Abstract
Emotion regulation theories posit that strategies like reappraisal should impact both the intensity and duration of emotional responses. However, research on reappraisal to date has examined almost exclusively its effect on the intensity of responses while failing to examine its effect on the duration of responses. To address this, we used inverse logit functions to estimate the height and duration of hemodynamic responses to negative pictures when individuals with recent life stress were instructed to use reappraisal either to decrease their negative emotion or to increase their positive emotion (relative to unregulated viewing of negative pictures). Several emotion-generative regions such as the amygdala, thalamus and midbrain exhibited decreases in duration of activation, even when controlling for differences in height of activation. In addition, whereas the amygdala exhibited both decreased activation height and duration when participants reappraised to decrease their negative emotion, it only exhibited decreased duration when participants reappraised to increase their positive emotion. These results indicate that emotion regulation alters the temporal dynamics of emotional responding and that models of reappraisal should accommodate whether reappraisal influences the height of activation, duration of activation or both, which may change based on the goal of the reappraisal strategy being used.
Keywords: emotion regulation, amygdala, temporal dynamics, medial prefrontal cortex, reappraisal, stress
While emotions often facilitate adaptive responses (Tooby and Cosmides, 1990), there are times when our emotions are of the wrong type or intensity for a given situation, and therefore require that we regulate them (Gross, 2015). Cognitive reappraisal is one such emotion regulation strategy that involves re-thinking, re-evaluating or re-interpreting a potentially emotional situation in order to change its meaning (Lazarus and Folkman, 1984; Giuliani and Gross, 2009). Cognitive reappraisal can be used to successfully decrease many aspects of emotional responding, including self-reported negative affect (Gross, 1998; Jackson et al., 2000), peripheral psychophysiological responding (Giuliani et al., 2008; McRae et al., 2012; Jamieson et al., 2013), and activation of emotion-generative brain regions (Ochsner et al., 2004; Goldin et al., 2008; McRae et al., 2010; Ochsner et al., 2012). For example, several studies show that the use of cognitive reappraisal is associated with reduced activation in both amygdala and insula regions (Ochsner et al., 2012; Buhle et al., 2014). Cognitive reappraisal has also been shown to engage cognitive control regions like the medial prefrontal cortex (MPFC) and lateral PFC (Buhle et al., 2014; Ochsner et al., 2012).
Our understanding of the effects of reappraisal on emotion is almost entirely based on how it changes one characteristic of the emotions: the peak intensity of an emotional response. However, emotions unfold over time (Davidson, 1998; Hemenover, 2003; Verduyn et al., 2009), and a primary role of emotion regulation should be to shorten the duration of emotional responding by decreasing its intensity over time (Thompson, 1990). Supporting this formulation, a few behavioral studies have shown that certain types of emotion regulation, such as reflecting on a negative experience from the perspective of an observer, can decrease the duration of self-reported emotional episodes (Verduyn et al., 2012). In this study, we investigate how reappraisal impacts the duration of responding in the brain as measured by the blood-oxygen level dependent signal (BOLD). This approach has several advantages over behavioral studies of emotion duration including better temporal resolution, not needing to rely on self-reports of emotion duration, and the ability to measure the duration of both emotional processes (as represented in core emotional regions) and emotion-regulatory processes (as represented in reappraisal-related regions).
Importantly, although the duration of the BOLD response and its height (or peak, amplitude) are related to the overall magnitude of a response, they can be dissociated (Lindquist and Wager, 2007), and recent studies have demonstrated the importance of measuring the duration of emotional responses in the brain in addition to the height of those responses. For example, investigators found that differences in people’s self-reported intensity of an emotional stimulus was better predicted by the duration than by the height of activity in the rostral medial prefrontal cortex (rMFC; Waugh et al., 2010). Furthermore, the duration of emotion-related neural responding predicts consequential individual differences in emotionality such as neuroticism (Schuyler et al., 2014) and depression (Siegle et al., 2002). Indeed, in the Schuyler et al., 2014 study, neuroticism better predicted the duration of neural responding in the amygdala than it did the initial height of responding in the amygdala when viewing emotional faces.
Despite these recent advances in assessing the duration of emotion-related neural measures, assessing duration has been relatively rare in the neuroscience of emotion regulation literature. One study found that one form of emotion regulation—strategically maintaining an emotional state—increased the duration of activation in emotion-regulatory regions such as the dorsomedial prefrontal cortex and lateral prefrontal cortex (Waugh et al., 2014). However, there have been no neuroimaging studies yet that test a fundamental principle of emotion regulation theory—that using emotion regulation to decrease emotion decreases the duration of responding in regions of the brain associated with emotional reactivity. We test that hypothesis in the current study. In addition, we test whether the emotion-regulatory processes involved in reappraisal lead to similar increases in duration in cognitive control regions of the MPFC and lateral PFC as was found when people maintained their emotional states (Waugh et al., 2014).
Reappraisal can refer to a great number of relatively heterogeneous processes (Ochsner et al., 2004; McRae et al., 2012). One important distinction in the reappraisal of negative stimuli is the emotional goal: whether or not individuals are reappraising to minimize their negative emotional responses to the situation or to maximize their positive emotional responses to the situation (i.e., positive reappraisal or reframing; McRae et al., 2012; Shiota and Levenson, 2012). The few studies that have distinguished between these goals show that using reappraisal to increase positive emotion is as effective as using reappraisal to decrease negative emotion at reducing self-reported negative emotion, but that increasing positive emotion does not decrease emotional arousal as measured by skin conductance responses (McRae et al., 2012) and cardiac reactivity (Shiota and Levenson, 2012). Thus, relative to using reappraisal to decrease negative emotion, when participants use reappraisal to increase positive emotion, we might expect to see fewer decreases in the height and/or duration of activation in arousal-related regions (e.g. amygdala, anterior cingulate cortex, insula; Critchley et al., 2001; Craig, 2002; Phan et al., 2004; Critchley, 2005).
In this study, we used inverse logit tools (Lindquist and Wager, 2007) to model the hemodynamic response so that we could estimate the duration (width) of the neural response in multiple regions of the brain. These represent the first tests of the effect of reappraisal on the duration of the hemodynamic response to an emotional stimulus. We also examined the differential impact of two types of reappraisal on the height and duration of the hemodynamic response in emotion-generative and emotion-regulatory regions. We predicted that cognitive reappraisal would decrease the duration of activation in emotion-responsive regions such as the amygdala and insula and increase the duration of activation in emotion-regulatory cognitive control regions such as the MPFC and lateral PFC. We also predicted that using an increase positive regulation strategy would lead to a smaller reduction in the height and or width of activation in arousal-related regions (e.g. amygdala, insula, ACC) than would using a decrease negative regulation strategy. Lastly, we conducted exploratory correlations to examine whether reappraisal-induced changes in height or duration of activation better predict reappraisal-induced changes in behavioral responses (e.g. decreased negative affect, increased positive affect).
