Abstract
Background
Esophageal cancer (EC) occurs commonly, especially in Asia, and is the sixth leading cause of cancer deaths worldwide. Recently, great progress has been made in research on the etiology and prevention of EC.
Summary
The major risk factors for esophageal squamous cell carcinoma (ESCC) are tobacco smoking and alcohol drinking, which act synergistically. Dietary parameters, including dietary carcinogens and insufficiency of micronutrients, could also be important risk factors in certain areas. A common etiological factor for both EC and some other cancers are low levels of intake of fruits and vegetables. With improvements in diet and drinking water in developing countries, the incidence of ESCC decreased. However, in economically well-developed countries, the incidence of esophageal adenocarcinoma (EAC) has markedly increased in the past 40 years. The major etiological factor for EAC is gastroesophageal reflux, which is also an etiological factor for gastric cardia adenocarcinoma (GCA). In certain areas of China, the occurrence of GCA is closely related to ESCC. Susceptibility genes for EC are starting to be discovered, and this may help to identify high-risk groups that have more need for preventive measures. Mitigation of the risk factors, early detection and treatment of precancerous lesions are effective approaches for prevention. Smoking cessation, avoidance of excessive alcohol, meat and caloric consumption, increasing physical activity and frequent consumption of vegetables and fruits are prudent lifestyle modifications for the prevention of EC as well as other diseases.
Key Message
The etiology of EC includes tobacco smoking, alcohol drinking, low levels of intake of fruits and vegetables as well as gastroesophageal reflux and susceptibility genes.
Practical Implications
A healthy lifestyle including smoking cessation, increasing physical activity, consumption of vegetables as well as reduction of alcohol intake and caloric consumption are major approaches to the prevention of EC.
Key Words: Esophageal cancer, Etiology, Prevention
Introduction
Esophageal cancer (EC) is the sixth leading cause of cancer death globally [1]. The epithelium of the esophagus has historically been the site of malignant neoplasia, and is still a major site of cancer formation. EC has been particularly prevalent in many areas of Asia - for example, in the Taihang Mountain ranges of Northern China. Traditionally, most of the EC are esophageal squamous cell carcinomas (ESCC), and this is still true for Asia. In Western countries, however, the incidence of esophageal adenocarcinoma (EAC) has risen sharply during the past 40 years and has become the major type of EC. EAC is mostly caused by gastroesophageal reflux manifested as gastroesophageal reflux disease. Associated with the increased incidence of EAC is the increase in gastric cardia adenocarcinoma (GCA) in Western countries. In some other geographic areas, such as in certain areas of Northern China, the occurrence of GCA is associated with ESCC.
There is a vast body of literature dealing with the involvement of different environmental and genetic factors in ESCC and EAC. Many prevention studies further illustrate the etiological factors and suggest effective preventive measures for these diseases. This paper reviews studies on the etiology and prevention of ESCC and EAC, relying heavily on recent review articles, meta-analyses and the authors' own work to draw conclusions on the causative factors. Perspectives on the effective prevention of EC are also discussed.
Epidemiology and Etiology of EC
Historically, most EC were derived from the squamous epithelia of the middle and lower thirds of the esophagus and therefore named ESCC. In recent decades, EAC, derived from islands of columnar cells near the gastroesophageal junction, has become the major type of EC in Western societies. These two types of EC are apparently caused by different etiological factors and are discussed separately in the following sections.
Esophageal Squamous Cell Carcinoma
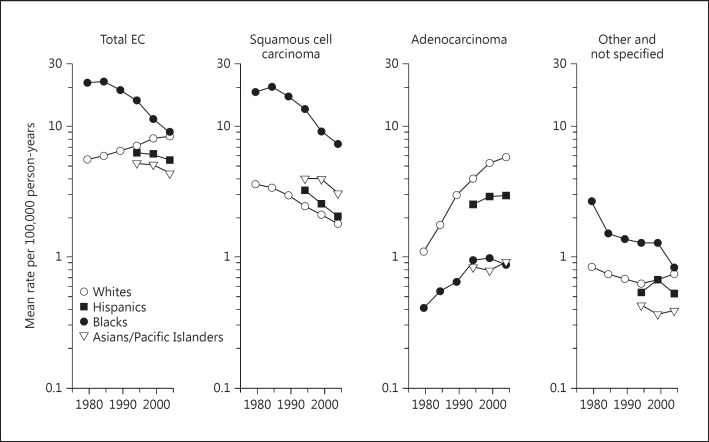
In Western countries, the incidence of ESCC is on the decline. Similarly, in China, the age-standardized death rates of EC decreased from 23.19/100,000 in 1973-1975 to 13.73/100,000 in 2004-2005, showing a decline of 59% [2]. The trend in EC incidence is clearly illustrated in an article by Cook et al. [3], which reviews the incidence of ESCC and EAC in the USA by race and sex for the period 1977-2005. The data on males are shown in figure 1. In the USA, the incidence rate of ESCC in African Americans was much higher than in European Americans, and the rate for both groups declined during the past decades. Such a decline was also found in Asian/Pacific Islander and Hispanic groups; their ESCC rates are lower than those for African Americans, but higher than those for European Americans. There is a large gender difference in EC incidence. In males, the incidence rates for both ESCC and EAC are 2- to 3-fold higher than in females; however, the patterns of a decrease in ESCC and an increase in EAC were similar [3].
Fig. 1.
EC incidence trends for men in the USA [1].
It has been well established that the major risk factors for ESCC are tobacco smoking and alcohol drinking [4,5,6,7,8,9,10]. These and other etiological factors are summarized in table 1. The ESCC risk is estimated to increase 3- to 7-fold in current smokers [5,6,7,8] and 3- to 5-fold if due to the consumption of alcoholic beverages [8,9,10]; these two risk factors are multiplicative in smokers who are also frequent alcohol consumers. That is, these two factors act synergistically to induce ESCC. Tobacco smoke is known to contain polycyclic aromatic hydrocarbons, nitrosamines and many other carcinogens. Some of these compounds are known esophageal carcinogens. Among the different carcinogens tested in animal models, nitrosamines have been shown to be particularly effective in inducing EC in rats, the most commonly used animal species for EC research [11]. Cigarette smoke also contains a large number of pro-oxidative substances and generates reactive oxygen species, which can initiate and promote carcinogenesis. Although it has been proposed that acetyl aldehyde, a metabolite of ethanol, may cause cellular damage and have a carcinogenic effect, the exact roles of ethanol and acetyl aldehyde in esophageal carcinogenesis have not been clarified [12]. A commonly accepted interpretation of the synergy between ethanol and tobacco smoke is that ethanol dissolves and facilitates the transport of tobacco carcinogens to cells, making the cells more susceptible to carcinogenesis. Dietary factors have also been suggested as etiological factors of ESCC for US populations, especially urban African Americans, whose intake of fruits and vegetables was lower than that of other ethnic groups [10,13]. Historically, African Americans were more likely to smoke than European Americans [10]. However, smoking prevalence and alcohol consumption cannot fully explain the racial differences or the decline in ESCC during the past 40 years.
