Abstract
Previous functional magnetic resonance imaging (fMRI) studies in healthy controls (HC) and pain-free migraine patients found activations to pain-related words in brain regions known to be activated while subjects experience pain. The aim of the present study was to identify neural activations induced by pain-related words in a sample of chronic back pain (CBP) patients experiencing current chronic pain compared to HC. In particular, we were interested in how current pain influences brain activations induced by pain-related adjectives. Subjects viewed pain-related, negative, positive, and neutral words; subjects were asked to generate mental images related to these words during fMRI scanning. Brain activation was compared between CBP patients and HC in response to the different word categories and examined in relation to current pain in CBP patients. Pain-related words vs. neutral words activated a network of brain regions including cingulate cortex and insula in subjects and patients. There was stronger activation in medial and dorsolateral prefrontal cortex (DLPFC) and anterior midcingulate cortex in CPB patients than in HC. The magnitude of activation for pain-related vs. negative words showed a negative linear relationship to CBP patients’ current pain. Our findings confirm earlier observations showing that pain-related words activate brain networks similar to noxious stimulation. Importantly, CBP patients show even stronger activation of these structures while merely processing pain-related words. Current pain directly influences on this activation.
Keywords: chronic back pain, semantic processing, current pain, fMRI
1. Introduction
Processing and perceptual evaluation of noxious events and their underlying neural substrates are strongly modulated by psychological variables such as attention [1,2,3,4], emotion [5,6,7,8,9,10], expectation [11,12], and learning [13,14]. Furthermore, several studies indicate that environmental semantic and visual pain-related cues can induce activity in structures of the brain that process, among others, nociceptive information [15,16,17] even when no noxious stimulus is applied [18,19]. Based on Hebb’s concept of cell assemblies, it can be assumed that whenever we experience pain, its semantic and emotional representations are activated simultaneously with neural structures that process noxious events and constitute the experience of pain [20,21]. Consequently, words that are used to describe pain-related experiences were found to alter pain itself [22,23], and to activate brain structures engaged in the processing of noxious stimuli, e.g., anterior cingulate cortex, insula, secondary somatosensory cortex (SII), prefrontal cortex, and parietal cortex [24,25].
It has been shown that chronic back pain (CBP) patients differ from healthy controls (HC) in structural [26], functional [27,28], and neurochemical brain parameters [29,30]. In CBP patients, pain is a common everyday experience and pain has been expressed hundreds of times throughout the course of chronic pain development. It was suggested that CBP patients as chronic pain sufferers should have developed a strong pain network and a strong link of this network to their lexicon of pain terms. Accordingly, chronic pain patients exhibit larger event-related potentials (ERPs) to painful stimuli [31], larger late ERP amplitudes when noxious stimuli were primed by pain-related words [14], and stronger blood oxygenation level dependent (BOLD) responses even when pain was not attended [32,33,34]. Furthermore, chronic pain patients showed larger late ERP magnitudes in response to pain-related words and rated such words more negatively than neutral words, indicating that pain-related words draw similar attention and information processing as if an actually painful stimulus would have been processed [35]. In summary, the processing of pain-related words leads to enhanced behavioral and neuronal responses even when semantic processing is precluded from conscious access.
The present study aimed to extend previous findings by investigating the processing of pain-related words in CBP patients with actually ongoing (current) back pain. It was expected that the presence of current pain would activate the brain regions important for the analysis of pain and, thereby, enhance the processing of pain-related words. These assumptions lead to the following hypotheses: H1—CBP patients exhibit different valence, arousal, and pain relevance to pain-related words compared to HC; H2—CBP patients vs. HC show a stronger activation during the processing of pain-related than non-pain-related word categories and to negative words; and H3—There is a linear relationship between the strength of current pain and the measured BOLD response during the processing of pain-related words in CBP patients.
2. Materials and Methods
2.1. Patients and Controls
Participants of this study were recruited by advertisement at the university or by personal contact. Thirteen patients with CBP (2 men; 23–56 years old, mean age = 44.3 years) and thirteen pain-free HC (2 men; 24–58 years old, mean age = 46.5 years), matched for gender, age, and education, participated in this study as paid volunteers. Sociodemographic and clinical characteristics of participants are summarized in Table 1. The CBP patients have been examined by physicians and met the following criteria: (1) minimum duration of low back pain: for 6 months; (2) pain classified as ‘non-specific low back pain’ (no indication for nerve root problems and radiation to foot or toes, numbness and/or paraesthesia; straight leg raising test caused no leg pain); (3) magnetic resonance imaging (MRI) of the spine only indicated age-related wear and tear but no spinal disorders or disc pathology; (4) no psychiatric disorders, no disease associated to small fiber pathology (e.g., diabetes mellitus), no other chronic disorder; (5) no use of medication (except contraceptive) for at least 48 h prior to the experiment (requested before scanning). All participants were native German speakers and right-handed as assessed by the Edinburgh Handedness Inventory (EHI) [36]. None of the healthy controls reported former subacute or chronic pain episodes (longer than one month), any neurological, psychiatric or other chronic disorder. Because depression may alter the processing of pain-related words [37], depressive symptoms were assessed with a German version of the Beck Depression Inventory-II (BDI-II) [38]. For the assessment of catastrophizing thoughts and persuasions, all subjects completed the Pain Catastrophizing Scale (PCS, [39]; German version: [40]). In accordance with the Declaration of Helsinki, written informed consent was obtained from each participant before the study, and the Ethics Committee of the Friedrich Schiller University approved the experiment.
Table 1.
