Abstract
OBJECTIVES
Pancreatic ductal adenocarcinoma (PDAC) remains a highly lethal disease. Diabetes mellitus (DM) is both a risk factor for and a sequela of PDAC. Metformin is a commonly prescribed biguanide oral hypoglycemic used for the treatment of type II DM. We investigated whether metformin use before PDAC diagnosis affected survival of patients with DM, controlling confounders such as diabetic severity.
METHODS
We used the Surveillance, Epidemiology, and End Results registry (SEER)-Medicare linked database to identify patients with PDAC diagnosed between 2007 and 2011. The diabetic comorbidity severity index (DCSI) controlled for DM severity. Inverse propensity weighted Cox Proportional-Hazard Models assessed the association between metformin use and overall survival adjusting for relevant confounders.
RESULTS
We identified 1,916 patients with PDAC and pre-existing DM on hypoglycemic medications at least 1 year before cancer diagnosis. Of these, 1,098 (57.3%) were treated with metformin and 818 (42.7%) with other DM medications. Mean survival for those on metformin was 5.5 months compared with 4.2 months for those not on metformin (P<0.01). After adjusting for confounders including DCSI, Charlson score, and chronic kidney disease (CKD), patients on metformin had a 12% decreased risk of mortality compared with patients on other medications (hazard ratio (HR): 0.88, 95% confidence interval (CI): 0.81–0.96, P<0.01). In stratified analysis, differences persisted regardless of the Charlson score, the DCSI score, the presence of kidney disease, or the use of insulin/other hypoglycemic medications (P<0.01 for all).
CONCLUSIONS
Metformin is associated with increased survival among diabetics with PDAC. If confirmed in a prospective study, then these results suggest a possible role for metformin as an adjunct to chemotherapy among diabetics with PDAC.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) remains a highly lethal disease. The 5-year survival rate for PDAC is 6.5%, increased only slightly from 2.4% four decades ago (1). In 2015, 48,960 cases were diagnosed in the United States, with 40,560 deaths (2). This slow progress has yielded increased interest over the last several years to explore the anti-neoplastic effects of already-approved medications as potential adjunctive therapies for pancreatic cancer. These medications have established safety profiles, making them particularly appealing from a drug repurposing development perspective. Metformin is a commonly prescribed biguanide oral hypoglycemic used for the treatment of type II diabetes mellitus (DM). It works mechanistically by increasing insulin sensitivity and decreasing hepatic glucose production.
Large studies have confirmed that patients with DM are at an increased risk of developing several cancers including pancreatic, colon, liver, breast, and lung (3–9). Furthermore, diabetic patients with cancer tend to have worse survival, and metformin has been shown to both decrease the risk of cancer and improve survival among diabetics with cancer (3,4,10–12).
The aim of our study was to use a nationally representative population-based cancer database to investigate whether metformin use before PDAC diagnosis affected survival of patients with known DM, controlling for diabetic severity and concurrent use of other diabetic medications.
METHODS
The analysis was performed using the Surveillance, Epidemiology, and End Results registry (SEER) linked to Medicare claims (13). Included patients were 65 years or older and diagnosed with histologically confirmed PDAC between 2007 and 2011 (Figure 1). This date range was chosen as Medicare Part D data, which provide information on medication use, are only available from 2007. Patients over age 65 were included because this is the age of enrollment in Medicare coverage in the United States. We excluded patients with more than one primary cancer to eliminate the effect of synchronous or metachronous lesions on prognosis. Individuals without Part B Medicare coverage, which covers outpatient visits, Part D Medicare coverage, or those in HMO (health-care maintenance organizations), were further excluded for incomplete claims (14). Adenocarcinoma was selected for using the International Classification of Diseases for Oncology, Second Edition (ICD-O-2, 1992) histology codes 8000, 8010, 8140, 8500, 8550, and 8560.
Figure 1.
PDAC cohort selection. HMO, health-care maintenance organizations; PDAC, pancreatic ductal adenocarcinoma.
Sociodemographic characteristics and comorbidities
Sociodemographic information was obtained from both the SEER and Medicare-linked databases. We controlled for comorbidities such as cardiovascular disease and chronic kidney disease (CKD), using the Deyo adaption of the Charlson comorbidity index (15–17). Both inpatient and outpatient hospital claims (Medicare Provider Analysis and Review, Outpatient Standard Analytical File) as well as diagnoses on claims submitted by individual physicians (Carrier file) were included to increase the possibility of identifying more comorbid conditions (14,17).