Table 1.
Etiology of ESCC
| Tobacco smoking and drinking of alcoholic beverages; these 2 factors work synergistically |
| Low intake of fruits and vegetables and associated marginal deficiencies in vitamins and trace elements |
| Carcinogens, e.g., nitrosamines, polyaromatic hydrocarbons |
| Thermal irritation from consumption of hot beverages, soup and food; physical irritation due to loss of teeth (poor oral hygiene) |
| Betel quid chewing in Taiwan and India |
| Extremely high salt intake in some populations |
| HPV 16 and 18 in some areas – results inconsistent |
| Genetic susceptibility, e.g., loci at PLCE1, C20orf54, ADH1B and ALDH2, coupled with alcohol consumption and smoking |
In many economically less-developed areas, such as Linxian in Northern China [14] and Golestan in Northeastern Iran [15], dietary factors, including dietary carcinogens and insufficiencies of micronutrients, were found to be even more important risk factors than smoking and drinking. One of the authors (C.S.Y.) spent a substantial amount of time in the 1980s working on the etiology and prevention of EC in Linxian (now renamed Linzhou City), and the occurrence of EC in this area will be discussed more extensively to serve as an example. EC has historically been an endemic disease in this area. Extensive epidemiological studies had been conducted in this area and nearby countries since 1959, and these studies were reviewed in 1980 [14]. On the southeast side of the Taihang Mountain range, where the three provinces of Henan, Hebei and Shanxi border on and center around Linxian and Yangchen, the EC mortality rates were >80/100,000 per year, surrounded by areas with gradually lower rates of 40-80 and then 20-40/100,000, forming irregular concentric belts. Outside of these belts, the rates were much lower; for example, the mortality rate for Datong (Shanxi Province) was 2.8/100,000.
The incidence rate of EC (including GCA) was 108/100,000 and the mortality rate was 99.8/100,000 in Linxian (data from 1959 to 1970), and the rates in Yangchong were even higher [reviewed in [14]]. The general observation in 1980 was that the populations at high risk of ESCC had been consuming a monotonous diet consisting mainly of corn. Consumption of vegetables and fruits was seasonal and low. Meat consumption was even lower; fish and dairy products were rarely consumed. Dietary intake of vitamins and trace elements were estimated to be low [14,16], and biochemical analysis confirmed that the population in Linxian had a low nutritional status in terms of vitamins A, C and E, riboflavin and carotenoids [16,17,18]. The conditions are referred to as micronutrient ‘marginal deficiencies’ or ‘insufficiencies’, because overt signs of deficiency were not observed [14,16]. Corns were often moldy, and picked vegetables were infested by different microorganisms. Water supply has historically been a major problem; pond water was used as drinking water. Among the causative carcinogens, nitrosamines have received the most attention in Linxian. The existence of preformed nitrosamines in the diet in Linxian, in the 1970s and 1980s, has been reported; however, the quantities of their occurrence and their contributions to the causation of ESCC have not been clearly established [reviewed in [14]]. The possible contribution of endogenously formed nitrosamines to the causation of EC has also been discussed extensively. The formation of nitrosamines from nitrite and secondary amines occurs favorably under the acidic pH in the stomach or in tissues with microorganism infections. Indeed, it was documented that the people at risk at Linxian had notably higher levels of nitrate and nitrite in their drinking and cooking water. These nitrates and nitrites came from the oxidation of nitrogenous organic materials in the water supply, usually a pond. Secondary amines were shown to exist in moldy food, such as moldy corn. The formation of esophageal carcinogens, diethylnitrosamine and methylbenzylnitrosamine as well as a novel nitrosamine, N-1-methylacetonyl-N-3-methylbutylnitrosamine, formed by the nitrosation of moldy corn in vitro, was reported [reviewed in [14]]. Inhibition of the nitrosation reaction by polyphenols and ascorbic acid in fruits and vegetables was proposed as a mechanism for the protective roles of fruits and vegetables against ESCC polycyclic aromatic hydrocarbons, which are produced by the burning of wood or coal for cooking or room heating in poorly ventilated rooms and were suggested as possible etiological factors [14]. However, clear evidence for the involvement of nitrosamines and polycyclic aromatic hydrocarbons is still lacking. Consumption of pickled vegetables had been suspected to be a risk factor for EC in Linxian [reviewed in [14]], but subsequent studies [19,20] did not confirm this widely publicized idea.
Consumption of beverages and food at high temperatures, which causes thermal damage to the esophageal epithelium, has been documented to increase the risk of ESCC [21]. Similarly, swallowing hard food without sufficient chewing due to dental problems may cause physical irritation to the esophageal epithelium and increase the risk of cancer. In certain areas, such as Taiwan and India, betel quid chewing, which irritates the oral and esophageal mucosa, increases the risk of ESCC, especially when used in combination with tobacco [22]. The involvement of human papillomavirus in EC has been studied extensively; a strong association was found in some reports, whereas no association was seen in other reports [4]. This inconsistency could be due to geographical variations or due to contaminations or artifacts. A recent meta-analysis showed an association between human papillomavirus infection and ESCC in the Chinese population [23]. This topic, however, has been controversial and remains to be further investigated.
