MicroAbstract
This study evaluated the use of lobectomy and sublobar resection for clinical stage IA non-small cell lung cancer (NSCLC) in the National Cancer Data Base. A total of 39,403 patients were included for analysis, of whom 9,667 (24.5%) underwent sublobar resection. Lobectomy was associated with superior survival, however among sublobar resection patients, lymph node sampling was associated with improved outcomes although was performed in a minority of sublobar patients. This study emphasizes the need for nodal sampling when performing sublobar resection even for the earliest stages of NSCLC.
Background
This study evaluated the use of lobectomy and sublobar resection for clinical stage IA non-small cell lung cancer (NSCLC) in the National Cancer Data Base (NCDB).
Methods
The National Cancer Data Base from 2003–2011 was analyzed to determine factors associated with the use of a sublobar resection versus a lobectomy for the treatment of clinical stage IA NSCLC. Overall survival was assessed using the Kaplan-Meier method and Cox proportional hazards modeling.
Results
Among 39,403 patients included for analysis, 29,736 (75.5%) received a lobectomy and 9,667 (24.5%) received a sublobar resection: 84.7% wedge resection (n=8192); 15.3% segmental resection (n=1475). Lymph node evaluation was not performed in 2,788 (28.8%) of sublobar resection patients, and 7,298 (75.5%) of sublobar resections were for tumors ≤2cm. Following multivariable logistic regression, older age, higher Charlson/Deyo comorbidity scores, smaller tumor size, and treatment at lower-volume institutions were associated with sublobar resection (all p<0.001). Overall, lobectomy was associated with significantly improved 5-year survival compared to sublobar resection (66.2% vs. 51.2%, p<0.001, adjusted hazard ratio 0.66, p<0.001). However among sublobar resection patients, nodal sampling was associated with significantly better 5-year survival (58.2% vs. 46.4%, p<0.001).
Conclusions
Despite adjustment for patient and tumor related characteristics, a sublobar resection is associated with significantly reduced long-term survival as compared to a formal surgical lobectomy among patients with NSCLC, even for stage 1A tumors. For patients who cannot tolerate lobectomy and are treated with sublobar resection, lymph node evaluation is essential to help guide further treatment.
Keywords: lung cancer, NSCLC, stage IA, lobectomy, sublobar resection
Introduction
A formal surgical lobectomy is the recommended treatment for patients diagnosed with early stage non-small cell lung cancer (NSCLC), based on level I data.1,2 Despite these findings, some have begun to question whether this procedure is necessary for all subsets of patients presenting with this disease, most notably patients with Stage IA tumors.3–6 Several retrospective studies have found no significant differences in survival between patients treated with a sublobar resection versus a formal lobectomy for stage I NSCLC tumors less than 2 cm in size.7–9 Other studies have suggested that the survival benefit of lobectomy over sublobar resection diminishes with age, likely secondary to the higher perioperative mortality in these patients as well as the competing risk of mortality from other comorbidities.8,10 In addition, one study found that local recurrence as well as overall and recurrence-free survival after sublobar resection were similar to lobectomy when lymph nodes (LN) were sampled at the time of sublobar resection.11 A prospective, randomized, multi-institutional phase III trial (Cancer and Lymphoma Group B [CALGB] 140503) that compares survival after lobectomy and intentional sublobar resection for peripheral tumors less than or equal to 2 cm in size is currently being conducted.12 This study, which requires complete mediastinal and hilar node sampling prior to subsequent randomization to lobectomy or sublobar resection if frozen section analyses of the lymph nodes are negative for malignancy, will likely provide strong evidence to guide clinical practice but the results are not likely to be available for several years.
The purpose of the current study was to use a large, nationwide cancer database to examine current practice patterns in the surgical management of patients with clinical stage IA NSCLC to both identify predictors of surgical management with sublobar resection versus lobectomy, and also to evaluate the extent of pathologic lymph node assessment performed in association with sublobar resections in a non-clinical trial setting. A secondary goal was to compare long-term survival between the two treatment approaches.
Material and Methods
The Duke University Institutional Review Board approved this retrospective review of patients diagnosed with clinical stage IA (T1N0M0) NSCLC cancer from 2003 to 2011 in the National Cancer Database (NCDB). This study period was chosen based on the availability of data in the NCDB at the time of analysis. The NCDB collects data from over 1,500 centers in the United States and Puerto Rico, is estimated to capture approximately 70 percent of all newly diagnosed cases of cancer nationwide. The NCDB records clinical stage using the American Joint Committee on Cancer (AJCC) staging criteria concordant with the year of diagnosis, and the AJCC 5th through 7th editions were therefore used to identify patients for inclusion. While the AJCC 7th edition further distinguished T1 tumors into T1a and T1b, there were no changes across editions in the overall definition of T1 or N0 disease, and as such no attempts to manually re-code the clinical staging data were attempted.