Demographic and clinical characteristics as well as behavioral data of chronic back pain patients (CBP) and healthy controls (HC).
| CBP | HC | ||||
|---|---|---|---|---|---|
| Sex | |||||
| Male/Female | 2/11 | 2/11 | |||
| Age (in years) | 44.31 ± 12.15 | 46.46 ± 10.19 | |||
| Range | 23–56 | 24–58 | |||
| Pain history | |||||
| 6–12 months | N = 2 | N = 0 | |||
| 2–5 years | N = 4 | N = 0 | |||
| >5 years | N = 7 | N = 0 | |||
| Pain intensity | t | df | p | ||
| Mean pain intensity (VAS a recent 4 weeks) | 3.31 ± 1.83 | 0.09 ± 0.30 | 5.72 | 10.53 | <0.001 |
| Strongest pain (VAS recent 4 weeks) | 5.14 ± 1.85 | 0.27 ± 0.90 | 6.27 | 12.76 | <0.001 |
| Current pain (VAS post scanning) | 1.72 ± 1.34 | 0.05 ± 0.15 | 4.15 | 10.25 | 0.002 |
| BDIbscore | 7.77 ± 5.13 | 2.62 ± 1.76 | 3.50 | 12.12 | 0.004 |
| Pain Catastrophizing Scale (PCS) | 14.08 ± 6.11 | 11.82 ± 7.04 | 0.61 | 24 | 0.550 |
| Rumination | 5.46 ± 3.46 | 5.09 ± 3.27 | 0.12 | 24 | 0.905 |
| Helplessness | 4.85 ± 3.11 | 4.00 ± 2.61 | 0.49 | 24 | 0.632 |
| Magnification | 3.77 ± 2.32 | 2.73 ± 2.19 | 0.98 | 24 | 0.351 |
| Task difficultyc | 1.38 ± 1.50 | 1.38 ± 1.50 | 0 | 24 | 1 |
| χ2 | |||||
| Correct word categorizationd | 15.66 ± 0.79 | 15.45 ± 1.65 | 0.04 | 1 | 0.865 |
Note: Values are mean ± SD; a Visual Analogue Scale (VAS): 0 = ‘‘no pain’’, 10 = ‘‘strongest pain imaginable”; b BDI = Beck Depression Inventory; c Visual Analogue Scale (VAS): 0 = “very easy”, 10 = “very difficult”; d correct categorizations out of 16 judgments.
2.2. Verbal Stimuli
Verbal stimuli included pain-related and non-pain-related negative, neutral, and positive adjectives. In a pilot study, 40 words were selected, and rated for valence, arousal, and pain relevance. Pain-related adjectives, affectively negative adjectives, and positive adjectives were matched for arousal. In addition, pain-related and negative adjectives were also matched for valence. Furthermore, word categories were matched according to the number of syllables and frequency in German language (COSMAS II database, http://www.ids-mannheim.de/cosmas2/). For a more detailed description of stimulus selection and the stimulus set, see Richter, Eck, Straube, Miltner and Weiss [32].
2.3. Experimental Procedure
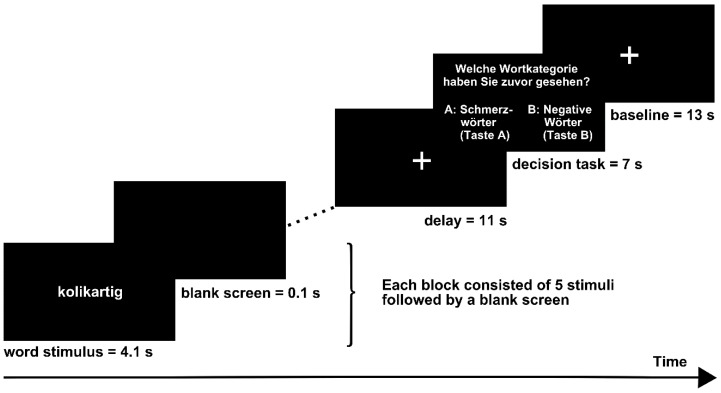
Examples of each word category were presented while participants were familiarized with the experimental procedure prior to the experiment. A video beamer projected the stimuli onto a screen mounted on the head coil of the MRI scanner. The experimental design is displayed in Figure 1. Subjects were instructed to focus on the semantics of the words by generating a mental image of a situation associated with the word. To increase compliance, subjects were told that they would be asked for examples of their imaginations after the experiment. All subjects were able to associate appropriate mental images to the words. For example, neutral word “traubenförmig” (“aciniform”) was frequently associated with wine grapes and positive word “wärmend” (“warming”) was commonly associated with an oven. Word stimuli were presented in 16 blocks (4 blocks of each word category). Each block consisted of five words (belonging to one word category); each word was displayed for 4.1 s and was followed by a blank screen for 0.1 s. Each block was followed by a delay phase in which a fixation cross was presented for 11 s and a subsequent interval of 7 s. During this interval, subjects were requested to choose the correct word category from two categories presented (e.g., A = pain-related, B = negative). Subjects responded by a MRI-compatible button response box fixed below their right hand. After the selection, a fixation cross was presented for 13 s. Each word was presented twice throughout the experiment. The order of the words within each block and the order of blocks were pseudo-randomized with the restriction that the same word category was not presented twice in succession. The whole fMRI run lasted 14 min.
Figure 1.
Stimulus protocol.
After the scanning session, participants rated the mean valence, arousal, and pain relevance of each word category on a 10-point numerical rating scale (NRS), with 0 = “negative/no arousal/not relevant”, and 10 = “positive/maximum arousal/highly relevant”. Following the scanning procedure, all subjects also rated their current back pain on a VAS (visual analogue scale, = “no pain”, 10 = “worst pain imaginable”). The pain ratings were obtained at the end of the experiment to avoid any mutual influence between the rating of words and the pain rating. Furthermore, a rating of task difficulty was requested using a VAS (0 = “very easy”, 10 = “very difficult”). During the study, we introduced the vividness of imagination scale [41] measuring emotional imagination, i.e., the ability to create emotional scenarios in mind. Higher ratings are associated with enhanced vividness of imagination.
2.4. Analysis of Behavioral Data
All statistical calculations were carried out using IBM SPSS Statistics 19 (IBM, Armonk, NY, USA). Normal distribution of behavioral data was determined by Kolmogorov-Smirnov Test. Levene’s test was applied to assess the equality of variances across the two groups. Variables were statistically analyzed using Student’s t- test if they were distributed normally; otherwise, χ2-tests were applied. Welch’s t-test was used for variables with unequal variances across groups. Differences between CBP patients and HC were evaluated for the ratings of arousal, valence, pain relevance, and vividness of imagination of word material [41].
To test differences of word category between patients and HC, separate two-way repeated measurements ANOVAs for mixed experimental design (between-subject factor Group and within-subject factor Word Category) were conducted. We considered values of p < 0.05 to be statistically significant.
