Abstract
Merlin, encoded by the NF2 gene, is a tumor suppressor that acts by inhibiting mitogenic signaling and is mutated in Neurofibromatosis type II (NF2) disease, although its molecular mechanism is not fully understood. Here, we observed that Merlin inhibited Wnt/β-catenin signaling by blocking phosphorylation of LRP6, which is necessary for Wnt signal transduction, whereas mutated Merlin in NF2 patients did not. Treatment with Wnt3a enhanced phosphorylation of Ser518 in Merlin via activation of PAK1 in a PIP2-dependent manner. Phosphorylated Merlin dissociated from LRP6, allowing for phosphorylation of LRP6. Tissues from NF2 patients exhibited higher levels of β-catenin, and proliferation of RT4-D6P2T rat schwannoma cells was significantly reduced by treatment with chemical inhibitors of Wnt/β-catenin signaling. Taken together, our findings suggest that sustained activation of Wnt/β-catenin signaling due to abrogation of Merlin-mediated inhibition of LRP6 phosphorylation may be a cause of NF2 disease.
Wnt/β-catenin signaling has essential roles in the regulation of embryonic development and maintenance of homeostasis in adult tissues.1, 2, 3 Fine tuning of Wnt/β-catenin signaling is achieved by multiple modulators, including components of other signaling pathways.4, 5, 6 Wnt/β-catenin signaling is involved in diverse biological processes, and misregulation of this signaling pathway causes various human diseases such as cancer, osteoporosis, and neurodegenerative diseases.7, 8 Therefore, identification and functional analysis of modulators of Wnt/β-catenin signaling should be performed to select specific modulators as therapeutic targets rather than core components in order to reduce potential side effects.8
The main function of Wnt/β-catenin signaling is regulation of the cytoplasmic β-catenin level. In the absence of Wnt, cytoplasmic β-catenin is phosphorylated by GSK3β and CK1α in a destruction complex that includes Axin, APC (adenomatous polyposis coli), and others.2, 3 Phosphorylated β-catenin is then recognized and ubiquitinated by β-TrCP E3 ligase for subsequent proteasomal degradation, which reduces the level of cytoplasmic β-catenin. On the other hand, binding of Wnt to Frizzled and its co-receptor, LRP5/6, leads to increased levels of phosphatidylinositol 4,5 bisphosphate (PIP2) and phosphorylation of the C-terminal region of LRP5/6 by GSK3β and CK1γ.9, 10 Phosphorylated LRP5/6 then recruits Axin to the plasma membrane, resulting in stabilization of cytoplasmic β-catenin.11, 12 The accumulated cytoplasmic β-catenin is then translocated into nuclei to activate transcription factor TCF/LEF1 and induces expression of target genes involved in the regulation of cell proliferation and cell fate decisions in a context-dependent manner.1, 2, 3
Neurofibromatosis type II (NF2) disease is an autosomal, dominantly inherited familial cancer syndrome. Manifestation of this disease mainly involves benign tumors such as bilateral vestibular schwannomas, ependymomas, and meningiomas in the central or peripheral nervous system.13 NF2 gene responsible for NF2 disease encodes Merlin, a tumor suppressor protein belonging to the band 4.1 superfamily, which is known to have homology with the ERM (Ezrin, Radixin, and Moesin) protein family.14 Merlin is composed of an N-terminal FERM domain, a central α-helical domain, and C-terminal FERM-binding domain. Merlin is mainly localized to the plasma membrane and exerts its tumor suppressor function via regulation of mitogenic receptors.14 Merlin is also known to accumulate in the nucleus and mediates tumor suppressor activity by inhibiting the activity of E3 ubiquitin ligase CRL4 (DCAF1).15, 16 In Drosophila, Merlin cooperates with another FERM domain protein, Expanded, to accelerate endocytosis of specific receptors, including mitogenic receptors.17 In addition, the role of Merlin in Hippo signaling, which is responsible for control of cell proliferation and organ size, has been intensively studied.18, 19 At low cell density, the key effector protein YAP/TAZ is localized to nuclei and enhances expression of target genes related to anti-apoptosis and proliferation. However, at high cell density, Merlin activates MST1/2 to phosphorylate LATS, which in turn phosphorylates YAP/TAZ. Phosphorylated YAP/TAZ is then sequestered by 14-3-3 protein and subsequently degraded in a proteasome-dependent manner, which results in inhibition of target gene expression.