Patients undergoing either a lobectomy (Facility Oncology Registry Data Standards [FORDS] codes 30, 33) or a sublobar resection (FORDS codes 21–22) for non-small cell lung cancer were included. Patients receiving induction therapy were excluded. Patients were then grouped by procedure type, and baseline characteristics were compared using Pearson’s chi-square test on categorical variables and Student’s t-test on continuous variables. To estimate predictors of receiving a lobectomy versus a sublobar resection, a multivariable logistic regression model was created which included the following variables based on clinical significance: patient age at diagnosis, sex, race, insurance (insured vs. uninsured), education, income, Charlson/Deyo comorbidity score, tumor size (by centimeter), and hospital volume (by quartile). A similar model was performed to estimate predictors of lymph node sampling among patients treated with sublobar resection. The Kaplan-Meier method and Cox proportional hazards modeling were utilized to estimate the association between surgical approach (lobectomy vs. sublobar resection) with long-term survival, after adjusting for patient age, sex, Charlson/Deyo comorbidity score, tumor size, and facility volume. Long-term survival was assessed for patients diagnosed from 2003–2006, as survival data was not available in the NCDB for 2007 and later at the time of analysis.
To explore whether any apparent long-term survival advantage of lobectomy was confounded by variability in lymph node sampling among the patients treated with a sublobar resection, we then conducted a series of subgroup analyses. First, survival among the cohort of patients treated with sublobar resection was examined, stratified by whether the procedure included lymph node sampling. We then compared survival among patients treated with formal lobectomy versus those patients treated with sublobar resection that included lymph node sampling. Lastly, the comparison of patients treated with formal lobectomy versus those patients treated with sublobar resection that included lymph node sampling was repeated, but only among patients who were found to have negative node pathology following resection.
An affirmative decision was made to control for type I error at the level of the comparison, and p-values less than 0.05 were considered statistically significant for all comparisons. Missing data were handled with complete-case analysis, given the substantial completeness of the NCDB data for the years included in this study. All analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, North Carolina).
Results
Of 39,403 patients who underwent resection for clinical stage IA NSCLC in the database, 29,736 (75.5%) were treated with anatomic lobectomy and 9,667 (24.5%) underwent a sublobar resection. Among the sublobar resection patients, wedge resection was performed in 84.7% (n=8192) and segmental resection was performed in 15.3% (n=1475). Patients treated with sublobar resection were generally older, had higher average Charlson/Deyo comorbidity scores, had tumors that were slightly smaller, and were more likely to be treated at lower-volume institutions (Table 1). Following adjustment with multivariable logistic regression, younger age, lower Charlson/Deyo scores, larger tumor size, and higher facility case volume were predictive of patients being treated with lobectomy (Table 2).
Table 1.
Baseline patient, tumor and treatment characteristics, comparing lobectomy versus sublobar resection for 2003–2011.
| Lobectomy (N=29736) | Sublobar (N=9667) | Total (N=39403) | p value | |
|---|---|---|---|---|
| Patient Age | <0.0001 | |||
| Mean (SD) | 66.7 (9.7) | 69.6 (9.5) | 67.4 (9.8) | |
| Median | 67.0 | 70.0 | 68.0 | |
| Sex | 0.18 | |||
| Male | 12970 (43.6%) | 4142 (42.8%) | 17112 (43.4%) | |
| Female | 16766 (56.4%) | 5525 (57.2%) | 22291 (56.6%) | |
| Race | <0.0001 | |||
| White | 26491 (89.1%) | 8775 (90.8%) | 35266 (89.5%) | |
| Black | 2365 (8.0%) | 713 (7.4%) | 3078 (7.8%) | |
| Other | 880 (3.0%) | 179 (1.9%) | 1059 (2.7%) | |
| Insurance | 0.0019 | |||
| Uninsured | 511 (1.7%) | 122 (1.3%) | 633 (1.6%) | |
| Insured | 29225 (98.3%) | 9545 (98.7%) | 38770 (98.4%) | |
| Income | 0.11 | |||
| < $30,000 | 3858 (13.0%) | 1271 (13.1%) | 5129 (13.0%) | |
| $30,000 – $34,999 | 5645 (19.0%) | 1867 (19.3%) | 7512 (19.1%) | |
| $35,000 – $45,999 | 8621 (29.0%) | 2677 (27.7%) | 11298 (28.7%) | |