2.5. fMRI-Data Acquisition and Analysis
MRI was obtained by a 3-Tesla magnetic resonance scanner (Tim Trio, Siemens, Medical Systems, Erlangen, Germany). For fMRI, 305 volumes were recorded using a T2* weighted echo-planar sequence (time to echo (TE) = 30 ms, flip angle = 90°, matrix = 64 × 64, field of view (FOV) = 192 mm, scan repeat time (TR) = 2.8 s). Each volume was comprised of 40 axial slices (thickness = 3 mm, no gap, in-plane resolution = 3 × 3 mm) parallel to the intercommissural plane (AC–PC-plane). Additionally, a high-resolution T1-weighted anatomical volume was obtained based on 192 slices with TE = 5 ms, matrix = 256 × 256 mm and resolution = 1 × 1 × 1 mm. Imaging data were pre-processed and analyzed using BrainVoyagerQX, Version 2.8 (Brain Innovation, Maastricht, The Netherlands) and NeuroElf V0.9 (Jochen Weber, SCAN Unit, Columbia University, New York City, NY, USA, http://www.neuroelf.net).
All volumes were realigned to the first volume in order to minimize the effects of head movements on data analysis. Further data pre-processing comprised spatial (6 mm full-width half-maximum isotropic Gaussian kernel) as well as temporal smoothing (high pass filter: 3 cycles per run). Anatomical and functional images were co-registered and normalized to the Talairach space [42]. Statistical analysis of fMRI-data was performed by multiple linear regression of the signal time course at each voxel. The expected blood oxygen level-dependent (BOLD) signal change for each event type (predictor) was modeled by a canonical hemodynamic response function (modified gamma function). A random-effects General Linear Model was used to identify associated brain activity in all acquired slices. To balance between type I and type II errors, we tested whether the detected clusters survived a correction for multiple comparisons. We used the approach as implemented in Brain Voyager which is based on a 3D extension of the randomization procedure described by Forman and colleagues [43,44]. First, voxel-level threshold was set at p < 0.05 (uncorrected). Threshold maps were then submitted to a correction for multiple comparisons for each contrast. The correction criterion was based on the estimate of the map’s spatial smoothness and on a Monte Carlo simulation (1000 iterations) for estimating cluster-level false-positive rates. The minimum cluster size threshold yielding a cluster level false-positive rate of 1% was applied to the statistical maps of each contrast [45]. All clusters reported in this article survived this control of multiple comparisons. Main effects were analyzed for the contrast between pain-related words vs. baseline (hypothesis 1; H1). Separate interaction analyses including the factor Group were performed for the relevant contrasts between word categories according to H2: pain-related (weighted 3 times according to the other word categories) vs. all other word categories (negative, neutral, and positive words) and pain-related vs. negative words. For the comparison between CBP patients and HC, the variance of depression (BDI-II) served as covariate in the General Linear Model.
In the next step, we analyzed correlations between VAS pain ratings after the scanning procedure with the relevant differences of parameter estimates (difference: pain vs. negative) for the group of the CBP patients only (HC were excluded because they had no pain, so there is no variance in these parameters allowing a correlative analysis) according to H3. Voxel-level threshold was set at p < 0.01 (uncorrected). The map was submitted to a correction for multiple comparisons (see above). After 1000 iterations, the minimum cluster size threshold yielding a cluster level false-positive rate of 1% was applied to the statistical maps.
3. Results
3.1. Questionnaire and Behavioral Data
Questionnaire data. On average, CBP patients reported significantly higher current pain ratings (M = 1.69, SD = 1.36) than healthy controls (HC) (M = 0.04, SD = 0.14), Welch’s t (12.25) = 4.34, p < 0.001 (Table 1). In addition, total BDI-2 scores of CBP patients (M = 7.77, SD = 5.13) were significantly higher than those of HC (M = 2.62, SD = 1.76), Welch’s t(14.78) = 3.424, p = .004 (Table 1). According to BDI-scores, only one patient expressed a clinically meaningful depression (score of 20; [38]). Since results remained essentially unchanged after exclusion of this subject, we kept this subject in all further analyses. BDI showed no linear relationship to VAS ratings for current pain (r(26) = 0.353, p = 0.77). fMRI group differences were calculated with BDI II values as a covariate. There was no significant difference between groups in pain catastrophizing according to the Pain Catastrophizing Scale (PCS; Table 1).
H1: Behavioral Effects of Group and Word Category
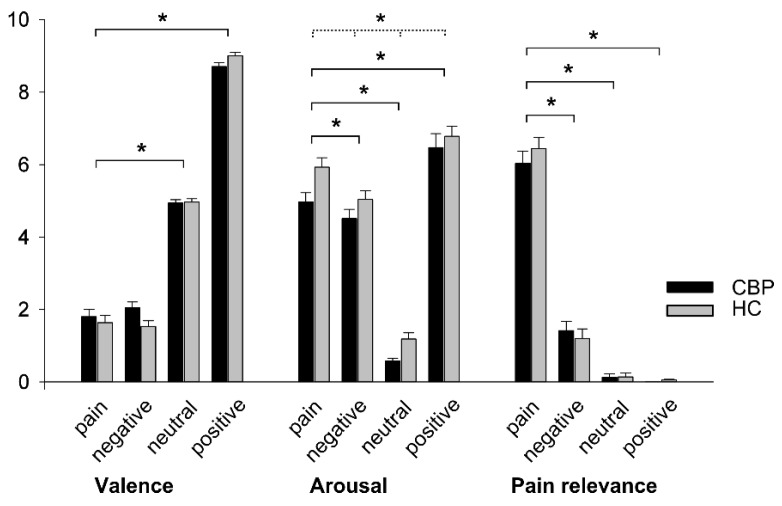
During the experiment, all participants categorized the words properly (MCBP = 15.66 and MHC = 15.45 correct out of 16 judgments, see Table 1). ANOVA results of the differences between CBP patients’ and HC subjects’ ratings (regarding post-scanning arousal, valence, and pain relevance) of the word categories are depicted in Figure 2.
Figure 2.
Mean ratings (standard errors) of valence, arousal, and pain relevance of each word category for CBP patients and HC. Valence (0 = “negative”; 10 = “positive”), arousal (0 = “no arousal”; 10 = “maximal arousal”), and pain relevance (0 = “not relevant”; 10 = “highly relevant”). Asterisks (*) indicate significant contrasts of Word Category (black line) and significant main effects of Group (dotted line).