Diabetes and diabetic regimen
Diabetic patients were identified according to validated algorithm for Medicare claims-based data requiring the presence of International Classification of Diseases, 9th Revision (ICD-9), code “250.xx” (18,19). Inclusion in our DM cohort required either one hospital claim or two outpatient claims more than 1 year but less than 2 years before PDAC diagnosis. We excluded patients diagnosed with DM in the year before PDAC diagnosis to minimize the effect of pancreaticogenic (type 3c) diabetes (reverse causation). Use of DM medications was extracted from Medicare Part D claims. Patients were considered to have used a specific DM medication if the pharmacy claim was submitted within 6 months before cancer diagnosis. Patients without any DM medications during this period were excluded. Specific categories of anti-hyperglycemics studied were metformin, insulin, sulfonylurea, thiazolidinediones (TZDs), dipeptidyl peptidase-4 inhibitors (DPP4-i), meglitinides, and alpha glucosidase inhibitors. Patients on combination medications were considered users of both drugs. DM severity, as manifested by end-organ damage and diabetes-related complications, was controlled for using the diabetic comorbidity severity index (DCSI). The DCSI is a validated, claims-based severity score (20,21). As it is disease specific to DM, it may predict additional or disparate health outcomes that may not be captured through generic risk adjustment tools such as the Charlson score (20,21).
Tumor characteristics and treatment
Tumor characteristics such as histology and stage, as well as treatment characteristics such as cancer-directed surgery and radiation therapy, were ascertained from the general SEER database. Chemotherapy administration was identified from the Medicare claims (Medicare Provider Analysis and Review, Carrier, Outpatient Standard Analytical File) using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) procedure code 99.23 and the Healthcare Common Procedure Coding System codes (HCPCS) Q0083, Q0084, Q0085, J7150, J3253, J3259, and J9000–J9999 after the cancer diagnosis (22,23).
Study outcome
The primary outcome of the study was overall survival. Survival times were calculated as the period from the date of diagnosis of PDAC to the date of death. Subjects alive on 15 December 2011 were censored.
Statistical analysis
Demographic characteristics were compared between diabetics on metformin and those on other diabetic medications using the chi-squared test for categorical variables and Student’s T-test for continuous variables.
Propensity score methods were used to control for potential allocation bias, as DCSI and comorbidity status (e.g., CKD) may influence a physician’s decision to prescribe metformin. The propensity score thus represents the likelihood that a patient will receive metformin based on his/her known characteristics (24). We used a logistic regression model to calculate propensity scores based on each patient’s sociodemographic characteristics (sex, marital status, income quartile), comorbidity status (Charlson score, CKD), DCSI, and DM medications (insulin, sulfonylurea, TZD, meglitinide, DPP4-i, alpha-glucosidase inhibitor).
Survival analysis was performed using Kaplan–Meier methods to test survival differences between DM patients on metformin compared with DM patients on medications other than metformin, as well as for patients on each of the other DM medications (insulin, sulfonylurea, TZD, meglitinide, DPP4-i, alpha-glucosidase inhibitor) compared with patients not on those medications. Cox proportional-hazards modeling was then employed using inverse propensity weighting to adjust for confounders such as propensity score, stage, and treatment characteristics (cancer-directed surgery, radiation therapy, and chemotherapy). Finally, we conducted stratified analysis by comorbidity status (Charlson score, CKD), diabetic severity (DCSI score), insulin use, sulfonylurea use, and length of time on metformin. All analyses were performed using SAS 9.3 (SAS, Cary, NC).
RESULTS
We identified 1,916 subjects with pancreatic adenocarcinoma and a diagnosis of DM at least 1 year before cancer diagnosis (Table 1). Of these, 1,098 (57%) patients were treated with metformin in the 6 months before cancer diagnosis. The remaining 818 (43%) patients were treated with diabetic medications other than metformin.
Table 1.