Genetic susceptibility to ESCC has been studied extensively for years. A previous genome-wide association study (GWAS) in Japan showed that functional variants in alcohol dehydrogenase IB and aldehyde dehydrogenase 2, coupled with alcohol drinking and smoking, synergistically enhanced the risk of EC [24]. A recent, large GWAS in China identified susceptibility loci at PLCE1 (regulating cell growth, differentiation, apoptosis and angiogenesis) and C20orf54 (involved in riboflavin transport) for both ESCC and GCA [25]. The shared susceptibility locus in PLCE1 for ESCC and GCA in the Chinese population was also observed by Abnet et al. [26]. A recent study showed that two novel variants on 13q22.1 were associated with the risk of ESCC as well as GCA, but not with non-cardia gastric cancer and colorectal cancer [27]. A joint analysis of three GWAS of ESCC in Chinese populations revealed two new loci at 5q31.2 (synonymous SNP TMEM173) and 17p13.1 (intronic SNP in ATP1B2, near TP53). In addition, a locus in the HLA class II region at 6p21.32 was found in populations at the highest risk of ESCC [28]. A recent genomic analysis by whole-genome or whole-exome sequencing revealed several frequently mutated genes (TP53, Notch1, CDKN2A, PIK3CA, RB1, FBXW7, PTCH1, SUFU and NFE2L2), indicating low activities of cell cycle regulation and Notch signaling, as well as high activities of PI3K signaling and Hedgehog signaling [29,30,31,32]. This genetic information could be used to develop effective means for early detection, prevention and personalized therapy [33].
Esophageal Adenocarcinoma
With the improvement in diet and nutrition in many populations, the incidence of ESCC has gradually decreased in the past decades. However, in economically well-developed areas, such as the USA and Western European countries, the incidence of EAC markedly increased in the past 40 years [34,35,36]. As shown in figure 1, in the USA, the incidence of EAC increased in both European Americans and African Americans - at a much higher rate in the former group than in the latter group. Similar rates of increase were also noted among females, albeit at lower incidence rates than in males [3].
The major etiological factor in EAC is gastroesophageal reflux manifested as gastroesophageal reflux disease [37,38,39,40]. This and other etiological factors are summarized in table 2. The gastric juice in the refluxate, which also contains bile acids and enzymes from the intestine, can cause irritation and inflammation of the mucosa of the lower esophagus, leading to the formation of islands of columnar cells among the squamous epithelium, commonly known as Barrett's esophagus (BE). EAC develops over time from these lesions. Animal studies have demonstrated that gastroesophageal reflux, induced by surgical procedures such as esophagoduodenal anastomosis and esophagogastroduodenal anastomosis, is sufficient to induce EAC [41,42,43,44]. Furthermore, the formation of EAC in these models is enhanced by oxidative stress induced by iron administration and is partially inhibited by the antioxidant nutrient vitamin E [42,43,44,45]. Studies in animal models suggest that oxidative stress and aberrant arachidonic acid metabolism are the two major key events that promote inflammation and induce gene mutations for the development of EAC [44]. Cyclooxygenase-2, 5-lipoxygenase and leukotriene A4 hydrolase, which catalyze the formation of proinflammatory eicosanoids (prostaglandin E2 and leukotriene B4), are overexpressed in both rat and human EAC samples [46,47,48]. Inhibitors of these enzymes, such as celecoxib, zileuton and bestatin, have been shown to inhibit the formation of EAC in the animal model [46,47,48], and may be considered for future human studies.
Table 2.
Etiology of EAC
| Gastroesophageal reflux disease, Barrett's esophagus |
| Abdominal obesity, which increases gastroesophageal reflux and inflammatory cytokines |
| Cigarette smoking; cigarette smoke contains carcinogens and promotes inflammation |
| Low intake of fruits, vegetables and cereal fibers; associated deficiencies in micronutrients may promote inflammation and carcinogenesis |
| Alcoholic beverages – results inconsistent |
| Genetic susceptibility |
Abdominal obesity is a related prominent risk factor [49,50,51,52]. For example, the risk of EAC increased 3-fold for individuals with body mass indices >30 [50]. In another study, abdominal obesity was found to be more important, as the risk of BE was more closely associated with waist-to-hip ratio than with body mass index [51]. A large waist circumference was also found to be a risk factor for reflux esophagitis [52]. Such types of obesity not only physically increase the episode of gastroesophageal reflux, but may also contribute to carcinogenesis by enhancing inflammation.
Although cigarette smoking and alcohol consumption have been established as the leading causative factors in ESCC in Western societies, the involvement of these two factors in EAC has not been conclusive. Cook et al. [53] conducted a pooled analysis of ten population-based case-control studies and two cohort studies from the International Barrett's Esophagus and Esophageal Adenocarcinoma Consortium and found strong associations between cigarette smoking and EAC as well as esophagogastric junction adenocarcinoma in Caucasians [53]. There was a strong dose-response association, and longer smoking cessation was associated with a decreased risk of all adenocarcinomas. On the other hand, the association between alcohol consumption and EAC and/or BE is still unclear. El-Serag and Lagergren [54] have reviewed three studies showing that the odds ratio for wine drinking and risk of EAC/BE was <1. The authors pointed out that the observed protective effect of a modest intake of wine could have resulted from confounding factors and suggested that additional studies, particularly cohort studies, are needed in order to reach a clear conclusion.
Case-control studies have found that a reduced risk of BE is associated with frequent intake of fruits and vegetables as well as the consumption of omega-3 fatty acids and fiber [55,56]. In a large prospective study in the USA on meat consumption and the risk of EC, red meat intake was positively associated with ESCC. The risk of EAC and GCA was found to be associated with the intake of heme iron and 2-amino-3,4,8-trimethylimidazo[4, 5-f]quinoxaline, a polycyclic amine formed during cooking of meat [57].
Genetic susceptibility to EAC has been studied for years. One puzzling issue is why a Caucasian background is a strong risk factor for both BE and EAC. The major hurdle in understanding the mechanisms of the racial disparity regarding BE and EAC lies in the small sample sizes of BE and EAC among other racial/ethnic groups [58]. GWAS in Europe and the USA have shown that the shared polygenic effects due to many genetic variants with a small effect contribute to the development of EAC [59]. Several significant SNPs are associated with FOXP1, BARX1, FOXF1, CRTC1, CDKN2A and TP53 [60,61,62]. Interestingly, genetic risk variants for common cancers and microRNA-related variants are seemingly not associated with the risk of EAC [63,64]. Recent genomic analysis by whole-genome or whole-exome sequencing revealed several frequently mutated genes (e.g., TP53, CDKN2A, SMAD4, ARID1A and PIK3CA) in human EAC [65]. BE itself is found to be polyclonal and highly mutated. Most mutations have already taken place in the absence of dysplasia. There is often surprisingly little overlap between the mutation spectra of EAC and its adjacent BE [66,67]. Not many mutations (e.g., TP53 and SMAD4) are specific for late-stage lesions such as high-grade dysplasia and adenocarcinoma [68]. In contrast, genomic catastrophes frequently lead to oncogene amplification through chromothripsis-derived double-minute chromosome formation or the breakage-fusion-bridge cycle [69].
