TAZ could promote cell proliferation and inhibit Celastrol-induced cell apoptosis. Up-regulation of B-cell lymphoma-2 (Bcl-2), down-regulation of Bcl-2 associated X protein (Bax) and activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway may be the mechanism underlying anti-apoptosis of TAZ.
Keywords: anti-apoptosis, Celastrol, proliferation, transcriptional co-activators with PDZ-binding motif (TAZ)
Abstract
Hippo pathway is a highly conservative signalling pathway related to the development of organisms, which has been demonstrated to be strongly linked to the tumorigenesis and tumour progression. As the major downstream effector of Hippo pathway, yes-associated protein (YAP), is a transcriptional activator of target genes that are involved in cell proliferation and survival. As an oncogene, YAP can promote cell growth and inhibit cell apoptosis. Another major downstream effector of Hippo pathway, transcriptional co-activators with PDZ-binding motif (TAZ), is nearly 60% homologous with YAP. In the present study, we assume that TAZ probably has the similar function to YAP. To test this issue, we established an inducible and a stable expression system of TAZ in T-Rex-293 and HEK293 cells respectively. The results of cell growth curves, colony formation assay and tumour xenograft growth showed that overexpression of TAZ could promote cell growth in vitro and in vivo. Meanwhile, we found that up-regulated expression of TAZ could partially restore Celastrol-induced cell apoptosis. Induced overexpression of TAZ could up-regulate its target genes including ankyrin repeat domain-containing protein (ANKRD), cysteine-rich 61 (CYR61) and connective tissue growth factor (CTGF), increase the expression of B-cell lymphoma-2 (Bcl-2), decrease the expression of Bcl-2 associated X protein (Bax) and activate the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway, which may be the mechanism underlying anti-apoptosis of TAZ. All these findings indicated that TAZ acts as an oncogene that could be a key regulator of cell proliferation and apoptosis.
INTRODUCTION
Hippo pathway, first discovered in Drosophila, aims at adjusting the organ size, maintaining the dynamic balance of cell proliferation, apoptosis and other functions [1]. With the abnormal Hippo pathway, the cells will proliferate excessively or apoptosis insufficiently, and the organ will grow out of control, which will eventually lead to the tumorigenesis and tumour progression [2]. The cores of Hippo pathway consist of the mammalian sterile20-like kinases serine/threonine kinases 1/2 (MST1/2), the large tumour suppressor serine/threonine protein kinases 1/2 (LATS1/2), as well as their scaffold proteins Salvador homologue 1 (SAV1; also called WW45) and Mps one binder kinase activator proteins (MOBs). Mechanistically, MST1/2 phosphorylate and activate LATS1/2 complexed with SAV1 and MOB1 [3,4]. Then, activated LATS1/2 phosphorylate the transcriptional regulators, including yes-associated protein (YAP) and transcriptional co-activators with PDZ-binding motif (TAZ). Phosphorylated YAP/TAZ lose their activation by sequestering in the cytoplasm via interaction with 14-3-3 proteins, then are degraded through the ubiquitin-proteasome pathway [5]. On the other hand, as the transcriptional regulators, YAP/TAZ bind to multiple transcription factors, such as TEADs, Smads, PAXs, TBX5 and RUNX2 [6], and regulate genes involved in stemness, differentiation, proliferation and apoptosis via nuclear translocation.
Besides, the abnormal high expression of YAP/TAZ have been detected in various tumours, such as hepatocellular carcinoma (HCC) [7], breast cancer [8], prostate cancer [9], non-small cell lung cancer [10] and ovarian cancer [11]. An increasing number of studies have confirmed that YAP promotes the tumorigenesis and tumour progression in a various of ways, including promoting cell growth and inhibiting cell apoptosis [12]. One report has described that YAP (not TAZ) could suppress tumour in breast cancer [13]. However, the similar function of TAZ have not been well demonstrated. Allowing for the homology of TAZ with YAP, we hypothesis that TAZ probably get functionality similar to YAP. To assess this issue, we established an inducible expression system of TAZ in T-Rex-293 cells and a stable expression system of TAZ in HEK293 cells. Our study proved that TAZ could act as an oncogene to promote cell proliferation and inhibit cell apoptosis.
MATERIALS AND METHODS
Cell culture and reagents
T-Rex-293 cell line was presented by Professor Quan Chen (Chinese Academy of Sciences, Beijing, China). T-Rex-293 cells and HEK293 cells were cultured in RPMI 1640 medium (BIOROC). All mediums were supplemented with 10% FBS and were grown in a humidified environment at 37°C with 5% CO2. Doxcycline (Dox) was purchased from Sigma–Aldrich and was prepared as a 2 mg/ml stock solution in sterile ddH2O. Giemsa was purchased from Solarbio. Celastrol (purity >98%) was purchased from Mingrui. Stock solutions of Celastrol were prepared in DMSO, stored at −20°C and freshly diluted to the desired concentration before use. The maximal final concentration of DMSO in the culture medium was lower than 0.1%.
Plasmid transfection and inducible expression system/stable cell line establishment
To establish the inducible/stable TAZ expression cell line, the open reading frame of human TAZ was cloned into the eukaryotic expression vector pcDNA4/TO/myc-His B (Invitrogen). The primers for TAZ clone are as follows: Forward: 5′-CCCAAGCTTATGAATCCGGCCTCGGCG-3′, Reverse: 5′-CGGAATTCCGGTACAGCCAGGTTAGAAAGGGC-3′. Transfections were performed in 70–80% confluent T-Rex-293 cells and HEK293 cells using LipofectAMINE2000 Reagent (Invitrogen), according to the manufacturer's protocol. Then the cells were selected with 100 μg/ml Zeocin (Invitrogen). The resistant mono-clone was verified by western blot and designated 293-TR/TAZ and HEK293/TAZ, and their corresponding control cells were designated 293-TR/control and HEK293/control.
Cell growth curve and cell colony formation assay
For the cell growth curve assay, the indicated cells in exponential growth were plated at a density of 50 cells/mm2 in triplicate. 293-TR/control and 293-TR/TAZ cells were treated with Dox or not for 7 days, and T-Rrex-293 cells were treated with Celastrol (0.5 μM) or not for 6 days. Cell numbers were counted as indicated with a haematocytometer and an Olympus inverted microscope.
