Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is a continuously increasing cause of chronic liver disease and a health burden in all populations affected by the obesity and metabolic syndrome pandemic. Cirrhotic alterations or hepatocellular carcinoma developing from NAFLD may require liver transplantation (LTx).
Methods
Current literature was screened for data on LTx in the setting of NAFLD.
Results
NAFLD-associated LTx is expected to increase in number and relevance during the next decade. NAFLD is part of the metabolic syndrome and thus connected to various metabolic alterations and comorbidities such as diabetes or hyperlipidemia. Moreover, NAFLD comprises an independent risk factor for cardiovascular and chronic kidney disease, which again are important risk factors for outcome of surgical interventions. Postoperative immunosuppression, possible steatosis of the liver graft, and a continued presence of metabolic alterations may lead to early recurrence of steatosis or even non-alcoholic steatohepatitis. Currently, no data are available on combined approaches of weight loss and LTx for NAFLD.
Conclusion
Specific guidelines on how to manage NAFLD-associated LTx are lacking. This particular situation requires close monitoring of metabolic syndrome-associated comorbidities. NAFLD represents a novel challenge to established LTx procedures.
Key Words: Non-alcoholic fatty liver disease, NAFLD, Non-alcoholic steatohepatitis, NASH, Alcoholic steatohepatitis, ASH, Transplantation
Metabolic Syndrome and NAFLD
Metabolic syndrome, which affects approximately 25% of our population [1], is a constellation of different disease states including obesity, arterial hypertension, hyperglycemia, and hyperlipidemia, and is characterized by a state of low-grade chronic inflammation. Metabolic syndrome is associated with an increased risk of myocardial infarction and stroke (not however diabetes as it is part of the metabolic syndrome), and the combination of excessive caloric intake (particularly through junk food) and a sedentary lifestyle is a major risk factor for developing metabolic syndrome.
As a pivotal organ for whole-body metabolism, the liver is also affected by and plays an important role in metabolic syndrome. Unsurprisingly, non-alcoholic fatty liver disease (NAFLD) is now a leading cause of chronic liver disease among industrialized nations. As individuals with NAFLD have been reported to develop liver cancer even in the absence of overt liver cirrhosis, it is likely that the incidence of NAFLD-associated hepatocellular carcinoma (HCC) will also increase over time. These factors in concert will lead to NAFLD being a leading indication for liver transplantation in the near future.
The diagnosis of NAFLD encompasses a spectrum of disease states which range from fatty infiltration of the liver (simple steatosis) to a more progressive form characterized by inflammation and hepatocyte injury with ballooning (steatohepatitis) with or without liver fibrosis. Despite intensive research efforts, our understanding of the underlying pathophysiological mechanisms that drive disease progression remains incomplete.
Western Lifestyle Is Associated with the Development of NAFLD and Alcoholic Liver Disease (ALD/ASH)
The Western diet/lifestyle typically involves an excess amount of calories in the form of excess food and alcohol. Contrary to current dogma, recent studies suggest that steatosis is not benign. Instead, the presence of liver steatosis would predispose an individual to subsequent hepatotoxic injury. For example, a fatty liver would increase sensitivity to viral, alcohol, and drug toxicities [2]. Acetaminophen and most antibiotics, whilst safe in a healthy individual, may lead to liver injury in those with NAFLD.
Among individuals who drink 40-50 g of alcohol per day, over 50% will develop alcohol-induced fatty liver disease. A smaller number (approximately 35%) will develop the more advanced stage of acute alcoholic liver disease (ALD) known as alcoholic steatohepatitis (ASH), and this risk is significantly increased in those who are obese [3]. Interestingly, although red wine has been recommended by cardiologists as a protective agent in coronary heart disease, it may be detrimental to those with obesity [4]. Indeed, an average of just 1 glass of red wine per day (2-3 units) may lead to liver cirrhosis in obese individuals, even without overt features of alcohol abuse. These individuals therefore are at risk of developing non-alcoholic steatohepatitis (NASH) and ASH disease.