Demographic characteristics of diabetic cohort stratified by metformin use
| Demographic characteristic | Metformin (n=1,098) | No metformin (n=818) | P value | Adjusted P valuea |
|---|---|---|---|---|
| Mean age; years (s.d.) | 76.2 (6.8) | 78.0 (7.2) | <0.01 | 0.97 |
| Race | ||||
| Caucasian | 876 (80.1%) | 618 (75.7%) | 0.07 | 0.52 |
| African-American | 106 (9.7%) | 128 (15.7%) | ||
| Other | 112 (10.2%) | 70 (8.6%) | ||
| Male | 433 (39.4%) | 319 (39.0%) | 0.85 | 0.99 |
| Married at diagnosis | 484 (45.8%) | 336 (42.7%) | 0.19 | 0.99 |
| Charlson comorbidity score | ||||
| 0 | 16 (1.5%) | 19 (2.3%) | <0.01 | 0.86 |
| 1 | 562 (51.2%) | 245 (30.0%) | ||
| 2 | 273 (24.9%) | 191 (23.4%) | ||
| ≥3 | 246 (22.4%) | 362 (44.3%) | ||
| Diabetic comorbidity severity index (DCSI) | ||||
| 0 | 452 (41.2%) | 193 (23.6%) | <0.01 | 0.94 |
| 1 | 221 (20.2%) | 122 (14.9%) | ||
| 2 | 217 (19.8%) | 167 (20.4%) | ||
| ≥3 | 207 (18.9%) | 335 (41.0%) | ||
| CKD | 342 (20.0%) | 387 (47.3%) | <0.01 | 0.74 |
| Income group | ||||
| 1 | 830 (75.6%) | 635 (77.6%) | 0.20 | 0.91 |
| 2 | 208 (18.9%) | 158 (19.3%) | ||
| 3 | 60 (5.5%) | 25 (3.0%) | ||
CKD, chronic kidney disease.
Reflects differences between groups after adjusting for propensity score for metformin.
After propensity weighting, a statistical method described above that represents the likelihood that a patient will receive metformin, there were no differences with regard to demographics characteristics or medication usage between the two groups (Tables 1 and 2). Specifically, patients treated with metformin were equally matched in terms of comorbidities and diabetic severity compared with patients not on metformin (P=0.96 and P=0.94, respectively) (Table 1). Similarly, patients on metformin were equally likely to be taking insulin or a sulfonylurea compared with patients not on metformin (P=0.62 and P =0.98, respectively) (Table 2). With regard to treatment, patients on metformin received more cancer-directed treatment (surgery, radiation, and chemotherapy) than patients on other medications (P<0.01) (Table 2); however, these differences were controlled for in the multivariate survival analysis model.
Table 2.
Tumor and treatment characteristics of diabetic cohort stratified by metformin use
| Tumor/treatment characteristic | Metformin (n=1,098) | No metformin (n=818) | P value | Adjusted P valuea |
|---|---|---|---|---|
| American Joint Committee on Cancer stage | 0.45 | NA | ||
| Stage I | 81 (8.3%) | 60 (8.8%) | ||
| Stage II | 241 (24.8%) | 171 (25.1%) | ||
| Stage III | 82 (8.4%) | 68 (10.0%) | ||
| Stage IV | 567 (58.3%) | 382 (56.1%) | ||
| Cancer-directed surgery | 145 (13.3%) | 74 (9.2%) | <0.01 | NA |
| Radiation | 148 (13.6%) | 72 (9.0%) | <0.01 | NA |
| Chemotherapy | 342 (31.2%) | 185 (22.6%) | <0.01 | NA |
| Specific medication | ||||
| Sulfonylurea | 448 (40.8%) | 279 (34.1%) | <0.01 | 0.98 |
| Insulin | 328 (29.9%) | 494 (60.4%) | <0.01 | 0.62 |
| TZD | 288 (26.2%) | 198 (24.2%) | 0.31 | 0.99 |
| Meglitinide | 45 (4.1%) | 56 (6.9%) | <0.01 | 0.98 |
| DPP4 inhibitor | 152 (13.8%) | 87 (10.6%) | 0.04 | 0.98 |
| Survival; months (s.d.) | 5.5 (8.2) | 4.2 (7.2) | <0.01 | NA |
DPP4, dipeptidyl peptidase-4; TZD, thiazolidinediones.