For cell colony formation assay, 293-TR/control and 293-TR/TAZ cells were plated 500 cells/well in six-well plates in triplicate and treated with Dox or not respectively. T-Rex-293 cells with Celastrol (0.5 μM) or not were plated 800 cells/well in six-well plates in triplicate. After approximately 2 weeks, the colonies were washed with PBS, fixed with formaldehyde and stained with Giemsa staining solution. Colonies larger than 100 μm in diameter were counted under an Olympus inverted microscope.
Tumour xenograft model
Equal numbers (2×106) of HEK293/control and HEK293/TAZ cells were harvested by trypsinization, washed with 1× PBS and resuspended in 0.1 ml of saline. A total number of five (6-week old) female nude mice were given bilateral subcutaneous injections with HEK293/control and HEK293/TAZ cells. The mice were kept in pathogen-free environments and checked every 2 days. The mice were sacrificed 30 days after injection. The weights of the tumour xenografts were measured at the end of the experiment.
MTT assay
For dose-dependent and time-dependent proliferation assay, nearly 20000 of T-Rex-293 cells were seeded into 96-well culture plate each well treated with Celastrol at different concentrations (0.25–5 μM). Each concentration was plated in 4 wells, with medium only wells used as the controls. The cells were cultured for 24, 48 and 72 h. Four hours before the end of the incubation, 20 μl MTT (5 mg/ml) were added to each well and 150 μl of DMSO were added to stop the reaction. Viable cell numbers were measured at a wavelength of 570 nm with the Model 680 Microplate Reader (Bio-Rad Laboratories).
TdT-mediated dUTP Nick end labelling assay
Apoptotic cells were confirmed with the in situ cell death detection kit, Alkaline Phosphatase (Roche Applied Science), in accordance with the manufacturer's instructions. T-Rex-293, 293-TR/control and 293-TR/TAZ cells were grown on coverslips. The following process was performed according to the manufacturer's protocol. Cells were mounted cell side downwards on a microscope slide, and the apoptotic cells (brown staining) were counted under a microscope. Three fields were randomly counted for each sample.
Western blot
Cells were harvested and lysed in RIPA buffer (Cell Signaling Technology), then western blot analysis was performed with the use of conventional protocols as described previously [14]. Total proteins were separated by SDS/PAGE, and transferred on to nitrocellulose membranes. The antibodies were used including β-actin (1:5000, Sigma–Aldrich), PARP (1:1000, Cell Signaling Technology), TAZ (1:1000, Santa Cruz Biotechnology), protein kinase B (Akt; 1:1000, Cell Signaling Technology), pho-Akt (Ser473; 1:1000, Cell Signaling Technology), B-cell lymphoma-2 (Bcl-2; 1:1000, Santa Cruz Biotechnology), Bcl-2 associated X protein (Bax; 1:1000, Santa Cruz Biotechnology). After extensively washed, the membranes were incubated with anti-mouse or anti-rabbit IgG-horseradish peroxidase conjugate antibody (Zhongshan Golden Bridge Biotechnology Company) for 1 h at room temperature and developed with a Luminol Chemiluminescence Detection Kit (Zhongshan Golden Bridge Biotechnology Company). Membranes were reprobed for β-actin antibodies for normalization and accurate quantification. Each result for western blot was repeated three times at least.
Immunohistochemistry
Sections of 5 μm thickness were dewaxed in xylene and rehydrated in serial dilutions of ethanol. Antigen retrieval was carried out in 0.01 M sodium citrate buffer (pH 6.0) for 10 min by microwave oven heating. Then endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 20 min, and nonspecific staining was blocked by 5% BSA for 1 h. The slides were incubated with anti-TAZ (1:100, Santa Cruz Biotechnology) and anti-PCNA (1:1000, Santa Cruz Biotechnology) overnight at 4°C and then incubated with secondary antibody for 30 min, followed by incubation with streptavidin peroxidase for 15 min. 3,3′-Diaminobenzidine tetrachloride (DAB) was applied to identify peroxidase activity. Each incubation step was performed at room temperature and was followed by sequential washed for 3 min each for three times in PBS. Finally, sections were dehydrated in alcohol and cleared in xylene.
Quantitative real-time PCR (q-PCR)
Total RNA was extracted with TRIZOL Reagent (Invitrogen), and then reverse-transcribed to cDNA with M-MLV Reverse Transcriptase (Promega). Q-PCR was performed with triplicate samplings of the reverse-transcribed cDNAs on a StepOnePlus Real-Time PCR System (Applied Biosystems) with SYBR Green PCR core reagents (Applied Biosystems), and analysed with StepOne Software. The primers used were as follows:
ANKRD (human):
5′-AGTAGAGGAACTGGTCACTGG-3′/5′-TGGGCTA-GAAGTGTCTTCAGAT-3′;
CYR61 (human):
5′-CCTTGTGGACAGCCAGTGTA-3′/5′-ACTTGGGC-CGGTATTTCTTC-3′;
CTGF (human):
5′-AGGAGTGGGTGTGTGACGA-3′/5′-CCAGGCAGT-TGGCTCTAATC-3′;
GAPDH (human):
5′-CTCCTGCACCACCAACTGCT-3′/5′-GGGCCATC-CACAGTCTTCTG-3′;
Statistical analysis
The results were expressed as mean±S.D. or ± S.E.M. A Student's two-tailed non-paired t-test was used to determine significant differences between treatment and control groups in all experiments. P<0.05 was considered statistically significant.
