Abstract
The neurobiological alterations resulting from adverse childhood experiences that subsequently may lead to neglectful mothering are poorly understood. Maternal neglect of an infant’s basic needs is the most prevalent type of child maltreatment. We tested white matter alterations in neglectful mothers, the majority of whom had also suffered maltreatment in their childhood, and compared them to a matched control group. The two groups were discriminated by a structural brain connectivity pattern comprising inferior fronto-temporo-occipital connectivity, which constitutes a major portion of the face-processing network and was indexed by fewer streamlines in neglectful mothers. Mediation and regression analyses showed that fewer streamlines in the right inferior longitudinal fasciculus tract (ILF-R) predicted a poorer quality of mother–child emotional availability observed during cooperative play and that effect depended on the respective interactions with left and right inferior fronto-occipital fasciculi (IFO-R/L), with no significant impact of psychopathological and cognitive conditions. Volume alteration in ILF-R but not in IFO-L modulated the impact of having been maltreated on emotional availability. The findings suggest the altered inferior fronto-temporal-occipital connectivity, affecting emotional visual processing, as a possible common neurological substrate linking a history of childhood maltreatment with maternal neglect.
Keywords: maternal neglect, white matter connectivity, emotional availability, childhood maltreatment
Introduction
Maternal neglect is the most prevalent form of child maltreatment, as well as the most pervasive across time, accounting for 79.5% of all child maltreatment cases in the USA (Department of Health and Human Services, 2015). Neglectful mothers typically provide insufficient or inadequate care to fulfill the child’s cognitive, emotional, educational and physical protection needs (Dubowitz et al., 2011). Yet, despite its prevalence, the neurological bases that underpin and sustain maternal neglectful behavior remain less explored than their short- and long-term consequences. Early neglect or abuse alter the child’s gray and white matter (WM) brain organization (see Hart and Rubia, 2012 for a review; Hanson et al., 2013), produces cognitive impairments in executive function and self-regulation skills and affects the body’s stress response (Pechtel and Pizzagalli, 2011). Adolescents and young adults who have experienced childhood maltreatment have disrupted WM connections among frontal, temporal and occipital regions; the cingulum bundle projecting to the hippocampus and the splenium of the corpus callosum (Daniels et al., 2013). However, those studies do not link WM alterations to subsequent inadequate mothering.
Neglectful mothers are more likely to have been neglected or maltreated in their infancy (Petersen et al., 2014). Therefore, altered tracts in neglectful mothers may overlap with altered tracts of historically maltreated individuals. In line with this proposal, there is preliminary evidence for an association between childhood maltreatment and adults’ neural responses during emotional face processing. Adults’ exposure to emotional abuse and/or neglect in early childhood was associated with enhanced bilateral amygdala reactivity to emotional adult faces in general and independent of psychiatric status (van Harmelen et al., 2013). Degree of exposure to childhood maltreatment in adults was correlated positively with activity in the face processing areas (bilateral fusiform gyri and the left hippocampus) during viewing of novel compared to familiar adult faces (Edmiston and Blackford, 2013). The face-responsive brain area comprises a highly interconnected functional network, hence implicating aspects of WM connectivity (Mori et al., 2005; Fairhall and Ishai, 2007; Gschwind et al., 2012). Therefore, the search for neural correlates of altered face processing can provide critical insights into the altered WM tracts presumably affected in maternal neglect.
Neglectful mothers typically show a low sensitivity to the child’s demands (Petersen et al., 2014), which are mainly conveyed by the child’s emotional signals, such as crying. This is a further reason to expect altered brain structures and functions associated with emotional processing of faces in neglectful mothers. Evidence from neuroimaging studies has shown that mothers displaying more sensitive care towards their child exhibited greater activation to their own infant’s cry in the right frontal pole, inferior frontal gyrus and left hippocampal regions, whereas mothers who displayed more intrusive behaviors showed greater activation in the left anterior insula and temporal pole (Musser et al., 2012). More sensitive as compared to intrusive mothers showed a differential recruitment of the nucleus accumbens and the amygdala in response to viewing videos of their own vs an unknown infant (Atzil et al., 2011). Mothers in response to infant videos showed greater own-infant response in the middle frontal gyrus associated with higher quality play interactions, such as less directive parent behavior and more interactive infant behavior (Wan et al., 2014). In turn, evidence from electrophysiological studies has shown that neglectful mothers displayed a lack of increased face-specific N170 amplitude in response to crying vs laughing and neutral infant expressions and an attenuated brain response in the late positive potential to infant faces and emotional stimuli in general (Rodrigo et al., 2011; León et al., 2014). In addition, an atypical fronto-occipital oscillatory pattern observed in neglectful mothers has been suggested as further evidence of impaired top-down signaling during emotional processing (León et al., 2014).
The above literature suggests that the emotional processing of visual stimuli (i.e. infant faces) is impaired in neglectful mothers, probably implicating altered WM connectivity among frontal, temporal and occipital face-responsive regions. However, we favor a whole brain analytic, bottom-up approach over making specific hypotheses focused solely on the tracts that are known to be involved with face processing. First, there is a lack of direct evidence for the association between specific WM tracts presumably disrupted in adults who have suffered childhood maltreatment and the commission of child neglect in mothers. Second, it is not clear which, if any, of these anomalies in connectivity subsequently undermines the establishment of positive mother–child interactions. Therefore, if we examined only tracts involved with face processing, we could dismiss the evidence of other tracts that could characterize neglectful mothers or those associated with failures in the mother–child bonding interactions.