NAFLD/NASH and Liver Transplantation
The prevalence of NAFLD is expected to increase in parallel with the obesity and type 2 diabetes mellitus (T2DM) epidemics, bringing about an inevitable increase in the demand for liver transplantation. Indeed, by 2020, the number of individuals with NAFLD cirrhosis is set to overtake that of those with hepatitis B- and C-related cirrhosis, and NAFLD/NASH will be the leading indication for liver transplantation within developed countries [5].
Since 2001, the number of young individuals (up to age 40) requiring liver transplantation for end-stage NASH cirrhosis has been increasing [6]. Despite improvements in surgical and post-transplant care, as well as better immunosuppression protocols, the long-term outcome of NASH transplants in this cohort remains unsatisfactory. Only 58% are alive at the last follow-up after their first transplantation, and about 12% will require re-transplantation for NASH recurrence [6]. The latter is most likely related to an increased prevalence of obesity and (difficult to control) T2DM that occurs as a consequence of steroid and calcineurin inhibitor use after a liver transplant [6,7].
NAFLD will not only impact at an individual level but also at a societal level, because livers from individuals with NAFLD (i.e. fatty livers) are considered as ‘second’ choice by many physicians, and may not even be suitable for use as donor livers for transplantation [8,9]. Indeed, a recent analysis of the UNOS database found that donor livers which were significantly steatotic (greater than 30% macrovesicular steatosis) exhibited impaired 1-year graft survival [10]. Nevertheless, the true long-term clinical impact of steatotic donor livers remains uncertain as there have been contradictory reports, and small animal models of liver regeneration have actually demonstrated a beneficial effect of mild to moderate liver steatosis in promoting hepatocyte proliferation [11]. Therefore, until additional research proves otherwise, concerns regarding NAFLD donor liver use will remain.
NAFLD/NASH and Coronary Heart Disease
Patients with diabetes and NAFLD have an increased risk of developing atherosclerosis [12] and coronary artery disease (CAD). Recent data suggest that patients with NAFLD alone (i.e. without T2DM) are also at risk of developing CAD. A long-term study carried out over 21 years showed cardiovascular events or malignancy to be the main causes of death in patients with NAFLD [13]. In this study, individuals with simple steatosis (NAFL) and NASH are at an increased risk of cardiovascular events. These data were used to determine whether typical serum biomarkers for liver damage could be used to predict cardiovascular events. Gamma-glutamyl transferase (GGT) is a promising marker for hepatobiliary dysfunction and, traditionally, for increased alcohol consumption. GGT promotes the oxidation of low-density lipoproteins and is located in foam cells in human atherosclerotic plaque, suggesting that it may play a role in atherogenesis [14].
In fact, serum GGT levels are significantly associated with established cardiovascular risk factors, such as triglycerides, body mass index (BMI), and cholesterol. Furthermore, elevated levels of GGT in the serum are linked to a range of cardiovascular risk factors and the incidence of metabolic syndrome. In patients with acute coronary syndrome, GGT levels can be used to predict cardiovascular events and mortality. Alanine aminotransferase (ALT), another specific marker for liver damage particularly in those with NAFLD, is also being considered as a biomarker for cardiovascular and metabolic diseases. An epidemiological assessment (Hoorn study) concluded that there was a significant association between raised ALT levels and CAD, even in the absence of metabolic syndrome and atherosclerotic lesions [15]. We recently showed that besides age and troponin, liver transaminases (ALT and aspartate aminotransferase) and alkaline phosphatase were highly important for the discrimination of patients with a stenosis diameter (SD) ≥ 50% and SD < 50% [16].
NAFLD Is a Risk Factor for Hepatocellular Carcinoma
Most chronic liver diseases (viral hepatitis: hepatitis B virus (HBV) and hepatitis C virus (HCV), alcohol) are characterized by progression from fibrosis to cirrhosis and finally tumor development. NAFLD is no different, although the actual incidence of HCC in this setting is still unclear [17]. A recent US study reported that NASH-HCC is the fastest growing indication for liver transplantation [18], with the number of NASH-HCC liver transplants having increased by nearly fourfold since 2002 while HCV-HCC numbers increased by twofold. Even if the incidence of HCC development in the setting of NAFLD (NASH) is low (compared with ALD, HBV, or HCV), the rising prevalence of NAFLD will lead to an increased NAFLD-HCC transplant burden [18,19].
