Abstract
Self-control is key to success in life. Initial acts of self-control temporarily impair subsequent self-control performance. Why such self-control failures occur is unclear, with prominent models postulating a loss of a limited resource vs a loss of motivation, respectively. Here, we used functional magnetic resonance imaging to identify the neural correlates of motivation-induced benefits on self-control. Participants initially exerted or did not exert self-control. In a subsequent Stroop task, participants performed worse after exerting self-control, but not if they were motivated to perform well by monetary incentives. On the neural level, having exerted self-control resulted in decreased activation in the left inferior frontal gyrus. Increasing motivation resulted in a particularly strong activation of this area specifically after exerting self-control. Thus, after self-control exertion participants showed more prefrontal neural activity without improving performance beyond baseline level. These findings suggest that impaired performance after self-control exertion may not exclusively be due to a loss of motivation.
Keywords: self-control, depletion, motivation, fMRI, prefrontal cortex
Introduction
Self-control is the ability to control impulses, emotions and thoughts. It is key to achieving long-term goals such as academic achievement, stable social relationships and maintaining good health (Duckworth, 2011; Moffitt et al., 2011). However, exerting self-control is effortful and costly. The strength model of self-control (Muraven and Baumeister, 2000; Baumeister, 2014) postulates that self-control relies on a limited and domain-independent resource that becomes depleted with use. When self-control resources are reduced, individuals are in a temporary state of ‘self-control depletion’ that is associated with an increased risk of self-control failures (see Hagger et al., 2010, for a meta-analysis; but also Carter et al., 2015; Inzlicht et al., 2016, for recent discussions).
Several theoretical accounts have challenged the assumption of a limited resource, proposing instead that changes in motivation are responsible for decreases in self-control over time (Beedie and Lane, 2012; Kurzban, Duckworth et al., 2013; Kool and Botvinick, 2014). According to the process model of self-control (Inzlicht and Schmeichel, 2012; Inzlicht et al., 2014), initial exertions of self-control shift motivation away from exerting further control and let individuals strive to pursue inherently gratifying and enjoyable behaviors. As a consequence, attention shifts away from cues signaling the need to control and toward cues signaling immediate reward.
On the behavioral level, increasing task motivation eliminates the effects of previous self-control exertion (Muraven and Slessareva, 2003). How individuals accomplish to counteract depletion effects remains unknown, however. From the perspective of the strength model, depletion can only be overcome by additional effort to compensate for the reduction of resources (Baumeister and Vohs, 2007). In contrast, the process model interprets this finding as evidence that there is no self-control resource. Once individuals who have exerted self-control previously have overcome their drop in motivation, they are equally well prepared to master new self-control tasks as individuals who did not exert self-control before (Inzlicht and Schmeichel, 2012). These competing predictions are hard to distinguish with behavioral data alone—both models predict an elimination of the decline in performance through increased motivation. Complementary measurement of brain activity may provide valuable information on the question of whether a boost in motivation affects depleted and nondepleted individuals in similar ways.
Few studies have examined the neural correlates of self-control depletion. An EEG study found a reduced error-related negativity after self-control exertion, indicating impaired conflict detection by the anterior cingulum (Inzlicht and Gutsell, 2007). Two studies using functional magnetic resonance imaging (fMRI) found reduced brain activity in the inferior frontal gyrus (IFG) after the initial exertion of self-control during cognitive control tasks (Friese et al., 2013; Persson et al., 2013). In addition, after self-control exertion the amygdala and orbitofrontal cortex showed increased reactivity to emotional and rewarding stimuli, respectively, while the connectivity with the IFG was reduced (Wagner et al., 2013; Wagner and Heatherton, 2013). However, no neuroimaging studies have investigated how individuals manage to overcome self-control depletion.
In the present study, self-control depletion and task motivation was manipulated independently. Effects on behavioral performance in a task requiring self-control (Stroop task) were observed, and brain activity during task performance was measured. On the behavioral level, we expected poorer self-control performance after depletion, but not for participants who were highly motivated by monetary incentives to perform well (Muraven and Slessareva, 2003). On the neural level, we hypothesized that self-control depletion would reduce activity in lateral prefrontal areas implicated in cognitive control processes (Friese et al., 2013; Persson et al., 2013) in regularly motivated participants. Regarding neural correlates of increased task motivation, we tested two competing hypotheses derived from the process model and the strength model of self-control:
(1) Process model: reduced activity in lateral prefrontal areas reflects decreased task motivation. Increasing task motivation abolishes depletion-dependent differences in brain activity, leading to similar brain activity in highly motivated, depleted participants as in nondepleted participants.
(2) Strength model: reduced activity in lateral prefrontal areas does not only reflect decreased task motivation. Increasing task motivation results in the recruitment of additional brain regions and/or a particularly strong activation in certain brain regions in highly motivated, depleted participants to compensate for a partial lack of self-control resources.
Methods
Participants
Following the guidelines by Simmons et al. (2011) we aimed at obtaining at least 20 participants per condition in the final data set. To account for potential problems with fMRI measurements and expected data loss due to excessive motion we decided to recruit 100 participants.
Because of technical problems with fMRI data acquisition, five participants had to be excluded from the analyses. Three participants had to be excluded due to excessive movement during the scanning periods (>3 mm in any direction) and two participants because of claustrophobia in the MRI scanner or color blindness. Three further participants were detected as outliers on Stroop interference based on boxplot inspections. The final sample consisted of 88 participants (41 female) with a mean age of 23.76 years (s.d. = 3.78). Participants were randomly assigned to one of four conditions: depletion-high motivation (n = 21), depletion-regular motivation (n = 25), control-high motivation (n = 21), and control-regular motivation (n = 21). Written informed consent was obtained from all participants and they received CHF 25/h (∼US $25). The ethics committee of the Canton of Zürich, Switzerland, approved the study.