Methods
Participants
Forty-seven female participants between the ages of 19 and 35 (M = 27.98, SD = 4.22) were recruited in the Denver Metropolitan Area through postings on online bulletins for a larger study on stress and completed the entire experimental procedure. As part of a separate project examining the influence of recent stress on emotion regulation, participants were recruited if they had experienced a stressful life event (e.g. loss of a loved one, job or home; severe illness or injury; divorce, separation or breakup of a serious romantic relationship; see Supplementary Table 1 for a breakdown of the types of stressors reported by the participants) in the 6 weeks prior to screening. Potential participants were not included in the study if they (a) had been hospitalized for emotional reasons in the past six months, (b) had attempted suicide in the past six months, (c) had drug dependency, (d) had past diagnosis of any disorders with psychotic features, (e) were below age 18 or above age 35 or (f) had any non-MRI compatible conditions (e.g. metal in body, left-handed). Although portions of these data were featured in another published study (see Davis et al., 2014 for more information about this sample), the current study addressed a substantially different theoretical question, which required the analysis of additional experimental conditions, and the use of a different analytical technique (the inverse logit analyses).
Table 1.
Activation in emotion regulation task
| Regions identified in baseline localizer | x | y | z | voxels | LNg > LNu | DNg > LNg | UPo > LNg | DNg > UPo |
|---|---|---|---|---|---|---|---|---|
| Negative > Neutral: Height | ||||||||
| L Posterior Superior Temporal Gyrus/Fusiform Gyrus | −41 | −46 | −8 | 2746 | + Hw | − hW | − hW | |
| R Posterior Superior Temporal Gyrus/Fusiform Gyrus | 43 | −52 | −8 | 1472 | + Hw | − H | + H | |
| L Superior Temporal Gyrus | −51 | 4 | −16 | 82 | + Hw | − hW | − W | − h |
| R Middle Insula | 35 | 10 | −12 | 93 | − hW | − hW | ||
| L Middle Frontal Gyrus/Inferior Frontal Gyrus | −43 | 28 | 10 | 1572 | + Hw | |||
| Thalamus/Midbrain | −11 | −16 | −4 | 499 | + hW | − W | − W | |
| L Middle Insula | −33 | 4 | −14 | 78 | + hw | − hW | − hW | |
| R Middle Frontal Gyrus/Inferior Frontal Gyrus | 37 | 34 | −6 | 433 | + hw | + Hw | + Hw | |
| Rostral Medial Prefrontal Cortex | 1 | 52 | 28 | 1007 | + Hw | + H | ||
| Posterior Cingulate Cortex | −3 | −44 | 24 | 659 | + hw | |||
| R Middle Frontal Gyrus | 41 | 28 | 20 | 169 | + hW | + Hw | + H | |
| Anterior Insula/Inferior Frontal Gyrus | 33 | 14 | 16 | 44 | + H | |||
| Neutral > Negative: Height | ||||||||
| L Posterior Superior Temporal Gyrus/Fusiform Gyrus | −51 | −44 | −14 | 434 | + Hw | − W | − hW | |
| R Posterior Superior Temporal Gyrus/Fusiform Gyrus | 47 | −52 | 0 | 200 | + Hw | |||
| Midbrain | −3 | −14 | −12 | 52 | + HW | − W | − W | |
| Ventral Medial Prefrontal Cortex | 3 | 46 | −10 | 46 | − hW | |||
| Posterior Cingulate Cortex | 1 | −40 | 22 | 113 | + hW | + H | ||
| Negative > Neutral: Width | ||||||||
| L Posterior Superior Temporal Gyrus/Fusiform Gyrus | −43 | −46 | −6 | 1475 | + Hw | − hW | − hW | |
| R Posterior Superior Temporal Gyrus/Fusiform Gyrus | 39 | −50 | −16 | 244 | + HW | − H | + H | |
| R Middle Frontal Gyrus/Inferior Frontal Gyrus | 39 | 32 | −8 | 94 | + hw | + Hw | + Hw | |
| L Inferior Frontal Gyrus/Orbitofrontal Cortex | −45 | 28 | 12 | 735 | + Hw | |||
| Midbrain | −3 | −18 | −6 | 89 | + hW | −W | ||
| Nucleus Accumbens | −17 | 2 | −8 | 64 | + hW | − hw | −w | |
| R Posterior Temporal Gyrus | 45 | −50 | 6 | 335 | + Hw | |||
| Thalamus | −5 | −4 | 6 | 60 | + hW | −W | ||
| Rostral Medial Prefrontal Cortex | 1 | 54 | 30 | 593 | + HW | + Hw | ||
| R Middle Frontal Gyrus/Inferior Frontal Gyrus | 45 | 30 | 18 | 104 | + hW | + Hw | + H | |
| Posterior Cingulate Cortex | −1 | −44 | 24 | 427 | + hW | + H | ||
| Dorsal Medial Prefrontal Cortex | −9 | 24 | 46 | 48 | + hW | + Hw | + Hw | |
| Neutral > Negative: Width | ||||||||
| L Fusiform Gyrus | −33 | −50 | −26 | 115 | + HW | − hw | + h | |
| Ventral Medial Prefrontal Cortex | 1 | 50 | −10 | 243 | − HW | |||
| L Posterior Superior Temporal Gyrus/Fusiform Gyrus | −51 | −38 | −4 | 120 | + Hw | − hW | − hW | |
| Lingual Gyrus | 11 | −68 | −6 | 42 | + Hw | − H | + h | |
| Posterior Cingulate Cortex | 1 | −42 | 22 | 85 | + hW | + H | ||
| A priori ROIs | ||||||||
| R. Amygdala | 29 | 0 | −20 | 367 | + hW | − hW | − W | |
| L. Amygdala | −23 | −2 | −18 | 323 | + hw | − hW | − W | |
Regions that were selected from the baseline functional localizer task (as well as two amygdala ROIs) and then assessed for their responsiveness to the regulation task. + = positive, − = negative, H = significant height of activation when controlling for width, h = significant height of activation, but rendered non-significant when controlling for width, W = significant width of activation when controlling for height, w = significant width of activation, but rendered non-significant when controlling for height.
Procedure
Participants first completed an initial phone screening interview to assess eligibility. Next, eligible participants completed a battery of questionnaires online that assessed personality, emotional habits and demographic information. Next, participants came to the fMRI session, which took place at The University of Colorado Health Sciences Center's Brain Imaging Center. Participants first completed a practice reappraisal task outside of the scanner. In the scanner, they completed a baseline functional localizer task followed by a reappraisal task. Upon completion of the session, participants were debriefed and paid $40. All procedures were in compliance with the local Institutional Review Board.