One practical issue in preventing EAC is how to stratify cancer risk among patients with BE. The current clinical strategy involves regular endoscopic examinations and biopsy pathology. This approach is controversial due to lack of specificity of a pathological diagnosis of dysplasia, and recent data suggest that routine endoscopic surveillance of BE is not effective for early detection of EAC. Recent studies have shown that molecular biomarkers in biopsy or exfoliative cells are potential solutions for this problem. Multiple techniques have been used for this purpose and shown great promise, such as fluorescence in situ hybridization for several marker genes [70], gene arrays for expression patterns of 90 genes [71] and genome sequencing [68].
Gastric Cardia Adenocarcinoma
The etiology of GCA is strongly associated with gastroesophageal reflux. In fact, this cancer is usually studied together with EAC, showing a similar increase in incidence to that of EAC during the past decades [3], and they share many of the etiological factors [3,35,57]. A similar increase in GCA has also been observed in Asian countries; for example, in Singapore, the incidence of GCA has increased each decade since 1968 from 6.3 to the recent 16.2% [72].
In certain cases, such as in Linxian, the etiology of GCA is closely related to that of ESCC. Historically, GCA and ESCC have both been referred to as EC, occurring at a ratio of 4:6. As will be discussed later, the only significant decrease in mortality rate in the Linxian Nutritional International Trial (LNIT) was found in gastric cancer (mainly GCA) by the daily supplementation of a combination of selenium, α-tocopherol and β-carotene [73]. Nested case-control studies have demonstrated that lower blood levels of α-tocopherol and selenium are associated with an increased risk of GCA and ESCC [18,74]. Low selenium nutritional status, as reflected in the low toenail selenium content, was also associated with risk of GCA and ESCC in the Netherlands Cohort Study [75]. In this area, some genetic susceptibility factors are shared by GCA and ESCC, as described above [25,26].
Common Risk and Protective Factors for EC
In ESCC and EAC as well as in gastric cancer, there is a ‘male predominance’. For example, the incidence rates for ESCC and EAC in men are 3- to 8-fold higher than in women [76]. One contributing factor is tobacco smoking, an etiological factor that is more prevalent among males. Another risk factor for ESCC, alcohol consumption, is also more prevalent among men. A biological basis for this male predominance, supported by results from animal models, is that estrogen is an inhibitor of esophageal carcinogenesis [77]. Abdominal obesity, which occurs more frequently in men than women, also increases the incidence of gastroesophageal reflux and contributes to the higher incidence of EAC and gastric cardia cancer [76]. Another common etiological factor for both EC and gastric cardia cancer, as discussed previously, is the low level of intake of fruits and vegetables (and associated insufficiencies of micronutrients) by the populations at risk.
Prevention of Esophageal and Gastric Cardia Cancers
As discussed above, the major etiological factors for EC and GCA are well studied. Eradication of these risk factors and early detection of precancerous lesions are the most logical approaches for preventions. Smoking cessation, avoidance of excessive alcohol consumption and reducing the intake of salt and salted food are all sound public health guidelines for the reduction of EC (table 3). Lifestyle changes are difficult to implement, and few studies on these approaches toward cancer prevention have been reported. The success in smoking prevention and cessation in the USA, which has been followed by a decreased incidence of lung and other cancers, indicates that behavioral changes are possible when enough effort is devoted to such public health initiatives. On the other hand, difficulties have been encountered in the prevention of EAC by reducing gastroesophageal reflux through changing dietary habits and reducing obesity. Medical treatments with antireflux drugs and proton pump inhibitors have not yielded consistent results [78].
Table 3.
Prevention of EC
| Do not smoke |
| Consume alcoholic beverages in moderation, if at all |
| Increase your intake of fruits and vegetables; this will provide vitamins, trace element nutrients and fiber for gastrointestinal health |
| Avoid salted and pickled vegetables as well as processed meats |
| Do not overeat to prevent esophageal reflux and obesity |
| Exercise regularly to maintain a healthy body weight and help with gastrointestinal functions |
| Treat any Helicobacter pylori infection, especially in high-risk areas for gastric cancer |
Intervention Studies in Linxian
The insufficient intake of vegetables and fruits, and the associated nutritional insufficiencies of micronutrients, is a common etiological factor in both EC and gastric cancer. The hypothesis that supplementation with micronutrients can prevent EC was tested in a large-scale intervention study, the Chinese-US cooperative trial LNIT. In the poor rural population of Linxian, there was a low intake of micronutrients, and insufficiencies of some micronutrients were indicated by blood nutrient analyses [16,17]. Because many micronutrients had been shown to be associated with EC, the nutrients were divided into four groups: (A) retinol and zinc; (B) riboflavin and niacin; (C) ascorbate and molybdenum, and (D) α-tocopherol, β-carotene and selenium, in a factorial design with eight groups: placebo, AB, AC, BC, AD, BD, CD and ABCD. The study involved 29,584 adults (aged 40-69 years) who were given supplementations as daily pills for 63 months (1985-1991). There were 2,127 deaths during the trial period; 32% were due to esophageal and gastric cancer. The study showed that daily supplementation with a combination of α-tocopherol (50 μg), β-carotene (15 mg) and selenium (50 μg) to the general population (>40 years old) for 63 months decreased mortality due to gastric cancer (mainly GCA) by 20% and total cancer mortality by 13% [73]. This result is consistent with the results of the nested case-control studies, which showed that the blood levels of α-tocopherol and selenium were inversely associated with cancer risk [18,74]. Other nutrient combinations, however, did not show any effects on the end points measured.
Follow-up studies found that 10 years after the cessation of the nutrient supplementation, the preventive effect of the supplementation with α-tocopherol/β-carotene/selenium on gastric cancer still remained, and a new observation was that the supplementation reduced ESCC mortality by 17% in subjects who started the supplementation at an age ≤55 years, but increased the ESCC mortality by 14% among those >55 years of age [79]. These results suggest that the supplementation was beneficial to subjects at a younger age or before the onset (or at the early stage) of esophageal precancerous lesions, but supplementation to older subjects with severe lesions was not effective. Consistent with this concept is the result of a parallel nutritional intervention trial in Linxian on adults with esophageal dysplasia in which a combination of 14 vitamins and 12 minerals or placebo was used [80]. The cumulative ESCC and gastric cancer death rates were only 8% lower and the total cancer death rate was 4% lower in the supplemented group; both of these reductions were statistically insignificant. This concept is also in agreement with the result of another randomized trial conducted later on the same cohort with selenomethionine, in which a beneficial effect was observed only in patients with mild esophageal dysplasia, but not in those with severe esophageal dysplasia [81]. Celecoxib had no effect on squamous histology in this trial.