This study addressed these knowledge gaps by exploring in 18 main tracts (Catani and de Schotten, 2012) the existence of structural WM anomalies in neglectful mothers, with the goal of understanding how these anomalies might impact the quality of mother–child bonding interactions. For our fist aim, we investigated altered patterns of WM organization in 22 neglectful compared with 22 control (non-neglectful) mothers, using diffusion tensor imaging analysis (DTI) (Basser et al., 1994; Wakana et al., 2004). We examined the combination of tracts that best discriminates between the two groups. For our second aim, we examined the extent to which the WM alterations in neglectful mothers, indexed by the number of streamlines for each fiber tract, would predict the mother–child emotional availability (EA) observed in a cooperative play task. EA, a proxy to the quality of bonding interactions, involves the ability to read and respond appropriately to each other’s emotional signals in mother–child daily interactions (Easterbrooks and Biringen, 2005). Neglectful mothers have been described as exhibiting lower levels of emotional expression and less understanding of their children’s emotional displays (Edwards et al., 2005). Therefore, we expected that the disrupted tracts in neglectful mothers, presumably connecting face-responsive brain areas, would predict their lower EA observed during play interactions with their child. However, we also checked for the existence of other tracts also predicting EA. For our third aim, in search of a deeper understanding of the explanatory factors associated with neglectful behavior, we examined whether the mothers’ history of childhood maltreatment and related psychopathological and cognitive conditions (Brietzke et al., 2012) contributed to the impact of the affected connections on the mother–child play interactions. Altogether, this study provides new evidence of the neurobiological links between maltreatment during childhood and the expression of maternal neglectful behavior towards offspring.
Materials and methods
Participants
Participants were 44 mothers (22 neglectful mothers and 22 control mothers), recruited through the same Primary Health Centers in Tenerife, Spain. Written consent was obtained from all the participants according to the protocol approved by the Ethics Committee of the University of La Laguna. In the neglectful group, the sole concern was substantiated neglect of a child younger than 5-year old in the previous 12 months, leading to referral recorded by the Child Protective Services. All mothers in the control group had a confirmed absence of Child Protective Services or Preventive Services records for the family. None of the neglected or control infants had been placed in foster care at any point in their history, nor had they been born prematurely or suffered perinatal or postnatal medical complications. The mean ages of the mothers in both groups were in the early 30s, with the control group being significantly older than the neglectful group. Average number of children in the neglectful and the control group was 2.04 (0.8) and 1.7 (0.6), and the mean age of the target child was 2.5 (1.3) and 2.3 (1.7) years, respectively, with no significant number of children or age differences. All mothers shared similar low-income backgrounds, lived in urban environments and were poorly educated and unemployed. Social workers reported on the presence of factors contributing to the mothers’ risk profiles: most mothers in the neglectful group compared to the control group had a history of childhood maltreatment or neglect (of the mother when she was a child) and scored positively in poor household management, disregard child’s needs and rigid/inconsistent norms (see Supplementary Methods, recruitment procedure and Table S1).
The groups differed significantly in most psychiatric disorders searched (See Supplementary Methods, Table S2), showing worse results in the neglectful group mainly in Major Depressive Disorder, Dysthymia, Hypo/Manic Episode, General Panic Disorder, Generalized Anxiety Disorder and Antisocial Personality. The neglectful group also scored significantly lower in Cognitive Integrity. None of the mothers in each group were medicated for psychiatric disorders by the time of testing.
Behavioral measures
Impairments in cognitive function were assessed using the Spanish version of the Mini-Mental State Examination (MMSE; Blesa et al., 2001). The Spanish version of the Mini International Neuropsychiatric Interview (M.I.N.I 6.0; Ferrando et al., 2000), including 15 major psychiatric disorders was also applied. EA was measured at home in the context of mother–child free-play by using the EA Scale: Infancy to Early Childhood Version (Easterbrooks and Biringen, 2005). EA signifies the quality of emotional exchanges, focusing on partners’ accessibility to each other and their ability to read and respond appropriately to each other’s communications. The scale operationalizes parental and child behavior in six scales that were factorized in one factor (see Supplementary Methods, for descriptions of the scales and testing details). Two external observers blind to the mothers’ groupings made the ratings from the videos and the inter-rater reliability of the ratings in each scale was adequate (Table 1). A principal component analysis performed with the six standardized scales yielded a single factor structure, given the existence of high inter-correlations among the factors in the mother–child dyad, KMO = 0.81, eigenvalue = 4.35 with an explained variance of 72% (Table 1). Therefore, the coefficient scores in this factor were used as a dependent variable.
Table 1.