Valence: Valence of word categories was rated differently as indicated by a significant main effect of Word Category (F3, 54 = 940.31, p < 0.001, η2 = 0.981). Contrasts were performed by comparing the pain-related word category to the remaining categories: This analysis revealed significant contrasts for neutral vs. pain-related words (F1, 18 = 315.49, p < 0.001, η2 = 0.946) and positive vs. pain-related words (F1, 18 = 1803.89, p < 0.001, η2 = 0.990). The contrast negative vs. pain-related words were not significant (F1, 18 = 0.139, p = 0.714, η2 = 0.008) indicating similar valence of these word categories. No significant main effect of Group (F1, 18 = 494, p = 0.491, η2 = 0.027) and no significant interaction of Word Category*Group (F3, 54 = 2.41, p = 0.077, η2 = 0.118) was observed on any valence rating.
Arousal: Mauchly’s test indicated that the assumption of sphericity was violated for the main effect of Word Category, (χ2(5) = 11.66, p = 0.040). Therefore, degrees of freedom were corrected using the Greenhouse–Geisser estimate of sphericity (ε = 0.67). As expected, there was a significant main effect of the factor Word Category on arousal ratings (F2.02, 36.35 = 181.14, p < 0.001, η2 = 0.910), with pain-related words being rated as more arousing than neutral words (F1, 18 = 505.98, p < 0.001, η2 = 0.966). A significant contrast was observed for the comparison between pain-related and positive words (F1, 18 = 13.32, p = 0.002, η2 = 0.425) as well as between pain-related and negative words (F1, 18 = 11.87, p = 0.003). Likewise, there was a significant main effect of Group on arousal ratings (F1, 18 = 13.26, p = 0.002, η2 = 0.424) with CBP patients showing lower arousal ratings than HC. No significant interaction Word Category × Group was observed (F2.02, 36.35 = 0.53, p = 0.597, η2 = 0.028).
Pain Relevance: Mauchly’s test indicated that the assumption of sphericity was violated for the main effect of Word Category, (χ2(5) = 25.57, p < 0.001). Therefore, degrees of freedom were corrected using Greenhouse–Geisser estimate of sphericity (ε = 0.65). A repeated measure ANOVA confirmed the effect of Word Category on the rating scores of pain relevance (F1.94, 34.85 = 413.15, p < 0.001, η2 = 0.958). Contrasts of the factor Word Category confirmed that pain-related words were rated as more pain relevant than negative (F1, 18 = 487.09, p < 0.001, η2 = 0.947), neutral (F1, 18 = 763.58, p < 0.001, η2 = 0.977), and positive words (F1, 18 = 714.04, p < 0.001, η2 = 0.975). There was no significant main effect of Group (F1, 18 = 0.13, p = 0.722, η2 = 0.007) and no significant interaction between Word Category × Group (F1.94, 34.85 = 0.83, p = 0.440, η2 = 0.044).
There was also no significant main effect of Vividness of Imagination (F3, 36 = 1.626, p = 0.2, η2 = 0.119) between word categories and no significant interaction Word Category × Group (F3, 36 = 0.463, p = 0.71, η2 = 0.037). Average vividness ratings for both groups were 5.58 for neutral words, 5.85 for positive words, 5.65 for negative words and 5.19 for pain-related words. Higher ratings are associated with enhanced vividness of imagination. These data show that both groups were similarly able to generate the requested emotive images associated with the presented words.
3.2. Imaging
CBP patients and HC showed activations in a similar network of brain regions in response to viewing pain-related words vs. a fixation cross (baseline). This network includes—among others—the striate and extrastriate cortex of the occipital lobe extending into the fusiform gyrus, widely distributed activations in the frontal lobe bilaterally, bilateral activations of the supplementary motor area (SMA) and pre-SMA, the primary motor cortex (MI) and the anterior cingulate cortex (ACC) (Supplementary Materials Table S1). These activations are in line with previous research [32,34].
3.2.1. H2: Effects of Group and Word Category
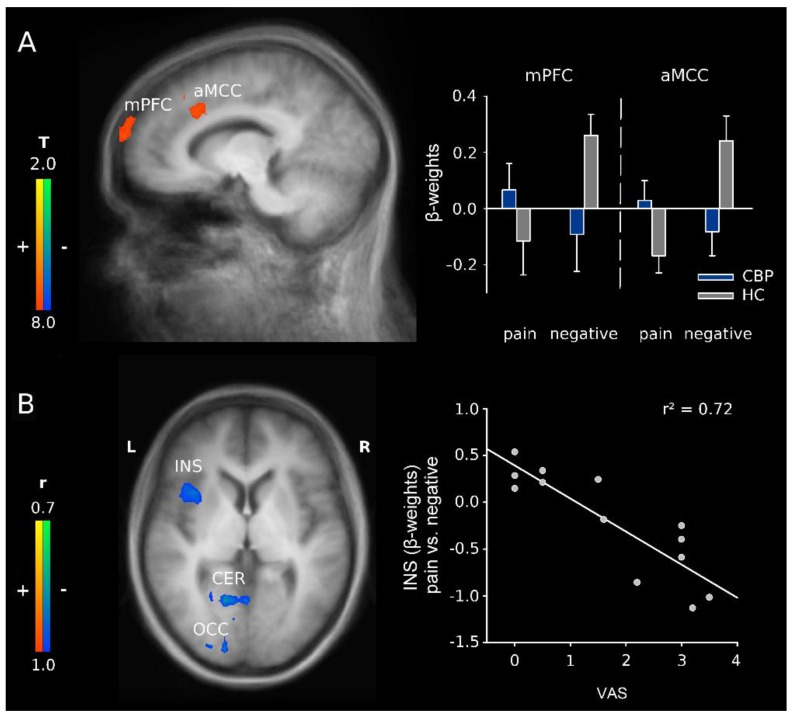
The interaction between Group and Word category (pain-related vs. negative words) revealed increased activations in CBP patients in the medial prefrontal cortex (mPFC), the anterior midcingulate cortex (aMCC), and in the dorsolateral prefrontal cortex (DLPFC; Figure 3A and Table 2). These results are in line with H2. For separate main effects of word category (pain-related words versus negative words), see Supplementary Materials Tables S3 and S4.
Figure 3.