Interestingly, recent studies have reported that HCC may develop in a fatty liver even without underlying cirrhosis [20,21]. In our own study, less than 50% of the patients with NAFLD-induced HCC were cirrhotic, compared to 70-99% of those suffering from other chronic liver diseases [22]. The mechanisms involved in NAFLD-HCC development are largely unknown but are likely to be different from other chronic liver diseases [23]. Tumor suppressor genes such as KLF6 and PTEN, which have been shown to regulate lipid and glucose metabolism, are possible candidates in the interplay between an altered metabolism and the promotion of a cancer phenotype in NAFLD.
NAFLD as a Risk Factor for Chronic Kidney Damage
Patients with NAFLD and diabetes have a higher prevalence of chronic kidney damage than patients with diabetes but no NAFLD [24], and this increase in incidence is independent of sex, age, BMI, waist circumference, blood pressure, smoking habits, diabetes, microalbuminuria, glomerular filtration rate, and medication [25]. This observation was supported by a study from Korea which found NAFLD to be an independent risk factor for chronic kidney disease [26]. In another report from the USA, investigators noted that the rate of combined liver-kidney transplants was highest among those with NAFLD (NASH) cirrhosis [27]. Significantly, NAFLD individuals who received combined liver-kidney transplants were at greater risk of developing renal dysfunction compared to those with other forms of chronic liver disease such as ALD or primary biliary cholangitis [27]. This suggests that NAFLD could lead to subclinical kidney injury without obvious signs of chronic kidney damage but still representing a relevant risk for surgical settings.
Bariatric Surgery and Liver Transplantation
Obese individuals are at increased risks of morbidity and mortality after bariatric surgery compared with those with a lower BMI [28]. Similarly, liver transplantation outcomes are dictated by the presence or absence of medical comorbidities [7]. In a series of 306 obese liver transplant recipients, LaMattina et al. [29] found that patient and graft survival, blood transfusion requirements, length of intensive care unit stay, and postoperative biliary complications were all significantly higher in obese patients compared to their non-obese counterparts. These findings are unsurprising as obesity is associated with coronary heart disease and chronic kidney disease, both independent determinants of liver transplant outcomes as mentioned above. In our study, we found that NASH patients with a higher BMI have an increased mortality rate [9]. Our findings and the described connections between NAFLD, CAD, and chronic kidney damage might imply that in NAFLD subclinical damage of the heart and kidney may be present which cannot be detected with current routine methods. Despite the absence of symptoms, heart and kidney injury due to NAFLD could confer a significant inter- and post-surgical risk for patients. This is in line with observed outcomes in liver transplantation for NASH and in obese patients.
As the vast majority of individuals with NAFLD are likely to be obese and/or harbor metabolic risk factors, patients undergoing liver transplantation for NASH cirrhosis will need a comprehensive risk evaluation. Management of obese individuals undergoing liver transplantation should also include the consideration of weight-reducing strategies [30] such as bariatric surgery as well as the timing (i.e. before, during, or after liver transplant) and type of such surgery (e.g. sleeve gastrectomy). These discussions are necessary because patients with decompensated liver disease have a much higher risk of dying following bariatric surgery (16.3%) compared to those with compensated liver disease (0.9%) or no liver disease (0.3%) [31]. The complex interplay of organs affected by metabolic alterations and associated risk factors in NASH-associated liver transplantation are outlined in figure 1. Future studies are needed to help clinicians identify patients who are likely to become decompensated during bariatric surgery, and those who may be more suitable for bariatric surgery during or after a liver transplant.
Fig. 1.