Two previous studies revealed that Merlin inhibits Wnt/β-catenin signaling. One study showed that loss of Merlin increases Wnt reporter activity via upregulation of Rac activity, which enhances nuclear localization of β-catenin via JNK.20 The other study showed that activated Src phosphorylates Tyr654 in β-catenin, which promotes dissociation of the membrane portion of β-catenin, resulting in accumulation of active β-catenin in nuclei of Merlin-deficient human schwannoma cells.21
In this report, we provide a novel mechanism for the inhibition of Wnt/β-catenin signaling by Merlin. Merlin interacts with and inhibits phosphorylation of LRP6 by blocking the interaction between Axin and LRP6. As evidence of this, we observed that Wnt3a-conditioned media activated PAK1 in a PIP2-dependent manner and promoted phosphorylation of Merlin, leading to dissociation of Merlin from LRP6. Analysis of ectopic expression of Merlin or injection of xMerlin morphorino into Xenopus embryos and immunohistochemical analysis of NF2 patients strongly confirmed that Merlin inhibits Wnt/β-catenin signaling by blocking LRP6 phosphorylation.
Results
Interaction of active form of Merlin with LRP6 is disrupted by treatment with Wnt3a-CM
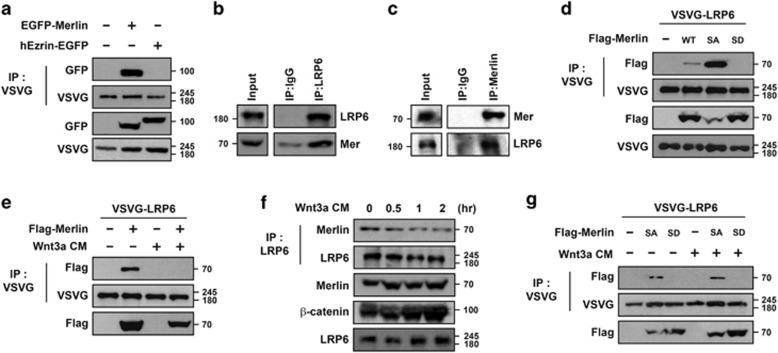
To determine whether or not LRP6 is a binding partner of Merlin, VSVG- LRP6 and EGFP-Merlin were co-expressed in HEK293T cells. Immunoprecipitation analysis revealed that VSVG-LRP6 specifically interacted with EGFP-Merlin but not with hEzrin-EGFP, although both belong to the ERM protein family (Figure 1a). Strong endogenous interaction between LRP6 and Merlin suggests that these two proteins interacted under physiological conditions (Figures 1b and c). Analysis using deletion constructs showed that Merlin interacted with LRP6 via its N-terminal FERM domains, which are necessary for other ERM proteins to interact with transmembrane proteins (Supplementary Figures S1A and B). Merlin exists as either a closed growth-suppressive or open growth-permissive protein, and phosphorylation of Ser518 is known to induce conformational changes from closed to open form.22, 23 However, a recent biochemical study suggested that phosphorylation converts Merlin into a less active and more closed form.24 To avoid confusion, however, we defined the unphosphorylated form as closed and active (see Discussion). Interestingly, S518A mutant of Merlin (Merlin-SA, mimetic of unphosphorylated) interacted more strongly with LRP6 than did wild-type Merlin, whereas S518D mutant (Merlin-SD, phospho-mimetic) showed almost no interaction (Figure 1d). As LRP6 is a co-receptor of Wnt, we next tested whether or not their interaction could be regulated by Wnt itself. Interactions between exogenously expressed VSVG-LRP6 and Flag-Merlin were severely blocked when HEK293T cells were incubated with Wnt3a-conditioned media (Wnt3a-CM) (Figure 1e). Endogenous interaction between LRP6 and Merlin in HEK293T cells was also inhibited by incubation with Wnt3a-CM in a time-dependent manner (Figure 1f), whereas interaction between Merlin-SA and VSVG-LRP6 was unaffected (Figure 1g). These data led us to hypothesize that inhibition of the interaction between Merlin and LRP6 could be caused by phosphorylation of Merlin induced in the presence of Wnt (see Figure 5).