(A) activation maps illustrating the interaction between group (CBP patients vs. HC) and word category (pain-related vs. negative adjectives) with activations in the medial prefrontal cortex (mPFC) and anterior midcingular cortex (aMCC) including the dorsolateral prefrontal cortex (DLPFC); x = −10. Right: schematic overview of the β-weights for the aforementioned structures; mean + Standard Error; and (B) correlation of current pain (VAS) with the differences in parameter estimates for the contrast pain-related vs. negative adjectives in CBP patients in insula (INS), cerebellum (CER) and occipital cortex (OCC); z = 4. Activations are superimposed on a Talairach template (average of all subjects). Right: correlation plot for the relation of current pain (VAS) and differences in parameter estimates for the contrast pain-related vs. negative adjectives for the anterior insula.
Table 2.
Activations to pain-related versus negative words in the comparison between CBP patients and HC.
| x | y | z | Cluster Size | t-Value | Brain Region | Laterality | Brodmann Area |
|---|---|---|---|---|---|---|---|
| −20 | 13 | 35 | 76 | 4.92 | anterior cingulate cortex/dorsolateral prefrontal cortex | R/L | 32 |
| −10 | −76 | 64 | 54 | 3.97 | medial prefrontal cortex | L | 10 |
Listed are clusters of activation with an uncorrected cluster threshold of p < 0.05. Talairach coordinates are provided for the maxima of the respective cluster. The corresponding neuroanatomical regions, the Brodmann areas, and the laterality (L, left; R, right) are described.
We also tested the interaction between Group and Word category for pain-related words and all other word categories [32,34]. This comparison revealed activations in the subgenual anterior cingulate cortex (sACC), the MCC, the posterior cingulate cortex (PCC), bilaterally in the posterior insula, the primary somatosensory cortex (SI) and MI, the fusiform gyrus, the posterior parietal cortex, the mPFC, and in the limbic parahippocampal gyrus (Supplementary Materials Table S2). These results confirm H2.
3.2.2. H3: Correlation Analyses of Word Category in CBP Patients
To test whether the current pain affects the processing of adjectives (H3), a correlation analysis between ratings (VAS) of current pain and the differences in activation between pain-related vs. negative words was performed for the group of CBP patients. We found clusters of negatively correlated activity in several regions including MI and the anterior insula (Figure 3B and Table 3).
Table 3.
Correlations between current pain ratings (VAS) and the differences in activation between pain-related vs. negative words in CBP patients.
| x | y | z | Cluster Size | r-Value | Brain Region | Laterality | Brodmann Area |
|---|---|---|---|---|---|---|---|
| 39 | 13 | 2 | 48 | −0.83 | insula | R | 13/44 |
| 45 | −65 | 21 | 56 | −0.84 | medial temporal cortex | R | 39 |
| −16 | −62 | −11 | 108 | −0.84 | cerebellum | L | |
| 5 | −87 | 18 | 193 | −0.88 | occipital cortex | R | 17/18/23 |
| −43 | −15 | 41 | 40 | −0.90 | precentral cortex (MI) | L | 4 |
| −4 | −77 | 39 | 106 | −0.91 | parietal cortex/occipital cortex | L | 19/7 |
| 14 | −55 | −8 | 211 | −0.96 | cerebellum | R | 19 |
Listed are clusters of activation with an uncorrected cluster threshold of p < 0.01. Talairach coordinates are provided for the maxima of the respective cluster. The corresponding neuroanatomical regions, the Brodmann areas, and the laterality (L, left; R, right) are described.
4. Discussion
The present fMRI study revealed several important results. Firstly, our results support previous findings [32,34] that showed an increase of activation during the processing of pain-related words in several regions of the brain including parts of brain areas that also become activated when exposed to painful stimuli. Secondly, patients suffering from CBP showed stronger activations than HC for pain-related vs. other word categories in several brain structures including the insula and parts of the cingulate cortex. Thirdly, data revealed linear relationships between patients’ current pain and brain activations in CBP patients in a variety of brain structures that are known to be involved in the processing of pain. Behavioral data showed only one effect including factor Group, i.e., a main effect of Group on arousal.
4.1. H1: Behavioral Effects of Group and Word Category
Behavioral data show the only effect including factor Group as main effect or interaction for arousal evoked by the different word categories. This effect results from lower arousal ratings in CBP patients compared to HC. There are several reasons that might account for this effect as well as for the absence of significant differences with respect to valence and pain relevance. First, in our sample, CBP patients had relatively low chronic back pain. We discuss this point extensively in Section 4.4 (Study Limitations). Second, ratings of CBP were especially low when the experiment took place. This has previously been reported and might result from distraction and excitement along with the scanning procedure [2,46]. Third, these partly unexpected behavioral results might be due to the permanent exposure to CBP and CBP-related stimuli that the patients suffer from. As a result, habituation to pain-related stimuli may have taken place. Fifth, we also have to take into account that pain-related words were well matched with the other word categories, but they were not specific for CBP; instead, they were referred to pain in general. This might have lowered the impact on our patients specifically suffering from CBP. Nevertheless, there were clear main effects of factor Word Category demonstrating that the expected valence and arousal were specific for each of the word categories. This might serve as a manipulation check of the stimulus material. Moreover, as there were no interaction effects of Word Category × Group on valence, arousal, or pain relevance, these effects could not account for the fMRI results.
4.2. H2: Effects of Group and Word Category
CBP patients showed stronger activations than HC during the processing of pain-related words vs. negative words in the medial prefrontal cortex and in a cluster including the aMCC and the DLPFC. In comparison, HC showed stronger brain activation during the processing of negative words and lower brain activation to pain-related words in these structures. Thus, a general difference in processing of pain-related verbal material was observed in CBP patients. Frontal lobe activity during the experience of pain was regularly observed and is generally linked to attention and cognitive processes [47,48]. The mPFC was found to be activated whenever contextual information was used to guide behavior [49]. This structure additionally exerts top-down pain modulation when cognitively demanding tasks interfere with the pain sensation [12,50,51,52]. Thus, activity in the mPFC seems to be strongly modulated during expectation of painful events [12]. In the sense of a priming mechanism, the pain-associated adjectives might have pre-activated a network of structures that were associated with the neuromatrix of pain in the past as a larger neural network. Thus, the cluster including the face area of M1 might be pre-activated due to painful facial expressions [53,54] according to current pain. In a broader sense, similar priming effects have also been shown for action verbs and activation of the sensorimotor cortex [55,56,57]. In the sense of such a priming mechanism and as the mPFC is involved in the recall of recent and remote memory traces [58], we suggest that the processing of painful words is associated with pain-related memories in CBP patients. The DLPFC is known to mediate the cognitive dimension of pain [18,59,60,61,62]. In previous studies, a stronger activation of the DLPFC was found in response to pain-related words as well [32,34]. In the present study, the activation in DLPFC is stronger in CBP patients than in HC, presumably, because these patients are prone to perceive pain more frequently and more seriously than HC. Activity of the aMCC has repeatedly been found for the subjective experience of pain [63]. More specifically, the aMCC seems to integrate pain processing and motor function [64,65]. Thus, structures that are commonly activated when CBP patients are exposed to current painful stimuli seem to be equally activated by words that indicate or connote pain.