Reflects differences between groups after adjusting for propensity score for metformin
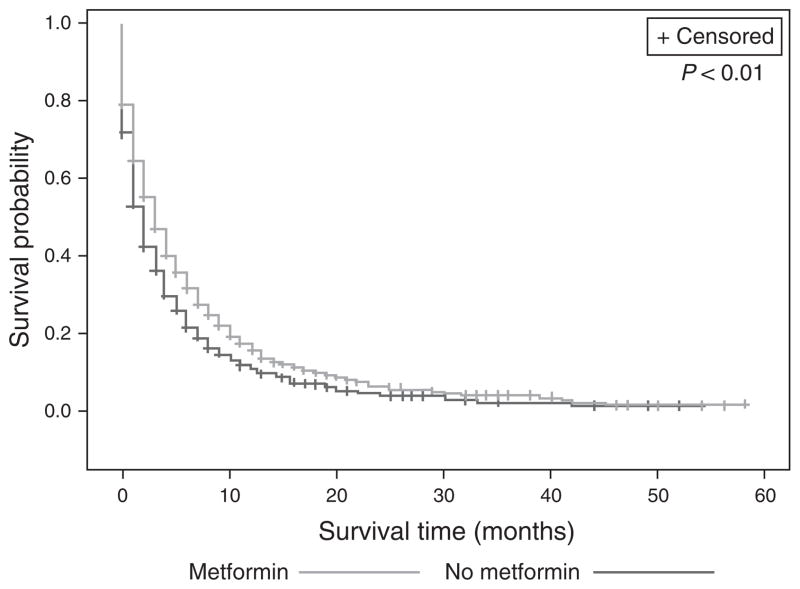
On Kaplan–Meier analysis, patients on metformin had improved survival compared with DM patients on medications other than metformin (overall survival 5.5 vs. 4.2 months, log-rank P<0.01) (Figure 2). When stratified by stage, this result persisted for all stages, except Stage 1 PDAC (Table 3). Furthermore, after adjusting for confounders including stage and treatment, as well as medication use, diabetic severity, and comorbidities by inversely weighting our Cox-Proportional Hazard model by the propensity score, these survival differences remained. Patients on metformin had a 12% decreased risk of mortality compared with patients on medications other than metformin (hazard ratio (HR) 0.88, 95% confidence interval (CI) 0.81–0.96, P<0.01) (Table 4).
Figure 2.
Metformin use increases survival in diabetics with PDAC. Overall survival 4.2 (s.d. 7.2) vs. 5.5 (s.d. 8.2) months, P<0.01. PDAC, pancreatic ductal adenocarcinoma.
Table 3.
Metformin survival by stage
| Stage | Mean survival (metformin vs. no metformin) | Log-rank P value |
|---|---|---|
| Overall | 5.5 vs. 4.2 months | <0.01 |
| Stage I | 9.2 vs. 6.5 months | 0.08 |
| Stage II | 9.3 vs. 7.6 months | <0.01 |
| Stage III | 7.4 vs. 4.5 months | 0.02 |
| Stage IV | 2.9 vs. 2.4 months | <0.01 |
Table 4.
Propensity score adjusted Cox-proportional hazards model for metformin
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| Metformin | 0.88 (0.81–0.96) | <0.01 |
| Stage | ||
| I (ref) | ||
| II | 1.19 (1.01–1.40) | 0.03 |
| III | 1.43 (1.18–1.73) | <0.01 |
| IV | 2.40 (2.07–2.78) | <0.01 |
| Surgery | 0.41 (0.34–0.49) | <0.01 |
| Radiation | 0.56 (0.48–0.65) | <0.01 |
| Chemotherapy | 0.39 (0.35–0.43) | <0.01 |
CI, confidence interval; CKD, chronic kidney disease; DCSI, diabetic comorbidity severity index; DPP4, dipeptidyl peptidase-4; TZD, thiazolidinediones.
Variables included in propensity analysis for metformin: age, sex, marital status, DCSI, Charlson Score, CKD, income quartile, insulin, sulfonylurea, TZD, meglitinide, DPP4, alpha glucosidase inhibitor.
On univariate Kaplan–Meier analysis, patients on insulin had worse survival than patients not on insulin (4.3 vs. 5.5 months, P <0.01). No other diabetic medications were associated with survival differences (Table 5). Adjusted analyses were not performed for these medications.