Procedure
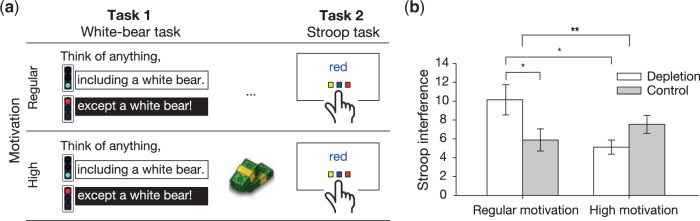
Participants practiced the self-control manipulation task and the Stroop task outside of the fMRI scanner. Next, they were positioned in the scanner and their head was secured in the coil. Participants performed either a thought suppression task (depletion condition) or an analogous control task not requiring self-control (control condition) followed by the Stroop task as the dependent variable. Preceding the Stroop task, the motivation manipulation was introduced. Half of participants were told that they could earn up to CHF 10 (∼US$10) in addition to their regular payment based on their performance in the Stroop task (high motivation condition). The other half of participants did not receive any information linking performance and additional payment (regular motivation condition). An overview of the study procedure is provided in Figure 1A. After scanning, participants filled out questionnaires including control questions, a manipulation check, and demographic data. All participants received additional payment based on their performance in the Stroop task, even if this had not been announced before (regular motivation condition). Participants were then debriefed and thanked.
Fig. 1.
Study Procedure and Behavioral Outcomes. (a) Participants performed two tasks. The first task consisted of either a thought suppression task (self-control depletion condition) or an analogous control task not requiring self-control (control condition). All participants performed the same Stroop task as the second task. Before the Stroop task, half of the participants were motivated to perform well by monetary incentives (high-motivation condition), whereas the other half was not (regular motivation condition). This resulted in a 2 (self-control: depletion vs. control) × 2 (motivation: regular vs high) between-subjects factorial design. (b) Within the regular motivation condition, the typical effect after self-control exertion occurred such that participants who had exerted self-control in the initial task performed worse in the subsequent Stroop task. High motivation resulted in a significant reduction in Stroop interference scores for participants in the depletion condition, enabling them to perform as well as control participants. Within the high motivation condition, self-control depletion had no significant effect on Stroop performance. Higher Stroop interference scores point to poorer self-control. Error bars indicate ± SEM. *P < 0.05, **P < 0.01.
Tasks
Thought suppression task. Self-control depletion was manipulated with a thought suppression task (Wegner, 1989; Mitchell et al., 2007). The task consisted of three different types of blocks, indicated by traffic lights of different colors. A green traffic light signaled to participants that they were allowed to think freely of anything in the following block, whereas a red light signaled that they had to suppress any thoughts about a white bear. Red and green traffic lights were presented for 2 s and were followed by a black screen of 40 s duration. During both blocks, participants were instructed to press a button whenever they thought of a white bear. If that happened during a red-light block, they were instructed to redirect their thoughts away and think about something other than a white bear. The third block type was a brief reaction time task to keep vigilance high. It consisted of four to six consecutively presented amber traffic lights and participants were instructed to press a button as quickly as possible each time an amber traffic light appeared. Amber traffic lights were presented until the button was pressed or until 3 s had passed, with a black screen presented in between. The depletion group engaged in 14 red, 7 green and 6 amber light blocks. The control group engaged in 21 green and 6 amber light blocks. Blocks in both conditions were presented in a pseudorandomized order. Total task duration was 17 min.
Stroop task. Color words appeared in the center of the screen written in blue, red or yellow letters. Participants were instructed to indicate the color of the letters by button press and ignore the semantic meaning of the word. Each trial was either congruent or incongruent, such that the semantic meaning of the word did or did not match the color of the letters, respectively. Stimuli remained on the screen until a button was pressed or until 1500 ms had passed. The duration of a following fixation cross was adjusted such that each trial was 2200 ms long (response time after stimulus presentation plus fixation cross). The task consisted of 246 trials (54 incongruent trials, 192 congruent trials) including 6 practice trials in a pseudorandomized order for a total of 9 min. We have previously used an almost identical task (Friese et al., 2013).
Functional imaging
MRI scanning was performed on a Philips Intera 3 T wholebody MR unit equipped with an eight-channel Philips SENSE head coil at the University Hospital of Zurich, Switzerland. Functional time series were acquired with a sensitivity encoded, single-shot echo-planar sequence (SENSE-sshEPI) sensitive to BOLD contrast (T2* fast field echo with the following acquisition parameters: TR (repetition time) = 2500 ms, TE (echo time) = 35 ms, FOV (field of view) = 0.22 cm, acquisition matrix = 80 × 80, interpolated to 128 × 128, voxel size: 2.75 × 2.75 × 3.30 mm3, no gap and SENSE acceleration factor R = 2.0). By using a midsagittal scout image, 40 contiguous axial slices were placed to the anterior–posterior commissure plane covering the entire brain. The first two acquisitions were discarded to allow for T1 saturation. The Stroop task consisted of approximately 240 functional scans. Participants viewed stimuli through a mirror mounted on top of the head coil.
Data analysis
Behavioral data were analyzed with SPSS. A subjective demand score was calculated based on four manipulation-check items indicating how exhausting the thought suppression task was and how much they had to control themselves (Cronbach’s α = 0.78). Mood was assessed based on two items prior to and after scanning (Supplementary Table S1). Stroop interference scores served as indicators of self-control performance and were calculated as the difference in the relative frequencies of errors on incongruent vs congruent trials.