RESULTS
TAZ promoted cell growth in vitro and in vivo
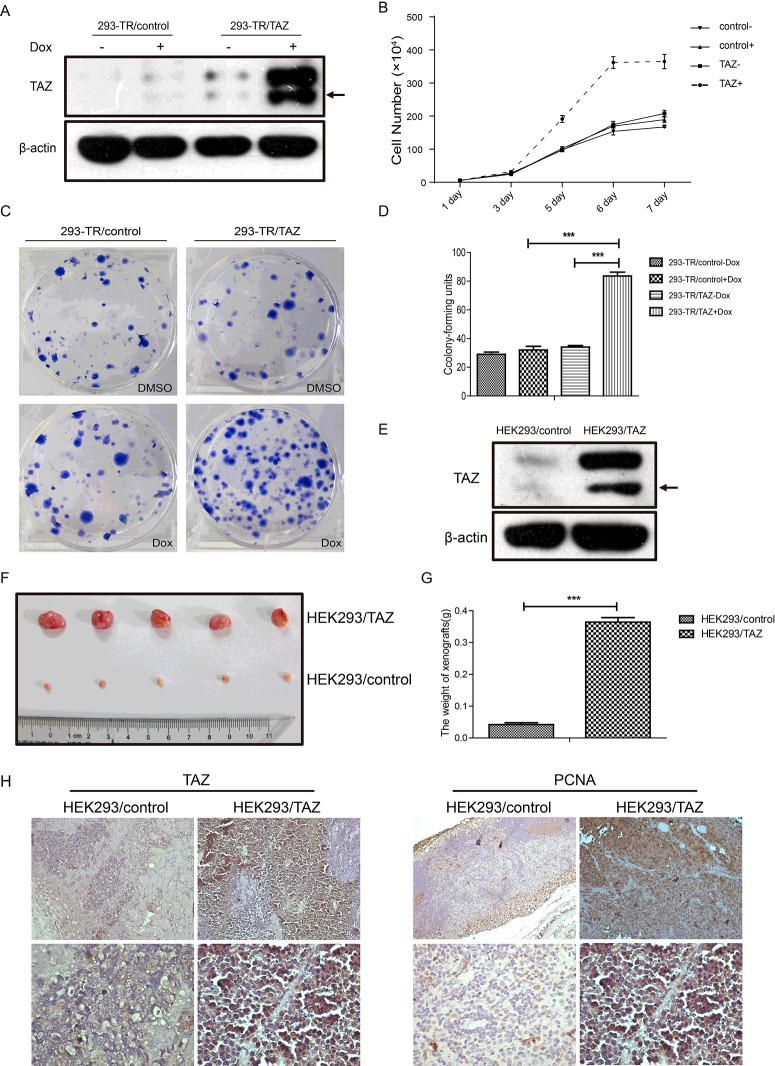
We utilized the T-Rex-293 system to establish an inducible 293-TR/TAZ cell line. As shown in Figure 1(A), only when treated with Dox for 24 h, the exogenous protein level of TAZ increased significantly in 293-TR/TAZ cell line, whereas the expression of TAZ in 293-TR/control cell line (treated with Dox or not) was hardly detected. To better investigate the effect of TAZ on T-Rex-293 cells, cell growth curve and cell colony formation assay were performed. The cell growth curves showed that 293-TR/TAZ cells grew obviously faster when treated with Dox, and the growth rate of the other three groups (293-TR/TAZ cells without Dox and 293-TR/control cells with Dox or not) were nearly the same (Figure 1B). Furthermore, 293-TR/TAZ cells with Dox treatment could form more colonies than the cells without Dox, also than 293-TR/control cells with or without Dox (Figures 1C and 1D). To further address the effect of TAZ in vivo, we established a stable expression system of TAZ in HEK293 cells. The resistant mono-clone was designated HEK293/control or HEK293/TAZ. The protein level of TAZ in HEK293/TAZ cells was obviously higher than HEK293/control cells (Figure 1E). The result of tumour xenograft growth in nude mice showed that HEK293/TAZ cells led to larger tumour formation compared with HEK293/control cells (Figures 1F and 1G). As shown in Figure 1(H), tumour analysis showed the protein levels of TAZ and Proliferating Cell Nuclear Antigen (PCNA) were increased in the tumour xenograft of HEK293/TAZ cells, as compared with the control group. All these data indicated that overexpression of TAZ could significantly promote cell growth and proliferation in vitro and in vivo.
Figure 1. TAZ promoted cell growth in vitro and in vivo.
(A) The protein levels of TAZ in 293-TR/control and 293-TR/TAZ cells with or without Dox treatment for 24 h were evaluated by western blot. β-Actin was used as a loading control. (B) The cell growth curves of 293-TR/control and 293-TR/TAZ cells. Cells were treated with or without Dox, and cell numbers were counted as indicated. Results represent means±S.D. (n=3). (C) The colony formation of 293-TR/control and 293-TR/TAZ cells. Five hundred cells/well were seeded into six-well plates and treated with Dox or not for 2 weeks. (D) The cell colony numbers of 293-TR/control and 293-TR/TAZ cells were analysed. Values are means±S.E.M. (n=3). ***P<0.001. (E) The protein levels of TAZ in HEK293/control and HEK293/TAZ cells were evaluated by western blot. β-Actin was used as a loading control. (F) HEK293/control and HEK293/TAZ cells were injected bilateral subcutaneously into nude mice. The mice were killed 30 days after injection (n=5 for each group). (G) The weights of tumour xenografts were measured. Results represent means±S.E.M. ***P<0.001. (H) The expression of TAZ and PCNA in the tumour xenograft of HEK293/control cells and HEK293/TAZ cells were detected by immunohistochemistry.
Celastrol suppressed cell growth and induced cell apoptosis in T-Rex-293 cells
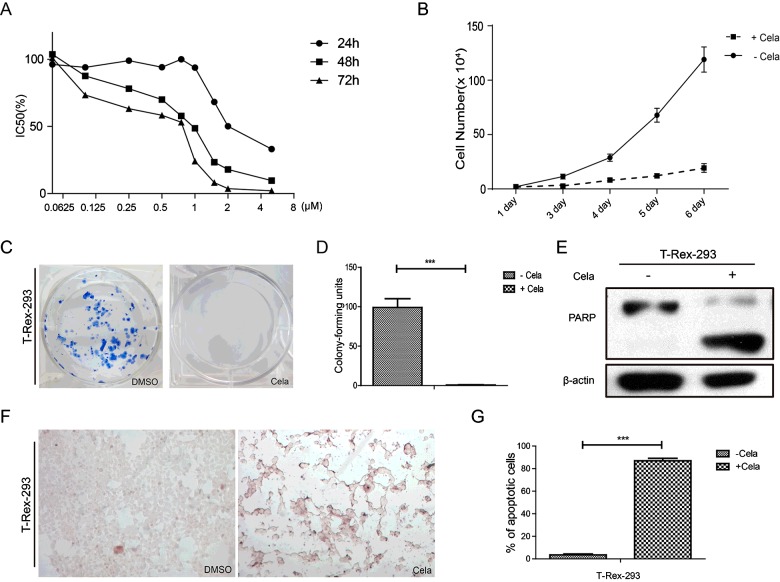
Celastrol is derived from the Chinese medicinal plant Tripterygium wilfordii [15] and can inhibit cell proliferation [16], induce cell apoptosis [17], suppress invasion/migration [18] and angiogenesis [19]. In our study, T-Rex-293 cells were treated with Celastrol at different concentrations (0.25–5 μM) as indicated for 24, 48 and 72 h respectively. As shown in Figure 2(A), the growth of T-Rex-293 cells was significantly inhibited by Celastrol in a dose- and time-dependent manner. The cell growth curve showed that Celastrol obviously inhibited the growth of T-Rex-293 cells (Figure 2B). Moreover, the colony numbers of T-Rex-293 cells treated with Celastrol was much less than the cells without treatment (Figures 2C and 2D). To further identify whether Celastrol-induced cell growth inhibition was partially due to increased apoptosis, western blot and TdT-mediated dUTP Nick end labelling (TUNEL) assay were performed. As shown in Figure 2(E), the PARP cleavage was definitely observed in T-Rex-293 cells treated with Celastrol compared with the untreated cells. TUNEL assays showed that approximately 87.0% cells were TUNEL-positive in Celastrol treatment group, compared with 3.5% in control group (Figures 2F and 2G). In summary, these data demonstrated that Celastrol inhibited cell growth and induced cell apoptosis in T-Rex-293 cells.