Table 5.
Univariate survival among diabetics by class of medication
| Medication | Mean survival (medication vs. no medication) | Log-rank P value |
|---|---|---|
| Metformin | 5.5 vs. 4.2 months | <0.01 |
| Sulfonylurea | 5.1 vs. 4.9 months | 0.81 |
| Insulin | 4.3 vs. 5.5 months | <0.01 |
| TZD | 5.3 vs. 4.8 months | 0.66 |
| Meglitinide | 6.1 vs. 4.9 months | 0.44 |
| DPP4 inhibitor | 4.5 vs. 5.0 months | 0.26 |
| Alpha glucosidase inhibitor | 3.5 vs. 5.0 months | 0.41 |
DPP4, dipeptidyl peptidase-4; TZD, thiazolidinediones.
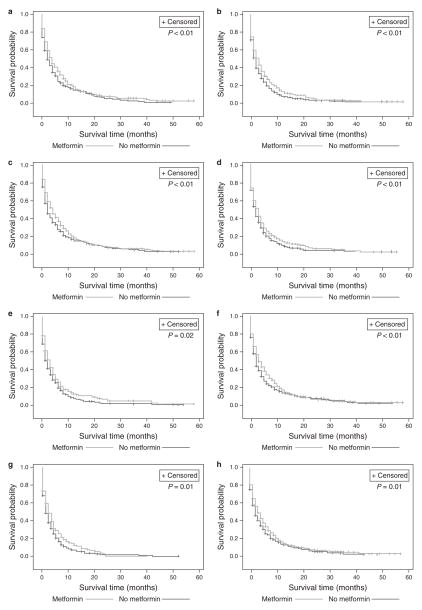
Sensitivity analyses were performed, in addition to the propensity analysis and multivariate survival analysis, in order to confirm that the survival advantage seen with metformin was not due to inherent differences between the two study groups. Sensitivity analyses stratified by the Charlson score, the presence of CKD, and the DCSI confirmed the significant survival advantage to metformin use compared with patients on diabetic medications other than metformin (all P<0.02) (Figure 3). Even for subjects on insulin, metformin use provided survival advantage (P=0.02, Figure 3e).
Figure 3.
Stratified Kaplan–Meier Curves showing persistent survival advantage to metformin, regardless of comorbidity status or other medication use. (a) Patients with Charlson score 0–1; (b) patients with Charlson score ≥2; (c) patients with DCSI 0–1; (d) patients with DCSI ≥2; (e) patients treated with insulin; (f) patients treated with other oral medications; (g) patients with CKD; and (h) patients without CKD. CKD, chronic kidney disease; DCSI, diabetic severity comorbidity index.
Finally, we performed additional sensitivity analyses to stratify patients by duration of metformin use. Both patients who received their first dose of metformin <6 months before diagnosis and those who were on metformin for >2 years before diagnosis had improved survival compared with those patients not on metformin. The HR for death from PDAC among those taking metformin for >2 years was 0.88 (95% CI 0.79–0.98, P=0.02) compared with those not on metformin. The HR for those on metformin <6 months was 0.87 (95% CI 0.75–1.01, P=0.07) compared with those not on metformin.
DISCUSSION
DM is a known-risk factor for PDAC, and recent pooled case–control studies suggest that metformin use at any time may reduce the incidence of PDAC (3,25,26). Nevertheless, little is known regarding the prognosis of diabetic patients taking metformin at the time of cancer diagnosis compared with those on other medications. We used a population-based data set to show that, among PDAC patients with pre-existing DM, those taking metformin at the time of cancer diagnosis have improved survival compared with patients taking diabetic medications other than metformin, controlling for comorbidities, diabetic severity, sociodemographic, and treatment characteristics.