Inter-rater reliabilities and factor loadings of the EA scales
| Scales | Kappa coefficients | Component loadings |
|---|---|---|
| Mother | ||
| Sensitivity | 0.94 | 0.874 |
| Structuring | 0.90 | 0.927 |
| Non-intrusiveness | 0.87 | 0.823 |
| Non-hostility | 0.92 | 0.727 |
| Child | ||
| Responsiveness | 0.92 | 0.881 |
| Involvement | 0.86 | 0.863 |
DTI and T1 acquisition
For each subject, an eight-channel phased-array surface coil standard scheme of diffusion gradients was acquired, using a General Electric 3T scanner. Using a single echo planar imaging sequence, 55 diffusion-weighted images were obtained (b = 1200 s/mm2) as well as six reference T2-weighted images (b0 image) with no diffusion weighting (b = 0 s/mm2). The acquisition parameters were: 57 contiguous sagittal slices, 2.4-mm thick, with an acquired matrix = 128 × 128, corresponding to a resolution in the axial plane of 2.3984 × 2.3984 mm2, echo time/repetition time = 90.9/17.000 ms. In order to correct the distortions caused by magnetic field inhomogeneities in the series of diffusion weighted images, phase and magnitude maps were also obtained. A T1 anatomical image was also recorded with the following characteristics: 196 contiguous sagittal slices, 1-mm thick, field of view = 256 × 256 mm2, corresponding to a resolution in the sagittal plane of 0.97 × 0.97 mm2, echo time = 1.736 ms, repetition time) = 8.716 ms.
Processing diffusion tensor images
Eddy current corrections were made with a linear recording of diffusion-weighted images to b0. Also, magnetic field inhomogeneities were corrected, using magnitude and phase images and the Unwarping package of SPM5 toolbox (http://www.fil.ion.ucl.ac.uk/spm/).
Estimation of the diffusion tensor and fiber tracking
The toolbox DTI and Fiber Tools v.3.0 (Kreher et al., 2008) was used to estimate the six elements of the diffusion tensors and associated fractional anisotropy and mean diffusivity maps. Three-dimensional tract reconstruction, using Fiber Assignment by Continuous Tracking, a deterministic tractography method (Mori et al., 1999), was performed to evaluate the existence of alterations in the main matter fiber bundles (18 tracts), given the absence of previous DTI studies in neglectful mothers compared to control (non-neglectful) mothers. The number of reconstructed streamlines (NS), which represent a surrogate marker of tract volume, was measured.
The fiber tracking was performed in all brain voxels, in accordance with the values of FA and the critical angle for starting and stopping tracking recommended in the toolbox (Kreher et al., 2008). Using the T1 of each subject, a set of Regions of Interest (ROIs) was also defined, replicating the ones predefined by Hua et al. (2008). Fibers that penetrated the ROIs were assigned to the specific tracts associated with them. ROIs were defined for the following tracts: anterior thalamic radiation, cingulum associated to cingulate gyrus, cingulum associated to hippocampal gyrus, corticospinal tract, inferior fronto-occipital fasciculus (IFO-L/R), inferior longitudinal fasciculus (ILF-L/R), superior longitudinal fasciculus, uncinate fasciculus, forceps major (F-major) and forceps minor (F-minor) of the corpus callosum. The resulting paths of these tracts were visually inspected and corrected when necessary, by the exclusion of fibers that did not belong anatomically to tracts. Finally, for each subject and fiber tract, the number of streamlines was normalized, dividing by the total amount of whole-brain streamlines. The number of streamlines (NS) resulting from this ratio was the dependent measure used in our analyses.
Results
The results of the analyses were organized as follows: discriminant analyses to identify the combination of altered tracts in neglectful mothers (first aim); regression analyses to examine whether the altered tracts predicted mother–child interaction (second aim) and discriminant, mediation and regression analyses to ascertain how childhood maltreatment and related psychopathological and cognitive conditions affected the mother–child interaction (third aim). All statistical analyses were conducted using R program (Team RC, 2015).
Combination of altered WM tracts in neglectful mothers
For our first aim, we used a linear discriminant analysis to determine the combination of WM tracts (extracted from the 18 major WM tracts) that reliably distinguished between neglectful and control mothers. The adjusted NS for each fiber tract was measured, as an index of tract volume. A significant function was obtained (Wilks’ lambda = 0.61, F(1,42) = 26.4, P < 0.001) that correctly classified 86% of the neglectful group and 82% of the control group. To guarantee the robustness and reproducibility of the results, leave-one-out cross validations and bootstrap tests were also performed. Cross validation yielded similar results (86% and 77%, respectively). A combination of the right inferior longitudinal fasciculus (ILF-R), connecting the occipital and temporal lobes, and the left and right inferior fronto-occipital fasciculi (IFO-L and IFO-R), connecting the occipital lobe and the orbitofrontal cortex, significantly contributed to the discriminant function. Table 2 lists the typical coefficients (indicating the relative importance of each variable in predicting group assignment from the function), the structure coefficients (indicating the correlations between each variable and the discriminant function score) and the discriminant function obtained in the comparison of neglectful and control groups. Number of streamlines in the ILF-R and IFO-L contributed independently to the discriminant function (both structure and typical coefficients were similar). In contrast, IFO-R contributed in a suppression (not in a redundant) way through the other two tracts to the discriminant function (high typical and low structure coefficients), indicating that its contribution was made through its relation to the other two tracts. Centroid values were negative for the control group and positive for the neglectful group.
Table 2.
Typical and structure coefficients and bootstrap confidence intervals of the discriminant function analysis for the neglectful and control groups on number of streamlines
| Tracts | Typical coefficients | Structure coefficients | Lower CI | Upper CI |
|---|---|---|---|---|
| ILF-R | −0.642 | −0.581 | −41.159 | −1.591 |
| IFO-L | −0.779 | −0.523 | −29.499 | −6.214 |
| IFO-R | 0.730 | 0.295 | 2.047 | 23.754 |
Note. Centroids of the function: control group = −0.7748; neglectful group = 0.7748.