In line with previous findings [32], the interaction between pain-related and other word categories revealed enhanced activation in several brain regions including sACC, the aMCC, the ventral posterior cingulate gyrus, the fusiform gyrus, the parahippocampal gyrus, and the posterior insula. Most of these brain areas are implicated in the experience of pain. For example, sACC is involved in the processing of emotional aspects of pain [66], but also in the processing of anxiety and stress [67]. The stronger activation of this structure in CBP patients suggests that pain-related words bear an elevated level of emotional salience and increased stress relatively to non-pain-related words of negative valence for CBP sufferers. It thus might be that their attention is more frequently focused on potential painful threats in the environment.
4.3. H3: Relationship to Current Pain
The correlation between current pain (VAS) and differences in activation between pain-related vs. negative words revealed negative correlations in MI and the anterior insula. This is in contrast to our hypothesis H3, i.e., we only found structures where the difference in activation between pain-related and negative words correlated negatively with VAS, but no structure with a positive linear correlation. This result might be due to several reasons. One possible explanation might be that current pain results in a constant activation of these structures. With fMRI, it is not possible to demonstrate this activation as the statistics belong to differences. However, if a pre-activation exists, then the activation of these structures by pain-related words might become less efficient due to pre-activation. Thus, the anterior insula might be pre-activated as a result of current pain due to its significance for the processing of salience information [17,68], which is a core feature of pain [15]. An alternative interpretation might result from the pain-inhibiting pain effect [69,70]. It is well known that two pain stimuli interact by different mechanisms influencing each other [52]. In this sense, chronic pain might result in a lower activation to a pain-related stimulus, even when this stimulus is a pain-related word.
4.4. Study Limitations
One limitation of the present study is its relative small number of participants. Strict inclusion criteria and exact matching of participants’ gender and age in both groups made recruitment difficult. However, even in this rather small sample of 13 subjects per group, we revealed significant differences in fMRI activations. Another important limitation of our study is that the CBP patients showed a comparatively low CBP intensity and a relatively low tendency to pain catastrophizing. These characteristics of our CBP patients might result from our inclusion criteria that were already at the advertisement, namely the request not to use any medication (beside contraceptive) 48 h prior to the experiment. This might have deterred more seriously affected CBP patients from participating in our experiment. This limitation is not only important with respect to explaining part of the behavioral results, but it should also be taken into account for the generalizability of the fMRI results. Nevertheless, the magnitudes of pain intensity ratings differed highly significantly from HC. In addition, CBP patients were significantly more depressed than HC, indicating an impairment of everyday life due to chronic pain states. Therefore, in the sense of generalizability, we would expect that our results rather underestimate the effect of CBP. Another limitation is that fMRI group differences were calculated using depression (BDI values) as covariates. Furthermore, our subjects were requested to attend to the presented words and to produce images in their mind with respect to these words. However, we were not able to control whether subjects fulfilled the requests. Future research might investigate whether the present results will remain stable when subjects do not attend or are not requested to imagine related scenes, as well as when CBP patients are more affected than those patients of our sample.
5. Conclusions
In summary, the present results revealed that CBP patients compared to HC show enhanced activations to pain-related words in brain structures commonly activated while processing painful events and while processing words with strong associations to pain. Thus, processing of verbal pain-related information is emphasized and particularly meaningful for chronic pain sufferers. However, as differences in brain activations to verbal expressions of pain vs. negative words became smaller, the stronger the current pain was in the CBP patients. These results are in accordance with the associative network theory [56], indicating a significant and systematic interplay between word and pain processing that is enhanced during chronic pain states.
Acknowledgments
This study was supported by the German Federal Ministry of Education and Research BMBF (01EC1003).
Abbreviations
The following abbreviations are used in this manuscript:
| CBP | chronic back pain |
| HC | healthy control |
| fMRI | functional magnetic resonance imaging |
| BOLD | blood oxygen level-dependent |
| DLPFC | dorsolateral prefrontal cortex |
| ERP | event-related potential |
| VAS | visual analogue scale |
| NRS | numerical rating scale |
| mPFC | medial prefrontal cortex |
| INS | insula |
| CER | cerebellum |
| OCC | occipital cortex |
| SMA | supplementary motor area |
| MI | primary motor cortex |
| ACC | anterior cingulate cortex |
| PCC | posterior cingulate cortex |
| MCC | midcingular cortex |
| sACC | subgenual anterior cingulate cortex |
| aMCC | anterior midcingulate cortex |
Supplementary Materials
The following are available online at www.mdpi.com/2227-9032/4/3/54/s1, Table S1: Activations to pain-related words versus baseline for CBP patients and HC, Table S2: Activations to pain-related versus all other word categories in the comparison between CBP patients and HC, Table S3: Activations to pain-related words versus negative words for HC, Table S4: Activations to pain-related words versus negative words for CBP patients.