Figure 1.
Interaction of Merlin with LRP6 is disrupted upon Wnt signaling. (a) Merlin bound to LRP6 under overexpression conditions. VSVG-LRP6 together with EGFP-Merlin or hEzrin-EGFP was transfected into HEK293T cells, subjected to immunoprecipitation with anti-VSVG, and immunoblotted with the indicated antibodies. (b and c) Merlin bound to LRP6 at endogenous level. MDCK cells were lysed, subjected to immunoprecipitation with anti-LRP6 (b), anti-Merlin (c), or control mouse IgG antibodies, and immunoblotted with the indicated antibodies. (d) Phospho-mimetic form of Merlin did not interact with LRP6. VSVG-LRP6 was co-transfected with wild-type (WT), S518A (SA), or S518D (SD) Merlin as indicated, subjected to immunoprecipitation with anti-VSVG, and immunoblotted with the indicated antibodies. (e) Interaction between Merlin and LRP6 was abrogated in the presence of Wnt. VSVG-LRP6 with or without Flag-Merlin-expressing HEK293T cells was treated with Wnt3a-CM overnight, followed by immunoprecipitation with anti-VSVG antibody and western blotting. (f) Interaction between endogenous Merlin and LRP6 was reduced upon treatment with Wnt3a-CM in a time-dependent manner. HEK293T cells were treated with Wnt3a-CM for the indicated time, followed by immunoprecipitation with anti-LRP6 antibody and western blotting with the indicated antibodies. (g) Interaction between unphospho-mimetic form of Merlin and LRP6 was not abrogated upon treatment with Wnt3a-CM. HEK293T cells, transfected with indicated plasmids, were incubated with or without Wnt3a-CM, followed by immunoprecipitation with anti-VSVG antibody and western blotting with the indicated antibodies. All western blotting and immunoprecipitation experiments were performed more than thrice and these data are representative of them
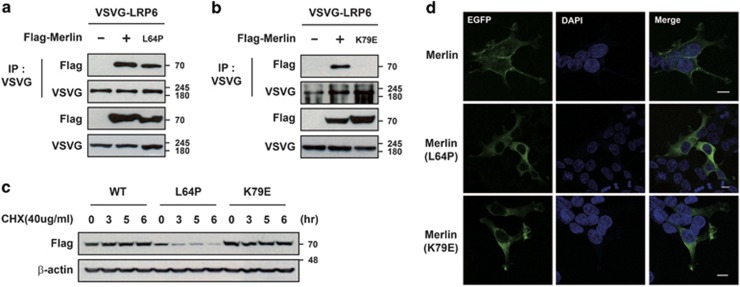
Numerous missense as well as non-sense mutations in Merlin have been identified in NF2 patients.25 Most missense mutations are reportedly localized to the N-terminal FERM domain or C-terminal tail of Merlin, as amino-acid substitutions in this region inhibit head-to-tail interactions due to an abnormal conformation and charge distribution, which may block interactions with transmembrane proteins.26 We thus tested whether or not the mutant forms of Merlin identified in NF2 patients (Merlin-L64P and Merlin-K79E) have disruptive effects on interactions with LRP6 (Figure 2). Merlin-K79E did not interact with LRP6, whereas overexpressed Merlin-L64P did interact with LRP6 but Merlin-L64P was very unstable (Figures 2a–c). Wild-type Merlin mainly localized to the plasma membrane while two mutant forms of Merlin were distributed broadly throughout the cytoplasm as shown previously,27 which can lead to reduced interactions between endogenous mutated Merlin and LRP6 in disease situations (Figure 2d). Overall, these data raise the possibility that genetic deletions or point mutations in Nf2/Merlin cause NF2 disease by inhibiting interactions with LRP6, which in turn elevates Wnt/β-catenin signaling.