The effect of the herbal preparation Antitumor B (a mixture of 6 herbs, newly carrying the commercial name of Zeng Sheng Ping) was studied extensively in individuals with precancerous lesions in Linxian in the 1980s [82]. After treatment with Antitumor B for 1, 3 or 5 years, the incidence of EC had significantly decreased [82]. In this trial, the randomization process, blinding, side effects and potential variation in the chemical compositions of the herbs were not sufficiently addressed.
The results of the LNIT increased our understanding of etiology. It appears that a low antioxidant nutritional status could make the upper gastrointestinal epithelial tissues more prone to inflammation. Inflammation of the esophagus (esophagitis) was reported to occur widely in this population [14], and it could be an important causative factor for EC. A suspected low dietary methionine and choline (both methyl donors) status may also make these subjects prone to epigenetic changes such as the silencing of tumor suppressor and receptor genes by promoter hypermethylation [83]. Recent observational studies have shown that as the population's diet has improved, with increased availability of fresh vegetables and fruits and other food items to replace the traditional monotonous corn-based diet, the incidence rates of ESCC and GCA have significantly decreased [84].
Other Studies
Chemoprevention with pharmaceutical agents has been tested for EC. Nonsteroidal anti-inflammatory drugs are associated with a reduced risk of EAC and other gastroesophageal cancers [reviewed in [4]]. Several agents with relatively specific mechanisms of action have been identified for future clinical studies. For example, statins, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, ursodeoxycholic acid and difluoromethylornithine are all promising agents against EAC [85]. However, it is difficult to test their chemopreventive efficacy through randomized clinical trials. Results from early studies are not encouraging; for example, celecoxib at 200 mg twice a day for 48 weeks failed to prevent the progression of Barrett's dysplasia to cancer [86].
The possible effect of physical activity in the prevention of EC was studied in a large cohort of US men and women followed for a period of 7-8 years. A protective effect against GCA and EAC was observed, but there was no association with ESCC [87]. Possible mechanisms involve decreased chronic inflammation, levels of IGF and leptin, as well as increased insulin sensitivity [87]. The effect was seen in non-cardia gastric cancer and patients infected with Helicobacter pylori[88].
As for early diagnosis of precancerous lesions, endoscopic screening in areas at high risk of EC and gastric cancer is a reliable approach, and some large-scale screenings have been successfully conducted in China. A recent study showed that endoscopic screening and intervention significantly reduced mortality caused by EC. Early detection and treatment of preneoplastic lesions also led to a reduction in the incidence of ESCC [89]. Similar to this, multiple studies have shown that treatment of dysplasia in BE patients - for example, with radiofrequency ablation (RFA) - reduces the risk of EAC [90,91,92]. RFA is an FDA-approved endoscopic technique in which diseased tissue is exposed to heat energy and destroyed. This technique has been proven to be safe and durable at least through medium-term follow-up [93,94]. In a multicenter RFA study in the USA, among 4,982 patients with RFA treatment, the incidence of EAC was markedly lower than in other studies of disease progression [91]. A general consensus has been reached among gastroenterologists in Europe and the USA that endoscopic resection and ablation is the most effective therapy for high-risk BE [95]. Furthermore, some recent studies also have shown that mucosal neoplasia arising from BE can be successfully treated with endoscopic mucosal resection (EMR) followed by RFA [96]. A nationwide study of BE patients treated with RFA followed by EMR has demonstrated that the procedure is effective and safe [97,98]. A further study showed that undergoing EMR prior to RFA was associated with an increased likelihood of maintaining a durable eradication of mucosal neoplasia [99].
Concluding Remarks
As reviewed above, the trends toward declining incidence rates of ESCC, combined with the rapidly rising rates of EAC and GCA during the past several decades, strongly suggest that lifestyle and other environmental factors are the major etiological factors for esophageal and gastric cancers. The ‘genetic makeup’ of populations has not changed. Studies on genetic susceptibility, especially using the recently developed GWAS approach, could generate useful information for understanding why certain individuals within a specific population are more susceptible to certain environmental factors. Some promising leads are beginning to appear; for example, polymorphisms of alcohol dehydrogenase and aldehyde dehydrogenase are linked to alcohol consumption for increasing the risk of ESCC. The recently identified, possible polymorphism in enzymes involved in riboflavin transport could be linked to marginal riboflavin nutrition. Much is still unknown concerning the racial differences in the incidence of EC, for example, the different patterns of EC in African Americans and European Americans. Based on the etiological factors discussed above, it is logical to conclude that most of the EC are preventable. Lifestyle modifications, such as smoking cessation, reducing alcohol consumption, decreasing salt intake and avoiding overeating, will significantly reduce the risk of these cancers. Consuming a diet that is rich in vegetables, fruits and fiber-containing foods would avoid nutrient insufficiency-enhanced carcinogenesis and promote healthy functions of the gastrointestinal tract. These recommendations, plus increased physical activity, in fact are guidelines for the maintenance of good health and prevention of cancer and many other diseases.
