The case fatality rate was higher in individuals aged ≥75 years compared with individuals aged <75 years [34.3% (95% CI 25.1-43.5) vs. 14.9% (95% CI 8.4-21.3)]. There was no significant trend over time in 30-day case fatality rates adjusted for age and sex [OR in 2012 vs. 1995: 0.83 (95% CI 0.27-2.52)] (fig. 3; table 3). There was no interaction between age and time (p = 0.57), or sex and time (p = 0.21), suggesting that the trends did not differ by age or sex.
Discussion
We observed no significant change in incidence and case fatality rates of ICH over time. Incidence rates of ICH increased steeply with increasing age, and were higher in men compared with women. Previous studies have reported higher, however not always statistically significant, incidence rates of ICH among men [4,27]. In line with our study, one review showed that the male predominance in stroke incidence decreased with increasing age [27].
Incidence of ICH trends differ by country income level, with increasing incidence rates in low- to middle-income countries and decreasing rates in high-income countries [20]. However, over the last three decades, results from high-income Western countries have shown diverging results. Two European population-based studies reported stable incidence rates [7,8], whereas one Australian and one study from the USA reported a significant decrease in incidence rates [11,12]. One population-based study from the Greater Cincinnati/Northern Kentucky region reported a significant increase in ICH rates from 1988 to 1999, driven by a change between 1988 and 1993/94 [15]. A subsequent publication from the same region showed stable incidence rates between 1993/94 and 2005 [9]. Three large register studies from the USA, Australia and Canada showed stable [10], decreasing [14] and increasing [16] admission rates, respectively.
Case fatality rates vary between studies, with reported case fatality rates ranging between 13 and 61%, the lowest rates reported in publications from Japan [4]. The case fatality rates in our cohort are in the lower range, and lower compared to two previously published studies from Norway [28,29]. There was no significant change in case fatality rates over time, which is in line with results from a meta-analysis of studies published in the period 1980 and 2008 [4].
Strengths and Limitations
The major strengths of our study is the longitudinal, population-based design, high attendance rates, and rigorous case validation. Our study is one of few studies which provide knowledge about trends in incidence of ICH in a population within a well-defined geographical area over a long-time span, including the last decade.
There are, however, some limitations. The number of ICHs is low, leading to limited power to detect statistically significant changes in incidence and case fatality rates, especially in subgroup analyses. Cohort studies carry a risk of both selection bias and bias due to loss to follow-up. Although attendance rates in the Tromsø Study have been high, lower attendance rates have been among the youngest, among men and nonmarried individuals [23]. In addition, lower attendance rates among the elderly and diseased may have influenced incidence and case fatality rates to some degree. Legal restrictions have prohibited the possibilities of detailed analyses of morbidity and mortality according to attendance. We regard the follow-up of our participants as close to complete. Participants are followed up from the date of first attendance (independently of attendance to later surveys) until the first event, death or upon moving away from the municipality.
However, as case identification was retrospective, not hot pursuit, we may have missed some nonhospitalized, nonfatal cases. In addition, some nonhospitalized fatal cases of ICH may have been coded as nonhemorrhagic due to lack of imaging or autopsy, leading to an underestimation of the true incidence rates. There is a possibility that a higher focus on treatment of stroke during the last decades may have led to higher admission rates to the hospital, resulting in relative underestimation of incidence rates in the first part of the observation period. There is also a possibility for a higher utilization of CT/MRI scanning in the diagnostics of stroke patients during the last decades. However, CT scan has been available at our hospital since 1977, and is routinely performed as a screening procedure in all patients admitted for stroke or transient ischemic attack.
Studies from the UK and France suggest that stable incidence rates may be explained by a shift in the risk factor profile during the last decades with a decrease in ICHs associated with hypertension and a concomitant increase in ICHs associated with antithrombotic treatment in the elderly [30,31]. Information on the use of antithrombotic treatment was unfortunately not available in our study.
Conclusion
We observed no significant change in incidence and case fatality rates in the period from 1995 through 2012.
Disclosure Statement
The authors have no conflict of interest to disclose.