MATLAB Release 2012b and SPM8 were used to analyze fMRI data. Functional images were corrected for differences in acquisition time between slices and realigned to correct for head movement. Next, images were normalized into a standard stereotaxic space (2 × 2 × 2 mm3 voxels) based on the SPM8 EPI template. Finally, images were spatially smoothed using an 8 mm FWHM Gaussian kernel. A 128-s-cutoff high-pass filter was added to the confound partition of the design matrix to account for low-frequency drifts, and a correction for intrinsic autocorrelations was included in the analysis.
For every subject, a GLM was set up with four regressors of interest (correctly answered incongruent trials, correctly answered congruent trials, incorrectly answered trials, button presses), which were convolved with a canonical hemodynamic response function, and six movement parameters derived from realignment correction as regressors of no interest. Contrast images for each participant comparing correctly answered incongruent trials vs congruent trials were used for all analyses. To identify areas commonly activated during the Stroop task, a one-sample t-test for the whole sample was calculated. We then looked for effects of our experimental manipulation in a 2 × 2 analysis of covariance (ANCOVA) with the factors self-control depletion and motivation, controlling for Stroop interference. Stroop interference was chosen as a covariate to receive a pure measure of self-control performance which is not confounded by error-related cognitive processes. Correction for multiple comparisons was employed by family wise error (FWE) correction for the whole brain based on the random field theory with a threshold at P < 0.05. To search specifically for reward-related activity, a region-of-interest (ROI) analysis was conducted in the nucleus accumbens (NAcc). WFU pickatlas was used to define the area anatomically (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2004). Anatomical labels were assigned with the AAL toolbox to the reported results (Tzourio-Mazoyer et al., 2002).
Results
Manipulation check
Participants rated the thought suppression task (subjective demand sum score: M = 16.55, SEM = 0.80) as more demanding than the control task [11.98 ± 0.56; F(1, 84) = 21.20, P < 0.001, partial η2 = 0.20], suggesting that the self-control manipulation was successful. As expected, neither the main effect of motivation nor the interaction between self-control condition and motivation was significant (Fs < 1, Ps > 0.250).
The self-control manipulation did not affect mood. In a 2 × 2 × 2 ANOVA with the within-subjects factor time (before and after the experiment) and the between-subjects factors self-control condition (depletion vs control) and motivation (regular vs high), no interactions with the experimental conditions were found (Fs < 1, Ps > 0.250).
Stroop performance
As expected, increasing task motivation eliminated the effect of self-control depletion on Stroop task performance. The two-way ANOVA of Stroop interference scores yielded a highly significant interaction between the self-control and motivation conditions [F(1, 84) = 7.49, P = 0.008, partial η2 = 0.08], depicted in Figure 1B. Follow-up analyses confirmed our hypotheses. In the regular motivation condition, depleted participants (M = 10.14, SEM = 1.60) performed worse than control participants [5.88 ± 1.18; t(84) = 2.52, P = 0.014, d = 0.62]. However, highly motivated, depleted participants performed as well as regularly motivated control participants [5.12 ± 0.76; t(84) = 1.05, P > 0.250], and significantly improved when compared with depleted participants with no motivational boost [t(84) = −2.97, P = 0.004, d = 0.81]. Finally, highly motivated control participants (7.54 ± 0.95) did not differ from regularly motivated control participants [(t(84) = −0.94, P > 0.250] nor from highly motivated depleted participants [t(84) = −1.38, P = 0.173]. These analyses indicate that initial self-control efforts led to a decrement in subsequent self-control performance in the regular motivation condition, whereas increased task motivation eliminated this effect. See the Supplementary Results for analyses of Stroop reaction time.
Neural activity associated with self-control
Preliminary analyses. Stroop task processing increased activity in widespread prefrontal areas including the dorsolateral prefrontal, parietal and the cingulate cortices (Supplementary Table S2). This pattern is in line with previous studies on inhibition tasks and specifically the Stroop task (Derrfuss et al., 2005; Nee et al., 2007). Furthermore, activity in frontal, parietal and occipital areas and the cerebellum were increased in the high when compared with the low-motivation condition (Supplementary Table S3). These are areas that are commonly active during Stroop task performance (Nee et al., 2007), thus providing evidence that the promise of reward for good performance enhanced task-relevant processing in the high motivation condition.