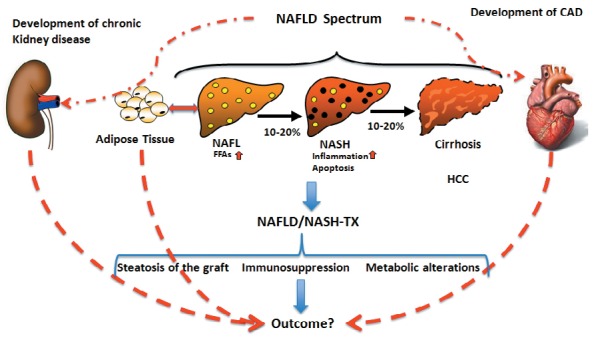
Complex interplay of organ systems relevant to outcome in non-alcoholic steatohepatitis (NASH)-associated liver transplantation. Metabolic alterations, in particular adipose tissue hypertrophy, lead to non-alcoholic fatty liver disease (NAFLD). NAFLD encompasses different disease states from non-alcoholic fatty liver/steatosis, NASH, and cirrhosis to tumor formation (HCC). NAFLD promotes the development of cardiovascular diseases (CAD) and chronic kidney disease. Upon liver transplantation for NAFLD or NASH, many factors influence the outcome, including CAD and chronic liver disease initially promoted by NAFLD itself. In addition, the persisting hypertrophic adipose tissue may again promote the development of NAFLD.
NASH Recurrence after Liver Transplantation
NASH recurrence is common; approximately 33% of patients develop steatohepatits in the allograft [32]. Multiple factors contribute to disease recurrence and/or disease progression, including the individual's genetic predisposition, the type of immunosuppression used, and the degree of graft steatosis on the index biopsy [7]. Studies in humans and mice suggest that ongoing metabolic dysregulation leading to elevated free fatty acids, hyperglycemia, and sarcopenia is responsible [33]. After all, visceral adipose tissue hypertrophy seems to be a crucial risk factor for NAFLD [34,35], remaining present after liver transplantation without concurrent weight reduction.
Research has continued to help physicians refine the (immediate and long-term) immunosuppression regimens used after liver transplantation. Corticosteroids, which remain the mainstay of treatment, decrease insulin production, suppress peripheral glucose utilization, and increase hepatic glucose production, thus promoting a diabetic state [7]. Similar effects are seen with calcineurin inhibitors (such as tacrolimus and cyclosporine). Therefore, patients who undergo transplantation for NASH should be managed on the least obesogenic and diabetogenic protocols.
The impact of donor steatosis on graft and patient outcomes remains poorly understood, but cumulative evidence suggests that the severity of donor steatosis may influence post-transplant outcomes [36]. For example, donor steatosis is associated with a threefold increased risk of post-transplant NAFLD [37].
Conclusion
NAFLD is a challenging and multifactorial disease with high socioeconomic impact. It is clear that over the next two decades, the prevalence of NASH will increase in parallel with obesity and T2DM, and NASH cirrhosis or HCC will be leading indications for liver transplantation. The management of patients with NAFLD is complicated because NAFLD is associated with metabolic risk factors, and may independently promote extrahepatic complications such as heart and kidney diseases. NAFLD recurrence after liver transplantation is common, and the development of recurrent disease may be dictated in part by the presence of metabolic comorbidities, post-transplant immunosuppression regimens, and the degree of steatosis in the donor liver. Future research should focus on refining immunosuppression protocols and clarifying the role of bariatric surgery in this complex patient group.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Moebus S, Hanisch J, Bramlage P, Lösch C, Hauner H, Wasem J, Jöckel K-H. Regional differences in the prevalence of the metabolic syndrome in primary care practices in Germany. Dtsch Arztebl Int. 2008;105:207–213. doi: 10.3238/artzebl.2008.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canbay A, Chen S-Y, Gieseler RK, Malago M, Karliova M, Gerken G, Broelsch CE, Treichel U. Overweight patients are more susceptible for acute liver failure. Hepatogastroenterology. 2005;52:1516–1520. [PubMed] [Google Scholar]
- 3.Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3:1260–1268. doi: 10.1016/s1542-3565(05)00743-3. [DOI] [PubMed] [Google Scholar]