Disclosure Statement
No potential conflicts of interest are disclosed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Chen WQ. Incidence, mortality, distribution characteristics and trends of tumor in China. In: Zhao P, Wang LD, Li JY, editors. Preventive Oncology. Beijing: People's Medical Publishing House; 2015. pp. 127–152. [Google Scholar]
- 3.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br J Cancer. 2009;101:855–859. doi: 10.1038/sj.bjc.6605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27–57. doi: 10.1016/j.gtc.2009.01.004. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years' observations on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin JK, Hrubec Z, Blot WJ, Fraumeni JF., Jr Smoking and cancer mortality among US veterans: a 26-year follow-up. Int J Cancer. 1995;60:190–193. [PubMed] [Google Scholar]
- 7.Ishikawa A, Kuriyama S, Tsubono Y, Fukao A, et al. Smoking, alcohol drinking, green tea consumption and the risk of esophageal cancer in Japanese men. J Epidemiol. 2006;16:185–192. doi: 10.2188/jea.16.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman ND, Abnet CC, Leitzmann MF, Mouw T, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424–1433. doi: 10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 9.Boffetta P, Garfinkel L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology. 1990;1:342–348. doi: 10.1097/00001648-199009000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Brown LM, Hoover R, Silverman D, Baris D, et al. Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. Am J Epidemiol. 2001;153:114–122. doi: 10.1093/aje/153.2.114. [DOI] [PubMed] [Google Scholar]
- 11.Yang CS. Nitrosamines and other etiological factors in the esophageal cancer in northern China. In: Magee PN, editor. Banbury Report 12: Nitrosamines and Human Cancer. New York: Cold Spring Harbor Laboratory; 1982. pp. 487–501. [Google Scholar]
- 12.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 13.Freedman ND, Park Y, Subar AF, Hollenbeck AR, et al. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int J Cancer. 2007;121:2753–2760. doi: 10.1002/ijc.22993. [DOI] [PubMed] [Google Scholar]
- 14.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633–2644. [PubMed] [Google Scholar]
- 15.Islami F, Kamangar F, Nasrollahzadeh D, Møller H, et al. Oesophageal cancer in Golestan Province, a high-incidence area in northern Iran - a review. Eur J Cancer. 2009;45:3156–3165. doi: 10.1016/j.ejca.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Yang CS, Miao J, Yang W, Huang M, et al. Diet and vitamin nutrition of the high esophageal cancer risk population in Linxian, China. Nutr Cancer. 1982;4:154–164. doi: 10.1080/01635588209513751. [DOI] [PubMed] [Google Scholar]
- 17.Yang CS, Sun Y, Yang QU, Miller KW, et al. Vitamin A and other deficiencies in Linxian, a high esophageal cancer incidence area in northern China. J Natl Cancer Inst. 1984;73:1449–1453. [PubMed] [Google Scholar]
- 18.Taylor PR, Qiao YL, Abnet CC, Dawsey SM, et al. Prospective study of serum vitamin E levels and esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1414–1416. doi: 10.1093/jnci/djg044. [DOI] [PubMed] [Google Scholar]
- 19.Tran GD, Sun XD, Abnet CC, Fan JH, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 20.Li JY, Ershow AG, Chen ZJ, Wacholder S, et al. A case-control study of cancer of the esophagus and gastric cardia in Linxian. Int J Cancer. 1989;43:755–761. doi: 10.1002/ijc.2910430502. [DOI] [PubMed] [Google Scholar]
- 21.Islami F, Boffetta P, Ren JS, Pedoeim L, et al. High-temperature beverages and foods and esophageal cancer risk - a systematic review. Int J Cancer. 2009;125:491–524. doi: 10.1002/ijc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen CP, Tsai MK, Chung WS, Hsu HL, et al. Cancer risks from betel quid chewing beyond oral cancer: a multiple-site carcinogen when acting with smoking. Cancer Causes Control. 2010;21:1427–1435. doi: 10.1007/s10552-010-9570-1. [DOI] [PubMed] [Google Scholar]
- 23.Liyanage SS, Rahman B, Gao Z, Zheng Y, et al. Evidence for the aetiology of human papillomavirus in oesophageal squamous cell carcinoma in the Chinese population: a meta-analysis. BMJ Open. 2013;3:e003604. doi: 10.1136/bmjopen-2013-003604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui R, Kamatani Y, Takahashi A, Usami M, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. 2009;137:1768–1775. doi: 10.1053/j.gastro.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 25.Wang LD, Zhou FY, Li XM, Sun LD, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies a susceptibility locus at PLCE1. Nat Genet. 2010;42:759–763. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 26.Abnet CC, Freedman ND, Hu N, Wang Z, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010;42:764–767. doi: 10.1038/ng.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang J, Wei L, Miao X, Yu D, et al. Two novel variants on 13q22.1 are associated with risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2015;24:1774–1780. doi: 10.1158/1055-9965.EPI-15-0154-T. [DOI] [PubMed] [Google Scholar]
- 28.Wu C, Wang Z, Song X, Feng XS, Abnet CC. Joint analysis of three genome-wide association studies of esophageal squamous cell carcinoma in Chinese populations. Nat Genet. 2014;46:1001–1006. doi: 10.1038/ng.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Zhou Y, Cheng C, Cui H, et al. Genomic analyses reveal mutational signatures and frequently altered genes in esophageal squamous cell carcinoma. Am J Hum Genet. 2015;96:597–611. doi: 10.1016/j.ajhg.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao YB, Chen ZL, Li JG, Hu XD, et al. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097–1102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 31.Lin DC, Hao JJ, Nagata Y, Xu L, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46:467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y, Li L, Ou Y, Gao Z, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 33.Kang X, Chen K, Li Y, Li J, et al. Personalized targeted therapy for esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:7648–7658. doi: 10.3748/wjg.v21.i25.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 35.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 36.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 38.Wu AH, Tseng CC, Bernstein L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer. 2003;98:940–948. doi: 10.1002/cncr.11568. [DOI] [PubMed] [Google Scholar]
- 39.Jankowski JA, Provenzale D, Moayyedi P. Esophageal adenocarcinoma arising from Barrett's metaplasia has regional variations in the west. Gastroenterology. 2002;122:588–590. doi: 10.1053/gast.2002.31599. [DOI] [PubMed] [Google Scholar]