| $46,000 + | 11612 (39.1%) | 3852 (39.8%) | 15464 (39.2%) | |
| Education (% of patients in census tract without HS education) | 0.79 | |||
| >=29% | 4663 (15.7%) | 1508 (15.6%) | 6171 (15.7%) | |
| 20–28.9% | 7268 (24.4%) | 2361 (24.4%) | 9629 (24.4%) | |
| 14–19.9% | 7381 (24.8%) | 2447 (25.3%) | 9828 (24.9%) | |
| < 14% | 10424 (35.1%) | 3351 (34.7%) | 13775 (35.0%) | |
| Charlson/Deyo Score | <0.0001 | |||
| 0 | 14615 (49.1%) | 3823 (39.5%) | 18438 (46.8%) | |
| 1 | 11053 (37.2%) | 4057 (42.0%) | 15110 (38.3%) | |
| ≥2 | 4068 (13.7%) | 1787 (18.5%) | 5855 (14.9%) | |
| Facility Volume (quartiles) | <0.0001 | |||
| First quartile (lowest volume) | 2279 (7.7%) | 888 (9.2%) | 3167 (8.0%) | |
| Second quartile | 3296 (11.1%) | 1193 (12.3%) | 4489 (11.4%) | |
| Third quartile | 6549 (22.0%) | 2157 (22.3%) | 8706 (22.1%) | |
| Fourth quartile (highest volume) | 17612 (59.2%) | 5429 (56.2%) | 23041 (58.5%) | |
| Size of Tumor (cm) | <0.0001 | |||
| Mean (SD) | 2.0 (0.9) | 1.7 (0.7) | 2.0 (0.9) | |
| AJCC Pathologic T | <0.0001 | |||
| 0/IS | 23 (0.1%) | 7 (0.0%) | 30 (0.1%) | |
| 1 | 23456 (79.4%) | 7586 (79.3%) | 31042 (79.4%) | |
| 2 | 4318 (14.6%) | 946 (9.9%) | 5264 (13.5%) | |
| 3 | 347 (1.2%) | 101 (1.1%) | 448 (1.1%) | |
| 4 | 402 (1.4%) | 116 (1.2%) | 518 (1.3%) | |
| AJCC Pathologic N | <0.0001 | |||
| 0 | 25477 (86.3%) | 6396 (67.6%) | 31873 (81.8%) | |
| 1 | 1811 (6.1%) | 115 (1.2%) | 1926 (4.9%) | |
| 2 | 950 (3.2%) | 155 (1.6%) | 1105 (2.8%) | |
| 3 | 2 (0.0%) | 2 (0.0%) | 4 (0.0%) | |
| X | 1268 (4.3%) | 2788 (29.5%) | 4056 (10.4%) | |
| Regional Lymph Nodes Examined | <0.0001 | |||
| Mean (SD) | 8.6 (6.6) | 2.9 (4.6) | 7.1 (6.7) | |
| Median | 7.0 | 1.0 | 6.0 | |
| Surgical Margins | <0.0001 | |||
| R0 | 29075 (97.8%) | 9061 (93.7%) | 38136 (96.8%) | |
| R1 | 327 (1.1%) | 250 (2.6%) | 577 (1.5%) | |
| R2 | 13 (0.0%) | 24 (0.2%) | 37 (0.1%) | |
| Residual tumor, NOS | 153 (0.5%) | 146 (1.5%) | 299 (0.8%) | |
| Unknown | 168 (0.6%) | 186 (1.9%) | 354 (0.9%) | |
| Adjuvant Chemotherapy | 2559 (8.6%) | 798 (8.2%) | 3357 (8.5%) | 0.28 |
| Adjuvant Radiation Therapy | 2475 (8.3%) | 921 (9.5%) | 3396 (8.6%) | 0.0002 |
Table 2.
Variables associated with treatment (lobectomy versus sublobar resection), following multivariable logistic regression, 2003–2011.
| Predictor | Odds ratio | 95% Wald Confidence Limits | P value | |
|---|---|---|---|---|
| Age (per decade) | 0.69 | 0.67 | 0.71 | <.0001 |
| Female vs Male | 0.98 | 0.94 | 1.03 | 0.51 |
| Race | ||||
| Black vs White | 0.99 | 0.91 | 1.09 | 0.90 |
| Other vs White | 1.45 | 1.23 | 1.71 | <.0001 |
| Insured vs Uninsured | 1.11 | 0.91 | 1.37 | 0.31 |
| Median census income (per quartile) | 0.99 | 0.96 | 1.02 | 0.59 |
| Median census education (per quartile) | 1.03 | 1.00 | 1.06 | 0.06 |
| Charlson/Deyo comorbidity score | ||||
| 1 vs 0 | 0.74 | 0.70 | 0.78 | <.0001 |
| ≥2 vs 0 | 0.62 | 0.58 | 0.66 | <.0001 |
| Tumor size (per centimeter) | 1.90 | 1.83 | 1.97 | <.0001 |
| Facility volume (per quartile) | 1.09 | 1.07 | 1.12 | <.0001 |
Patients who underwent lobectomy had positive margins recorded in 1.6% (n=493) of cases, while those undergoing a sublobar resection were recorded as having positive margins in 4.3% (n=420) of cases (p<0.001). Regarding pathologic nodal status, patients treated with lobectomy were node positive in 2,763 (9.3%) cases, while patients undergoing sublobar resection were node positive in 272 (2.8%) cases (p<0.001). However, an additional 2,788 (29.5%) patients who had sublobar resection were designated as pNX due to the absence of LN sampling as compared to only 1,268 (4.3%) lobectomy patients. Of the patients treated with a sublobar resection, 7,298 (75.5%) had tumors ≤2 cm and 2,369 (24.5%) had tumors >2 cm, and wedge resection was associated with substantially lower odds of LN sampling compared to segmental resection (OR: 0.32, p<0.001). Similarly, older age, lower median census income, higher Charlson/Deyo comorbidity scores, and lower facility volume were associated with significantly lower odds of LN sampling being performed (Table 3A). Of patients who had a sublobar resection, lower facility volume and smaller tumor size predicted that a wedge resection was performed rather than a segmentectomy (Table 3B). Among patients in whom nodes were sampled, lobectomy was associated with significantly more nodes retrieved compared to sublobar resection (mean 8.8 vs. 5.4, respectively, p<.0001).