- 4.Bechmann LP, Zahn D, Gieseler RK, Fingas CD, Marquitan G, Jochum C, Gerken G, Friedman SL, Canbay A. Resveratrol amplifies profibrogenic effects of free fatty acids on human hepatic stellate cells. Hepatol Res. 2009;39:601–608. doi: 10.1111/j.1872-034X.2008.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 6.Alkhouri N, Hanouneh IA, Zein NN, Lopez R, Kelly D, Eghtesad B, Fung JJ. Liver transplantation for nonalcoholic steatohepatitis (NASH) in young patients. Transpl Int. 2016;29:418–424. doi: 10.1111/tri.12694. [DOI] [PubMed] [Google Scholar]
- 7.Merola J, Liapakis A, Mulligan DC, Yoo PS. Non-alcoholic fatty liver disease following liver transplantation: a clinical review. Clin Transplant. 2015;29:728–737. doi: 10.1111/ctr.12585. [DOI] [PubMed] [Google Scholar]
- 8.Dutkowski P, Schlegel A, Slankamenac K, Oberkofler CE, Adam R, Burroughs AK, Schadde E, Müllhaupt B, Clavien P-A. The use of fatty liver grafts in modern allocation systems: risk assessment by the balance of risk (BAR) score. Ann Surg. 2012;256:861–868. doi: 10.1097/SLA.0b013e318272dea2. discussion 868-869. [DOI] [PubMed] [Google Scholar]
- 9.Heuer M, Kaiser GM, Kahraman A, Banysch M, Saner FH, Mathé Z, Gerken G, Paul A, Canbay A, Treckmann JW. Liver transplantation in nonalcoholic steatohepatitis is associated with high mortality and post-transplant complications: a single-center experience. Digestion. 2012;86:107–113. doi: 10.1159/000339344. [DOI] [PubMed] [Google Scholar]
- 10.Spitzer AL, Lao OB, Dick AAS, Bakthavatsalam R, Halldorson JB, Yeh MM, Upton MP, Reyes JD, Perkins JD. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010;16:874–884. doi: 10.1002/lt.22085. [DOI] [PubMed] [Google Scholar]
- 11.Sydor S, Gu Y, Schlattjan M, Bechmann LP, Rauen U, Best J, Paul A, Baba HA, Sowa J-P, Gerken G, Canbay A. Steatosis does not impair liver regeneration after partial hepatectomy. Lab Invest. 2013;93:20–30. doi: 10.1038/labinvest.2012.142. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui MS, Sterling RK, Luketic VA, Puri P, Stravitz RT, Bouneva I, Boyett S, Fuchs M, Sargeant C, Warnick GR, Grami S, Sanyal AJ. Association between high-normal levels of alanine aminotransferase and risk factors for atherogenesis. Gastroenterology. 2013;145:1271–9. doi: 10.1053/j.gastro.2013.08.036. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed MH, Barakat S, Almobarak AO. Nonalcoholic fatty liver disease and cardiovascular disease: has the time come for cardiologists to be hepatologists? J Obes. 2012;2012:483135. doi: 10.1155/2012/483135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emdin M, Passino C, Michelassi C, Titta F, L'abbate A, Donato L, Pompella A, Paolicchi A. Prognostic value of serum gamma-glutamyl transferase activity after myocardial infarction. Eur Heart J. 2001;22:1802–1807. doi: 10.1053/euhj.2001.2807. [DOI] [PubMed] [Google Scholar]
- 15.Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CDA, Heine RJ, Diamant M. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191:391–396. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Baars T, Neumann U, Jinawy M, Hendricks S, Sowa J-P, Kälsch J, Riemenschneider M, Gerken G, Erbel R, Heider D, Canbay A. In acute myocardial infarction liver parameters are associated with stenosis diameter. Medicine (Baltimore) 2016;95:e2807. doi: 10.1097/MD.0000000000002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 18.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 19.Charlton M. Evolving aspects of liver transplantation for nonalcoholic steatohepatitis. Curr Opin Organ Transplant. 2013;18:251–258. doi: 10.1097/MOT.0b013e3283615d30. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: growing evidence of an epidemic? Hepatol Res. 2012;42:1–14. doi: 10.1111/j.1872-034X.2011.00872.x. [DOI] [PubMed] [Google Scholar]