Prior studies examining the effects of metformin among diabetic patients with PDAC are conflicting. With regard to incidence, case–control and cohort studies have suggested that metformin use at any time reduces incidence of PDAC among diabetics, whereas insulin and insulin secretagogues increase the risk (3,25–27). However, a recent meta-analysis of 11 studies and 1,770 cases of PDAC in 730,664 patients with DM found no significant association between metformin, insulin, or TZDs and the risk of developing PDAC (28). With regard to survival of diabetics with PDAC, two studies on metformin use have been published. A single-center study of 302 patients with PDAC and DM found a 36% lower risk of death among patients with non-metastatic disease who received metformin; however, this study was not able to control for diabetic severity (29). Furthermore, recall bias may have been a significant issue in this study, as detailed medical information was obtained via personal interview for 76% of patients. A UK-based population cohort of 516 patients with stage IV PDAC and DM found no difference in survival among patients taking metformin, but this study did not control for race or concurrent use of other diabetic medications such as insulin (30). Our study is the first US-based population study to examine the effects of metformin on DM patients while controlling for diabetic severity and concurrent use of other DM medications. Our data demonstrate a protective effect of metformin for both metastatic and non-metastatic disease.
From a mechanistic point of view, metformin inhibits PDAC cell growth by lowering insulin and IGF-1 levels (31,32). These hormones stimulate neoplasia via their interaction with G-protein-coupled receptors that promote mitogenic signaling. Metformin also has a direct inhibitory effect on PDAC cells by activating AMP-activated protein kinase, which in turn inactivates the mammalian target of the rapamycin pathway—a regulatory pathway that promotes cell proliferation (33–36). Our data support this mechanism as diabetics on insulin in our cohort had worse survival, and even among patients on insulin, metformin had a protective effect (Figure 3e). Furthermore, no other diabetic medications improved survival, suggesting that this protective effect is specific to metformin.
Duration of metformin use may impact survival. To address this concern, we performed a sensitivity analysis comparing patients who had received metformin for >2 years before PDAC diagnosis with patients who received their first metformin prescription <6 months before diagnosis. Although the HR was significant only for those on metformin >2 years, the point estimate for the HR is similar (0.88 for those on metformin >2 years vs. 0.87 for those on metformin <6 months). This difference is likely due to diminished power to detect a difference in survival for those on metformin <6 months, as only 204 subjects met this criterion. In contrast, 459 subjects were on metformin for >2 years and 818 subjects were not on metformin. Part D (prescription) coverage for Medicare became available in the SEER-Medicare database in 2007, resulting in a limited sample size of diabetic PDAC subjects with long-term prescription information.
Increasing evidence suggests that there may be a bi-directional relationship between diabetes and PDAC. In addition to being a risk factor for PDAC, diabetes can also occur as a consequence of PDAC—either from insulin resistance and islet cell dysfunction, chronic obstruction of the pancreatic duct with upstream glandular atrophy and loss of islet cells, or simply loss of islet cells post resection (6,37,38). To mitigate the effect of type 3c DM (reverse causation), we included only subjects with a diagnosis of DM at least 1 year before PDAC diagnosis.
If PDAC cases were ascertained because of a new diagnosis of DM, then this may create lead-time bias and impact survival in our study. Exclusion of subjects diagnosed with DM <1 year before PDAC diagnosis also serves to minimize the impact of lead-time bias, which we expect to be minimal for cases of DM >1 year before PDAC diagnosis given the rapidly progressive nature of PDAC.