Figure 2. Celastrol suppressed cell growth and induced cell apoptosis in T-Rex-293 cells.
(A) Growth inhibition of T-Rex-293 cells treated with Celastrol at different concentration (0.25–5 μM) and different time-point (24, 48 and 72 h). (B) The cell growth curves of T-Rex-293 cells treated with or without Celastrol (0.5 μM 48 h). Cell numbers were counted as indicated. Results represent means±S.D. (n=3). (C) The colony formation of T-Rex-293 cells treated with or without Celastrol (0.5 μM 48 h). Eight hundred cells/well were seeded into six-well plates for 2 weeks. (D) The cell colony numbers of T-Rex-293 cells treated with or without Celastrol (0.5 μM 48 h) were analysed. Values are means±S.E.M. (n=3). ***P<0.001. (E) Western blot analysis of cleaved PARP in T-Rex-293 cells treated with or without Celastrol (0.5 μM) for 48 h. β-Actin was used as a loading control. (F) TUNEL assay was used to detect the apoptotic phenotype induced by Celastrol (0.5 μM 48 h). TUNEL-positive (apoptotic) cells were stained brown (magnification 100×). (G) The ratio of apoptosis cells was quantified. Values are means±S.E.M of three measurements in each group. ***P<0.001.
Overexpression of TAZ could attenuate Celastrol-induced cell apoptosis of T-Rex-293 cells
TAZ is nearly 60% homologous with YAP and YAP could inhibit cell apoptosis [12], so we next determined whether TAZ had the similar function. As Figure 3(A) shown, Dox-induced overexpression of TAZ could inhibit Celastrol-induced PARP cleavage in 293-TR/TAZ cells; When treated with Celastrol (0.5 μM) for 48 h, the percentage of TUNEL-positive cells was obviously reduced from 86.8 to 22.9% in 293-TR/TAZ cells after Dox induction, however, 293-TR/control cells with or without Dox treatment had almost the same ratio of TUNEL-positive cells (Figures 3B and 3C). Our data indicated that overexpression of TAZ could partially inhibit Celastrol-induced cell apoptosis. To investigate the possible mechanism by which TAZ attenuate Celastrol-induced cell apoptosis, we firstly detected the mRNA levels of several TAZ target genes, including ankyrin repeat domain-containing protein (ANKRD), cysteine-rich 61 (CYR61), connective tissue growth factor (CTGF) [20–23]. The results showed that the expressions of ANKRD, CYR61, CTGF were significantly up-regulated in 293-TR/TAZ cells after Dox induction, and were slightly decreased when treated with Celastrol (0.5 μM 48 h; Figure 3D). These data suggested that TAZ may resist cell apoptosis through its target genes. Furthermore, some studies demonstrated that suppression of Akt activation could partially contribute to Celastrol-induced cell apoptosis [24–26]. In the present study, we found Celastrol treatment did not influence the total Akt protein expression, but could decrease the phosphorylation of Akt and Dox-induced overexpression of TAZ increased the phosphorylation of Akt (Figure 3E). Moreover, western blot analysis showed that Celastrol treatment could increase the expression of Bax and decrease the expression of Bcl-2, but induced overexpression of TAZ could attenuate the effect that mentioned above. These data indicated that TAZ may resist Celastrol-induced cell apoptosis through activating phosphatidylinositol 3-kinase (PI3K)/Akt pathway and changing the balance of pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2.
Figure 3. Overexpression of TAZ could attenuate Celastrol-induced apoptosis in T-Rex-293 cells.