Tasks
Standardized laboratory paradigms were used to measure negative emotional reactivity and ability to regulate emotion with reappraisal while BOLD signal was being acquired (McRae et al., 2010). These procedures used standardized negative and neutral images from the International Affective Picture System (IAPS; Lang et al., 1997). Given that our participants were recently stressed and potentially more emotionally reactive to the most highly arousing IAPS images (e.g. those featuring gore, mutilation), we excluded these from our set and supplemented the set with another set of images normed on the same scale as the IAPS. These images were combined with the IAPS and normative ratings from both sets were used to ensure comparable overall valance and arousal ratings across conditions and counterbalanced orders. The valence (1—least pleasurable to 9—most pleasurable) and arousal ratings (1—lowest arousal to 9—highest arousal) of the images were as follows: negative image valence rating, M = 2.89, SD = 0.74; negative image arousal rating, M = 5.13, SD = 1.36; neutral image valence rating, M = 4.83, SD = 0.26; neutral image arousal rating, M = 2.58, SD = 1.39.
Cognitive reappraisal task.Before beginning, participants were trained to 1) look at, react naturally and not reappraise to images that followed the instruction to ‘LOOK’ 2) decrease their negative emotional response to images that followed the instruction to ‘CHANGE’ with a blue background or 3) increase their positive emotional response to images that followed the instruction to ‘CHANGE’ with a purple background. Examples of reappraisals were provided (e.g. ‘the situation is not as bad as it first seemed’ or ‘he/she is back to normal now’ for Decrease Negative and ‘he will be grateful that he had this experience’ or ‘their relationship will be stronger than before’ for Increase Positive). To ensure that they understood the instructions and to minimize learning effects during scanning, all participants were required to generate at least two correct reappraisals of each type before advancing. Participants also completed a practice block of each instruction type without the experimenter present and were given the opportunity to ask questions before beginning the task in the scanner.
During the reappraisal task in the scanner, for each trial, participants viewed an instruction screen followed by a neutral or negative image. Neutral images always followed the instruction to ‘LOOK’ (cued with a green colored border to indicate Look). One-third of the negative images followed the instruction to ‘LOOK’, another third of the negative images followed the instruction to ‘CHANGE’ (cued with a blue colored border to indicate Decrease Negative), and the final third of the negative images followed the instruction to ‘CHANGE’ (cued with a purple colored border to indicate Increase Positive). Although Look Neutral, Look Negative and Change Negative trials were pseudo-randomized within blocks, to minimize the burden of rapidly switching between Change types, the type of Change was consistent within blocks, with participants following instructions for the same regulation condition in blocks 1 and 3 and 2 and 4. Order of training and presentation (which were always matched) was counterbalanced across participants, as was assignment of particular negative images to the ‘LOOK’ and ‘CHANGE’ conditions.
For each trial, the instruction screen was presented for a variable duration with an average of 2 seconds (500–3000 ms), the image was presented for 7 s, the question ‘Rate current NEGATIVE feeling?’ and a four-point emotion rating scale was presented for 3.5 s (1 = not at all, 4 = very much), the question ‘Rate current POSITIVE feeling?’ four-point emotion rating scale was presented for 3.5 s and the question ‘Rate current AROUSAL?’ four-point emotion rating scale was presented for 3.5 s. Between trials, a slide directing participants to ‘RELAX’ was presented for 2 s. The variable duration of the instruction screen provided the inter-stimulus jitter necessary to model each picture viewing period (Goghari and MacDonald, 2008). One hundred and twelve trials (28 trials each of look neutral, look negative, decrease negative and increase positive) were presented for each participant. As a manipulation check, after the scanning session, participants reported the ease with which they were able to generate the appropriate reappraisals for the task (see Supplementary information for details).
Baseline emotional reactivity localizer.In the scanner, but before the reappraisal task began, participants completed a baseline task designed to provide an emotion functional localizer that would identify the emotion-sensitive regions that could then be used as ROIs in the reappraisal task. The baseline task was identical in trial structure to the reappraisal task detailed above except each picture was preceded by a fixation cross (e.g. +, cued by a black border) in place of an instruction (e.g. ‘LOOK’ or ‘CHANGE’). Participants completed 28 trials of the baseline task (14 negative-image trials; 14 neutral-image trials) and completed the same three ratings (negative, positive and arousal). Different negative images were used in the baseline task and the regulation task.
Scan parameters
During the baseline reactivity and regulation trials, whole-brain echo-planar images were obtained. Data were collected with a 3.0 T GE MR System scanner (General Electric Healthcare, Wakesha, WI) using a standard quadrature head coil. Functional images were acquired by using gradient-echo T2* blood oxygenation level-dependent (BOLD) contrast technique (TR = 2000ms, TE = 28 ms, matrix = 64 × 64, field of view = 22x22cm2). Each volume included 32 slices (4mm thick, no gap). High resolution SPGR scans were acquired following the completion of all functional scans for anatomical normalization.
Image preprocessing
Standard pre-processing steps were completed in AFNI. Functional images were corrected for motion across scans using the last functional scan as reference and then automatically co-registered to each subject’s high resolution anatomical. Anatomical images were then normalized to a structural template image, and normalization parameters were applied to the functional images. We used a Talairach template in AFNI for normalization. Images were re-sampled to a resolution of 2 × 2 × 2 mm, smoothed spatially with a 4-mm filter, converted to percent signal change, and then converted to statistical parametric mapping (SPM) format. Lastly, to aid the inverse logit analyses, each voxel’s time-series was temporally smoothed (6 s kernel; Waugh et al., 2014) and mean-centered. Because the trials were of variable duration, trials often started off-TR, however, for the logit analyses to work, we rounded the onset of each trial to the closest whole TR.
Functional analysis
Because we were interested in the effects of emotion regulation on emotional responding, we modeled the picture-viewing period for each trial. To assess the duration of BOLD activation, we used SPM8 (Wellcome Department of Cognitive Neurology) and custom scripts to conduct inverse logit (IL) modeling, a flexible time-varying hemodynamic response function (HRF) estimation procedure (Lindquist and Wager, 2007) that has been shown in several previous studies to more accurately model BOLD activation patterns that differ in their duration (Lindquist and Wager, 2007), including emotion-related activation (Waugh et al., 2010; Waugh et al., 2014). Three parameters (height, time to inflection and slope of inflection) were estimated for each of the IL functions using a fast deterministic optimization algorithm that minimized sum of squares error (see Waugh et al., 2014 for initial parameters). The resulting IL functions were then summed to create the HRF from which the height (H) and width at half-height (W) of the BOLD responses were estimated.