Figure 2.
Mutant forms of Merlin found in NF2 patients do not interact with LRP6. (a and b) Overexpressed Merlin mutants differentially interacted with LRP6. HEK293T cells were transfected with VSVG-LRP6 and the indicated plasmids, subjected to immunoprecipitation with anti-VSVG antibody, and immunoblotted with the indicated antibodies. Since Merlin-L64P was unstable, double the amount of plasmid for L64P Merlin was transfected in (a and d) to normalize ectopically expressed proteins. (c) Merlin-L64P is unstable. Stabilities of EGFP-Merlin, EGFP-Merlin-L64P, or EGFP-Merlin-K79E in HEK293T cells were measured after treatment of cells with cycloheximide. All experiments were performed more than three times, and these data are representative. (d) Mislocalization of mutant forms of Merlin. HEK293T cells were transfected with EGFP-Merlin, EGFP-Merlin-L64P, or EGFP-Merlin-K79E, fixed with 4% paraformaldehyde (PFA), and then examined for cellular distribution of EGFP signals. Scale bars indicate 10 μm
Active form of Merlin inhibits Wnt/β-catenin signaling
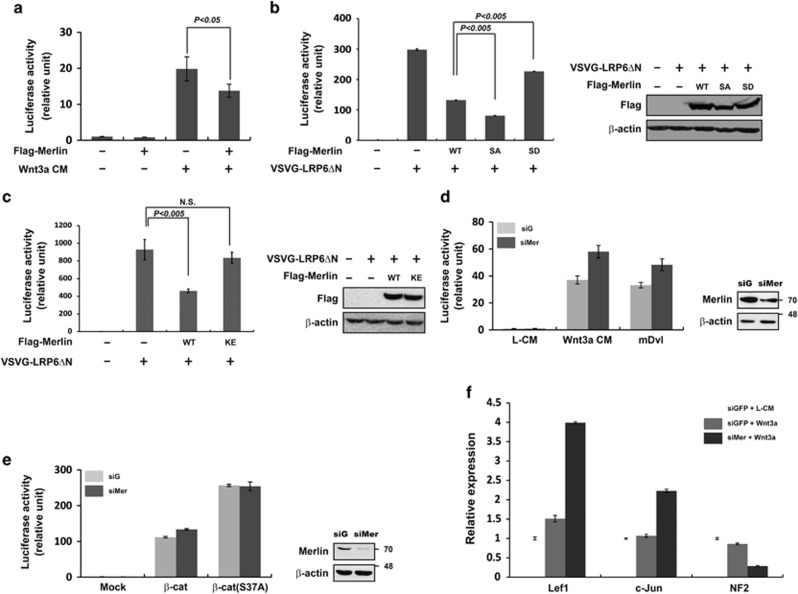
Previous studies have reported that Merlin possesses tumor suppressive activity.28 Thus, we hypothesized that Merlin may inhibit Wnt/β-catenin signaling via interaction with LRP6. Ectopic expression of Merlin inhibited reporter activity mediated by Wnt3a-CM or VSVG-LRP6ΔN, a constitutively active form with a deleted N-terminal extracellular domain (Figures 3a and b). Slightly higher expression of exogenous Merlin compared with endogenous Merlin was sufficient to significantly inhibit reporter activity induced by LRP6ΔN (Supplementary Figure S2A). Especially, Merlin-SA showed a stronger inhibitory effect than wild-type Merlin, whereas Merlin-SD and Merlin-K79E exhibited weaker effects (Figures 3b and c). Interestingly, ectopic expression of Merlin did not result in inhibition of reporter activity mediated by Wnt3a-CM under LRP6 knockdown conditions (Supplementary Figure S2B). Therefore, the inhibitory function of Merlin on Wnt/β-catenin signaling corresponded with its ability to interact with LRP6. Consistently, treatment with siRNA specific for endogenous Merlin enhanced Wnt reporter activity induced by Wnt3a-CM or overexpression of mouse Dvl-1 (Figure 3d), whereas knockdown of Merlin had little effect on reporter activity induced by ectopic expression of β-catenin or β-catenin S37A (constitutively active form of β-catenin) (Figure 3e), suggesting that Merlin acts upstream of β-catenin. Next, we determined whether or not knockdown of Merlin could increase endogenous Wnt target gene transcription in response to Wnt3a-CM. Real-time PCR analysis showed that Lef1, c-jun, c-Myc, and cyclin D1 transcription levels strongly increased upon Merlin depletion (Figure 3f and Supplementary Figure S2C). In summary, these results demonstrate that endogenous Merlin negatively regulates Wnt signaling by interacting with LRP6.
Figure 3.