Author Contributions
Thomas Weiss designed the experiment, Alexander Ritter, Thomas Weiss, Marcel Franz, Caroline Dietrich, Christian Puta and Wolfgang H. R. Miltner wrote the manuscript. Alexander Ritter and Marcel Franz analyzed the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kenntner-Mabiala R., Weyers P., Pauli P. Independent effects of emotion and attention on sensory and affective pain perception. Cogn. Emot. 2007;21:1615–1629. doi: 10.1080/02699930701252249. [DOI] [Google Scholar]
- 2.Villemure C., Slotnick B.M., Bushnell M.C. Effects of odors on pain perception: Deciphering the roles of emotion and attention. Pain. 2003;106:101–108. doi: 10.1016/S0304-3959(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 3.Seminowicz D.A., Davis K.D. Interactions of pain intensity and cognitive load: The brain stays on task. Cereb. Cortex. 2007;17:1412–1422. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- 4.Valet M., Sprenger T., Boecker H., Willoch F., Rummeny E., Conrad B., Erhard P., Tolle T.R. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—An FMR1 analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 5.Loggia M.L., Mogil J.S., Bushnell M.C. Empathy hurts: Compassion for another increases both sensory and affective components of pain perception. Pain. 2008;136:168–176. doi: 10.1016/j.pain.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Rainville P., Bao Q.V.H., Chretien P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain. 2005;118:306–318. doi: 10.1016/j.pain.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Godinho F., Magnin M., Frot M., Perchet C., Garcia-Larrea L. Emotional modulation of pain: Is it the sensation or what we recall? J. Neurosci. 2006;26:11454–11461. doi: 10.1523/JNEUROSCI.2260-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decety J., Jackson P.L., Brunet E., Meltzoff A.N. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Kenntner-Mabiala R., Pauli P. Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiology. 2005;42:559–567. doi: 10.1111/j.1469-8986.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 10.Singer T., Seymour B., O’Doherty J., Kaube H., Dolan R.J., Frith C.D. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 11.Coghill R.C., Koyama T., McHaffie J.G., Laurienti P.J. The subjective experience of pain: Where expectations become reality. Proc. Natl. Acad. Sci. USA. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wager T.D., Rilling J.K., Smith E.E., Sokolik A., Casey K.L., Davidson R.J., Kosslyn S.M., Rose R.M., Cohen J.D. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 13.Miltner W.H.R., Braun C., Arnold M., Witte H., Taub E. Coherence of gamma-band eeg activity as a basis for associative learning. Nature. 1999;397:434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- 14.Weiss T., Miltner W.H.R., Dillmann J. The influence of semantic priming on event-related potentials to painful laser-heat stimuli in migraine patients. Neurosci. Lett. 2003;340:135–138. doi: 10.1016/S0304-3940(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 15.Iannetti G.D., Hughes N.P., Lee M.C., Mouraux A. Determinants of laser-evoked EEG responses: Pain perception or stimulus saliency? J. Neurophysiol. 2008;100:815–828. doi: 10.1152/jn.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iannetti G.D., Mouraux A. From the neuromatrix to the pain matrix (and back) Exp. Brain Res. 2010;205:1–12. doi: 10.1007/s00221-010-2340-1. [DOI] [PubMed] [Google Scholar]
- 17.Legrain V., Iannetti G.D., Plaghki L., Mouraux A. The pain matrix reloaded: A salience detection system for the body. Prog. Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Apkarian A.V., Bushnell M.C., Treede R.D., Zubieta J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Melzack R. From the gate to the neuromatrix. Pain. 1999:S121–S126. doi: 10.1016/S0304-3959(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 20.Bower G.H. Mood and memory. Am. Psychol. 1981;36:129–148. doi: 10.1037/0003-066X.36.2.129. [DOI] [PubMed] [Google Scholar]
- 21.Hebb D.O. The Organization of Behavior: A Neuropsychological Theory. Wiley; New York, NY, USA: 1949. p. 335. [Google Scholar]
- 22.Dutt-Gupta J., Bown T., Cyna A.M. Effect of communication on pain during intravenous cannulation: A randomized controlled trial. Br. J. Anaesth. 2007;99:871–875. doi: 10.1093/bja/aem308. [DOI] [PubMed] [Google Scholar]
- 23.Ott J., Aust S., Nouri K., Promberger R. An everyday phrase may harm your patients the influence of negative words on pain during venous blood sampling. Clin. J. Pain. 2012;28:324–328. doi: 10.1097/AJP.0b013e3182321cc3. [DOI] [PubMed] [Google Scholar]
- 24.Gu X., Han S. Neural substrates underlying evaluation of pain in actions depicted in words. Behav. Brain Res. 2007;181:218–223. doi: 10.1016/j.bbr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Osaka N., Osaka M., Morishita M., Kondo H., Fukuyama H. A word expressing affective pain activates the anterior cingulate cortex in the human brain: An fMRI study. Behav. Brain Res. 2004;153:123–127. doi: 10.1016/j.bbr.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Apkarian A.V., Sosa Y., Sonty S., Levy R.M., Harden R.N., Parrish T.B., Gitelman D.R. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flor H., Braun C., Elbert T., Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci. Lett. 1997;224:5–8. doi: 10.1016/S0304-3940(97)13441-3. [DOI] [PubMed] [Google Scholar]
- 28.Baliki M.N., Chialvo D.R., Geha P.Y., Levy R.M., Harden R.N., Parrish T.B., Apkarian A.V. Chronic pain and the emotional brain: Specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J. Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wand B.M., Parkitny L., O’Connell N.E., Luomajoki H., McAuley J.H., Thacker M., Moseley G.L. Cortical changes in chronic low back pain: Current state of the art and implications for clinical practice. Man. Ther. 2011;16:15–20. doi: 10.1016/j.math.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Siddall P.J., Stanwell P., Woodhouse A., Somorjai R.L., Dolenko B., Nikulin A., Bourne R., Himmelreich U., Lean C., Cousins M.J., et al. Magnetic resonance spectroscopy detects biochemical changes in the brain associated with chronic low back pain: A preliminary report. Anesth. Analg. 2006;102:1164–1168. doi: 10.1213/01.ane.0000198333.22687.a6. [DOI] [PubMed] [Google Scholar]
- 31.Lutzenberger W., Flor H., Birbaumer N. Enhanced dimensional complexity of the eeg during memory for personal pain in chronic pain patients. Neurosci. Lett. 1997;226:167–170. doi: 10.1016/S0304-3940(97)00268-1. [DOI] [PubMed] [Google Scholar]