To constrain our analyses to emotion and emotion-regulation related regions, we employed a combination of a priori anatomical masking and functional localizer methods. Baseline functional localizer.First, to limit our regulation analyses to regions involved in emotion as exhibited by this sample of participants, we used participants’ responses to the baseline reactivity scan as a functional localizer. Those regions that exhibited differential activation to negative vs. neutral images during this scan served as ROIs when examining the responses during the emotion regulation scans. To do this, we created contrast images for both height and width estimates to examine the effect of negative reactivity [Look Negative vs. Look Neutral] during the baseline scans. For the group analysis, we used robust regression at the 2nd level (Wager et al., 2005), which minimizes the influence of outliers, to conduct random effects analyses on the contrasts. To further limit the regions found during the baseline reactivity scan to regions identified as responsive to emotion more generally (Waugh et al., 2010), we used a voxelwise analysis approach in regions defined by a large ‘emotional brain’ mask derived from a meta-analysis of fMRI activations combined across various emotion tasks (Wager et al., 2008a). In this voxelwise approach, results for the baseline reactivity contrast were thresholded using a Monte Carlo simulation that, accounting for spatial smoothness in the data (full-width half-maximum [FWHM] estimated using AFNI’s 3dFWHMx; Cox, 1996), calculated a cluster size of k = 42 needed for a per-voxel threshold of .001 to render a cluster-level corrected p-value of .05 (AlphaSim; Ward, 2000).
Regulation scans.We used those regions that showed differential activity during the baseline functional localizer scans as ROIs for the regulation scans. Because we were interested specifically in the amygdala, we also added the left and right amygdala as ROIs, which were defined anatomically using the WFU pickatlas (+1 dilation; Maldjian et al., 2003). For each of these ROIs derived from the functional localizer as well as for the amygdala ROIs, we extracted and averaged the height and width estimates across the voxels for each condition in the regulation scans: Look Neutral, Look Negative, Decrease Negative and Increase Positive. We then conducted pairwise t-tests on the average height and width estimates to determine if there were significant (P < 0 .05) effects of emotion regulation [Decrease Negative vs Look Negative] and [Increase Positive vs Look Negative] and differences between the two styles of emotion regulation [Decrease Negative vs Increase Positive].
Post-hoc analyses. For those clusters that exhibited significant differences between conditions in both height and width of activation, we sought to determine whether their height and/or width of activation differences remained significant when controlling for the other. To accomplish this, we first calculated difference scores between the conditions of interest (e.g. Decrease Negative—Look Negative) for both height and width of activation. Then, for each cluster, we regressed its height difference scores on its width difference scores. The resulting intercept from this regression equation represents whether height difference scores are significantly different from zero controlling for width difference scores (i.e., on the regression line where the width difference score is zero). Regressing width difference scores on height difference scores then provides a test of whether width differences are still significant when controlling for height differences.
Exploratory brain-behavior correlations. To explore possible brain-behavior correlations, we used a cluster-wise strategy that was consistent with the above analyses in which we used each cluster’s average height and width of activation as the unit of data. We first calculated difference scores between the conditions of interest for each of the three behavioral indices (positive valence, negative valence and arousal) and correlated these with the corresponding height and width activation contrasts for all of the clusters (e.g. Decrease Negative—Look Negative for negative affect correlated with Decrease Negative—Look Negative for height of activation in some region). We considered these difference scores to index reappraisal success, as they reflect either decreased negative emotion or increased positive emotion when participants were reappraising the stimuli relative to when they were instructed to just look at the stimuli. We conducted a permutation test in which for each permutation, participants’ scores (self-report, BOLD responses) were randomly assigned throughout the sample of participants and 216 correlations between the self-report and BOLD responses were calculated. We recorded the maximum correlation obtained for each of the 5000 permutations, which created a non-parametric distribution of possible correlations under the null hypothesis that the correlation between these responses is zero. With an alpha of .05, we determined what minimum absolute correlation (±0.50) would be needed to reject the null hypothesis.
Results
Self-reported affect and arousal
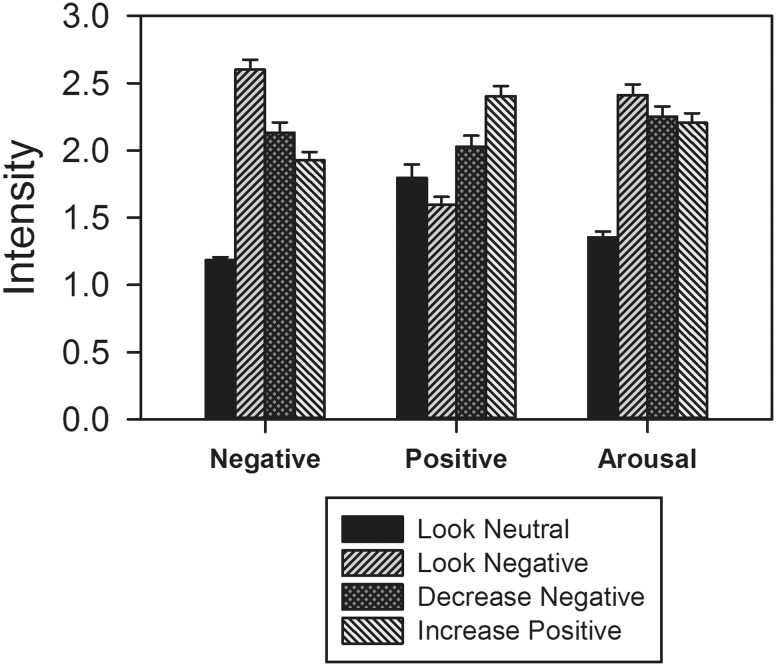
Negative affect. Participants successfully reduced their negative emotion (M = 2.13, SD = 0.08) in the decrease negative condition, compared to the look negative condition (M = 2.60, SD = 0.51), t(46) = 9.05, P < 0.001, as well as in the increase positive condition (M = 1.93, SD = 0.42) compared to the look negative condition, t(46) = 12.63, P < 0.001 (Figure 1). In addition, reductions in negative emotion were greater in the increase positive condition than in the decrease negative condition (t(46) = 5.51, P < 0.001).
Fig. 1.
Behavioral indices of regulation success. Error bars are SE.
Positive affect. Participants also successfully enhanced their positive emotion in the decrease negative condition (M = 2.03, SD = 0 .57), compared to the look negative condition (M = 1.59, SD = 0.41), t(46) = 8.10, P < 0.001, as well as in the increase positive condition (M = 2.40, SD = .51), compared to the look negative condition, t(46) = 14.74, P < 0.001 (Figure 1). In addition, increases in positive emotion were greater in the increase positive condition than in the decrease negative condition, t(46) = 8.11, P < 0.001.