Table 3.
(A) Variables associated with undergoing lymph node sampling (yes vs. no) among patients treated with sublobar resection and (B) independent predictors of wedge resection versus segmentectomy among patients treated with sublobar resection, following multivariable logistic regression, 2003–2011.
| A. | ||||
|---|---|---|---|---|
| Predictor | Odds ratio | 95% Wald Confidence Limits | P value | |
| Wedge vs. segmental resection | 0.32 | 0.28 | 0.37 | <0.0001 |
| Age (per decade) | 0.83 | 0.79 | 0.87 | <0.0001 |
| Median census income (per quartile) | 1.06 | 1.01 | 1.13 | 0.026 |
| Median census education (per quartile) | 0.97 | 0.92 | 1.02 | 0.27 |
| Charlson/Deyo comorbidity score | ||||
| 1 vs. 0 | 0.81 | 0.74 | 0.89 | <0.0001 |
| ≥2 vs. 0 | 0.77 | 0.68 | 0.87 | <0.0001 |
| Facility volume (per quartile) | 1.31 | 1.25 | 1.37 | <0.0001 |
| Tumor size (per cm) | 1.31 | 1.23 | 1.40 | <0.0001 |
| B. | ||||
|---|---|---|---|---|
| Predictor | Odds ratio | 95% Wald Confidence Limits | P value | |
| Tumor histology | ||||
| Squamous vs. Adeno | 1.05 | 0.92 | 1.20 | 0.447 |
| Other histology vs. Adeno | 1.25 | 1.04 | 1.50 | 0.017 |
| Age (per decade) | 1.01 | 0.96 | 1.08 | 0.640 |
| Median census income (per quartile) | 0.97 | 0.90 | 1.05 | 0.453 |
| Median census education (per quartile) | 0.96 | 0.90 | 1.03 | 0.297 |
| Charlson/Deyo comorbidity score | ||||
| 1 vs. 0 | 0.94 | 0.83 | 1.06 | 0.294 |
| ≥2 vs. 0 | 1.12 | 0.95 | 1.32 | 0.175 |
| Facility volume (per quartile) | 0.86 | 0.81 | 0.92 | <0.001 |
| Tumor size (per cm) | 0.79 | 0.73 | 0.84 | <0.001 |
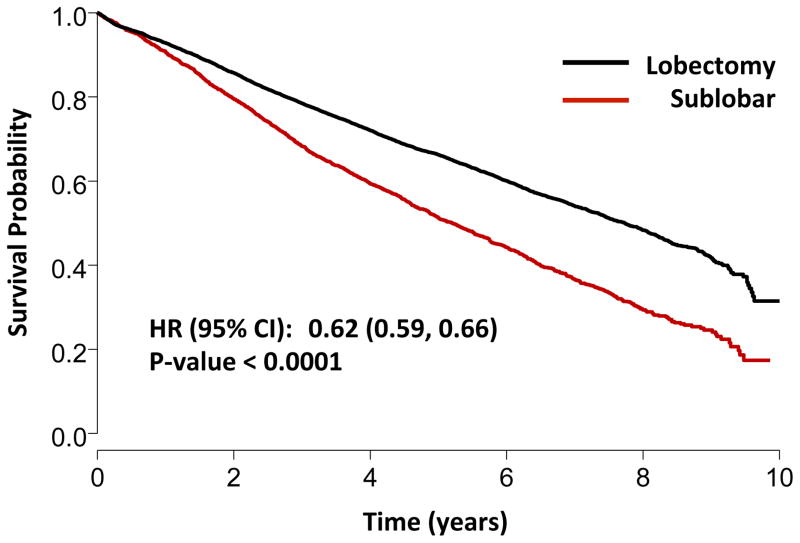
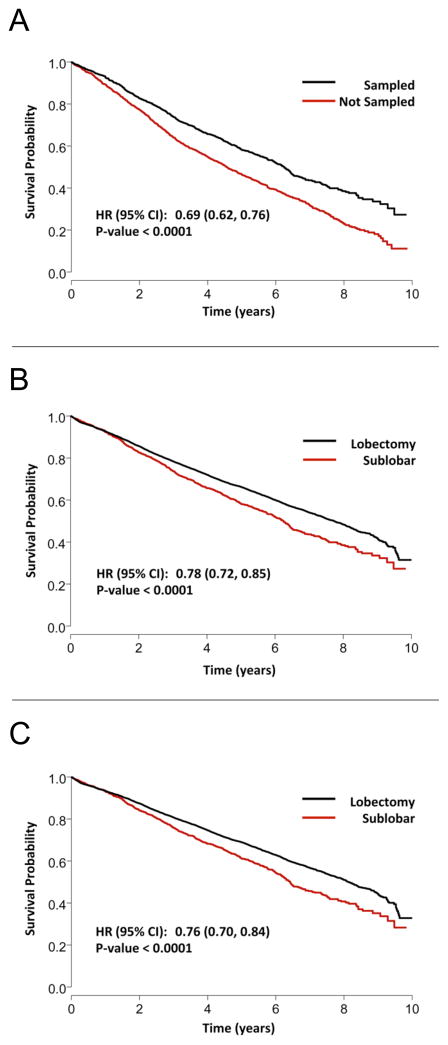
Out of the entire 39,403 patient cohort, 11,990 (30.4%) were reseccted between 2003 and 2006 and were thus included in the survival analysis. Median follow-up time was 6.3 years (IQR: 5.0–7.4 years). Lobectomy was associated with significantly improved 5-year survival compared to sublobar resection (Figure 1) on unadjusted analysis. Multivariable Cox proportional hazards modeling demonstrated that despite adjustment for patient and tumor related factors, lobectomy remained associated with a significant survival advantage as compared to sublobar resection. Additionally, younger age, female sex, lower Charlson comorbidity scores, smaller tumor size, and higher facility case volumes were associated with improved survival (Table 4). Among patients undergoing sublobar resection, patients who received a LN evaluation had significantly better 5-year survival as compared to patients who did not have LN sampling (Figure 2A).