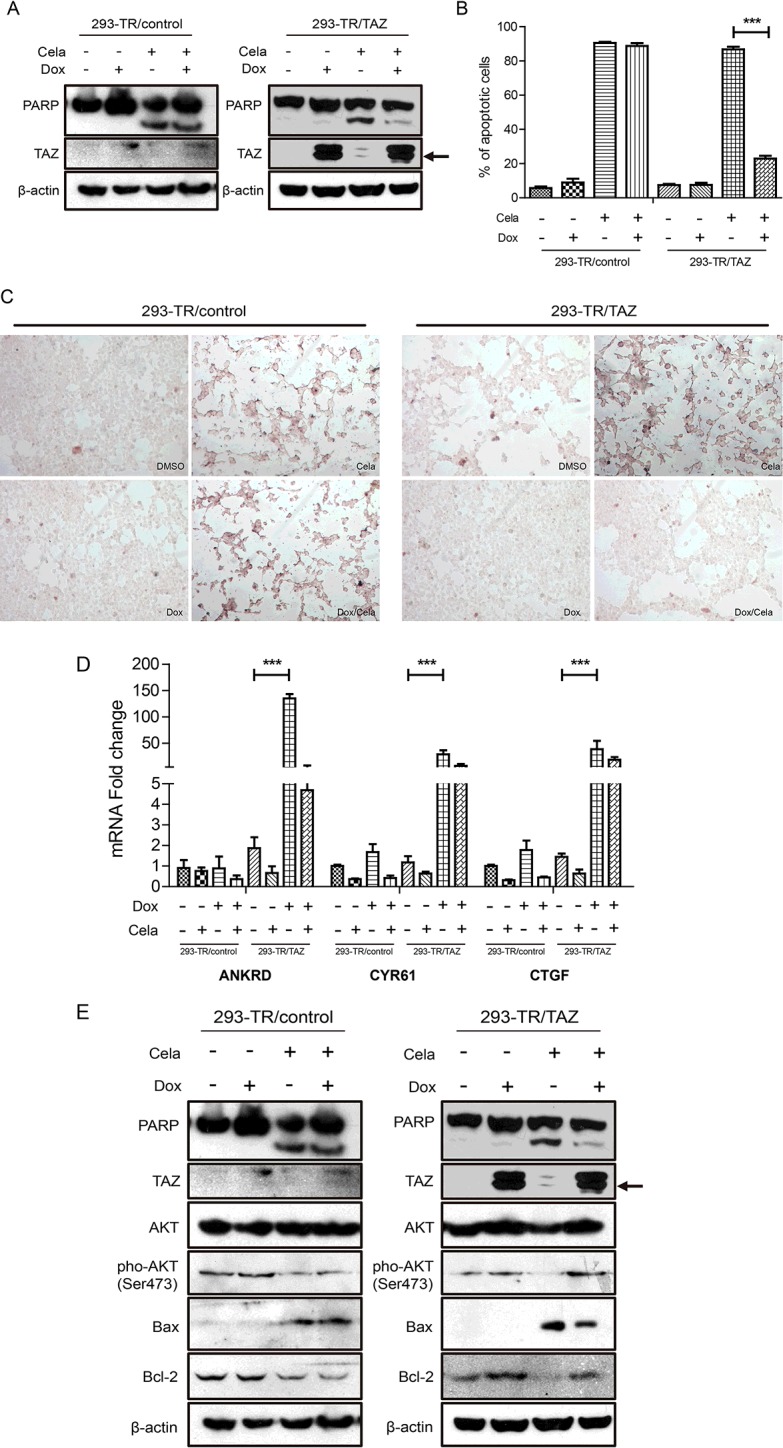
(A) Celastrol (0.5 μM) and DMSO were added to 293-TR/control and 293-TR/TAZ cells in the absence and presence of Dox for 48 h. TAZ and cleaved PARP were detected by western blot. β-Actin was used as a loading control. (B) Celastrol (0.5 μM) and DMSO were added to 293-TR/control and 293-TR/TAZ cells in the absence and presence of Dox for 48 h respectively. TUNEL assay was used to detect the apoptotic phenotype. TUNEL-positive (apoptotic) cells were stained brown (magnification 100 ×). (C) The ratio of apoptotic cells was quantified. Values are means±S.E.M. (n=3). ***P<0.001. (D) 293-TR/control cells and 293-TR/TAZ cells were treated with Celastrol (0.5 μM) and DMSO in the absence and presence of Dox for 48 h, then the mRNA expression of ANKRD, CYR61 and CTGF were detected by real-time PCR. Values are means±S.E.M. (n=3). ***P<0.001. (E) 293-TR/control cells and 293-TR/TAZ cells were treated with Celastrol (0.5 μM) and DMSO in the absence and presence of Dox for 48 h, then Akt, pho-Akt (Ser473), Bcl-2 and Bax were detected by western blot. β-Actin was used as a loading control.
DISCUSSION
Increasing evidence implicates deregulation of Hippo pathway in a variety of cancer. As the major downstream effectors of Hippo pathway, YAP and TAZ are homologous and previous studies demonstrate that most of their biological functions are the same. In the matter of regulating the cell or tissue property, YAP/TAZ play vital roles inapico-basal polarity, mechanotransduction, cell–cell adhesion and contact inhibition [27]. Besides, YAP/TAZ can regulate cell competition, stem cell maintenance, metastasis and regeneration [28]. As oncogenes, both of YAP and TAZ can promote the occurrence and development of tumour.
To date, there has been a considerable body of evidence that links YAP/TAZ to tumorigenicity in cancers. Overexpression of YAP is generally common in a wide variety of tumours, including colon cancer [29], lung cancer [10], gastric cancer [30], liver cancer [31] and breast cancer [8]. YAP can promote cell proliferation, inhibit cell apoptosis, lead to loss of cell contact inhibition and promote cell malignant transformation [32]. Importantly, activation of the LATS kinase results in an increased cytoplasmic phosphorylated YAP accompanied by an induction of apoptosis [33]; when LATS is inhibited, it results in an accumulation of nuclear YAP and then YAP-dependent transcriptional regulation, the consequence of YAP activation can inhibit cell apoptosis [34]. Moreover, the kinases JNKs can phosphorylate YAP to regulate cell apoptosis [35]. Intriguingly, YAP also can accelerate cell apoptosis in the condition of DNA damage. As a transcriptional activated cofactor, YAP can enhance the transcription of the apoptosis-relative genes such as p73 [36] and p53 [37]. YAP promotes apoptosis, which most often occurs in the chemotherapy-induced DNA damage. A previous study reported that phosphorylation of YAP is a crucial regulatory process for its function as a tumour suppressor in certain breast cancers [13]. All these shows that YAP has contradictory roles in cancer progression.
Although YAP and TAZ act as oncogenes, they still have some apparent differences [38]: YAP knockout animals are embryonic lethal [39], but TAZ null mice have renal cysts and lead to end stage kidney disease [40,41]; however, the deletion of both YAP and TAZ causes the exceptionally early death in mice, suggesting there is a potential synergy between YAP and TAZ [42]. Moreover, a study shows that the mRNA expression of TAZ is a prognostic indicator for colon cancer progression, but YAP is not [43].
Compared with TAZ, YAP has more significant effect on cell proliferation and apoptosis [44], but our results indicated that TAZ could also promote cell proliferation. In the present study, we chose T-Rex-293 cell line to construct an inducible expression system of TAZ. In accordance with our expectations, overexpression of TAZ accelerated cell growth in vitro. Moreover, the latency of tumour formation in nude mice injected with HEK293/TAZ cells was shorter than the tumours injected with HEK293/control cells (results not shown), which confirmed that TAZ could promote tumour formation in vivo. The immunohistochemical results of PCNA further confirmed that TAZ could promote cell proliferation. Furthermore, our cell immunochemistry results proved the same conclusion in vitro (results not shown). In addition, previous study demonstrated YAP/TAZ depletion suppress cell growth [32], which is consistent with our study.