Arousal. Participants successfully reduced their arousal in the decrease negative condition (M = 2.25, SD = 0.51), compared to the look negative condition (M = 2.41, SD = 0.55), t(46) = 3.14, P = 0.003, as well as in the increase positive condition (M = 2.21, SD = 0 .48), compared to the look negative condition, t(46) = 4.52, P < 0.001 (Figure 1). Arousal ratings were not significantly different between the increase positive and decrease negative conditions, t(46) = 1.31, P = 0.197.
Baseline functional localizer
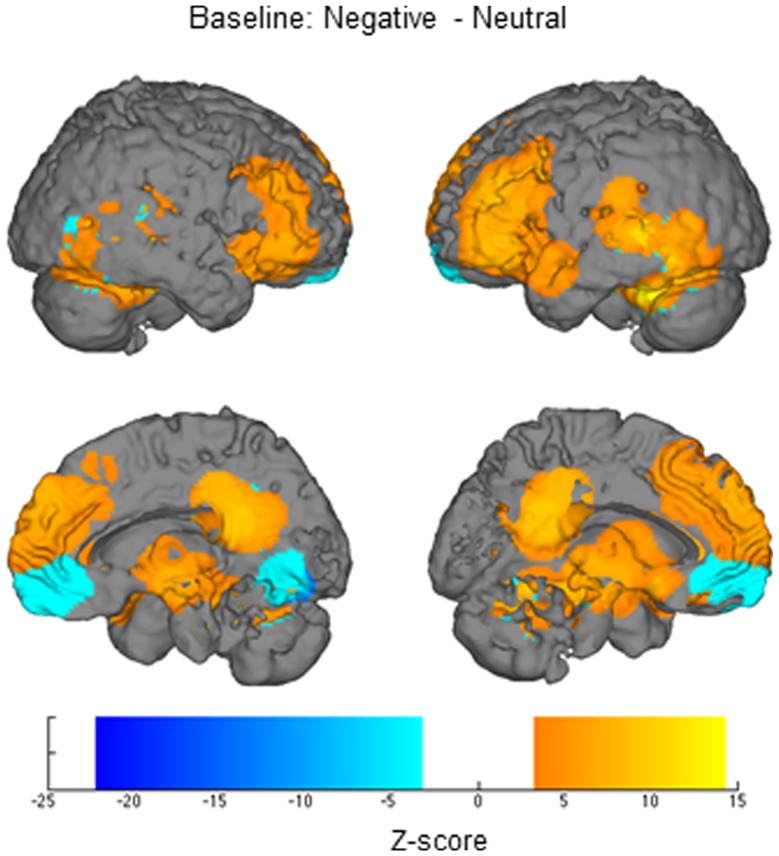
Negative vsneutral.Relative to viewing neutral images, when viewing negative images, participants exhibited greater activation (height and/or width) in many areas, including rostral and dorsal MPFC, posterior cingulate, occipital cortex, parahippocampal gyrus, right posterior insula, thalamus, lateral PFC (Figure 2) and the anatomical right amygdala ROI. For the reverse contrast, participants exhibited less activation when viewing negative images than when viewing neutral images in ventral MPFC, cuneus, posterior cingulate and superior temporal gyrus. These regions then formed the ROIs for examining height and width of activation differences as a function of reappraisal (Figure 2).
Fig. 2.
Regions identified when comparing responses to negative and neutral stimuli in the baseline functional localizer.
Emotion regulation fMRI
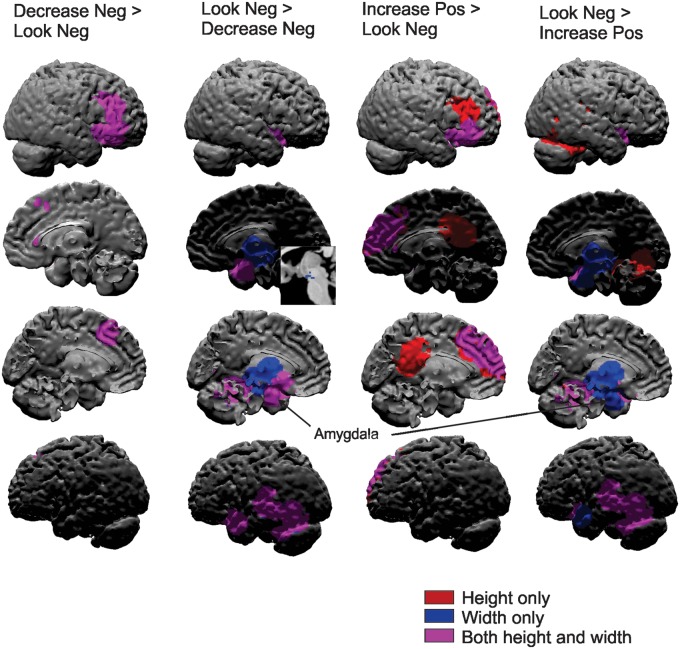
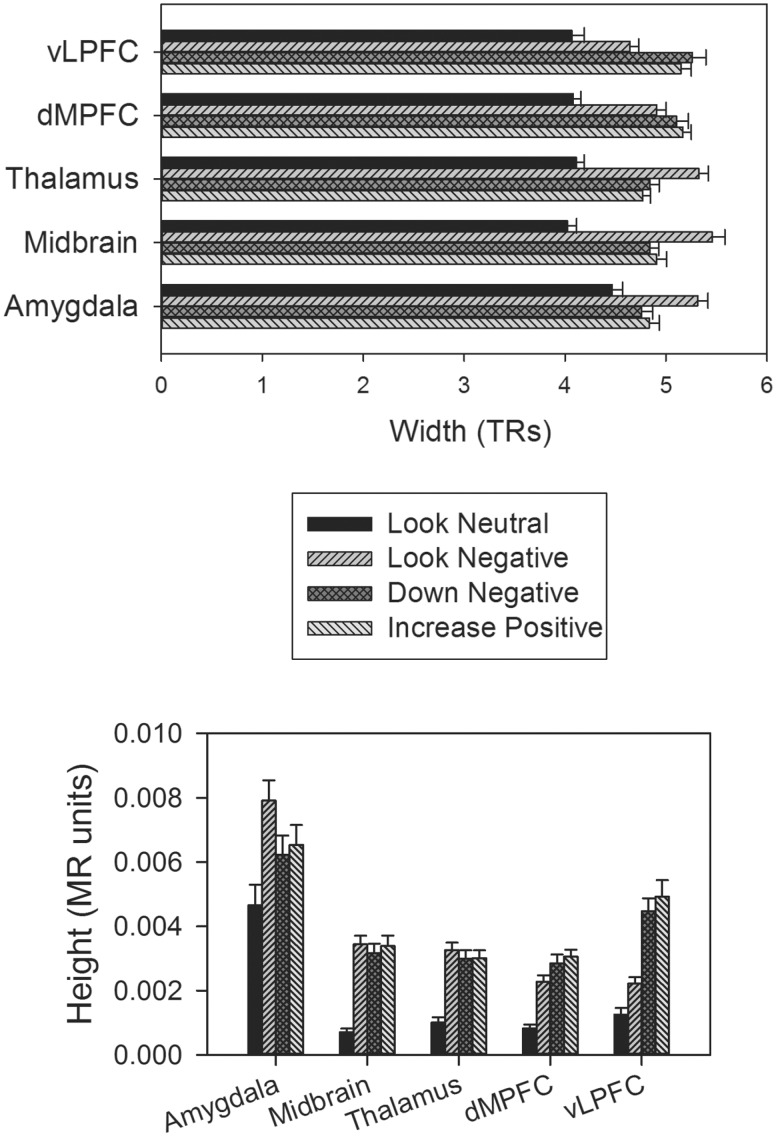
Decrease negative vslook negative.Relative to just viewing images in the look negative condition, when participants were instructed to decrease their negative emotion, they exhibited increased height and width of activation in the right middle/inferior frontal gyrus and dorsal MPFC (Figures 3 and 4; Table 1). For both of these regions, height of activation remained significant when controlling for width but not vice versa. For the reverse contrast, when decreasing their negative emotion, participants exhibited decreased height and width of activation in the bilateral amygdala ROI, nucleus accumbens, middle insula and left superior temporal gyrus/fusiform gyrus. For the amygdala, middle insula and superior temporal gyrus, width was the stronger predictor—remaining significant when controlling for height of activation (but not vice versa). Of note, participants exhibited only decreased width of activation (but not height) in the thalamus and midbrain (Figures 3 and 4; Table 1).