Figure 1.
Kaplan-Meier overall survival curve for lobectomy versus sublobar resection; stage IA NSCLC.
Table 4.
Factors associated with long-term survival, based on a multivariable Cox proportional hazards model, 2003–2006.
| Factor | HR | 95% Confidence Limits | P value | |
|---|---|---|---|---|
| Lobectomy vs. sublobar resection | 0.66 | 0.62 | 0.70 | <.0001 |
| Age (per decade) | 1.37 | 1.33 | 1.41 | <.0001 |
| Female vs. Male | 0.76 | 0.72 | 0.80 | <.0001 |
| Charlson/Deyo comorbidity score | ||||
| 1 vs. 0 | 1.21 | 1.14 | 1.29 | <.0001 |
| ≥2 vs. 0 | 1.56 | 1.44 | 1.68 | <.0001 |
| Tumor size (per centimeter) | 1.19 | 1.16 | 1.22 | <.0001 |
| Facility volume (per quartile) | 0.95 | 0.93 | 0.98 | 0.0006 |
Figure 2.
Kaplan-Meier survival curves demonstrating the association of lymph node sampling with outcomes among patients with stage IA NSCLC: (A) comparing patients treated with sublobar resection who underwent LN sampling versus those who did not; (B) comparing formal lobectomy versus sublobar resection that included LN sampling; and (C) for pathologic node-negative lobectomy versus pathologic node-negative sublobar resection.
To determine whether the infrequency of LN sampling during sublobar resection, and the subsequent lack of adequate staging data to guide further therapy, was a primary factor associated with worse long-term survival, sub-group analysis comparing lobectomy to only those patients treated with sublobar resection that included LN sampling was performed. When examining only these patients undergoing LN sampling, lobectomy maintained a significant long-term survival advantage over sublobar resection (Figure 2B). Similarly, when comparing lobectomy to sublobar resection with LN sampling only among patients who were found to be pN0, lobectomy continued to be associated with a long term survival benefit (Figure 2C). Among patients found to have a positive LN, there was no difference between the sublobar resection and lobectomy groups with respect to the rate of adjuvant chemotherapy use following resection (60.0 vs. 60.6%, p=0.86, respectively).
Discussion
In this study of almost 40,000 patients treated with surgical resection of clinical stage IA NSCLC in the United States from 2003–2011, we found that sublobar resection was used in approximately 25% of cases. Most sublobar resections consisted of a wedge resection, with segmentectomy performed in only 15% of cases. Over 25% of sublobar resections also did not involve a pathologic lymph node assessment. The use of sublobar resection was associated not only with patient characteristics (age, tumor size, comorbidity index) but also with provider (facility case volume) variables. Furthermore, a lobectomy was associated with significantly better 5-year survival compared to a sublobar resection despite adjustment for important prognostic factors such as patient age, gender, comorbidity index, and tumor size. Lastly, when a sublobar resection was performed, survival was significantly improved when lymph node evaluation was performed.
The Lung Cancer Study Group randomized trial that demonstrated a benefit to lobectomy compared with more limited resection for T1N0 NSCLC involved 276 patients who had surgery between 1982 and 1988. This trial demonstrated that limited resection was associated with nearly double rates of local recurrence and trends toward worse disease-specific and overall survival.2 Despite these findings, both diagnostic and staging modalities have clearly changed since this randomized trial was performed. The increased use of radiologic studies in general, and CT scans in particular, are likely leading to the identification of tumors of smaller sizes than what was typically seen in the 1980s and earlier. Prognosis for T1 tumors is related to tumor size, with survival appearing to be significantly better in patients whose tumors are less than 2 cm.13–17 Several single-institution and multi-institution studies have suggested that sublobar resection may have similar survival benefits to lobectomy for certain subsets of patients with early stage NSCLC, including patients with smaller tumors and older patients.7–9,18–21 More specifically, Okada et al reported that with respect to long-term survival for tumors less than 3cm, segmental resection was equivalent to lobectomy, while wedge resection was inferior.15 Limited resections also have the benefit of potentially less morbidity and more preservation of pulmonary function.22 However, ultimate completion of the ongoing prospective randomized CALGB 140503 trial that compares survival after lobectomy and intentional sublobar resection for small peripheral tumors will significantly improve the evidence available to guide treatment.