In contrast, TAZ acts as an oncogene to induce epithelial–mesenchymal transition (EMT), and increase cell migration and invasion [45], which is different from YAP. The mutation of TAZ in breast cancer patients with metastases is significantly more than the primary breast cancer patients, which suggests that TAZ may be involved in breast cancer metastasis [46]. Similarly, TAZ can enhance tumour cell metastasis and invasion in the lung cancer, colorectal cancer and glial cell sarcoma. However, there is no direct evidence prompting TAZ can inhibit cell apoptosis. A recent report only indicated that siRNA-mediated knockdown of TAZ could increase apoptosis [47]. Our study further proved that TAZ could have anti-apoptotic ability, which confirmed its oncogenetic roles, but the mechanism of TAZ anti-apoptosis remains to be elucidated.
As we know CYR61 and CTGF are direct target genes of TAZ. Previous studies have demonstrated that CYR61 is a metastatic biomarker and a driver of gastric cardia adenocarcinoma (GCA) [48]. Promotion of angiogenesis and cell proliferation is the major function of CYR61 in tumour progression [49]. CTGF is a matricellular protein which plays an important role in promoting fibrosis and scarring in the skin, kidney, liver, brain and lung [50,51]. Moreover, CTGF may be an adverse prognostic factor in male breast cancer (MBC) [22]. Our results suggested that the anti-apoptotic function of TAZ may be relevant with the up-regulation of CYR61 and CTGF and the decrease in Akt phosphorylation which leads to cell apoptosis [25,26,52,53] may be a mechanism for Celastrol-induced cell apoptosis. On the other hand, some studies indicated that CYR61 and CTGF could increase the phosphorylation of Akt [54–56]. Hence, TAZ may restore Celastrol-induced cell apoptosis by stimulating Akt signalling pathway activation via up-regulation of CYR61 and CTGF.
The Bcl-2 family containing pro-apoptotic member Bax and anti-apoptotic member Bcl-2, play a pivotal role in the regulation of apoptosis [57]. Our data also showed that Celastrol treatment increased the expression of Bax, decreased the expression of Bcl-2. But overexpression of TAZ could reverse the changes of above-mentioned proteins. It is very likely that the anti-apoptotic potential of TAZ is mediated through suppression of apoptotic gene expression (Bax) and induction of anti-apoptotic gene expression (Bcl-2).
In conclusion, overexpression of TAZ not only could promote cell growth in vitro and in vivo, it could also inhibit drug-induced apoptosis through the activation of PI3K/Akt signalling pathway and regulation of apoptosis-related proteins (Bax/Bcl-2). Our results reaffirm the oncogenetic role of TAZ and provide clue to improve our understanding of the functions of TAZ in cancers. In view of the above-mentioned roles of TAZ in cell fate determination, it may serve as a novel biomarker for cancers and an effective and selective target for cancer therapy.
Acknowledgments
We thank Professor Quan Chen for the parental T-Rex-293cells.
Abbreviations
- Akt/PKB
protein kinase B
- ANKRD
ankyrin repeat domain-containing protein
- Bax
B-cell lymphoma-2 associated X protein
- Bcl-2
B-cell lymphoma-2
- CTGF
connective tissue growth factor
- CYR61
cysteine-rich 61
- Dox
doxcycline
- LATS1/2
large tumour suppressor serine/threonine protein kinases 1/2
- MOB
Mps one binder kinase activator protein
- MST1/2
mammalian sterile20-like kinases serine/threonine kinases 1/2
- PI3K
phosphatidylinositol 3-kinase
- SAV1
also called WW45, Salvador homologue 1
- TAZ
transcriptional co-activators with PDZ-binding motif
- TUNEL
TdT-mediated dUTP Nick end labelling
- YAP
yes-associated protein
AUTHOR CONTRIBUTION
Shuren Wang conducted the experiments, analysed the data and wrote the manuscript. Kai Ma designed the experiments, discussed and analysed the data. Lechuang Chen, Hongxia Zhu and Shufang Liang designed the experiments and discussed the data. Mei Liu and Ningzhi Xu oversaw the project and worked to finalize the manuscript. All authors approved the final version of the manuscript.
FUNDING
This work was supported by the National Natural Science Foundation [grant numbers 81321091, 81472561 and 81302279]; the Postgraduate Innovation Fund of the Beijing Union Medical College [grant number 10023-0710-1015]; the National Key Research & Development (R&D) Plan [grant numbers 2016YFC0906000 and 2016YFC0906002]; and the 973 Program [grant number 2011CB910700].