Our study has several strengths. Notably, this is the only US-based population study to examine the effect of metformin use at diagnosis on survival among patients with DM and PDAC. Our sample size is significantly larger than previously-published studies, and we use validated methodology to extract claims from a widely-utilized national epidemiological database. However, several limitations inherent with the use of claims data must be addressed. First, our study was retrospective and therefore not randomized. To address this, we used propensity scoring to estimate the probability that patients would receive metformin based on his/her baseline characteristics. Propensity scores control for allocation bias that may occur as a result of differences in diabetic severity, comorbidities, or other factors that may influence a prescriber’s decision to use metformin in a particular patient (24,39). After applying this method, no significant differences remained between metformin users vs. non-users (Tables 1 and 2). We weighted our Cox models by the inverse of the propensity score and also controlled for additional confounders such as stage, and receipt of various treatments such as surgery, chemotherapy, and radiation to ensure that the two groups were equally matched. Second, DM patients not on metformin at the time of PDAC diagnosis appeared to be sicker, as suggested by higher Charlson and DCSI scores, and less likely to be on insulin (Table 1). To address this limitation, we not only performed a propensity analysis as above but also performed stratified analyses by comorbidity status, diabetic severity, and insulin use to investigate any confounding that may not have been controlled for in our multivariate survival analysis (Figure 3). We found that, regardless of Charlson, DCSI score, CKD, or insulin use, diabetic PDAC patients on metformin had improved survival compared with patients on medications other than metformin. A third limitation with any claims database is the concern for mis-classification bias. SEER-Medicare relies on physician coding and data entry, which may at times be driven by monetary concerns rather than medical indication. However, any misclassification is likely to be non-differential with respect to the outcome, and its effect is likely to underestimate differences between groups. Fourth, our study uses pharmacy claims as a proxy for diabetic medication use and therefore assumes patient adherence. Nevertheless, the lack of adherence would have biased our results toward the null and prescription claims data have been shown to be a reliable estimate of medication adherence in older people (40). Fifth, we were unable to control for certain established survival determinants of PDAC, such as smoking, ASA score, liver specific metastases, CA 19-9 levels, CEA levels, or daily pain score, as these variables are not available in the SEER-Medicare database (41–43). Nevertheless, we were able to control for several other established prognostic factors such as age, race, sex, American Joint Committee on Cancer stage, diabetic severity, comorbidity, and treatment received (chemo, radiation, surgery) in our propensity-weighted multivariate survival analysis (Table 4). Finally, we were unable to assess the survival benefit of metformin by dose response in this study. Future studies that consider metformin dose would provide stronger evidence of a survival benefit.
In conclusion, these data suggest that diabetic patients receiving metformin at the time of PDAC diagnosis have improved survival compared with patients on other medications. This effect of metformin in PDAC persists for both non-metastatic and metastatic disease, is consistent with that reported for other cancers, and accounts for comorbidity status, diabetic severity, treatment, and concurrent use of other diabetic agents such as insulin. Further prospective studies in conjunction with chemotherapy are needed to confirm these findings.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Pancreatic adenocarcinoma remains a highly lethal disease.
Metformin has been shown to both decrease the risk of cancer and improve survival among diabetics with cancer.
WHAT IS NEW HERE
Diabetic patients receiving metformin at the time of pancreatic cancer diagnosis have improved survival compared with diabetics on other medications.
This survival advantage persists regardless of comorbidity status, insulin use, or cancer treatment modality.
Metformin may have a role as an adjunct to chemotherapy among diabetics with pancreatic cancer.
Acknowledgments
Financial support: Departmental funds were used to purchase the SEER-Medicare data set.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: A.L. Lucas, MD, MSc.
Specific author contributions: Study planning, analysis of data, interpretation of data, manuscript drafting, and has approved the final draft of the submitted manuscript: S. Amin; study planning, analysis of data, interpretation of data, and has approved the final draft of the submitted manuscript: G. Mhango; study planning, interpretation of data, and has approved the final draft of the submitted manuscript: J. Lin, J. Wisnivesky, P. Boffetta; study planning and has approved the final draft of the submitted manuscript: A. Aronson; study planning, interpretation of data, manuscript drafting, and has approved the final draft of the submitted manuscript: A.L. Lucas.
Potential competing interests: None.
References
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: Apr, 2013. http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER website. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Lee M-S, Hsu C-C, Wahlqvist ML, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noto H, Tsujimoto T, Sasazuki T, et al. Significantly increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Endocr Pract. 2011;17:616–28. doi: 10.4158/EP10357.RA. [DOI] [PubMed] [Google Scholar]
- 5.Adami HO, Chow WH, Nyrén O, et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472–7. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 6.Song S, Wang B, Zhang X, et al. Long-term diabetes mellitus is associated with an increased risk of pancreatic cancer: a meta-analysis. PLoS One. 2015;10:e0134321. doi: 10.1371/journal.pone.0134321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–62. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 8.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 9.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928–37. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Noto H, Goto A, Tsujimoto T, et al. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z-J, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:707–10. doi: 10.1111/dom.12267. [DOI] [PubMed] [Google Scholar]
- 12.Currie CJ, Poole CD, Jenkins-Jones S, et al. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care. 2006;44:921–8. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 18.Rector TS, Wickstrom SL, Shah M, et al. Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health Serv Res. 2004;39:1839–57. doi: 10.1111/j.1475-6773.2004.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hebert PL, Geiss LS, Tierney EF, et al. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14:270–7. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 20.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 21.Joish VN, Malone DC, Wendel C, et al. Development and validation of a diabetes mellitus severity index: a risk-adjustment tool for predicting health care resource use and costs. Pharmacother J Hum Pharmacol Drug Ther. 2005;25:676–84. doi: 10.1592/phco.25.5.676.63594. [DOI] [PubMed] [Google Scholar]
- 22.Parmar AD, Vargas GM, Tamirisa NP, et al. Trajectory of care and use of multimodality therapy in older patients with pancreatic adenocarcinoma. Surgery. 2014;156:280–9. doi: 10.1016/j.surg.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Procedure Codes for SEER-Medicare Analyses [Internet] [cited 21 August 2015]. Available from http://healthcaredelivery.cancer.gov/seermedicare/considerations/procedure_codes.html.