- 21.Torres DM, Harrison SA. Nonalcoholic steatohepatitis and noncirrhotic hepatocellular carcinoma: fertile soil. Semin Liver Dis. 2012;32:30–38. doi: 10.1055/s-0032-1306424. [DOI] [PubMed] [Google Scholar]
- 22.Ertle J, Dechêne A, Sowa J-P, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn W-K, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436–2443. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 23.Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Yasui K, Sumida Y, Mori Y, Mitsuyoshi H, Minami M, Itoh Y, Kanemasa K, Matsubara H, Okanoue T, Yoshikawa T. Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metab Clin Exp. 2011;60:735–739. doi: 10.1016/j.metabol.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5:2166–2171. doi: 10.2215/CJN.05050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang Y, Ryu S, Sung E, Woo H-Y, Oh E, Cha K, Jung E, Kim WS. Nonalcoholic fatty liver disease predicts chronic kidney disease in nonhypertensive and nondiabetic Korean men. Metab Clin Exp. 2008;57:569–576. doi: 10.1016/j.metabol.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Hmoud B, Kuo Y-F, Wiesner RH, Singal AK. Outcomes of liver transplantation alone after listing for simultaneous kidney: comparison to simultaneous liver kidney transplantation. Transplantation. 2015;99:823–828. doi: 10.1097/TP.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 28.de Lorenzo A, Soldati L, Sarlo F, Calvani M, Di Lorenzo N, Di Renzo L. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol. 2016;22:681–703. doi: 10.3748/wjg.v22.i2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaMattina JC, Foley DP, Fernandez LA, Pirsch JD, Musat AI, D'Alessandro AM, Mezrich JD. Complications associated with liver transplantation in the obese recipient. Clin Transplant. 2012;26:910–918. doi: 10.1111/j.1399-0012.2012.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reino DC, Weigle KE, Dutson EP, Bodzin AS, Lunsford KE, Busuttil RW. Liver transplantation and sleeve gastrectomy in the medically complicated obese: new challenges on the horizon. World J Hepatol. 2015;7:2315–2318. doi: 10.4254/wjh.v7.i21.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:897–901. doi: 10.1016/j.cgh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Bhagat V, Mindikoglu AL, Nudo CG, Schiff ER, Tzakis A, Regev A. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15:1814–1820. doi: 10.1002/lt.21927. [DOI] [PubMed] [Google Scholar]
- 33.Schütz T, Hudjetz H, Roske A-E, Katzorke C, Kreymann G, Budde K, Fritsche L, Neumayer H-H, Lochs H, Plauth M. Weight gain in long-term survivors of kidney or liver transplantation - another paradigm of sarcopenic obesity? Nutrition. 2012;28:378–383. doi: 10.1016/j.nut.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 34.Park BJ, Kim YJ, Kim DH, Kim W, Jung YJ, Yoon JH, Kim CY, Cho YM, Kim SH, Lee KB, Jang JJ, Lee HS. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol. 2008;23:900–907. doi: 10.1111/j.1440-1746.2007.05212.x. [DOI] [PubMed] [Google Scholar]
- 35.Wree A, Schlattjan M, Bechmann LP, Claudel T, Sowa J-P, Stojakovic T, Scharnagl H, Köfeler H, Baba HA, Gerken G, Feldstein AE, Trauner M, Canbay A. Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients. Metab Clin Exp. 2014;63:1542–1552. doi: 10.1016/j.metabol.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Dumortier J, Giostra E, Belbouab S, Morard I, Guillaud O, Spahr L, Boillot O, Rubbia-Brandt L, Scoazec J-Y, Hadengue A. Non-alcoholic fatty liver disease in liver transplant recipients: another story of ‘seed and soil’. Am J Gastroenterol. 2010;105:613–620. doi: 10.1038/ajg.2009.717. [DOI] [PubMed] [Google Scholar]
- 37.Kim H, Lee K, Lee K, Yi N-J, Lee HW, Hong G, Choi Y, You T, Suh S-W, Jang JJ, Suh K-S. Histologically proven non-alcoholic fatty liver disease and clinically related factors in recipients after liver transplantation. Clin Transplant. 2014;28:521–529. doi: 10.1111/ctr.12343. [DOI] [PubMed] [Google Scholar]