Fig. 3.
Regions showing significant height and/or width of activation differences between the regulation (Decrease Neg and Increase Pos) and non-regulation conditions (Look Neg). Neg = negative, Pos = positive.
Fig. 4.
Width and height of activations for key regions across the regulation and non-regulation conditions. Amygdala (29, 0, −20), Midbrain (−3, −14, −12), Thalamus (−11, −16, −4), dMPFC = dorsal medial prefrontal cortex (−9, 24, 46), vLPFC = ventrolateral prefrontal cortex (37, 34, −6). Error bars are SE.
Increase positive vslook negative.Participants exhibited increased height and width of activation in several regions, most of which were also engaged during the decrease negative strategy (vs. look negative) including the middle frontal gyrus and dorsal MPFC. These regions also showed significant height activation differences when controlling for width, but not vice versa. Additional regions, not observed during the decrease negative strategy (vs look negative) included the posterior cingulate, which showed significantly increased height only (not width) to the increase positive regulation condition and a rostral MPFC region, which showed increased height and width of activation to the increase positive condition, but only height remained significant when controlling for the other (Figures 3 and 4; Table 1).
For the reverse contrast, regions that exhibited similar decreases in activation for the two strategies included the middle insula (height and width), superior temporal gyrus (height and width), midbrain (width only) and thalamus (width only). The middle insula and superior temporal gyrus again exhibited significant differences in width of activation when controlling for height, but not vice versa. Unlike in the decrease negative condition, when participants were using the increase positive regulation strategy the amygdala ROI exhibited only decreased width (and not height) of activation (Figures 3 and 4; Table 1).
Decrease negative vsincrease positive.When directly comparing the two strategies (decrease negative vs increase positive), only a few regions showed significant differences in activation. When decreasing negative emotion (vs increasing positive), participants exhibited increased height of activation in the lingual gyrus, right posterior superior temporal gyrus and left fusiform gyrus and decreased height of activation in the left superior temporal gyrus (Figures 3 and 4; Table 1).
Exploratory brain-behavior correlations
No clusters exhibited significant brain-behavior correlations.
Discussion
Emotion theorists have long posited that emotion regulation decreases the duration of emotional responding, and the current data provide the first evidence of this in the brain. Relative to unregulated viewing of negative images, when individuals were asked to change their emotional states using cognitive reappraisal, we observed a significant shortening of the response in the amygdala, insula and superior temporal gyrus and a significant lengthening of the response in dorsomedial and ventrolateral prefrontal cortices. In addition, there were more subtle height and duration of activation differences between when participants reappraised with the goal of decreasing negative emotion and when they reappraised with the goal of increasing positive emotion. Notably, whereas the amygdala exhibited decreased activation in both duration and height when participants reappraised to decrease their negative emotion, it only exhibited decreased duration when participants reappraised to increase their positive emotion.
Duration of the hemodynamic response in emotion generative regions
We report for the first time that the use of cognitive reappraisal results in significant shortening of the hemodynamic response in multiple regions implicated in emotion generation such as the amygdala, thalamus, midbrain and insula. Indeed, even for the regions that showed decreases in both height and duration of activation, a large majority of these regions (including the amygdala) exhibited significant decreases in duration of activation when controlling for height and height became non-significant when controlling for duration, suggesting that reappraisal may act primarily on reducing the duration of activation in these regions. Previous studies of the duration of the hemodynamic response indicate that the duration of the response may be related to the intensity of the emotional stimulus (Waugh et al., 2010), suggesting that shortening the duration in these regions may be related to successful down-regulation of the intensity of the stimulus. However, in the Waugh et al., 2010 study, only the insula and thalamus exhibited increased duration of activity in response to increasing intensity (not the amygdala or midbrain), and in the present study, there were no significant correlations between decreasing activation in these regions and decreasing emotional intensity. A more parsimonious explanation of these findings is that decreased duration of activation corresponds to decreased time spent engaging with the eliciting emotional stimulus, which may or may not be reflected in the self-reported intensity of that stimulus. In turn, this decreased duration of time spent engaging with the stimulus may have downstream effects on emotion-related behavior and physiology. For example, decreased duration of amygdala activation may translate into a shorter amount of time that the amygdala is sending modulatory signals to a number of peripheral physiological systems (heart rate, blood pressure, skin conductance; Phelps and LeDoux, 2005). Therefore, the ability to use reappraisal to shorten duration may correspond to lesser wear-and-tear on physiological systems.