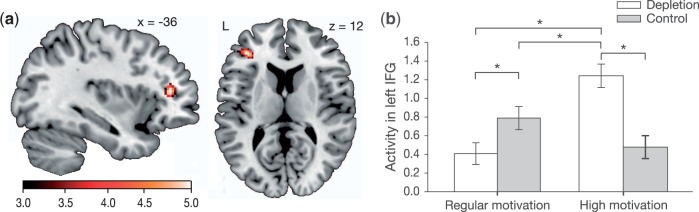
Main analyses. We tested the competing hypotheses concerning the effects of self-control depletion and task motivation on neural activity. If performance deficits after exerting self-control are purely due to diminished motivation to exert further self-control, elimination of these deficits by increased task motivation should be accompanied by a similar elimination of depletion-induced reductions in lateral prefrontal brain activity (Hypothesis 1). In contrast, if performance deficits after exerting self-control cannot be reduced to a lack of motivation, increased task motivation may affect depleted and control participants in different ways on the level of brain activity (Hypothesis 2). Consistent with Hypothesis 2, increased task motivation after self-control depletion resulted in a strong and highly significant increase in prefrontal activation. A 2 × 2 ANCOVA was calculated with the factors self-control condition and motivation, controlling for differences in behavioral performance between conditions. Family-wise error (FWE) correction was applied to the whole brain, which is a conservative method to control for multiple testing based on the random field theory. This analysis yielded a statistically robust interaction effect in the left IFG (pars triangularis) between self-control condition and motivation (BA 45, Figure 2A, Peak at [−36 38 12], Z = 4.90, P(FWE-corrected for the whole brain) = 0.026, Cohen’s d = 1.16). The left IFG is part of a large prefrontal cluster activated during the Stroop task (Supplementary Table S2), both in the complete sample as well as when analyzing the regular motivation conditions only. Thus, the brain area of the left IFG is (i) critically involved in processing of the Stroop task in general and (ii) sensitive to interacting effects of depletion and motivation. To examine activity in the left IFG further, the first eigenvariate from the activity cluster of the interaction effect was extracted (uncorrected threshold of P < 0.001, 108 voxels) and follow-up analyses were conducted (Figure 2B). In the regular motivation condition, the interaction cluster in the left IFG was significantly weaker activated in depleted participants (0.41 ± 0.12) than in control participants (0.79 ± 0.12; P = 0.031). In contrast, depleted participants in the high motivation condition (1.24 ± 0.13) compensated this depletion-induced decrease and instead showed significantly higher activity when compared with regularly motivated control participants (P = 0.011), regularly motivated depleted participants (P < 0.001), and highly motivated control participants (0.48 ± 0.12; P < 0.001). Finally in the control condition, there was an opposite trend toward stronger activity in the left IFG interaction cluster in regularly when compared with highly motivated participants (P = 0.079).
Fig. 2.
Prefrontal activity interacts with self-control exertion and motivation. (a) Based on the contrast “correct incongruent vs correct congruent trials” during performance of the Stroop task, an interaction cluster was found of which 80% lay within the IFG, and 20% within the middle frontal cortex [P(uncorrected) < 0.001]. Brain activity in this area is commonly associated with inhibition of dominant response tendencies (Derrfuss et al., 2005; Nee et al., 2007). Activation is superimposed on a canonical normalized image. (b) Activity in this cluster (arbitrary units), corrected for the covariate Stroop performance. Within the regular motivation condition, a depletion-related reduction of activity can be observed. After having exerted self-control, highly motivated depleted participants activate the area more strongly than participants in the other conditions. Error bars indicate ±SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
To search for interaction effects not withstanding whole-brain correction, we used a more lenient threshold and identified further interaction effects in the precentral gyrus and the Thalamus (Supplementary Table S4). In accordance with our prior study (Friese et al., 2013), we also found an interaction in the right lateral prefrontal cortex, but with weaker effects (see the Supplementary Results for further details). These interactions revealed similar activation patterns as the one identified in the left IFG. Meta-analytic results show that these areas are typically activated during the Stroop task (Nee et al., 2007). Furthermore, when the analysis was performed without controlling for task performance, the cluster in the IFG was also the area with the strongest interaction effect ([−38 38 12], Z = 4.44, P[uncorrected] < .001), but the effect did not withstand the conservative FWE correction for the whole brain (see Supplementary Table S5 and the Supplementary Figure S1 for the full results of the 2 × 2 ANOVA with the factors self-control condition and motivation).
Reward-related neural activity
The previous analyses revealed stronger brain activity in the left IFG after self-control exertion in participants in the high motivation condition than in any other condition, which is in line with the strength model of self-control, but difficult to reconcile with the process model. One way for the process model to explain this finding is by referring to its assumption that participants become particularly sensitive to signals of reward after exerting self-control (Inzlicht and Schmeichel, 2012). In the present study, higher reward-sensitivity could enhance the motivation to perform well to increase chances of obtaining the reward. From this perspective, differences in left IFG activity would reflect motivational differences in the Stroop task—reduced after exerting self-control in the regular motivation condition, but particularly high due to increased reward sensitivity after exerting self-control in the high-motivation condition. In contrast, if there were no differences in reward-sensitivity as a function of self-control condition, this would be incompatible with the view that left IFG activity reflected the motivation to perform well. To test this second set of competing predictions, an anatomically defined ROI analysis in the NAcc was conducted. The NAcc is one of the brain areas most consistently associated with reward-related processing (Cauda et al., 2011; Liu et al., 2011).
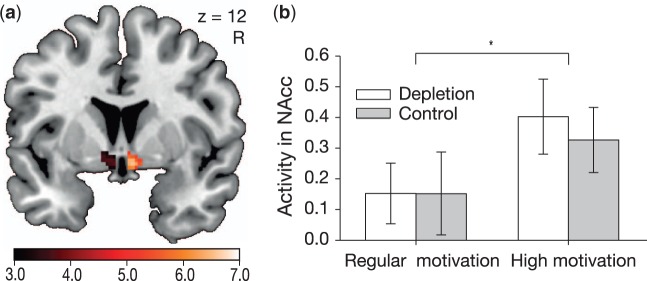
Analysis of NAcc activity during the Stroop task revealed a significant main effect of the motivation condition (Figure 3A). Highly motivated participants showed stronger activity in the NAcc bilaterally (Figure 3B) than regularly motivated participants who were unaware of a monetary reward for good performance (left NAcc: Peak at [−8 0 −6], Z = 3.94, P = 0.008, Cohen’s d = 0.90; right NAcc: Peak at [4 10 −8], Z = 3.88, P = 0.010, Cohen’s d = 0.90; small volume corrected (SVC) with FWE for a bilateral NAcc ROI). This main effect suggests that NAcc activity during task performance may serve as an indicator of reward-related processes in the present study. Importantly, there was no interaction effect of the motivation condition and the self-control condition, and no main effect of the self-control condition [both ps(SVC) > 0.250]. Follow-up analyses were conducted based on the extracted activity of the two NAcc. Separate tests for the two motivation conditions revealed neither a significant difference in the high motivation (depletion: 0.40 ± 0.12; control: 0.33 ± 0.11; t(84) = 0.46, P = 0.650) nor in the regular motivation condition (depletion: 0.15 ± 0.10; control: 0.15 ± 0.14; t(84) < −0.01, P = 0.996). Thus, there was no evidence for increased reward sensitivity on the neural level after exerting self-control. In addition to the NAcc analysis, a bilateral ROI of the dorsal part of the striatum was analyzed. Highly motivated participants showed marginally stronger activity in this area when compared with regularly motivated participants ([6 8 −10], Z = 3.70, P(FWE) = 0.072), but there were again no interaction effects or effects of self-control condition [both ps(SVC) > 0.250].