It is very important to note, however, that the randomized CALGB 14053 trial is restricted to tumors less than or equal to 2 cm and that the trial requires pathologic lymph node assessment prior to treatment randomization.12 One potential reason that anatomic resection with lobectomy was observed to have better locoregional control in the original randomized trial may have been due to resection of unsuspected intralobar tumor spread, which could be therapeutic in itself or also help direct adjuvant treatment, especially considering that in one study 23% of patients with clinical IA NSCLC were found to have pathologic lymph node involvement or intrapulmonary metastases.23 Lymph node sampling is therefore potentially very important when sublobar resection is performed. Indeed, one study showed that local recurrence rate and overall and recurrence-free survival after sublobar resection were similar to lobectomy when lymph nodes were sampled at the time of sublobar resection.11 Another study also has suggested that wedge resection with lymph node sampling is an adequate oncological procedure for non-small cell lung cancer in patients with stage IA tumors less than 2 cm in diameter.7
In our study, we found that among patients treated with sublobar resection, segmental resection was associated with substantially higher odds of LN sampling compared to wedge resection, as were younger age, fewer comorbidities and higher facility volume. We also found that lymph node sampling was associated with improved survival in sublobar patients. While it is possible that this was due to a therapeutic effect of lymph node sampling, it is more likely that this reflects the importance of obtaining pathologic nodal staging data, whether to select patients who should have a more formal anatomic lobectomy at the time of surgery, or to help guide appropriate postoperative therapy. Accordingly, the results of our current study and the other described studies essentially mandate that lymph node sampling be an integral part of the surgical management of NSCLC. However, pathologic lymph node assessment was not performed in over 25% of patients who had sublobar resections in our study. Even if CALGB 14503 ultimately shows that a sublobar resection is appropriate for small peripheral tumors, many surgeons will likely need to alter their surgical practice to include lymph node assessment. In fact, the ability to sample nodes is one very important potential advantage of the use of sublobar resection versus non-invasive ablative therapies such as stereotactic radiation.
Given that several studies have suggested that sublobar resection may not compromise oncologic efficacy for some early-stage NSCLC patients, the relatively common use of this approach in the NCDB is not necessarily surprising. However, this study does show that certain variables are associated with the use of lobectomy. The most powerful predictors for the use of lobectomy are patient-specific, such as age, tumor size, and patient co-morbidity index. Patient age and comorbidity may be in part related to whether patients were considered medically fit for a lobectomy, and tumor size may have been related to provider belief regarding whether sublobar resection is oncologically equivalent to lobectomy. These findings suggest that the treatment utilized for many patients is appropriately chosen based on individual patient factors. However, a non-medical patient characteristic - the education level of the census tract where patients live – was also found to have a strong trend towards significance (p=0.059) as a predictor for the use of lobectomy, although the association of this factor with lobectomy was much weaker than that of the important identified clinical factors. Perhaps more importantly, facility volume was also an important predictor for the extent of resection. Although the association of this non-patient variable with the extent of resection was also weaker than those of the above patient-specific variables, this finding raises concerns that treatment is not appropriately standardized and unnecessarily variable. In particular, less experienced centers may be choosing a less technically complex procedure for reasons not related to patient or tumor characteristics. Other studies have shown similar disparities in the treatment of patients with lung cancer,24–27 and do raise the possibility that treatment differences could be due to patient access to surgeons comfortable with performing an anatomic resection versus a potentially less technically demanding wedge resection. In the current study, it remains unclear as to whether the volume-outcomes relationship observed was due to observed differences in extent of resection, or other factors related to perioperative care, patient populations, and availability of multidisciplinary treatment teams.
We should note that in this current study of patients with cT1N0 disease across the United States, we did find that patients who received a lobectomy had significantly improved survival as compared to patients who received a sublobar resection. Furthermore, this difference remained even when comparing patients who received a lobectomy to patients who received a sublobar resection with a lymph node evaluation. Unfortunately, it is possible that the survival benefit seen among lobectomy patients could potentially be related to selection bias. Even though multivariable adjustment can correct for observed covariates, unobserved confounding cannot be excluded. For example, the NCDB does not identify if a sublobar resection was performed because a patient was felt to be unfit for lobectomy, or if the treatment was intentionally planned as a sublobar resection based on tumor characteristics. Furthermore, there is relatively limited information regarding patient comorbidities and functional status and no data on pulmonary function measurements, which are clearly important in predicting both survival and treatment. Importantly, the NCDB does not contain data on location of lymph node sampling, so while we were able to ascertain whether nodes were sampled, we do not have information regarding nodal stations, nor are we able to discriminate between hilar and mediastinal nodes. Also, some of the patients with more central tumors would not have been candidates for a sublobar resection, which could bias the results against the lobectomy group if the prognosis for a central tumor is not equivalent to that of a peripheral tumor. Still, although it is possible that the benefit of lobectomy over sublobar resection may not be present in some subsets of patients, our results should encourage surgeons to carefully consider sublobar resection in the absence of randomized data to guide that decision.