Acknowledgement
The Tromsø Study has been supported by the Research Council of Norway, the Norwegian Council on Cardiovascular Disease, the Northern Norway Regional Health Authority, the University of Tromsø, the Norwegian Foundation for Health and Rehabilitation and the Odd Berg Research foundation.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duerr A, Ellingsen CL, Egeland G, Tell G, Seliussen I, Igland J, et al. Hjerte og karregisteret. Rapport for 2012. Nasjonalt Folkehelseinstitutt. 2014 [Google Scholar]
- 3.Flaherty ML, Woo D, Broderick JP. The epidemiology of intracerebral hemorrhage. In: Carhuapoma JR, Mayer SA, Hanley DF, editors. Intracerebral Hemorrhage. Cambridge: Cambridge University Press; 2010. pp. 1–10. [Google Scholar]
- 4.Van Asch CJJ, Luitse MJA, Rinkel GJE, Van der Tweel I, Algra A, Klijn CJM. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 5.Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840–855. doi: 10.1111/ijs.12309. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Arima H, Al-Shahi Salman R, Woodward M, Heeley E, Stapf C, et al. Rapid blood pressure lowering according to recovery at different time intervals after acute intracerebral hemorrhage: pooled analysis of the INTERACT studies. Cerebrovasc Dis. 2015;39:242–248. doi: 10.1159/000381107. [DOI] [PubMed] [Google Scholar]
- 7.Benatru I, Rouaud O, Durier J, Contegal F, Couvreur G, Bejot Y, et al. Stable stroke incidence rates but improved case-fatality in Dijon, France, from 1985 to 2004. Stroke. 2006;37:1674–1679. doi: 10.1161/01.STR.0000226979.56456.a8. [DOI] [PubMed] [Google Scholar]
- 8.Palm F, Henschke N, Wolf J, Zimmer K, Safer A, Schröder RJ, et al. Intracerebral haemorrhage in a population-based stroke registry (LuSSt): incidence, aetiology, functional outcome and mortality. J Neurol. 2013;260:2541–2550. doi: 10.1007/s00415-013-7013-0. [DOI] [PubMed] [Google Scholar]
- 9.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41:1326–1331. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rincon F, Mayer SA. The epidemiology of intracerebral hemorrhage in the United States from 1979 to 2008. Neurocrit Care. 2013;19:95–102. doi: 10.1007/s12028-012-9793-y. [DOI] [PubMed] [Google Scholar]
- 11.Islam S, Anderson CS, Hankey GJ, Hardie K, Carter K, Broadhurst R, et al. Trends in incidence and outcome of stroke in Perth, Western Australia during 1989-2001: the Perth Community Stroke Study. Stroke. 2008;39:776–782. doi: 10.1161/STROKEAHA.107.493643. [DOI] [PubMed] [Google Scholar]
- 12.Zahuranec DB, Lisabeth LD, Sánchez BN, Smith MA, Brown DL, Garcia NM, et al. Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology. 2014;82:2180–2186. doi: 10.1212/WNL.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pajunen P, Pääkkönen R, Hämäläinen H, Keskimäki I, Laatikainen T, Niemi M, et al. Trends in fatal and nonfatal strokes among persons aged 35 to ≥85 years during 1991-2002 in Finland. Stroke. 2005;36:244–248. doi: 10.1161/01.STR.0000152945.28543.4a. [DOI] [PubMed] [Google Scholar]
- 14.Gattellari M, Goumas C, Worthington J. Declining rates of fatal and nonfatal intracerebral hemorrhage: epidemiological trends in Australia. J Am Heart Assoc. 2014;3:e00116. doi: 10.1161/JAHA.114.001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaherty ML, Kissela B, Woo D, Kleindorfer D, Alwell K, Sekar P, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116–121. doi: 10.1212/01.wnl.0000250340.05202.8b. [DOI] [PubMed] [Google Scholar]
- 16.Mayo NE, Nadeau L, Daskalopoulou SS, Côté R. The evolution of stroke in Quebec: a 15-year perspective. Neurology. 2007;68:1122–1127. doi: 10.1212/01.wnl.0000258664.12423.4c. [DOI] [PubMed] [Google Scholar]
- 17.Meretoja A, Kaste M, Roine RO, Juntunen M, Linna M, Hillbom M, et al. Trends in treatment and outcome of stroke patients in Finland from 1999 to 2007. PERFECT Stroke, a nationwide register study. Ann Med. 2011;43(suppl 1):S22–S30. doi: 10.3109/07853890.2011.586361. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M, Jacobsen JB, Johnsen SP, Bøtker HE, Sørensen HT. Eighteen-year trends in stroke mortality and the prognostic influence of comorbidity. Neurology. 2014;82:340–350. doi: 10.1212/WNL.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 19.Feigin VL, Lawes CMM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 20.Krishnamurthi RV, Moran AE, Forouzanfar MH, Bennett DA, Mensah GA, Lawes CM, et al. The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob Heart. 2014;9:101–106. doi: 10.1016/j.gheart.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Statistics Norway Population and quarterly population changes. The whole country counties and municipalities http//ssb.no/236794/population-and-quarterly-population-changes.the-whole-country-counties-and-municipalities (accessed September 15, 2015).
- 22.Central Bureau of Statistics . 1 January 1974; Statistical Yearbook of Norway 1974. Oslo: Central Bureau of Statistics; 1974. Resident Population of the Municipalities; pp. 7–9. [Google Scholar]
- 23.Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njølstad I. Cohort profile: the Tromsø Study. Int J Epidemiol. 2012;41:961–967. doi: 10.1093/ije/dyr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UiT The Arctic University of Norway The Tromsø Study https//en.uit.no/prosjekter/prosjekt?p_document_id=80172 (accessed July 12, 2015).
- 25.Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58:113–130. [PMC free article] [PubMed] [Google Scholar]
- 26.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 27.Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 28.Ellekjær H, Holmen J, Indredavik B, Terent A. Epidemiology of stroke in Innherred, Norway, 1994 to 1996. Incidence and 30-day case-fatality rate. Stroke. 1997;28:2180–2184. doi: 10.1161/01.str.28.11.2180. [DOI] [PubMed] [Google Scholar]
- 29.Tveiten A, Ljøstad U, Mygland Å, Thomassen L, Pripp AH, Naess H. Intracerebral hemorrhage in southern Norway - a hospital-based incidence study. Eur Neurol. 2012;67:240–245. doi: 10.1159/000336299. [DOI] [PubMed] [Google Scholar]
- 30.Lovelock CE, Molyneux AJ, Rothwell PM, on behalf of the Oxford Vascular Study Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol. 2007;6:487–493. doi: 10.1016/S1474-4422(07)70107-2. [DOI] [PubMed] [Google Scholar]
- 31.Béjot Y, Cordonnier C, Durier J, Aboa-Eboulé C, Rouaud O, Giroud M. Intracerebral haemorrhage profiles are changing: results from the Dijon population-based study. Brain. 2013;136:658–664. doi: 10.1093/brain/aws349. [DOI] [PubMed] [Google Scholar]