Fig. 3.
The nucleus accumbens showed an increased activity bilaterally in highly motivated participants when compared with regularly motivated participants for the contrast “correct incongruent vs correct congruent trials” during performance of the Stroop task (a). For illustration purposes a threshold at P < 0.005 was chosen and activation is superimposed on a canonical normalized image. (b) The motivation condition had a significant influence on activity in the nucleus accumbens, but there was no effect of the self-control condition or interaction between the two factors. Error bars indicate ±SEM. *P < 0.05.
NAcc activity was included as a covariate in the main analysis of brain activity in the cluster in the left IFG. The interaction of self-control condition and motivation condition in the left IFG remained significant (Peak again at [−36 38 12], Z = 5.00, P(FWE-corrected for the whole brain) = 0.019). Finally, we examined if NAcc activity may account for depletion effects on the behavioral level by including NAcc activity as a covariate in the analysis of Stroop interference scores. The covariate was insignificant (F(1, 83) = 2.52, P = .116) and the interaction effect of self-control condition and motivation remained highly significant (F(1, 83) = 7.93, P = .006). Thus, increased reward sensitivity as indicated by NAcc activity cannot account for the present findings on the behavioral or the neural level. In concert, the findings suggest that left IFG activity does not reflect motivation to perform well in the Stroop task.
To investigate effects of the experimental manipulations on functional brain connections, a psychophysiological-interaction (PPI) analysis was conducted with the NAcc and the cluster within the left IFG identified in the above analysis as seed areas. No changes in connection strength were found as a function of the experimental conditions when whole-brain correction was employed (see the Supplementary Results for additional analyses with a more lenient threshold).
Discussion
There is an ongoing debate whether the mechanisms underlying self-control depletion effects are caused by depletion of a (physical) self-control resource (Baumeister, 2014) or whether they can be exclusively explained by changes in motivation (Beedie and Lane, 2012; Kurzban et al., 2013; Inzlicht et al., 2014; Kool and Botvinick, 2014). In the present study, exerting self-control in a thought suppression task led to impaired subsequent Stroop task performance and decreased activity in the left IFG. On the behavioral level, this self-control depletion effect was eliminated by monetary incentives to perform well in the Stroop task. On the neural level, there was no similar elimination of the aftereffects of exerting self-control. Instead, highly motivated depleted participants activated the left IFG more strongly than any other group. These findings on aftereffects of exerting self-control on the neural level (i) are difficult to explain by task disengagement and a lack of motivation alone, and (ii) are compatible with a resource account of self-control (without providing direct support for a resource account).
Resource vs motivation
From the perspective of the strength model of self-control, the drop in activity after exerting self-control in the regular motivation condition reflects increased difficulty to recruit areas needed for control due to a partial depletion of resources (Persson et al., 2013). The strong IFG activity after exerting self-control in the high-motivation condition may suggest that depleted participants needed to recruit the IFG to a stronger degree to compensate for the partial loss of resources. This finding may indicate that these participants invested increased effort or that they changed their task strategy, which might have led to a shift in neural resources from other areas to the IFG. Further studies are needed to understand this mechanism better. The crucial point is that the motivation manipulation affected depleted and nondepleted participants differently.
Compensating effects associated with increased frontal brain activity have also been observed in the aging brain (Cabeza et al., 2002; Cox et al., 2014). In those studies elderly participants were able to maintain stable performance to a certain degree, but a sudden drop in performance followed when the compensation threshold was reached (Prvulovic et al., 2005). Likewise, in a series of behavioral experiments motivation counteracted intermediate levels, but not more severe levels of depletion (Vohs et al., 2013).
From the perspective of the process model, the evident assumption is that the drop in IFG activity in the regular motivation condition reflects a loss of task motivation. Increasing motivation should bring depleted and control participants on the same level and eliminate any behavioral and neural differences between these groups. This is also what other motivational accounts would predict, such as the labor/leisure tradeoff model of cognitive control (Kool and Botvinick, 2014) or the opportunity cost model of subjective effort and task performance (Kurzban et al., 2013). However, the motivation manipulation affected the depletion and the control group very differently; the depleted group showed much higher left IFG activity when highly motivated. This finding is difficult to reconcile with models that focus purely on a loss of motivation after depletion, because the incentive to perform well presumably has re-established motivation. Possibly, depletion reduced self-efficacy for the Stroop task and participants felt that they needed to exert additional effort to perform well when they were incentivized to do so (Chow et al., 2015).