Merlin inhibits Wnt/β-catenin signaling upstream of β-catenin. (a) Ectopic expression of Merlin is associated with reduced Wnt3a-mediated reporter activity. pSuperTop and pRL-TK reporter plasmids with Flag-Merlin as indicated were co-transfected into HEK293T cells. Cells were harvested 24 h after transfection, and luciferase activity was measured by the dual luciferase assay system. Wnt3a-CM was added to cells about 20 h before harvesting cells. In all subsequent reporter assays, the same reporter plasmids and assay system were used. Data represent average values from one representative experiment performed in triplicate. Error bars indicate standard deviations of triplicate. (b) Unphospho- or phosphor-mimetic forms of Merlin showed stronger or weaker inhibitory activity, respectively, than wild-type Merlin as determined by VSVG-LRP6ΔN-induced luciferase activity (left panel). Ectopic expression of Flag-Merlin was examined by western blotting (right panel). (c) Mutant form of Merlin (Merlin-K79E in NF2 patients) did not inhibit VSVG-LRP6ΔN-induced luciferase activity (left panel). Ectopic expression of Flag-Merlin was examined by western blotting (right panel). (d and e) Merlin inhibited Wnt3a-CM or Dvl but not β-catenin or the stabilized form of β-catenin as determined by reporter activity. Relative luciferase activities were measured in cells transfected with the indicated plasmids. Cells were treated with Wnt3a-CM for 20 h. Knockdown of Merlin was examined by western blotting (right panel). (f) Knockdown of Merlin enhanced expression of Wnt target genes. siGFP or siMerlin-transfected HEK293T cells were treated with L-CM or Wnt3a-CM overnight. Real-time PCR was performed to measure expression of Wnt target genes (Lef1 and c-Jun) and Merlin
Consistent with our biochemical results, a very high level of β-catenin was detected in all schwannomas isolated from clinically defined NF2 patients, whereas the level of β-catenin was low in normal adjacent tissue or malignant peripheral nerve sheath tumor isolated from defined NF1 patients (Supplementary Figure S3 and Table 1). NF1 is clinically different from NF2 and is produced by mutation of neurofibromin instead of Nf2/Merlin.29 However, contrary to the suggested role of Merlin in Hippo signaling,18, 30 the level of YAP or phospho-YAP was not significantly altered in NF2 patients (see Discussion). When Merlin was ectopically expressed in RT4-D6P2T schwannoma cells, which are commonly used to evaluate Merlin function due to their very low expression level of Merlin,31 the levels of LRP6 phosphorylation (a hallmark of activation of Wnt/β-catenin signaling) and β-catenin were significantly decreased (Supplementary Figure S4A). Treatment of RT4-D6P2T cells with chemical inhibitors of Wnt/β-catenin such as ICG-001 (blocks interaction between CBP and β-catenin) and IWP-2 (blocks Wnt secretion by inhibiting palmitoylation of Wnt proteins by porcupine, a critical acyltransferase)32, 33 reduced the growth rate of schwannoma cells (Supplementary Figure S3B). These results suggest that growth of schwannoma cells in NF2 disease may be caused by hyper-activation of Wnt/β-catenin signaling.