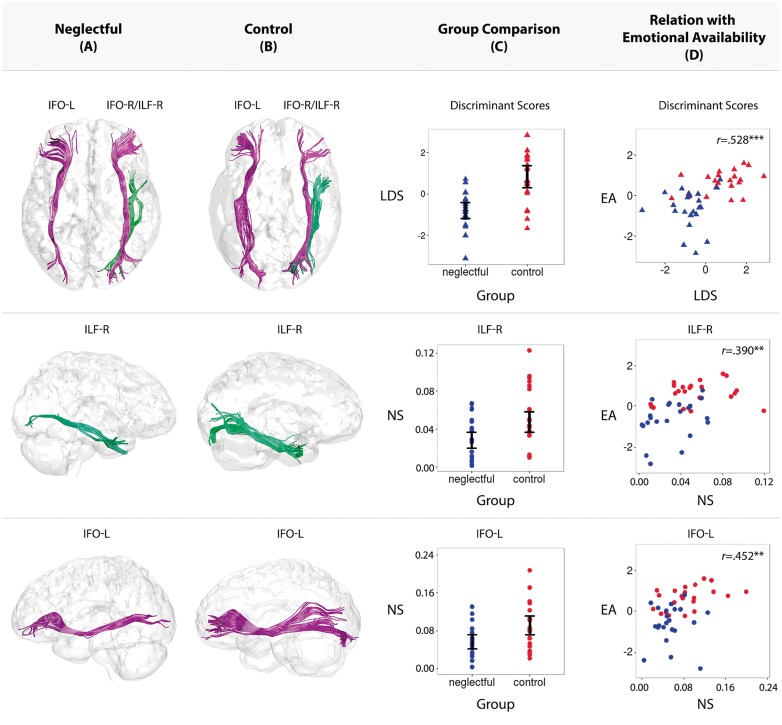
A reconstruction of the three WM fiber bundles that contributed to the discriminant function from two demonstrative mothers in the neglectful and control groups is in the upper part of Figure 1A and B, respectively. The mapping of the groups onto the discriminant function, indexed by each individual’s linear discriminant scores, is also shown in the upper part of Figure 1C. The next rows show the reconstruction of ILF-R and IFO-L (A, B) and the distribution of individual NS values, showing significant group comparisons (C): lower NS values in neglectful as compared to control mothers in ILF-R, t(42) = −2.988, P = 0.004, η2 = 0.17 and in IFO-L, t(42) = −2.716, P = 0.009, η2 = 0.15, but not in IFO-R, t(42) = −1.51, P > 0.10. All confidence intervals come from a Bayesian estimation of the highest density interval (95%) of the posterior distribution of each group means (Kruschke, 2013).
Fig. 1.
WM tracts, indexed by the number of streamlines (NS), discriminate between neglectful and control mothers and were related to behavioral mother–child bonding interactions (EA). (A, B) Reconstruction of WM fiber bundles in IFO-L, IFO-R and ILF-R, from two demonstrative mothers in the neglectful and control groups, respectively. (C) Comparison of neglectful (red n = 22) and control (blue n = 22) mothers performed with each individual’s linear discriminant scores (LDS) and the number of streamlines (NS) values at the identified tracts showing significant group differences and confident intervals. (D) Scatter diagrams plotting LDS and NS values on EA scores showing significant correlations.
Anomalies in WM tracts predict mother–child interaction in neglectful mothers
Figure 1D shows significant correlations between discriminant scores (first row) and NS scores in ILF-R and IFO-L (second and third rows) with EA. For our second aim, we used regression analyses to test whether the three tracts resulting from the discriminant function that differentiated between neglectful and control mothers predicted the EA in mother–child interactions. To rule out the existence of other tracts not contributing to the discriminant function but also predicting EA, a previous regression model was tested, comprising the whole set of 18 tracts on EA scores. No other tracts resulted from that regression, so we proceeded only with the three identified tracts. The target regression model was tested for the EA scores as predicted by the NS values in ILF-R, IFO-L and IFO-R, as well as by the group variable (neglectful and control mothers). We also entered maternal age in the model as a control factor, since there was a significant age difference by mother group. Results showed that the proposed model was significant (F(8,35) = 7.386, P < 0.001), explaining a large proportion of the variance (R2= 0.628; AdjR2= 0.543) and demonstrating robust and stable interactive effects (Table 3).
Table 3.
NS values in ILF and IFO tracts predict EA scores in neglectful and control mothers
| Groups Neglectful/control |
|||
|---|---|---|---|
| Predictors | Estimate | Standard error | t |
| (Constant) | 0.329 | 0.183 | 1.800 |
| ILF-R | 0.328 | 0.613 | 0.536 |
| IFO-R | −0.214 | 0.240 | −0.891 |
| IFO-L | 0.486 | 0.291 | 1.667 |
| Groups | −0.525 | 0.304 | −1.722 |
| Maternal age | 0.041 | 0.019 | 2.138* |
| ILF-R x IFO-R | −3.462 | 1.310 | −2.642** |
| ILF-R x IFO-L | 4.214 | 1.520 | 2.773** |
| ILF-R x group | 2.815 | 1.045 | 2.692** |
| IFO-R x age | — | — | — |
*P ≤ 0.05.