- 32.Richter M., Eck J., Straube T., Miltner W.H.R., Weiss T. Do words hurt? Brain activation during the processing of pain-related words. Pain. 2010;148:198–205. doi: 10.1016/j.pain.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Simon D., Craig K.D., Miltner W.H.R., Rainville P. Brain responses to dynamic facial expressions of pain. Pain. 2006;126:309–318. doi: 10.1016/j.pain.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 34.Eck J., Richter M., Straube T., Miltner W.H.R., Weiss T. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain. 2011;152:1104–1113. doi: 10.1016/j.pain.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Flor H., Knost B., Birbaumer N. Processing of pain- and body-related verbal material in chronic pain patients: Central and peripheral correlates. Pain. 1997;73:413–421. doi: 10.1016/S0304-3959(97)00137-1. [DOI] [PubMed] [Google Scholar]
- 36.Oldfield R.C. Assessment and analysis of handedness - edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 37.Nikendei C., Dengler W., Wiedemann G., Pauli P. Selective processing of pain-related word stimuli in subclinical depression as indicated by event-related brain potentials. Biol. Psychol. 2005;70:52–60. doi: 10.1016/j.biopsycho.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Hautzinger M., Kühner C., Keller F. Bdi-ii Beck-Depressions-Inventar. Harcourt Test Services; Frankfurt, Germany: 2006. [Google Scholar]
- 39.Sullivan M.J.L., Bishop S.R., Pivik J. The pain catastrophizing scale: Development and validation. Psychol. Assess. 1995;7:524–532. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- 40.Meyer K., Sprott H., Mannion A.F. Cross-cultural adaptation, reliability, and validity of the german version of the pain catastrophizing scale. J. Psychosom. Res. 2008;64:469–478. doi: 10.1016/j.jpsychores.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Guy M.E., Mccarter R.E. Scale to measure emotive imagery. Percept. Mot. Skill. 1978;46:1267–1274. doi: 10.2466/pms.1978.46.3c.1267. [DOI] [Google Scholar]
- 42.Talairach J., Tournoux P. Coplanar Stereotaxic atlas of the Human Brain. Thieme; Stuttgart, Germany: 1988. [Google Scholar]
- 43.Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic-resonance-imaging (fmri)—Use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 44.Goebel R., Esposito F., Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Straube T., Schmidt S., Weiss T., Mentzel H.J., Miltner W.H.R. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum. Brain Mapp. 2009;30:689–698. doi: 10.1002/hbm.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bantick S.J., Wise R.G., Ploghaus A., Clare S., Smith S.M., Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 47.Bornhovd K., Quante M., Glauche V., Bromm B., Weiller C., Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: A single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 48.Tolle T.R., Kaufmann T., Siessmeier T., Lautenbacher S., Berthele A., Munz F., Zieglgansberger W., Willoch F., Schwaiger M., Conrad B., et al. Region-specific encoding of sensory and affective components of pain in the human brain: A positron emission tomography correlation analysis. Ann. Neurol. 1999;45:40–47. doi: 10.1002/1531-8249(199901)45:1<40::AID-ART8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 49.Euston D.R., Gruber A.J., McNaughton B.L. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bingel U., Schoell E., Buchel C. Imaging pain modulation in health and disease. Curr. Opin. Neurol. 2007;20:424–431. doi: 10.1097/WCO.0b013e328259c34d. [DOI] [PubMed] [Google Scholar]
- 51.Petrovic P., Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95:1–5. doi: 10.1016/S0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- 52.Tracey I., Mantyh P.W. The cerebral signature and its modulation for pain perception. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Kunz M., Chen J.I., Lautenbacher S., Vachon-Presseau E., Rainville P. Cerebral regulation of facial expressions of pain. J. Neurosci. 2011;31:8730–8738. doi: 10.1523/JNEUROSCI.0217-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunz M., Lautenbacher S., LeBlanc N., Rainville P. Are both the sensory and the affective dimensions of pain encoded in the face? Pain. 2012;153:350–358. doi: 10.1016/j.pain.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 55.Pulvermuller F. Brain embodiment of syntax and grammar: Discrete combinatorial mechanisms spelt out in neuronal circuits. Brain Lang. 2010;112:167–179. doi: 10.1016/j.bandl.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Pulvermuller F., Fadiga L. Active perception: Sensorimotor circuits as a cortical basis for language. Nat. Rev. Neurosci. 2010;11:351–360. doi: 10.1038/nrn2811. [DOI] [PubMed] [Google Scholar]
- 57.Pulvermuller F. Brain reflections of words and their meaning. Trends Cogn. Sci. 2001;5:517–524. doi: 10.1016/S1364-6613(00)01803-9. [DOI] [PubMed] [Google Scholar]
- 58.Hugues S., Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn. Mem. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brighina F., De Tommaso M., Giglia F., Scalia S., Cosentino G., Puma A., Panetta M., Giglia G., Fierro B. Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J. Headache Pain. 2011;12:185–191. doi: 10.1007/s10194-011-0322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorenz J., Minoshima S., Casey K.L. Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 61.Ung H., Brown J.E., Johnson K.A., Younger J., Hush J., Mackey S. Multivariate classification of structural MRI data detects chronic low back pain. Cereb. Cortex. 2014;24:1037–1044. doi: 10.1093/cercor/bhs378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bushnell M.C., Ceko M., Low L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Misra G., Coombes S.A. Neuroimaging evidence of motor control and pain processing in the human midcingulate cortex. Cereb. Cortex. 2015;25:1906–1919. doi: 10.1093/cercor/bhu001. [DOI] [PubMed] [Google Scholar]
- 65.Peyron R., Faillenot I., Mertens P., Laurent B., Garcia-Larrea L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A pet study. Neuroimage. 2007;34:310–321. doi: 10.1016/j.neuroimage.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 66.Vogt B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peyron R., Laurent B., Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol. Clin. 2000;30:263–288. doi: 10.1016/S0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 68.Wiech K., Lin C.S., Brodersen K.H., Bingel U., Ploner M., Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. J. Neurosci. 2010;30:16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diers M., Koeppe C., Diesch E., Stolle A.M., Holzl R., Schiltenwolf M., van Ackern K., Flor H. Central processing of acute muscle pain in chronic low back pain patients: An EEG mapping study. J. Clin. Neurophysiol. 2007;24:76–83. doi: 10.1097/01.wnp.0000241093.00844.0e. [DOI] [PubMed] [Google Scholar]
- 70.Reinert A., Treede R., Bromm B. The pain inhibiting pain effect: An electrophysiological study in humans. Brain Res. 2000;862:103–110. doi: 10.1016/S0006-8993(00)02077-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.