Our study has other limitations, including that wedge resections and segmentectomies were considered together but may not be oncologically equivalent, nor were they performed in equal proportion with wedge being utilized substantially more frequently.15 Wedge resection in one trial was associated with a smaller parenchymal margin, and a lower yield of lymph nodes and rate of nodal upstaging when compared with segmentectomy.28 However the use of the NCDB does have the very significant strength of being able to investigate a specific cancer substage with particularly high power due to its population-based nature.
Conclusions
Sublobar resection is used relatively commonly for clinical stage IA NSCLC, and in our study use was primarily determined by clinical characteristics but also by facility volume. Surgeons that utilized sublobar resection often did not perform the pathologic lymph node assessment that is an integral part of an ongoing randomized trial comparing survival between lobectomy and sublobar resection for small, early-stage NSCLC. Based on the results of our current study, surgeons should be cautious when utilizing a sublobar resection for clinical stage IA NSCLC due to risk for reduced long-term survival, particularly in the absence of other compelling patient factors such as comorbidities or poor lung function. In addition, some degree of pathological lymph node evaluation should be universally employed when performing a sublobar resection. Until further prospective data are available, sublobar resections should be reserved for patients who are at risk for higher perioperative mortality and who may not tolerate a lobectomy.
Clinical Practice Points.
Lobectomy is currently recommended for early-stage non-small cell lung cancer (NSCLC) based on a randomized trial that showed sublobar resection was associated with higher local recurrence rates. Although no subsequent randomized trials have either confirmed or refuted those findings, studies have questioned whether lobectomy is necessary for early-stage NSCLC. Several retrospective studies have found no difference in survival between sublobar resection and lobectomy for stage I tumors less than 2 cm in size. The purpose of the current study was to use a large, nationwide cancer database to examine current practice patterns to both identify predictors of surgical management with sublobar resection versus lobectomy, and also to evaluate the extent of pathologic lymph node assessment performed in association with sublobar resections.
In this study of almost 40,000 patients treated with surgical resection of clinical stage IA NSCLC in the United States from 2003–2011, we found that sublobar resection was used in approximately 25% of cases. Most sublobar resections involved wedge resection, with segmentectomy performed fairly uncommonly. Over 25% of sublobar resections also did not involve pathologic lymph node assessment. Lobectomy was associated with significantly better survival compared to sublobar resection. However, survival after sublobar resection was significantly better when lymph nodes were pathologically sampled.
Our results mandate that lymph node sampling be an integral part of the surgical management of NSCLC, if for no other reason than to help guide appropriate treatment. Even if future randomized trials ultimately show that a sublobar resection is appropriate for small peripheral tumors, the results of this study suggest that many surgeons will likely need to alter their practice to include lymph node assessment. Our results should also caution surgeons to carefully consider sublobar resection in the absence of randomized data to guide that decision.
Acknowledgments
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators. In addition, this work was supported by the NIH funded Cardiothoracic Surgery Trials Network (B.C.G. and M.G.H.), 5U01HL088953-05.
Abbreviations
- NSCLS
non-small cell lung cancer
- NCDB
National Cancer Data Base
- AJCC
American Joint Committee on Cancer
- FORDS
Facility Oncology Registry Data Standards
- LN
lymph node
Footnotes
Conflicts of interest: One of the authors (T.A.D.) serves as a consultant for Scanlan International, Inc.
Sources of support and disclosures: This work was supported by the NIH funded Cardiothoracic Surgery Trials Network (M.F.B and M.G.H.). 5U01HL088953-05.
References
- 1.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, et al. Non-small cell lung cancer, version 2.2013. Journal of the National Comprehensive Cancer Network : JNCCN. 2013;11:645–53. doi: 10.6004/jnccn.2013.0084. quiz 53. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. The Annals of thoracic surgery. 1995;60:615–22. doi: 10.1016/0003-4975(95)00537-u. discussion 22–3. [DOI] [PubMed] [Google Scholar]
- 3.Altorki NK, Yip R, Hanaoka T, Bauer T, Aye R, Kohman L, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. The Journal of thoracic and cardiovascular surgery. 2014;147:754–62. doi: 10.1016/j.jtcvs.2013.09.065. Discussion 62–4. [DOI] [PubMed] [Google Scholar]
- 4.El-Sherif A, Gooding WE, Santos R, Pettiford B, Ferson PF, Fernando HC, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. The Annals of thoracic surgery. 2006;82:408–15. doi: 10.1016/j.athoracsur.2006.02.029. discussion 15–6. [DOI] [PubMed] [Google Scholar]
- 5.Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J. 2009;33(2):426–35. doi: 10.1183/09031936.00099808. [DOI] [PubMed] [Google Scholar]