- 40.Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729–735. doi: 10.1111/j.1440-1746.2009.05824.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Yang G, Ding WY, Bondoc F, et al. An esophagogastroduodenal anastomosis model for esophageal adenocarcinogenesis in rats and enhancement by iron overload. Carcinogenesis. 1999;20:1801–1808. doi: 10.1093/carcin/20.9.1801. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Mikhail SS, Ding YW, Yang G, et al. Effects of vitamin E and selenium supplementation on esophageal adenocarcinogenesis in a surgical model with rats. Carcinogenesis. 2000;21:1531–1536. [PubMed] [Google Scholar]
- 43.Chen X, Ding YW, Yang G, Bondoc F, et al. Oxidative damage in an esophageal adenocarcinoma model with rats. Carcinogenesis. 2000;21:257–263. doi: 10.1093/carcin/21.2.257. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Yang CS. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis. 2001;22:1119–1129. doi: 10.1093/carcin/22.8.1119. [DOI] [PubMed] [Google Scholar]
- 45.Hao J, Zhang B, Liu B, Lee M, et al. Effect of α-tocopherol, N-acetylcysteine and omeprazole on esophageal adenocarcinoma formation in a rat surgical model. Int J Cancer. 2009;124:1270–1275. doi: 10.1002/ijc.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Li N, Wang S, Hong J, et al. Aberrant arachidonic acid metabolism in esophageal adenocarcinogenesis, and the effects of sulindac, nordihydroguaiaretic acid, and α-difluoromethylornithine on tumorigenesis in a rat surgical model. Carcinogenesis. 2002;23:2095–2102. doi: 10.1093/carcin/23.12.2095. [DOI] [PubMed] [Google Scholar]
- 47.Chen X, Li N, Wang S, Wu N, et al. Leukotriene A4 hydrolase in rat and human esophageal adenocarcinomas and inhibitory effects of bestatin. J Natl Cancer Inst. 2003;95:1053–1061. doi: 10.1093/jnci/95.14.1053. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Wang S, Wu N, Sood S, et al. Overexpression of 5-lipoxygenase in rat and human esophageal adenocarcinoma and inhibitory effects of zileuton and celecoxib on carcinogenesis. Clin Cancer Res. 2004;10:6703–6709. doi: 10.1158/1078-0432.CCR-04-0838. [DOI] [PubMed] [Google Scholar]
- 49.Vial M, Grande L, Pera M. Epidemiology of adenocarcinoma of the esophagus, gastric cardia, and upper gastric third. Recent Results Cancer Res. 2010;182:1–17. doi: 10.1007/978-3-540-70579-6_1. [DOI] [PubMed] [Google Scholar]
- 50.Percik R, Stumvoll M. Obesity and cancer. Exp Clin Endocrinol Diabetes. 2009;117:563–566. doi: 10.1055/s-0029-1241870. [DOI] [PubMed] [Google Scholar]
- 51.Edelstein ZR, Farrow DC, Bronner MP, Rosen SN, Vaughan TL. Central adiposity and risk of Barrett's esophagus. Gastroenterology. 2007;133:403–411. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 52.Yasuhara H, Miyake Y, Toyokawa T, Matsumoto K, et al. Large waist circumference is a risk factor for reflux esophagitis in Japanese males. Digestion. 2010;81:181–187. doi: 10.1159/000235919. [DOI] [PubMed] [Google Scholar]
- 53.Cook MB, Kamangar F, Whiteman DC, Freedman ND, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102:1344–1353. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Serag HB, Lagergren J. Alcohol drinking and the risk of Barrett's esophagus and esophageal adenocarcinoma. Gastroenterology. 2009;136:1155–1157. doi: 10.1053/j.gastro.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson OM, Beresford SA, Kirk EA, Vaughan TL. Vegetable and fruit intakes and risk of Barrett's esophagus in men and women. Am J Clin Nutr. 2009;89:890–896. doi: 10.3945/ajcn.2008.26497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kubo A, Block G, Quesenberry CP, Jr, Buffler P, Corley DA. Effects of dietary fiber, fats, and meat intakes on the risk of Barrett's esophagus. Nutr Cancer. 2009;61:607–616. doi: 10.1080/01635580902846585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cross AJ, Freedman ND, Ren J, Ward MH, et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol. 2010;106:432–442. doi: 10.1038/ajg.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen TH, Thrift AP, Ramsey D, Green L, et al. Risk factors for Barrett's esophagus compared between African Americans and non-Hispanic Whites. Am J Gastroenterol. 2014;109:1870–1880. doi: 10.1038/ajg.2014.351. [DOI] [PubMed] [Google Scholar]
- 59.Ek WE, Levine DM, D'Amato M, Pedersen NL, et al. Germline genetic contributions to risk for esophageal adenocarcinoma, Barrett's esophagus, and gastroesophageal reflux. J Natl Cancer Inst. 2013;105:1711–1718. doi: 10.1093/jnci/djt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker J, May A, Gerges C, Anders M, et al. Supportive evidence for FOXP1 BARX1, and FOXF1 as genetic risk loci for the development of esophageal adenocarcinoma. Cancer Med. 2015;4:1700–1704. doi: 10.1002/cam4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buas MF, Levine DM, Makar KW, Utsugi H, et al. Integrative post-genome-wide association analysis of CDKN2A and TP53 SNPs and risk of esophageal adenocarcinoma. Carcinogenesis. 2014;35:2740–2747. doi: 10.1093/carcin/bgu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levine DM, Ek WE, Zhang R, Liu X, et al. A genome-wide association study identifies new susceptibility loci for esophageal adenocarcinoma and Barrett's esophagus. Nat Genet. 2013;45:1487–1493. doi: 10.1038/ng.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee E, Stram DO, Ek WE, Onstad LE, et al. Pleiotropic analysis of cancer risk loci on esophageal adenocarcinoma risk. Cancer Epidemiol Biomarkers Prev. 2015;24:1801–1803. doi: 10.1158/1055-9965.EPI-15-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buas MF, Onstad L, Levine DM, Risch HA, et al. miRNA-related SNPs and risk of esophageal adenocarcinoma and Barrett's esophagus: post genome-wide association analysis in the BEACON consortium. PLoS One. 2015;10:e0128617. doi: 10.1371/journal.pone.0128617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dulak AM, Stojanov P, Peng S, Lawrence MS, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45:478–486. doi: 10.1038/ng.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ross-Innes CS, Becq J, Warren A, Cheetham RK, et al. Whole-genome sequencing provides new insights into the clonal architecture of Barrett's esophagus and esophageal adenocarcinoma. Nat Genet. 2015;47:1038–1046. doi: 10.1038/ng.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stachler MD, Taylor-Weiner A, Peng S, McKenna A, et al. Paired exome analysis of Barrett's esophagus and adenocarcinoma. Nat Genet. 2015;47:1047–1055. doi: 10.1038/ng.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weaver JM, Ross-Innes CS, Shannon N, Lynch AG, et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat Genet. 2014;46:837–843. doi: 10.1038/ng.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nones K, Waddell N, Wayte N, Patch AM, et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat Commun. 2014;5:5224. doi: 10.1038/ncomms6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Timmer MR, Martinez P, Lau CT, Westra WM, et al. Derivation of genetic biomarkers for cancer risk stratification in Barrett's oesophagus: a prospective cohort study. Gut. 2015 doi: 10.1136/gutjnl-2015-309642. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varghese S, Newton R, Ross-Innes CS, Lao-Sirieix P, et al. Analysis of dysplasia in patients with Barrett's esophagus based on expression pattern of 90 genes. Gastroenterology. 2015;149:1511–1518. doi: 10.1053/j.gastro.2015.07.053. e5. [DOI] [PubMed] [Google Scholar]