References
- 1.Tapon N., Harvey K.F., Bell D.W., Wahrer D.C.R., Schiripo T.A., Haber D.A., Hariharan I.K. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/S0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 2.Halder G., Johnson R.L. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Praskova M., Xia F., Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 2008;18:311–321. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callus B.A., Verhagen A.M., Vaux D.L. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes. Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marti P., Stein C., Blumer T., Abraham Y., Dill M.T., Pikiolek M., Orsini V., Jurisic G., Megel P., Makowska Z., et al. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology. 2015;62:1497–1510. doi: 10.1002/hep.27992. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi H., Higashi T., Yokoyama N., Kaida T., Sakamoto K., Fukushima Y., Ishimoto T., Kuroki H., Nitta H., Hashimoto D., et al. An imbalance in TAZ and YAP expression in hepatocellular carcinoma confers cancer stem cell-like behaviors contributing to disease progression. Cancer Res. 2015;75:4985–4997. doi: 10.1158/0008-5472.CAN-15-0291. [DOI] [PubMed] [Google Scholar]
- 8.Hiemer S.E., Szymaniak A.D., Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor beta-induced tumorigenic phenotypes in breast cancer cells. J. Biol. Chem. 2014;289:13461–13474. doi: 10.1074/jbc.M113.529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L., Yang S.P., Chen X.C., Stauffer C., Yu F., Lele S.M., Fu K., Datta K., Palermo N., Chen Y.H., et al. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol. Cell. Biol. 2015;35:1350–1362. doi: 10.1128/MCB.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su L.L., Ma W.X., Yuan J.F., Shao Y., Xiao W., Jiang S.J. Expression of Yes-associated protein in non-small cell lung cancer and its relationship with clinical pathological factors. Chin. Med. J. (Engl) 2012;125:4003–4008. [PubMed] [Google Scholar]
- 11.Xia Y., Chang T., Wang Y., Liu Y., Li W., Li M., Fan H.Y. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One. 2014;9:e91770. doi: 10.1371/journal.pone.0091770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng X., Li W.B., Wang D.L., Chen K.H., Cao J.J., Luo Z., He J., Li M.C., Liu W.J., Yu C. YAP is closely correlated with castration-resistant prostate cancer, and downregulation of YAP reduces proliferation and induces apoptosis of PC-3 cells. Mol. Med. Rep. 2015;12:4867–4876. doi: 10.3892/mmr.2015.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan M., Tomlinson V., Lara R., Holliday D., Chelala C., Harada T., Gangeswaran R., Manson-Bishop C., Smith P., Danovi S.A., et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C.Q., Liu S., Zhou X.B., Xuez L.Y., Quan L.P., Lu N., Zhang G., Bai J.F., Wang Y.H., Liu Z.H., et al. Overexpression of human pituitary tumor transforming gene (hPTTG), is regulated by beta-catenin/TCF pathway in human esophageal squamous cell carcinoma. Int. J. Cancer. 2005;113:891–898. doi: 10.1002/ijc.20642. [DOI] [PubMed] [Google Scholar]
- 15.Salminen A., Lehtonen M., Paimela T., Kaarniranta K. Celastrol: molecular targets of thunder god vine. Biochem. Biophys. Res. Commun. 2010;394:439–442. doi: 10.1016/j.bbrc.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 16.Shao L., Zhou Z., Cai Y., Castro P., Dakhov O., Shi P., Bai Y., Ji H., Shen W., Wang J. Celastrol suppresses tumor cell growth through targeting an AR-ERG-NF-kappaB pathway in TMPRSS2/ERG fusion gene expressing prostate cancer. PLoS One. 2013;8:e58391. doi: 10.1371/journal.pone.0058391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.H., Won Y.S., Park K.H., Lee M.K., Tachibana H., Yamada K., Seo K.I. Celastrol inhibits growth and induces apoptotic cell death in melanoma cells via the activation ROS-dependent mitochondrial pathway and the suppression of PI3K/AKT signaling. Apoptosis. 2012;17:1275–1286. doi: 10.1007/s10495-012-0767-5. [DOI] [PubMed] [Google Scholar]
- 18.Yadav V.R., Sung B., Prasad S., Kannappan R., Cho S.G., Liu M.Y., Chaturvedi M.M., Aggarwal B.B. Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J. Mol. Med. 2010;88:1243–1253. doi: 10.1007/s00109-010-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni H.W., Zhao W.Z., Kong X.T., Li H.T., Ouyang J. Celastrol inhibits lipopolysaccharide-induced angiogenesis by suppressing TLR4-triggered nuclear factor-kappa B activation. Acta Haematol. 2014;131:102–111. doi: 10.1159/000354770. [DOI] [PubMed] [Google Scholar]
- 20.Mi W., Lin Q., Childress C., Sudol M., Robishaw J., Berlot C.H., Shabahang M., Yang W. Geranylgeranylation signals to the Hippo pathway for breast cancer cell proliferation and migration. Oncogene. 2015;34:3095–3106. doi: 10.1038/onc.2014.251. [DOI] [PubMed] [Google Scholar]
- 21.Pobbati A.V., Hong W. Emerging roles of TEAD transcription factors and its coactivators in cancers. Cancer Biol. Ther. 2013;14:390–398. doi: 10.4161/cbt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Benedetto A., Mottolese M., Sperati F., Ercolani C., Di Lauro L., Pizzuti L., Vici P., Terrenato I., Sperduti I., Shaaban A.M., et al. The Hippo transducers TAZ/YAP and their target CTGF in male breast cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Xia H., Ge X., Chen Q., Yuan D., Chen Q., Leng W., Chen L., Tang Q., Bi F. CD44 acts through RhoA to regulate YAP signaling. Cell Signal. 2014;26:2504–2513. doi: 10.1016/j.cellsig.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 24.Pang X.F., Yi Z.F., Zhang J., Lu B.B., Sung B., Qu W.J., Aggarwal B.B., Liu M.Y. Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res. 2010;70:1951–1959. doi: 10.1158/0008-5472.CAN-09-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han X., Sun S., Zhao M., Cheng X., Chen G., Lin S., Guan Y., Yu X. Celastrol stimulates hypoxia-inducible factor-1 activity in tumor cells by initiating the ROS/Akt/p70S6K signaling pathway and enhancing hypoxia-inducible factor-1alpha protein synthesis. PLoS One. 2014;9:e112470. doi: 10.1371/journal.pone.0112470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shrivastava S., Jeengar M.K., Reddy V.S., Reddy G.B., Naidu V.G. Anticancer effect of celastrol on human triple negative breast cancer: possible involvement of oxidative stress, mitochondrial dysfunction, apoptosis and PI3K/Akt pathways. Exp. Mol. Pathol. 2015;98:313–327. doi: 10.1016/j.yexmp.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Harvey K.F., Zhang X., Thomas D.M. The Hippo pathway and human cancer. Nat. Rev. Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 28.Han D., Byun S.H., Park S., Kim J., Kim I., Ha S., Kwon M., Yoon K. YAP/TAZ enhance mammalian embryonic neural stem cell characteristics in a Tead-dependent manner. Biochem. Biophys. Res. Commun. 2015;458:110–116. doi: 10.1016/j.bbrc.2015.01.077. [DOI] [PubMed] [Google Scholar]