**P ≤ 0.01.
Significant tract interactions in Table 1 showed that the positive contribution of ILF to EA was modulated differently by IFO-L and IFO-R (see Supplementary Results, Figure S1). The combined increases in volume (i.e. larger number of streamlines) in both ILF-R and IFO-L tracts predicted better mother–child functioning. This combination was less likely to occur in neglectful mothers, who had fewer streamlines in ILF-R and IFO-L. In turn, in the combination of ILF-R and IFO-R, the beneficial impact of increased volume in ILF-R on mother–child functioning was undermined by the increases in IFO-R volume.
The ILF-R x Group interaction showed that the positive influence of ILF-R on EA, indexed by number of streamlines, was significantly modulated by the groups. As illustrated in Figure 2, each value of the regression coefficients in ILF-R was used to predict the EA values linearly estimated for the control mothers (blue line) and neglectful mothers (red line). Increases in regression coefficients of NS values in ILF-R predicted a pattern characterized by a better interactive performance in EA in neglectful mothers only, suggesting that the increased volume in ILF-R is critical for neglectful mothers being able to establish a sensitive relationship with their child.
Fig. 2.
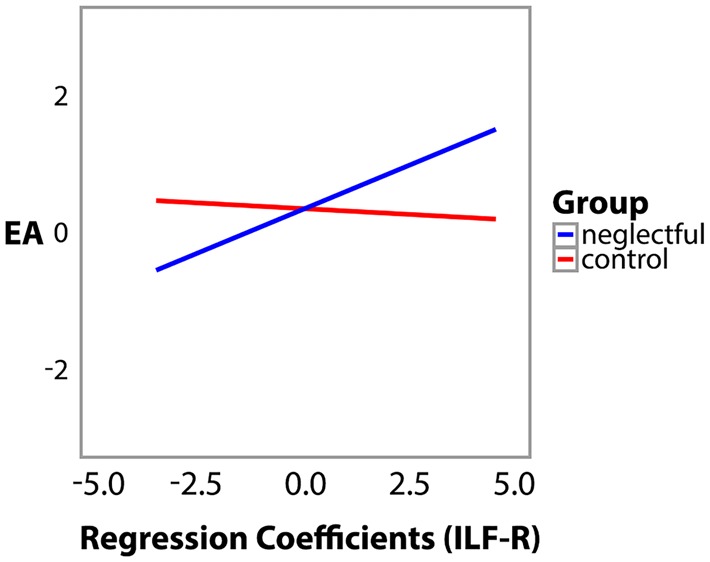
Increases in the ILF-R volume, indexed by number of streamlines, predict better mother–child bonding interactions in neglectful mothers. Each value of the regression coefficients of NS in ILF was used to predict the EA values linearly estimated for the control/neglectful group, Bcontrol = −0.214, 95% confidence interval (CI) [−0.71, 0.27]; Bneglectful = 2.810, 95% CI [0.69, 4.83].
WM alterations in mothers with childhood maltreatment predict mother–child interactions
Given that 80% of the neglectful mothers had suffered childhood maltreatment (see Supplementary Methods, Table S1), for our third aim we examined the contribution of childhood maltreatment to the association of WM anomalies with the EA. We first used discriminant analyses to determine the combination of WM tracts (extracted from the 18 major WM tracts) that reliably distinguished between maltreated and non-maltreated mothers as group levels (regrouping the classification of neglectful/control mothers into maltreated/non-maltreated mothers). Results showed robust and stable results for the ILF-R and IFO-L, whereas IFO-R was not included in the discriminant function (cross validation yielded results of 75% for maltreated and 60% for non-maltreated mothers (see Supplementary Results, Table S5).
Second, two mediation analyses were carried out to examine whether the ILF-R or IFO-L alterations mediated the impact of the mothers’ childhood maltreatment (CM) on EA (CM → WM → EA). Analysis on the first model with ILF-R as the mediator showed a significant direct effect of CM on EA scores (average direct effect, ADE = −0.85, P = 0.001); however, part of the variance on EA scores was explained by the mediation of the ILF-R (average causal mediation effects, ACME = −0.21, P = 0.05), indicating that this alteration mediate the relation between the condition of having been maltreated and EA. A second analysis with IFO-L as a mediator showed that CM had a significant direct impact on EA (ADE = −0.91, P = 0.003), but IFO-L that was affected by CM did not mediate this relation (ACME = −0.14, P = 0.22).
Third, given the mediating role of the ILF-R on EA and the interaction effects found between ILF-R and IFO tracts in the target regression model in Table 3, it seemed reasonable to test the combined influence of the ILF-R, IFO-L, IFO-R and Maternal age on EA scores, but this time entering the maltreatment classification as a Group variable instead of the neglectful classification (Table 4). The model was significant, F(2,41) = 7.987, P < 0.0001, R2= 0.692; AdjR2= 0.605, explaining a large proportion of the variance in EA and demonstrating robust and stable results.
Table 4.