- 24.Lin JJ, Gallagher EJ, Sigel K, et al. Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am J Respir Crit Care Med. 2015;191:448–54. doi: 10.1164/rccm.201407-1395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li D, Yeung SJ, Hassan MM, et al. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Currie CJ, Poole CD, Gale EAM. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 27.Bodmer M, Becker C, Meier C, et al. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol. 2012;107:620–6. doi: 10.1038/ajg.2011.483. [DOI] [PubMed] [Google Scholar]
- 28.Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:510–9. doi: 10.1038/ajg.2013.7. quiz 520. [DOI] [PubMed] [Google Scholar]
- 29.Sadeghi N, Abbruzzese JL, Yeung S-CJ, et al. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–12. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang AL, Haynes K, Hwang W-T, et al. Metformin and survival in pancreatic cancer: a retrospective cohort study. Pancreas. 2013;42:1054–9. doi: 10.1097/MPA.0b013e3182965a3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Sasajima J, Mizukami Y, et al. Hedgehog promotes neovascularization in pancreatic cancers by regulating Ang-1 and IGF-1 expression in bone-marrow derived pro-angiogenic cells. PLoS One. 2010;5:e8824. doi: 10.1371/journal.pone.0008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yue W, Yang CS, DiPaola RS, et al. Repurposing of metformin and aspirin by targeting AMPK-mTOR and inflammation for pancreatic cancer prevention and treatment. Cancer Prev Res (Phila PA) 2014;7:388–97. doi: 10.1158/1940-6207.CAPR-13-0337. [DOI] [PubMed] [Google Scholar]
- 34.Inoki K, Ouyang H, Zhu T, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 35.Fryer LGD, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 36.Kisfalvi K, Eibl G, Sinnett-Smith J, et al. Metformin disrupts crosstalk between G Protein–coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539–45. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JW, Jang J-Y, Kim E-J, et al. Effects of pancreatectomy on nutritional state, pancreatic function and quality of life. Br J Surg. 2013;100:1064–70. doi: 10.1002/bjs.9146. [DOI] [PubMed] [Google Scholar]
- 38.Muniraj T, Chari S. Diabetes and pancreatic cancer. Minerva Gastroenterol Dietol. 2012;58:331–45. [PMC free article] [PubMed] [Google Scholar]
- 39.Curtis LH, Hammill BG, Eisenstein EL, et al. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45:S103–7. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 40.Grymonpre R, Cheang M, Fraser M, et al. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44:471–7. doi: 10.1097/01.mlr.0000207817.32496.cb. [DOI] [PubMed] [Google Scholar]
- 41.Antwi SO, Oberg AL, Shivappa N, et al. Pancreatic cancer: associations of inflammatory potential of diet, cigarette smoking and long-standing diabetes. Carcinogenesis. 2016;37:481–90. doi: 10.1093/carcin/bgw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller MW, Friess H, Köninger J, et al. Factors influencing survival after bypass procedures in patients with advanced pancreatic adenocarcinomas. Am J Surg. 2008;195:221–8. doi: 10.1016/j.amjsurg.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Park JK, Yoon YB, Kim Y-T, et al. Survival and prognostic factors of unresectable pancreatic cancer. J Clin Gastroenterol. 2008;42:86–91. doi: 10.1097/01.mcg.0000225657.30803.9d. [DOI] [PubMed] [Google Scholar]