Alternatively, to reconcile the present findings with a motivational account such as the process model one could argue that the IFG is evaluating different goals, i.e. to disengage from the task or to gain the dangling reward. However, the current study and meta-analyses have identified the IFG as an area that is generally activated during Stroop task performance, independent of task motivation (Derrfuss et al., 2005; Nee et al., 2007). There are few studies which have reported both activation associated with inhibitory and reward processing in different parts of the IFG (Mullette-Gillman et al., 2011; Yamasaki et al., 2002). A comparison of peak coordinates revealed that the IFG activity in the present study lies closer to clusters associated with inhibition than with reward processing. Further studies point to the area’s role in inhibition of dominant responses and self-control in various domains (Aron et al., 2007; Hare et al., 2009; Kober et al., 2010; Berkman et al., 2011; Volkow and Baler, 2012; Tabibnia et al., 2014). Hence, the IFG is overwhelmingly implicated in inhibitory processes, not in processes reflecting task motivation. Furthermore, the present findings fit well with previous work on the aftereffects of self-control exertion. Persson et al. (2013) also observed reduced brain activity in the left IFG after exerting self-control. In an earlier study, we reported that self-control exertion reduced activity in the right inferior and middle frontal gyrus (Friese et al., 2013). Research has highlighted the importance of the right IFG (Aron et al., 2004) as well as the left IFG (Swick et al., 2014) for inhibition. While the IFG was activated bilaterally in the current task (Supplementary Table S2), the interaction effect was restricted to the IFG in the left hemisphere. It has been suggested that the left hemisphere is particularly important for the Stroop task because of the strong verbal nature of the task (Nee et al., 2007).
In addition to decreased task motivation, the process model assumes that self-control exertion renders participants more sensitive to signals of rewards and leads to changes in attention. Note that these potential consequences of depletion are likely contingent on changes in motivation. Changes in reward processing and attention seem unlikely and even disadvantageous for goal-attainment if motivation to perform well in a control task was still high. We will discuss the current findings with respect to these potential downstream consequences of depletion.
(a) Changes in reward processing
Is it possible that self-control exertion made participants more susceptible to monetary rewards and that they therefore activated the left IFG particularly strongly when motivated? Based on the NAcc findings, this possibility appears unlikely. The NAcc has been related to individuals’ motivation to earn a potential reward (Clithero et al., 2011). NAcc activity is usually measured during a trial anticipation phase, after participants were informed about the potential reward, and the reward value is varied across trials (e.g. Knutson et al., 2001). In the current study, in line with the original behavioral studies manipulating motivation and self-control exertion (Muraven and Slessareva, 2003), participants were informed about the potential reward at the beginning of the task. NAcc activity was recorded during ongoing Stroop task performance. Nevertheless, motivating participants with monetary incentives increased activity in the NAcc when mastering difficult incongruent when compared with congruent trials during the Stroop task. This suggests that the NAcc activity observed here reflects reward-related processes despite the procedural differences to typical studies focusing on NAcc activity. In other words, the observed main effect of the motivation manipulation on NAcc activity during Stroop task performance provides an internal validation of NAcc activity as reward-related processing. It rules out that the task-concomitant measurement rendered only uninterpretable noise in NAcc activity. Crucially, NAcc activity should have been particularly high in highly motivated depleted participants if they were particularly motivated to attain the reward. However, NAcc activity was similar in highly motivated depleted and control participants. Thus, the present findings suggest that depleted participants were not more strongly motivated to attain the potential reward for good performance than control participants.
(b) Changes in attention
The process model postulates shifts in attention toward immediately gratifying cues and away from cues signaling the need to control after self-control exertion. Could activity in the left IFG reflect such attentional processes? If IFG activity reflected attention toward immediately gratifying cues, this activity should have increased, not decreased, after self-control exertion in the regular motivation condition. It should have decreased, not increased, after self-control exertion in the high motivation condition, because these participants were interested in performing well, for which relegating attention to rewards would have been disadvantageous. Turning to attention to cues signaling the need to control, the process model predicts that increasing motivation reestablishes these processes after self-control exertion. The model does not predict that incentivized participants show particularly strong task-relevant attention after self-control exertion. Thus, even if the left IFG activity reflected attention processes, the current result pattern does not seem to fit the predictions of the process model.
Critique of the strength model
Even though the present findings are compatible with the strength model, it is important to realize two observations: first, compatibility with the strength model does not imply no other approach could also be compatible with the data pattern. We only focused on predictions of the strength model vs the process model here. Second, direct evidence for the strength model’s main assumption, the existence of a limited resource needed for self-control, is still missing. Previous research attempted to show that the self-control resource is blood glucose (Gailliot and Baumeister, 2007; Gailliot et al., 2007), but these efforts have been heavily disputed (Beedie and Lane, 2012; Molden et al., 2012; Sanders et al., 2012; Kurzban et al., 2013). Although more than 150 studies have delivered results compatible with the strength model, all evidence remains indirect until concrete testable hypotheses about the nature of the resource have been proposed. Neither behavioral nor fMRI studies are capable of directly measuring a resource and can therefore provide only indirect evidence for the model. This is different for the process model. Assumptions of reduced motivation, or increased reward sensitivity after self-control exertion can be directly tested. Measuring neurometabolites with positron emission tomography (PET) or magnetic resonance spectroscopy might reveal insights into the physical basis of the potential self-control resource.