- 72.Deans C, Yeo MS, Soe MY, Shabbir A, et al. Cancer of the gastric cardia is rising in incidence in an Asian population and is associated with adverse outcome. World J Surg. 2011;35:617–624. doi: 10.1007/s00268-010-0935-0. [DOI] [PubMed] [Google Scholar]
- 73.Blot WJ, Li J-Y, Taylor PR, Guo W, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–1491. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 74.Mark SD, Qiao YL, Dawsey SM, Wu YP, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92:1753–1763. doi: 10.1093/jnci/92.21.1753. [DOI] [PubMed] [Google Scholar]
- 75.Steevens J, Van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands Cohort Study. Gastroenterology. 2010;138:1704–1713. doi: 10.1053/j.gastro.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Chandanos E, Lagergren J. The mystery of male dominance in oesophageal cancer and the potential protective role of oestrogen. Eur J Cancer. 2009;45:3149–3155. doi: 10.1016/j.ejca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 77.Chandanos E, Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008;44:2397–2403. doi: 10.1016/j.ejca.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 78.García Rodríguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut. 2006;55:1538–1544. doi: 10.1136/gut.2005.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiao YL, Dawsey SM, Kamangar F, Fan JH, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507–518. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li JY, Taylor PR, Li B, Dawsey S, et al. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst. 1993;85:1492–1498. doi: 10.1093/jnci/85.18.1492. [DOI] [PubMed] [Google Scholar]
- 81.Limburg PJ, Wei W, Ahnen DJ, Qiao Y, et al. Randomized, placebo-controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology. 2005;129:863–873. doi: 10.1053/j.gastro.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 82.Lin P. Medicamentous inhibitory therapy of precancerous lesions of the esophagus - 3 and 5 year inhibitory effect of Antitumor B, retinamide and riboflavin (in Chinese) Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1990;12:235–245. [PubMed] [Google Scholar]
- 83.Xing EP, Nie Y, Song Y, Yang GY, et al. Mechanisms of inactivation of p14ARF p15INK4b, and p16INK4a genes in human esophageal squamous cell carcinoma. Clin Cancer Res. 1999;5:2704–2713. [PubMed] [Google Scholar]
- 84.Yang W-X, Lu X-X, Liu G-T, et al. Etiological prevention trial for esophageal cancer among high-risk population in Linzhou city, China. China Cancer. 2008;17:548–552. [Google Scholar]
- 85.Zeb MH, Baruah A, Kossak SK, Buttar NS. Chemoprevention in Barrett's esophagus: current status. Gastroenterol Clin North Am. 2015;44:391–413. doi: 10.1016/j.gtc.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 86.Heath EI, Canto MI, Piantadosi S, Montgomery E, et al. Secondary chemoprevention of Barrett's esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst. 2007;99:545–557. doi: 10.1093/jnci/djk112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leitzmann MF, Koebnick C, Freedman ND, Park Y, et al. Physical activity and esophageal and gastric carcinoma in a large prospective study. Am J Prev Med. 2009;36:112–119. doi: 10.1016/j.amepre.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang P, Zhou Y, Chen B, Wan HW, et al. Aspirin use and the risk of gastric cancer: a meta-analysis. Dig Dis Sci. 2010;55:1533–1539. doi: 10.1007/s10620-009-0915-0. [DOI] [PubMed] [Google Scholar]
- 89.Wei WQ, Chen ZF, He YT, Feng H, et al. Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal cancer in China. J Clin Oncol. 2015;33:1951–1957. doi: 10.1200/JCO.2014.58.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Small AJ, Araujo JL, Leggett CL, Mendelson AH, et al. Radiofrequency ablation is associated with decreased neoplastic progression in patients with Barrett's esophagus and confirmed low-grade dysplasia. Gastroenterology. 2015;149:567–576. doi: 10.1053/j.gastro.2015.04.013. e3; quiz e13-e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolf WA, Pasricha S, Cotton C, Li N, et al. Incidence of esophageal adenocarcinoma and causes of mortality after radiofrequency ablation of Barrett's esophagus. Gastroenterology. 2015;149:1752–1761. doi: 10.1053/j.gastro.2015.08.048. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 93.Orman ES, Kim HP, Bulsiewicz WJ, Cotton CC, et al. Intestinal metaplasia recurs infrequently in patients successfully treated for Barrett's esophagus with radiofrequency ablation. Am J Gastroenterol. 2013;108:187–195. doi: 10.1038/ajg.2012.413. quiz 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shaheen NJ, Overholt BF, Sampliner RE, Wolfsen HC, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology. 2011;141:460–468. doi: 10.1053/j.gastro.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bennett C, Vakil N, Bergman J, Harrison R, et al. Consensus statements for management of Barrett's dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology. 2012;143:336–346. doi: 10.1053/j.gastro.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haidry RJ, Lipman G, Banks MR, Butt MA, et al. Comparing outcome of radiofrequency ablation in Barrett's with high grade dysplasia and intramucosal carcinoma: a prospective multicenter UK registry. Endoscopy. 2015;47:980–987. doi: 10.1055/s-0034-1392414. [DOI] [PubMed] [Google Scholar]
- 97.Li N, Pasricha S, Bulsiewicz WJ, Pruitt RE, et al. Effects of preceding endoscopic mucosal resection on the efficacy and safety of radiofrequency ablation for treatment of Barrett's esophagus: results from the United States Radiofrequency Ablation Registry. Dis Esophagus. 2015 doi: 10.1111/dote.12386. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Vilsteren FG, Alvarez Herrero L, Pouw RE, Visser M, et al. Radiofrequency ablation and endoscopic resection in a single session for Barrett's esophagus containing early neoplasia: a feasibility study. Endoscopy. 2012;44:1096–1104. doi: 10.1055/s-0032-1325731. [DOI] [PubMed] [Google Scholar]
- 99.Strauss AC, Agoston AT, Dulai PS, Srivastava A, Rothstein RI. Radiofrequency ablation for Barrett's-associated intramucosal carcinoma: a multi-center follow-up study. Surg Endosc. 2014;28:3366–3372. doi: 10.1007/s00464-014-3629-0. [DOI] [PubMed] [Google Scholar]