Notably, whereas the amygdala exhibited decreases in both height and duration of response (at least in the decrease negative condition), the thalamus and midbrain only exhibited decreases in activation duration – patterns that would have either been overlooked or misinterpreted as changes in height using standard, time-inflexible canonical HRFs. The thalamus and midbrain (especially the peri-aqueductal gray; PAG) are commonly activated in response to emotional stimuli (Lindquist et al., 2012; Wager et al., 2008a; Wager et al., 2009) and sometimes during emotion regulation (Wager et al., 2008b), but not consistently (Buhle et al., 2014). The thalamus provides a relay through which sensory and other types of information can reach core emotion areas like the amygdala and orbitofrontal cortex and emotional regulation areas like the mPFC and ACC. Taken together, this suggests that decreasing the duration of activation in the thalamus during reappraisal might be an effective way of limiting emotion-related information from reaching core emotional regions. One of those regions receiving information from the thalamus is the midbrain (specifically, the PAG; Wager et al., 2009), which is consistent with this region also exhibiting decreased duration of activity during reappraisal.
Increasing duration of activation in emotion regulative regions
Reappraising negative stimuli also led participants to exhibit increased duration and height of activation in various regions associated with emotion regulation. Participants exhibited increased duration of activation in the right vLPFC and the dMPFC. The vLPFC is often engaged during emotion regulation studies and has been shown to be associated with labeling emotions (Lieberman et al., 2007), reappraisal of emotion (Ochsner et al., 2012) and correlated with reappraisal success (Wager et al., 2008a). Given that the vLPFC showed increased height and width of activation, this pattern most likely reflects overall increased activation while participants reappraised the stimuli. The dMPFC is also evoked often during emotion regulation (Ochsner and Gross, 2005; Waugh et al., 2014) and has been found to predict reappraisal success via functional connections with the nucleus accumbens (Wager et al., 2008a). Notably, the dMPFC has been found to increase in activation when participants are instructed to increase (Ochsner et al., 2004), maintain (Waugh et al., 2014) or decrease (McRae et al., 2010; Otto et al., 2014) their emotional states, suggesting that this region is not just a down-regulatory region, but perhaps monitors regulation success and/or conflict, whatever the regulation goal might be. If this ‘ongoing monitoring’ interpretation is appropriate, it would explain why the dMPFC reliably exhibits extended duration of processing when regulating emotion (Waugh et al., 2014).
Reappraising to decrease negative emotion vs reappraising to increase positive emotion
Although the amygdala exhibited decreased duration of activation regardless of reappraisal instruction type, it exhibited decreased height of activation only when participants were instructed to decrease their negative emotion, not when they were instructed to increase their positive emotion, a pattern that only emerges because we used HRFs that could separately estimate height and width. This result adds to previous psychophysiological studies that demonstrate that increasing positive (relative to decreasing negative) reduces negative affect, this strategy induces a less pronounced reduction in skin conductance response and an increased cardiac response associated with engagement with the stimulus (McRae et al., 2012; Shiota and Levenson, 2012). Together, these findings suggest that increasing positive emotion may involve more engagement with the emotional stimulus relative to decreasing negative emotion. Supporting this formulation is the current finding that the rostral MPFC exhibited increased activation only when participants were instructed to increase their positive emotion. The rostral MPFC has been theorized to be associated with the making (Roy et al., 2012) and elaboration (Waugh et al., 2014) of emotional meaning, which, in the current study, may be more prevalent in the increase positive reappraisal condition than the decrease negative condition. Although greater engagement with and elaboration on the stimulus might be expected to hinder successful emotion regulation, participants reported the strongest changes in negative and positive emotion while increasing positive affect. This suggests that a robust transformation of emotional meaning may be possible when individuals remain engaged with the stimulus for a longer period of time. This is consistent with observations that at least in some contexts, reappraisal is to generally more successful at achieving its emotional goal than other types of emotion regulation that involve disengaging from the stimulus, like distraction, even if reappraisal results in amygdala changes that are smaller in magnitude (McRae, 2010; but see Sheppes and Meiran, 2008).
Limitations and future directions
Currently, most methods of modeling the hemodynamic response do not separately estimate the height and width of the response. Therefore, prior to this study, it was unclear whether the reductions observed in emotion-related regions reflect decreases in the height of the response, decreases in the duration or both. However, although we were able to estimate the width of the hemodynamic response, it should be noted that the hemodynamic response is a cardiovascular response in the brain. Although the hemodynamic response is known to be somewhat coupled to action potentials in the nearby neural regions (Nir et al., 2007), we do not know whether or not the neural engagement actually lasted for a longer time when we observed a greater width. In addition, one limitation of the inverse logit method is that it only provides an estimate for positive-going responses, which precludes the discovery of reliable decreases in activation to the stimuli relative to a pre-stimulus baseline.
The participants in this study were originally recruited based on their having been exposed to recent life stressors (Davis et al., 2014). Experiencing a recent life stressor can impact people’s emotional (Eck et al., 1998; Pike et al., 1998) and physiological responses (Pike et al., 1998; Gump and Matthews, 1999) to unrelated acute stressors/emotional events. There is also evidence that an acute stressor can impact emotion regulation in the moment (Raio and Phelps, 2015) and that the controllability of recent life stressors can moderate the effectiveness of certain emotion regulation strategies (Troy et al., 2013). Although life stress was not a focus of the current study, the current findings may speak to the patterns of neural activation associated with emotion regulation after recent, common life stressors (e.g. divorce, unemployment; see Supplementary Table 1 for a breakdown of the types of stressors reported by the participants), when it might be especially important to regulate emotions effectively. Future studies will be necessary, however, to determine whether this pattern of activation is different in those without a recent life stressor. We also included only female participants in our study to control for potential inter-gender differences in neural responses to emotion and emotion regulation (McRae et al., 2008). Given these participant selection parameters, caution should be used before generalizing the findings from the current study to a broader population and future studies should seek to replicate these findings in a more representative sample.
This work also opens several avenues for future investigations. Much of the previous literature on the duration of affective responses has used continuous measures, for example of subjective emotion or peripheral physiology. Similar models could be used to independently estimate the height, width and delay of those responses. In addition, there are likely interesting individual differences in the height, width and delay of these responses, both when emotional responding is unregulated and when individuals are trying to influence their own response. Of course, the biggest avenue for future investigations is to continue to assess the various temporal dynamics of emotion and emotion regulation to more accurately form models of the underlying functions that may reliably vary as a function of time.
Acknowledgements
We would like to thank James J. Gross on his input on study conceptualization and design, as well as Victoria Floerke, E. Knight Wing, and Yenchen Chang for their support with data collection and management. We also thank Luke Chang and Martin Lindquist with their advice on some of the statistics presented here.
Funding
This research was supported by a grant to Iris B. Mauss and Kateri McRae from the John Templeton Foundation’s Positive Neuroscience project.
Conflict of interest. None declared.