Recently, the strength model of self-control has received a fair amount of criticism. A high-powered registered replication report (RRR) (Hagger et al., 2016) had 23 laboratories in different countries ran one particular computerized version of an ego-depletion study and found a null effect overall (see Baumeister and Vohs, 2016, for a commentary). This RRR raises fundamental questions about the depletion effect and the strength model of self-control. However, a RRR of one specific depletion manipulation with one specific dependent variable primarily allows conclusions about this particular experimental setup and is less suited to draw inferences about the general (non)existence of depletion effects. Meta-analyses summarizing the evidence of a broad array of experimental setups are more suited for this aim. A first meta-analysis revealed a mean effect size of d = 0.62 (Hagger et al., 2010), but a more recent meta-analysis found evidence for publication bias in the literature and reported effect sizes that were smaller or not distinguishable from zero (g = 0.43 for random effects model, g = 0.24 for trim-and-fill correction, b = 0.00 for PEESE or b = −0.27 for PET; Carter et al., 2015). In turn, the techniques used by Carter et al. (2015) to estimate and correct for publication bias have been heavily criticized (Gervais, 2015; Inzlicht et al., 2016). Thus, the magnitude of the depletion effect is currently unknown. More, preferably high-powered, pre-registered research on the topic is needed, including moderator studies that may help to gain a more differentiated understanding about the boundary conditions of the depletion effect.
In the present study, we relied on both a widely used depletion manipulation (thought suppression) and a widely used dependent variable (Stroop task). This combination led to a depletion effect in the regular motivation condition of d = 0.62. Previous research provided evidence for increased task motivation to counteract the depletion effect, similar to the present study (Muraven and Slessareva, 2003), and we have conceptually replicated this behavioral effect in our own laboratory. Thus, we are optimistic that the present findings rest on a robust empirical foundation, and we deem it important for future research to provide further evidence on this.
Conclusion
The present study suggests that decrements in self-control performance cannot be attributed to diminished motivation alone. While motivational processes play an important role in self-control depletion effects, they appear to interact with depletion-specific processes on the neural level. The nature of the depletion-specific processes currently remains unidentified, but evidence suggests that they may be related to use-dependent processes of specific prefrontal brain areas involved in self-control. Models of self-control should incorporate the idea that additional factors besides a loss of motivation are needed to explain self-control depletion effects.
Supplementary data
Supplementary data are available at SCAN online.
Authors’ contributions
M.F., B.R. and J.B. designed the experiment; J.B., P.B. and R.L. collected the data; R.L. and P.B. provided scripts for data preparation; M.L. performed the data analysis and interpretation, and wrote the manuscript, under supervision of M.F. and B.R. All authors approved the final version of the manuscript.
Funding
This work was supported by grants from the Swiss National Science Foundation to M.F. and B.R. (100014_138630), and to B.R. (100014_162388).
Supplementary Material
References
- Aron A.R., Durston S., Eagle D.M., Logan G.D., Stinear C.M., Stuphorn V. (2007). Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. Journal of Neuroscience, 27, 11860–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8, 170–7. [DOI] [PubMed] [Google Scholar]
- Baumeister R.F. (2014). Self-regulation, ego depletion, and inhibition. Neuropsychologia, 65, 313–9. [DOI] [PubMed] [Google Scholar]
- Baumeister R.F., Vohs K.D. (2016). Missguided effort with elusive implications. Perspectives on Psychological Science. http://doi.org/10.1017/CBO9781107415324.004. [DOI] [PubMed] [Google Scholar]
- Baumeister R.F., Vohs K.D. (2007). Self-regulation, ego depletion, and motivation. Social and Personality Psychology Compass, 1, 115–28. [Google Scholar]
- Beedie C.J., Lane A.M. (2012). The role of glucose in self-control: another look at the evidence and an alternative conceptualization. Personality and Social Psychology Review, 16, 143–53. [DOI] [PubMed] [Google Scholar]
- Berkman E.T., Falk E.B., Lieberman M.D. (2011). In the trenches of real-world self-control: neural correlates of breaking the link between craving and smoking. Psychological Science, 22, 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Anderson N.D., Locantore J.K., McIntosh A.R. (2002). Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage, 17, 1394–402. [DOI] [PubMed] [Google Scholar]
- Carter E.C., Kofler L.M., Forster D.E., McCullough M.E. (2015). A series of meta-analytic tests of the depletion effect: self-control does not seem to rely on a limited resource. Journal of Experimental Psychology: General, 144, 796–815. [DOI] [PubMed] [Google Scholar]
- Cauda F., Cavanna A.E., D’agata F., Sacco K., Duca S., Geminiani G.C. (2011). Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. Journal of Cognitive Neuroscience, 23, 2864–77. [DOI] [PubMed] [Google Scholar]
- Chow J.T., Hui C.M., Lau S. (2015). A depleted mind feels inefficacious: ego-depletion reduces self-efficacy to exert further self-control. European Journal of Social Psychology, 45, 754–68. [Google Scholar]
- Clithero J.A., Reeck C., Carter R.M., Smith D.V., Huettel S.A. (2011). Nucleus accumbens mediates relative motivation for rewards in the absence of choice. Frontiers in Human Neuroscience, 5, doi: 10.3389/fnhum.2011.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S.R., Bastin M.E., Ferguson K.J., et al. (2014). Compensation or inhibitory failure? Testing hypotheses of age-related right frontal lobe involvement in verbal memory ability using structural and diffusion MRI. Cortex, 63C, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J., Brass M., Neumann J., von Cramon D.Y. (2005). Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Human Brain Mapping, 25, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth A.L. (2011). The significance of self-control. Proceedings of the National Academy of Sciences of the United States of America, 108, 2639–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese M., Binder J., Luechinger R., Boesiger P., Rasch B. (2013). Suppressing emotions impairs subsequent Stroop performance and reduces prefrontal brain activation. Plos One, 8, e60385.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailliot M.T., Baumeister R.F. (2007). The physiology of willpower: Linking blood glucose to self-control. Personality and Social Psychology Review, 11, 303–27. [DOI] [PubMed] [Google Scholar]
- Gailliot M.T., Baumeister R.F., DeWall C.N., et al. (2007). Self-control relies on glucose as a limited energy source: willpower is more than a metaphor. Journal of Personality and Social Psychology, 92, 325–36. [DOI] [PubMed] [Google Scholar]
- Gervais W. (2015). Putting PET-PEESE to the test. Retrieved from http://willgervais.com/blog/2015/6/25/putting-pet-peese-to-the-test-1.