NS values in ILF and IFO tracts predict EA scores in the maltreated and non-maltreated mothers
| Groups Maltreated/Non-Maltreated |
|||
|---|---|---|---|
| Predictors | Estimate | Standard error | t |
| (Constant) | −0.073 | 0.175 | −0.417 |
| ILF-R | 38.210 | 8.276 | 4.617*** |
| IFO-R | −6.852 | 2.230 | −3.072** |
| IFO-L | 4.043 | 3.098 | 1.305 |
| Groups | −0.290 | 0.246 | −1.182 |
| Maternal age | 0.058 | 0.018 | 3.247** |
| ILF-R x IFO-R | −411.570 | 127.269 | −3.234** |
| ILF-R x IFO-L | 724.380 | 164.026 | 4.416*** |
| ILF-R x group | 44.310 | 10.665 | 4.155*** |
| IFO-R x age | −0.733 | 0.344 | −2.129* |
*P ≤ 0.05.
**P ≤ 0.01.
***P ≤ 0.001.
The model indicated independent and combined tract effects. Higher NS values in ILF-R predicted higher EA scores, whereas higher NS values in IFO-R predicted lower EA scores. The pattern of tract interaction effects was similar to that found in the neglectful/control mothers regression model, showing the differential effects of the increased volumes in IFO-L and IFO-R on the positive contribution of ILF-R to mother–child functioning (see Supplementary Results, Figure S1). The effect of ILF-R on EA was also modulated by the maltreatment classification in the same way as for the neglectful/control groups (see Supplementary Results, Figure S2). In line with the mediation model, increases in regression coefficients of NS values in ILF-R predicted a linear pattern characterized by better interactive functioning in EA in maltreated mothers only, suggesting again that increases in the ILF-R volume are crucial in promoting positive mother–child interactions in this group. Mediation and regression analyses showed that psychopathological and cognitive integrity measures did not contribute significantly to the regression model of WM anomalies on EA in neglectful mothers (see Supplementary Results, Additional Measures). The parameters of the mediation models were significant and fell outside the confidence intervals indicating that the results are not likely to be random. Moreover, the confidence intervals were generated using a bootstrap resampling procedure, which assures the consistency and robustness of the results.
Discussion
Our results provide the first evidence that neglectful mothers, compared to control mothers, presented disruptions in the structural organization of the most important connectors between the occipital lobe and the temporal and frontal lobes: ILF-R and bilaterally the inferior fronto-occipital fasciculi (IFO-R and IFO-L). Reduced volumes in ILF-R and IFO-L characterized neglectful mothers with respect to controls, whereas the contribution of IFO-R volume to the group classification, as revealed by the discriminant function, was made through its relation to the other two tracts. A difference in volume might be interpreted as changes in the degree of myelination or in the number of fibers, but any of these possibilities needs confirmation or convergence with other data.
It has been established that the ILF and IFO tracts connect face-responsive regions (the occipital face and fusiform face areas), with a significant right-hemisphere predominance (e.g. Gschwind et al., 2012). ILF extends from the posterior portion of the fusiform and lingual gyri and cuneus in the occipital lobe and appears to mediate the fast transfer of visual signals to the superior, inferior and middle temporal gyri, as well as the hippocampus complex and amygdala (Catani et al., 2003; Catani and de Schotten, 2012). In turn, the IFO, which is a tract unique to the human brain, is a bilateral association pathway connecting the ventro-medial occipital cortex (subserving object and face recognition areas) to emotion-related orbital and polar frontal areas, via rapid feed-forward propagation of visual input to anterior frontal regions and direct top–down modulation of early visual processing (Catani et al., 2002; Forkel et al., 2014). Therefore, WM alterations in the ILF-R and IFO tracts found in neglectful mothers suggest disruptions in the main components of visual cortical pathways, connected in turn with the temporal-limbic areas, which correspond to some of the mothers’ brain areas involved in the processing of infant faces (e.g. Musser et al., 2012). These alterations in the visual pathways may underlie the mothers’ disregard of child’s needs typical of neglectful mothers, since facial infant expressions, particularly infant crying, represent the main emotional cue that the caregiver should be able to ‘read’ to satisfy the infant’s needs and demands.
We also provided the first evidence that this atypical fronto-temporo-occipital pattern detected in neglectful mothers has an impact on the quality of mother–child bonding interactions, indexed by EA. Thus, positive mother–child interactions were predicted by increased volume in ILF-R; this effect was modulated positively by increases in volume in the contralateral IFO-L (leading to better mother–child functioning) and negatively by increases in volume in the ipsolateral IFO-R (leading to worse mother–child functioning). The combined influences found between ILF-R and bilateral IFO could be expected due to their participation in the functional connections established in the face processing system (Mori et al., 2005). What is new is that the two tracts work together in the potentiation of positive mother–child interactions. Neglectful mothers had fewer streamlines in ILF-R and IFO-L, which explains why they participated in dyads with lower EA. Moreover, increased volume in ILF-R appeared to represent a protective factor for neglectful mothers being able to establish a more sensitive and responsive interaction with their child, as shown by the interactive effects of ILF-R with neglectful and control mothers.