- 6.Fan J, Wang L, Jiang GN, Gao W. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol. 2012;19(2):661–8. doi: 10.1245/s10434-011-1931-9. [DOI] [PubMed] [Google Scholar]
- 7.McGuire AL, Hopman WM, Petsikas D, Reid K. Outcomes: wedge resection versus lobectomy for non-small cell lung cancer at the Cancer Centre of Southeastern Ontario 1998–2009. Canadian journal of surgery Journal canadien de chirurgie. 2013;56:E165–70. doi: 10.1503/cjs.006311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisnivesky JP, Henschke CI, Swanson S, Yankelevitz DF, Zulueta J, Marcus S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Annals of surgery. 2010;251:550–4. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 9.Yendamuri S, Sharma R, Demmy M, Groman A, Hennon M, Dexter E, et al. Temporal trends in outcomes following sublobar and lobar resections for small (≤ 2 cm) non-small cell lung cancers--a Surveillance Epidemiology End Results database analysis. The Journal of surgical research. 2013;183:27–32. doi: 10.1016/j.jss.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 10.Mery CM, Pappas AN, Bueno R, Colson YL, Linden P, Sugarbaker DJ, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest. 2005;128:237–45. doi: 10.1378/chest.128.1.237. [DOI] [PubMed] [Google Scholar]
- 11.Wolf AS, Richards WG, Jaklitsch MT, Gill R, Chirieac LR, Colson YL, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. The Annals of thoracic surgery. 2011;92:1819–23. doi: 10.1016/j.athoracsur.2011.06.099. discussion 24–5. [DOI] [PubMed] [Google Scholar]
- 12.Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2010;5:1583–93. doi: 10.1097/jto.0b013e3181e77604. [DOI] [PubMed] [Google Scholar]
- 13.Gajra A, Newman N, Gamble GP, Abraham NZ, Kohman LJ, Graziano SL. Impact of tumor size on survival in stage IA non-small cell lung cancer: a case for subdividing stage IA disease. Lung cancer (Amsterdam, Netherlands) 2003;42:51–7. doi: 10.1016/s0169-5002(03)00285-x. [DOI] [PubMed] [Google Scholar]
- 14.Koike T, Terashima M, Takizawa T, Watanabe T, Kurita Y, Yokoyama A. Clinical analysis of small-sized peripheral lung cancer. The Journal of thoracic and cardiovascular surgery. 1998;115:1015–20. doi: 10.1016/S0022-5223(98)70399-X. [DOI] [PubMed] [Google Scholar]
- 15.Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. The Journal of thoracic and cardiovascular surgery. 2005;129:87–93. doi: 10.1016/j.jtcvs.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Port JL, Kent MS, Korst RJ, Libby D, Pasmantier M, Altorki NK. Tumor size predicts survival within stage IA non-small cell lung cancer. Chest. 2003;124:1828–33. doi: 10.1378/chest.124.5.1828. [DOI] [PubMed] [Google Scholar]
- 17.Wisnivesky JP, Yankelevitz D, Henschke CI. The effect of tumor size on curability of stage I non-small cell lung cancers. Chest. 2004;126:761–5. doi: 10.1378/chest.126.3.761. [DOI] [PubMed] [Google Scholar]
- 18.Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest. 2011;139:491–6. doi: 10.1378/chest.09-2547. [DOI] [PubMed] [Google Scholar]
- 19.Kodama K, Doi O, Higashiyama M, Yokouchi H. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. The Journal of thoracic and cardiovascular surgery. 1997;114:347–53. doi: 10.1016/S0022-5223(97)70179-X. [DOI] [PubMed] [Google Scholar]
- 20.Koike T, Yamato Y, Yoshiya K, Shimoyama T, Suzuki R. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. The Journal of thoracic and cardiovascular surgery. 2003;125:924–8. doi: 10.1067/mtc.2003.156. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura H, Kawasaki N, Taguchi M, Kabasawa K. Survival following lobectomy vs limited resection for stage I lung cancer: a meta-analysis. British journal of cancer. 2005;92:1033–7. doi: 10.1038/sj.bjc.6602414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keenan RJ, Landreneau RJ, Maley RH, Singh D, Macherey R, Bartley S, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. The Annals of thoracic surgery. 2004;78:228–33. doi: 10.1016/j.athoracsur.2004.01.024. discussion -33. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki K, Nagai K, Yoshida J, Nishimura M, Nishiwaki Y. Predictors of lymph node and intrapulmonary metastasis in clinical stage IA non-small cell lung carcinoma. The Annals of thoracic surgery. 2001;72:352–6. doi: 10.1016/s0003-4975(01)02748-5. [DOI] [PubMed] [Google Scholar]
- 24.Berry MF, Worni M, Pietrobon R, D’Amico TA, Akushevich I. Variability in the treatment of elderly patients with stage IIIA (N2) non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:744–52. doi: 10.1097/JTO.0b013e31828916aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadgeel SM, Kalemkerian GP. Racial differences in lung cancer. Cancer metastasis reviews. 2003;22:39–46. doi: 10.1023/a:1022207917249. [DOI] [PubMed] [Google Scholar]
- 26.Hardy D, Liu C-C, Xia R, Cormier JN, Chan W, White A, et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115:2199–211. doi: 10.1002/cncr.24248. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer causes & control : CCC. 2003;14:761–6. doi: 10.1023/a:1026321923883. [DOI] [PubMed] [Google Scholar]
- 28.Kent M, Landreneau R, Mandrekar S, Hillman S, Nichols F, Jones D, et al. Segmentectomy versus wedge resection for non-small cell lung cancer in high-risk operable patients. The Annals of thoracic surgery. 2013;96:1747–54. doi: 10.1016/j.athoracsur.2013.05.104. discussion 54–5. [DOI] [PubMed] [Google Scholar]