- Hagger M.S., Chatzisarantis N.L.D., Alberts H., et al. (2016). A multi-lab pre-registered replication of the ego-depletion effect. Perspectives on Psychological Science. http://doi.org/10.1177/0956797614526415.Data. [DOI] [PubMed] [Google Scholar]
- Hagger M.S., Wood C., Stiff C., Chatzisarantis N.L.D. (2010). Ego depletion and the strength model of self-control: a meta-analysis. Psychological Bulletin, 136, 495–525. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324, 646–8. [DOI] [PubMed] [Google Scholar]
- Inzlicht M., Gervais W.M., Berkman E.T. (2016). Bias-correction techniques alone cannot determine whether ego depletion is different from zero: Commentary on Carter, Kofler, Forster, & McCullough, 2015. Retrieved from http://ssrn.com/abstract=2659409.
- Inzlicht M., Gutsell J.N. (2007). Running on empty: neural signals for self-control failure. Psychological Science, 18, 933–7. [DOI] [PubMed] [Google Scholar]
- Inzlicht M., Schmeichel B.J. (2012). What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspectives on Psychological Science, 7, 450–63. [DOI] [PubMed] [Google Scholar]
- Inzlicht M., Schmeichel B.J., Macrae C.N. (2014). Why self-control seems (but may not be) limited. Trends in Cognitive Sciences, 18, 127–33. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience, 21, RC159.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H., Mende-siedlecki P., Kross E.F., et al. (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America, 107, 14811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool W., Botvinick M.M. (2014). A labor/leisure tradeoff in cognitive control. Journal of Experimental Psychology: General, 143, 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzban R., Duckworth A.L., Kable J.W., Myers J. (2013). An opportunity cost model of subjective effort and task performance. Behavioral and Brain Sciences, 36, 661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35, 1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21, 450–5. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Heatherton T.F., Kelley W.M., Wyland C.L., Wegner D.M., Neil Macrae C. (2007). Separating sustained from transient aspects of cognitive control during thought suppression. Psychological Science, 18, 292–7. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E., Arseneault L., Belsky D., et al. (2011). A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the United States of America, 108, 2693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molden D.C., Hui C.M., Scholer A.A., et al. (2012). Motivational versus metabolic effects of carbohydrates on self-control. Psychological Science, 23, 1137–44. [DOI] [PubMed] [Google Scholar]
- Mullette-Gillman O. a., Detwiler J.M., Winecoff A., Dobbins I., Huettel S.A. (2011). Infrequent, task-irrelevant monetary gains and losses engage dorsolateral and ventrolateral prefrontal cortex. Brain Research, 1395, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M., Baumeister R.F. (2000). Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychological Bulletin, 126, 247–59. [DOI] [PubMed] [Google Scholar]
- Muraven M., Slessareva E. (2003). Mechanisms of self-control failure: motivation and limited resources. Personality and Social Psychology Bulletin, 29, 894–906. [DOI] [PubMed] [Google Scholar]
- Nee D.E., Wager T.D., Jonides J. (2007). Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective, & Behavioral Neuroscience, 7, 1–17. [DOI] [PubMed] [Google Scholar]
- Persson J., Larsson A., Reuter-Lorenz P.A. (2013). Imaging fatigue of interference control reveals the neural basis of executive resource depletion. Journal of Cognitive Neuroscience, 25, 338–51. [DOI] [PubMed] [Google Scholar]
- Prvulovic D., Van de Ven V., Sack A.T., Maurer K., Linden D.E.J. (2005). Functional activation imaging in aging and dementia. Psychiatry Research, 140, 97–113. [DOI] [PubMed] [Google Scholar]
- Sanders M.A., Shirk S.D., Burgin C.J., Martin L.L. (2012). The gargle effect: rinsing the mouth with glucose enhances self-control. Psychological Science, 23, 1470–2. [DOI] [PubMed] [Google Scholar]
- Simmons J.P., Nelson L.D., Simonsohn U. (2011). False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science, 22, 1359–66. [DOI] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken A.U. (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience, 9, 102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G., Creswell J.D., Kraynak T.E., Westbrook C., Julson E., Tindle H.A. (2014). Common prefrontal regions activate during self-control of craving, emotion, and motor impulses in smokers. Clinical Psychological Science, 2, 611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- Vohs K.D., Baumeister R.F., Schmeichel B.J. (2013). Erratum to Motivation, personal beliefs, and limited resources all contribute to self-control. Journal of Experimental Social Psychology, 49, 184–8. [Google Scholar]
- Volkow N.D., Baler R.D. (2012). To stop or not to stop? Science, 335, 546–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.D., Altman M., Boswell R.G., Kelley W.M., Heatherton T.F. (2013). Self-regulatory depletion enhances neural responses to rewards and impairs top-down control. Psychological Science, 24, 2262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.D., Heatherton T.F. (2013). Self-regulatory depletion increases emotional reactivity in the amygdala. Social Cognitive and Affective Neuroscience, 8, 410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner D.M. (1989). White Bears and Other Unwanted Thoughts. New York, NY: Viking/Penguin. [Google Scholar]
- Yamasaki H., LaBar K.S., McCarthy G. (2002). Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences of the United States of America, 99, 11447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.