Neglectful mothers had a higher probability of exposure to early adversity, higher vulnerability to psychopathologies (10 out of 15 clinical syndromes) and lower cognitive integrity compared to control mothers, all of which are considered as risk factors associated with child neglect (Dubowitz et al., 2011). As expected, an atypical pattern of WM alterations in ILF-R and IFO-L was observed when directly comparing maltreated and non-maltreated mothers, supporting previous evidence obtained in individuals suffering early adversity experiences (Choi et al., 2012; Huang et al., 2012). Furthermore, a substantial part of the impact of the neglectful mothers’ WM anomalies on the quality of mother–child bonding interactions (EA) was explained by their adverse experiences endured in early childhood. Mediation and regression results provide a clear-cut picture of the distinctive role played by ILF-R and IFO-L in that relation. Part of the effect of the early adverse experiences on the subsequent mother–child interaction was mediated by the alterations suffered in the tract ILF-R but not in the tract IFO-L. Critically, the increased volume in the ILF-R predicted a more positive EA only in the maltreated group. In turn, the influence of volume alterations in IFO-L on EA was mainly conveyed through working together with the ILF-R in the potentiation of positive mother–child interactions. A reduced volume in the ILF-R was associated with the neglectful mothers’ greater tendency to suffer General Panic Disorder and their lower cognitive integrity with respect to control mothers. However, only cognitive integrity mediated the impact of the ILF-R alteration on the mother–child interactions, though its effect was negligible in the full regression model tested. In sum, the influence of ILF-R on EA was mediated, whereas the influence of IFO-L was specifically or directly related to EA, since no mediation effect was found for any psychopathological or cognitive variable.
Despite the consistency and robustness of the results, some limitation of tractography per se must be taken in account. The effect of tract length in fiber reconstruction, due to the accumulation of noise error, and other factors could affect the tract reconstruction (e.g. narrowing of the tract, perturbation by crossing fibers), (Jones et al., 2013). Further studies using other fiber tractography algorithms will be useful with the purpose of making the current results robust to the choice of tracking method.
In conclusion, this study provides the first description of alterations in the inferior fronto-temporo-occipital connectivity in neglectful mothers, which may have a major impact on the processing of emotional face stimuli. The study also shows that a decrease in volume in the right temporo-occipital connectivity is only detrimental in establishing a more sensitive and responsive interaction with their child when this alteration is accompanied by a childhood maltreatment history. Although no causal links can be asserted from our cross-sectional design, results of our mediation analysis following the proper time course (CM → WM → EA) suggest that the own mothers’ history of childhood maltreatment is involved as a condition for the volume anomalies found in the right temporo-occipital connectivity and that this alteration subsequently undermined the establishment of positive mother–child interactions. The plausibility of this interpretation is supported by converging evidence. Studies in rats have shown epigenetic mechanisms related to alterations in a critical system for maternal care, the estrogen–oxytocin interaction and the differential methylation of hypothalamic estrogen receptor, mediating the behavioral transmission of postpartum behavior from mothers to female offspring (Champagne, 2008). Epidemiological and longitudinal studies also supported the link between childhood maltreatment and poor mothering to the next generation (Schofield et al., 2013; Plant et al., 2013). Therefore, the structural disruption observed in the visual pathways in neglectful mothers could be, at least, part of a common neurological substrate linking a history of childhood maltreatment with poor maternal care.
Finally, we have found that temporo-occipital connectivity plays a crucial role in modulating the contribution of fronto-occipital connectivity to positive mother–child bonding interactions in play settings. These results provide insights into the impact that alterations at broader levels of cerebral organization states could have on human behavior, particularly in the context of sensitive and insensitive mothering. Future studies may help reveal the brain alterations in gray matter in neglectful mothers, differences in face-responsive regions when specifically processing infant faces, as well as the plasticity of the neurological circuits involved. These studies would be very helpful for the design of intervention programs focused on improving maternal responses to infant signals. In sum, the study of the neurological bases of maternal neglect offers an interesting venue for investigating the long-term impact of brain alterations associated with early adverse experiences on the quality of maternal care, helping to broaden our understanding of the neurobiology of mothering.
Supplementary data
Supplementary data are available at SCAN online.
Acknowledgments
The data collection was performed thanks to an agreement with the Health Department of the Canarian Government, who granted us access to neglectful and control mothers attending their services. Special thanks for their helpful advice to Manuel de Vega, Yasser Iturria, Ileana Quiñones, Wael El Deredy and Yasser Alemán; and for his assistance in graphic design to Oscar Carballido.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness (MINECO) and the European Regional Development Fund (FEDER) under the Grant PSI2015-69971-R (MINECO/FEDER, to M.J.R. and I.L.).
Conflict of interest. None declared.