- 29.Wang L.J., Shi S.J., Guo Z.Y., Zhang X., Han S.X., Yang A.G., Wen W.H., Zhu Q. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One. 2013;8:e65539. doi: 10.1371/journal.pone.0065539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Ma L.G., Bian S.B., Cui J.X., Xi H.Q., Zhang K.C., Qin H.Z., Zhu X.M., Chen L. LKB1 inhibits the proliferation of gastric cancer cells by suppressing the nuclear translocation of Yap and beta-catenin. Int. J. Mol. Med. 2016;37:1039–1048. doi: 10.3892/ijmm.2016.2494. [DOI] [PubMed] [Google Scholar]
- 31.Yimlamai D., Fowl B.H., Camargo F.D. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J. Hepatol. 2015;63:1491–1501. doi: 10.1016/j.jhep.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamar J.M., Stern P., Liu H., Schindler J.W., Jiang Z.G., Hynes R.O. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wennmann D.O., Vollenbroker B., Eckart A.K., Bonse J., Erdmann F., Wolters D.A., Schenk L.K., Schulze U., Kremerskothen J., Weide T., et al. The Hippo pathway is controlled by angiotensin II signaling and its reactivation induces apoptosis in podocytes. Cell Death Dis. 2014;5:e1519. doi: 10.1038/cddis.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigneron A.M., Ludwig R.L., Vousden K.H. Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP. Genes Dev. 2010;24:2430–2439. doi: 10.1101/gad.1954310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomlinson V., Gudmundsdottir K., Luong P., Leung K.Y., Knebel A., Basu S. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 2010;1:e29. doi: 10.1038/cddis.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strano S., Monti O., Pediconi N., Baccarini A., Fontemaggi G., Lapi E., Mantovani F., Damalas A., Citro G., Sacchi A., et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol. Cell. 2005;18:447–459. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Reuven N., Adler J., Meltser V., Shaul Y. The Hippo pathway kinase Lats2 prevents DNA damage-induced apoptosis through inhibition of the tyrosine kinase c-Abl. Cell Death Differ. 2013;20:1330–1340. doi: 10.1038/cdd.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanai F., Marignani P.A., Sarbassova D., Yagi R., Hall R.A., Donowitz M., Hisaminato A., Fujiwara T., Ito Y., Cantley L.C., et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morin-Kensicki E.M., Boone B.N., Howell M., Stonebraker J.R., Teed J., Alb J.G., Magnuson T.R., O'Neal W., Milgram S.L. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol. Cell. Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makita R., Uchijima Y., Nishiyama K., Amano T., Chen Q., Takeuchi T., Mitani A., Nagase T., Yatomi Y., Aburatani H., et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am. J. Physiol. Renal. Physiol. 2008;294:F542–F553. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- 41.Hossain Z., Ali S.M., Ko H.L., Xu J.L., Ng C.P., Guo K., Qi Z., Ponniah S., Hong W.J., Hunziker W. Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1631–1636. doi: 10.1073/pnas.0605266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishioka N., Inoue K., Adachi K., Kiyonari H., Ota M., Ralston A., Yabuta N., Hirahara S., Stephenson R.O., Ogonuki N., et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Yuen H.F., McCrudden C.M., Huang Y.H., Tham J.M., Zhang X.Q., Zeng Q., Zhang S.D., Hong W.J. TAZ expression as a prognostic indicator in colorectal cancer. Plos One. 2013;8:e54211. doi: 10.1371/journal.pone.0054211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao B., Lei Q.Y., Guan K.L. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr. Opin. Cell Biol. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan S.W., Lim C.J., Guo K., Ng C.P., Lee I., Hunziker W., Zeng Q., Hong W. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- 46.Ding L., Ellis M.J., Li S., Larson D.E., Chen K., Wallis J.W., Harris C.C., McLellan M.D., Fulton R.S., Fulton L.L., et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikon N., Su B., Hsu F.F., Forte T.M., Ryan R.O. Exogenous cardiolipin localizes to mitochondria and prevents TAZ knockdown-induced apoptosis in myeloid progenitor cells. Biochem. Biophys. Res. Commun. 2015;464:580–585. doi: 10.1016/j.bbrc.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei J., Yu G., Shao G., Sun A., Chen M., Yang W., Lin Q. CYR61 (CCN1) is a metastatic biomarker of gastric cardia adenocarcinoma. Oncotarget. 2016;7:31067–31078. doi: 10.18632/oncotarget.8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuonen F., Secondini C., Ruegg C. Molecular pathways: emerging pathways mediating growth, invasion, and metastasis of tumors progressing in an irradiated microenvironment. Clin. Cancer Res. 2012;18:5196–5202. doi: 10.1158/1078-0432.CCR-11-1758. [DOI] [PubMed] [Google Scholar]
- 50.Shi-Wen X., Leask A., Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Jun J.I., Lau L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat. Rev. Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kannaiyan R., Manu K.A., Chen L., Li F., Rajendran P., Subramaniam A., Lam P., Kumar A.P., Sethi G. Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3 K/Akt signaling pathways. Apoptosis. 2011;16:1028–1041. doi: 10.1007/s10495-011-0629-6. [DOI] [PubMed] [Google Scholar]
- 53.Sha M., Ye J., Zhang L.X., Luan Z.Y., Chen Y.B., Huang J.X. Celastrol induces apoptosis of gastric cancer cells by miR-21 inhibiting PI3K/Akt-NF-kappaB signaling pathway. Pharmacology. 2014;93:39–46. doi: 10.1159/000357683. [DOI] [PubMed] [Google Scholar]
- 54.Gao W., Cai L., Xu X., Fan J., Xue X., Yan X., Qu Q., Wang X., Zhang C., Wu G. Anti-CTGF single-chain variable fragment dimers inhibit human airway smooth muscle (ASM) cell proliferation by down-regulating p-Akt and p-mTOR levels. PLoS One. 2014;9:e113980. doi: 10.1371/journal.pone.0113980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mu S., Kang B., Zeng W., Sun Y., Yang F. MicroRNA-143-3p inhibits hyperplastic scar formation by targeting connective tissue growth factor CTGF/CCN2 via the Akt/mTOR pathway. Mol. Cell Biochem. 2016;416:99–108. doi: 10.1007/s11010-016-2699-9. [DOI] [PubMed] [Google Scholar]
- 56.Lee K.B., Byun H.J., Park S.H., Park C.Y., Lee S.H., Rho S.B. CYR61 controls p53 and NF-kappaB expression through PI3K/Akt/mTOR pathways in carboplatin-induced ovarian cancer cells. Cancer Lett. 2012;315:86–95. doi: 10.1016/j.canlet.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 57.Tomita T. Apoptosis in pancreatic beta-islet cells in Type 2 diabetes. Bosn. J. Basic Med. Sci. 2016;16:162–179. doi: 10.17305/bjbms.2016.919. [DOI] [PMC free article] [PubMed] [Google Scholar]