References
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–73. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2002). Opinion: How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3, 655–66. [DOI] [PubMed] [Google Scholar]
- Critchley H.D. (2005). Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology, 493, 154–66. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Mathias C.J., Dolan R.J. (2001). Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron, 29, 537–45. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. (1998). Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion, 12, 307–30. [Google Scholar]
- Davis T.S., Mauss I.B., Lumian D., et al. (2014). Emotional reactivity and emotion regulation among adults with a history of self-harm: Laboratory self-report and functional MRI evidence. Journal of Abnormal Psychology, 123, 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Gross J.J. (2009). Reappraisal In: Sander D., Scherer K. R., editors. Oxford Companion to the Affective Sciences. New York: Oxford University Press. [Google Scholar]
- Giuliani N.R., McRae K., Gross J.J. (2008). The up- and down-regulation of amusement: Experiential, behavioral and autonomic consequences. Emotion, 8, 714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari V.M., MacDonald A.W. (2008). Effects of varying the experimental design of a cognitive control paradigm on behavioral and functional imaging outcome measures. Journal of Cognitive Neuroscience, 20, 20–35. [DOI] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63, 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. (1998). Antecedent and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74, 224–37. [DOI] [PubMed] [Google Scholar]
- Gross J.J. (2015). Emotion Regulation: Current status and future prospects. Psychological Inquiry, 26, 1–26. [Google Scholar]
- Hemenover S.H. (2003). Individual differences in rate of affect change: Studies in affective chronometry. Journal of Personality and Social Psychology, 85, 121–31. [DOI] [PubMed] [Google Scholar]
- Jackson D.C., Maimstadt J.R., Larson C.L., Davidson R.J. (2000). Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology, 37, 515–22. [PubMed] [Google Scholar]
- Jamieson J.P., Mendes W.B., Nock M.K. (2013). Improving acute stress responses: The power of reappraisal. Current Directions in Psychological Science, 22, 51–6. [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (1997). International Affective Picture System (IAPS): Technical Manual and Affective Ratings. NIMH Center for the Study of Emotion and Attention, University of Florida, Gainesville, FL. [Google Scholar]
- Lazarus R.S., Folkman S. (1984). Stress, Appraisal, and Coping. New York, NY: Springer Publishing Company, Inc. [Google Scholar]
- Lieberman M.D., Eisenberg N.I., Crockett M.J., Tom S.M., Pfeifer J.H., Way B.M. (2007). Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science, 18, 421–8. [DOI] [PubMed] [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. (2012). The brain basis of emotion: A meta-analytic review. Behavioral and Brain Sciences, 35, 121–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist M.A., Wager T.D. (2007). Validity and power in hemodynamic response modeling: A comparison study and a new approach. Human Brain Mapping, 28, 764–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. Neuroimage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- McRae K., Ciesielski B., Gross J.J. (2012). Unpacking cognitive reappraisal: Goals, tactics, and outcomes. Emotion, 12, 250–5. [DOI] [PubMed] [Google Scholar]
- McRae K., Hughes B., Chopra S., Gabrieli J.D.E., Gross J.J., Ochsner K.N. (2010). The Neural Bases of Distraction and Reappraisal. Journal of Cognitive Neuroscience, 22, 248–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K., Ochsner K.N., Mauss I.B., Gabrieli J.D.E., Gross J.J. (2008). Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes & Intergroup Relations, 11, 143–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y., Fisch L., Mukamel R., et al. (2007). Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Current Biology, 17, 1275–85. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage, 23, 483–99. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Year in Cognitive Neuroscience, 1251, E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., Misra S., Prasad A., McRae K. (2014). Functional overlap of top-down emotion regulation and generation: An fMRI study identifying common neural substrates between cognitive reappraisal and cognitively generated emotions. Cognitive Affective and Behavioral Neuroscience, 14, 923–38. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Taylor S.F., Welsh R.C., Ho S.H., Britton J.C., Liberzon I. (2004). Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage, 21, 768–80. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48, 175–87. [DOI] [PubMed] [Google Scholar]
- Roy M., Shohamy D., Wager T.D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16, 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler B.S., Kral T.R.A., Jacquart J., et al. (2014). Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Social Cognitive and Affective Neuroscience, 9, 176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G., Meiran N. (2008). Divergent cognitive costs for online forms of reappraisal and distraction. Emotion, 8, 870–4. [DOI] [PubMed] [Google Scholar]
- Shiota M.N., Levenson R.W. (2012). Turn down the volume or change the channel?: Emotional effects of detached versus positive reappraisal. Journal of Personality and Social Psychology, 103, 416–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G.J., Steinhauer S.R., Thase M.E., Stenger V.A., Carter C.S. (2002). Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry, 51, 693–707. [DOI] [PubMed] [Google Scholar]
- Thompson R.A. (1990). Emotion and self-regulation In: Thompson R.A. editor, Socioemotional Development: Nebraska Symposium on Motivation (Vol. 36, pp. 367–467). Lincoln, NE: University of Nebraska Press. [PubMed] [Google Scholar]
- Tooby J., Cosmides L. (1990). The past explains the present: Emotional adaptations and the structure of ancestral environments. Ethology and Sociobiology, 11, 375–424. [Google Scholar]
- Troy A.S., Shallcross A.J., Mauss I.B. (2013). A person-by-situation approach to emotion regulation: Cognitive reappraisal can either help or hurt, depending on the context. Psychological Science, 24, 2505–14. [DOI] [PubMed] [Google Scholar]
- Verduyn P., Van Mechelen I., Kross E., Chezzi C., Van Bever F. (2012). The relationship between self-distancing and the duration of negative and positive emotional experiences in daily life. Emotion, 12(6),1248–63. [DOI] [PubMed] [Google Scholar]
- Verduyn P., Van Mechelen I., Tuerlinckx F., Meers K., Van Coillie H. (2009). Intensity profiles of emotional experience over time. Cognition & Emotion 23(7),1427–43. [Google Scholar]
- Wager T.D., Barrett L.F., Bliss-Moreau E., et al. (2008a). The neuroimaging of emotion In Lewis M., Haviland-Jones J. M., Feldman-Barrett L. (Eds.), Handbook of Emotion. New York, NY: Guilford Press. [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008b). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Keller M.C., Lacey S.C., Jonides J. (2005). Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage, 26, 99–113. [DOI] [PubMed] [Google Scholar]
- Wager T.D., van Ast V.A., Hughes B.L., Davidson M.L., Lindquist M.A., Ochsner K.N. (2009). Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage, 47, 836–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B.D. (2000). Simultaneous inference for fMRI data. Retrieved August, 2007, from http://afni.nimh.nih.gov/afni/docpdf/AlphaSim.pdf.
- Waugh C.E., Hamilton J.P., Gotlib I.H. (2010). The neural temporal dynamics of the intensity of emotional experience. Neuroimage 49, 1699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Lemus M.G., Gotlib I.H. (2014). The role of the medial frontal cortex in the maintenance of emotional states. Social Cognitive and Affective Neuroscience, 9(12),2001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]