References
- Atzil S., Hendler T., Feldman R. (2011). Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology, 36, 2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., LeBihan D. (1994). MR diffusion tensor spectroscopy and imaging. Biophysical Journal, 66, 259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa R, et al. (2001). Clinical validity of the ‘mini-mental state’ for Spanish speaking communities. Neuropsychologia, 39, 1150–7. [DOI] [PubMed] [Google Scholar]
- Brietzke E, et al. (2012). Impact of childhood stress on psychopathology. Revista Brasileira De Psiquiatría, 34, 480–8. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Donato R. (2003). Occipito‐temporal connections in the human brain. Brain, 126, 2093–107. [DOI] [PubMed] [Google Scholar]
- Catani M., de Schotten M.T. (2012). Atlas of Human Brain Connections . Oxford (UK: ): Oxford University Press. [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. (2002). Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17, 77–94. [DOI] [PubMed] [Google Scholar]
- Champagne F.A. (2008). Epigenetic mechanisms and the transgenerational effects of maternal care. Frontiers in Neuroendocrinology, 29, 386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Jeong B., Polcari A., Rohan M.L., Teicher M.H. (2012). Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage, 59, 1071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels J.K., Lamke J.P., Gaebler M., Walter H., Scheel M. (2013). White matter integrity and its relationship to PTSD and childhood trauma—A systematic review and meta‐analysis. Depression and Anxiety, 30, 207–16. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. Administration on Children, Youth and Families. Children’s Bureau (2015). Child Maltreatment 2013. Washington, DC (U.S: ): Government Printing Office. [Google Scholar]
- Dubowitz H., Kim J., Black M.M., Weisbart C., Semiatin J., Magder L.S. (2011). Identifying children at high risk for a child maltreatment report. Child Abuse and Neglect, 35, 96–104. [DOI] [PubMed] [Google Scholar]
- Easterbrooks M., Biringen Z. (2005). The emotional availability scales: METHODOLOGICAL refinements of the construct and clinical implications related to gender and at‐risk interactions. Infant Mental Health Journal, 26, 291–4. [DOI] [PubMed] [Google Scholar]
- Edmiston E.K., Blackford J.U. (2013). Childhood maltreatment and brain response to novel faces in adults with inhibited temperament. Psychiatry Research, 212, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Shipman K., Brown A. (2005). The socialization of emotional understanding: A comparison of neglectful and nonneglectful mothers and their children. Child Maltreatment, 10, 293–304. [DOI] [PubMed] [Google Scholar]
- Fairhall S.L., Ishai A. (2007). Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex, 17, 2400–6. [DOI] [PubMed] [Google Scholar]
- Ferrando L.B.J., Gilbert J., Soto M., Soto O. (2000). Mini International Neuropychiatric Interview (M.I.N.I.). Versión en Español 5.0.0. Madrid: Instituto IAP.
- Forkel S.J., Thiebaut de Schotten M., Kawadler J.M., Dell’Acqua F., Danek A., Catani M. (2014). The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex 56, 73–84. [DOI] [PubMed] [Google Scholar]
- Gschwind M., Pourtois G., Schwartz S., Van De Ville D., Vuilleumier P. (2012). White-matter connectivity between face-responsive regions in the human brain. Cerebral Cortex, 22, 1564–76. [DOI] [PubMed] [Google Scholar]
- Hanson J.L., Adluru N., Chung M.K., Alexander A.L., Davidson R.J., Pollak S.D. (2013). Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Development, 84, 1566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H., Rubia K. (2012). Neuroimaging of child abuse: a critical review. Frontiers in Human Neuroscience, 6, 52.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, et al. (2008). Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage, 39, 336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Gundapuneedi T., Rao U. (2012). White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology, 37, 2693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. (2013). White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage, 73, 239–54. [DOI] [PubMed] [Google Scholar]
- Kreher B, et al. (2008). Connecting and merging fibres: pathway extraction by combining probability maps. Neuroimage 43, 81–9. [DOI] [PubMed] [Google Scholar]
- Kruschke J.K. (2013). Bayesian estimation supersedes the t test. Journal of Experimental Psychology: General, 142, 573–603. [DOI] [PubMed] [Google Scholar]
- León I, et al. (2014). Electrophysiological responses to affective stimuli in neglectful mothers. PLoS One, 9, e87808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Crain B.J., Chacko V., Van Zijl P. (1999). Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology, 45, 265–9. [DOI] [PubMed] [Google Scholar]
- Mori S., Wakana S., Nagae-Poetscher L.M., van Zijl P.C. (2005). MRI Atlas of Human White Matter. Amsterdam (The Netherlands: ): Elsevier. [Google Scholar]
- Musser E.D., Kaiser-Laurent H., Ablow J.C. (2012). The neural correlates of maternal sensitivity: an fMRI study. Developomental Cognitive Neuroscience, 2, 428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology, 214, 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A., Joseph J., Feit M. (2014). New Directions in Child Abuse and Neglect Research. Washington, DC (U.S: ): The National Academies Press. [PubMed] [Google Scholar]
- Plant D., Barker E.D., Waters C., Pawlby S., Pariante C. (2013). Intergenerational transmission of maltreatment and psychopathology: the role of antenatal depression. Psychological Medicine, 43, 519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo M.J., León I., Quiñones I., Lage A., Byrne S., Bobes M.A. (2011). Brain and personality bases of insensitivity to infant cues in neglectful mothers: an event-related potential study. Developmental Psychopathology, 23, 163–76. [DOI] [PubMed] [Google Scholar]
- Schofield T.J., Lee R.D., Merrick M.T. (2013). Safe, stable, nurturing relationships as a moderator of intergenerational continuity of child maltreatment: a meta-analysis. Journal of Adolescent Health, 53, 32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC (2015) R: A Language and Environment for Statistical Computing. Vienna (Austria: ): R Foundation for Statistical Computing. [Google Scholar]
- van Harmelen A.L., et al. (2013). Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience, 8, 362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Jiang H., Nagae-Poetscher L.M., Van Zijl P.C., Mori S. (2004). Fiber tract–based atlas of human white matter anatomy 1. Radiology, 230, 77–87. [DOI] [PubMed] [Google Scholar]
- Wan M.W., Downey D., Strachan H., Elliott R., Williams S.R., Abel K.M. (2014). The neural basis of maternal bonding. PLoS One, 9, e88436.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.