Table 1. Information for patients who provided tissues used in this work.
| Sex | Age | Tumor type | Location | |
|---|---|---|---|---|
| NF2 case 1 | M | 49 | Schwannoma | Tongue |
| NF2 case 2 | M | 27 | Schwannoma | Spine |
| NF2 case 3 | F | 21 | Schwannoma | Spine |
| NF2 case 4 | M | 37 | Meningioma | Cranium |
| NF1 case 1 | F | 34 | MPNST | Spine |
| NF1 case 2 | M | 52 | MPNST | Iliac bone |
| NF1 case 3 | F | 32 | MPNST | Chest wall |
| Control | M | 43 | Nerve tissue | Mesocolon |
Abbreviation: MPNST, malignant peripheral nerve sheath tumor
To further confirm the impact of Merlin on Wnt/β-catenin signaling and the phenotype of cancer cells by Merlin, we next evaluated the above findings in other glioblastoma cell lines, Merlin-expressing T98G and Merlin-deficient A172 cells.34 Similar to RT4-D6P2T cells, ectopic expression of Merlin in A172 cells clearly reduced the levels of LRP6 phosphorylation and β-catenin, and decreased the expression of Wnt target genes (Supplementary Figures S4D and E). When A172 cells were treated with XAV939, which stabilizes Axin and inhibits Wnt/β-catenin signaling,35 cell migration was strongly reduced (Supplementary Figure S4F). Conversely, knockdown of Merlin in T98G cells increased the levels of LRP6 phosphorylation, and β-catenin and enhanced cell proliferation and migration (Supplementary Figures S4G–I). Importantly, concomitant knockdown of β-catenin restored the enhanced cell growth and migration induced by Merlin depletion (Supplementary Figures S4F and I). Overall, these data strongly suggest that Merlin inhibits Wnt/β-catenin signaling and thereby controls the phenotype of glioblastoma cells.
Wnt3a-mediated phosphorylation of LRP6 is inhibited by Merlin
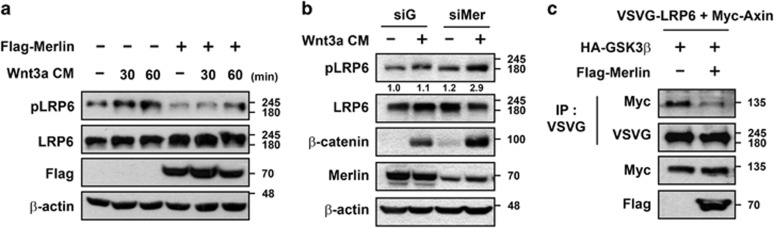
Given the results that Merlin interacted with LRP6 and inhibited Wnt/β-catenin signaling upstream of β-catenin and downstream of both Wnt3a and Dvl (Figures 1 and 3), we speculated that Merlin might inhibit LRP6 phosphorylation, which is necessary for activation of downstream signaling. As shown in Figure 4a, enhanced phosphorylation of LRP6 induced by treatment with Wnt3a-CM was clearly blocked by ectopic expression of Merlin. Interestingly, limited overexpression of exogenous Merlin compared with endogenous levels was sufficient to significantly inhibit phosphorylation of LRP6 induced by Wnt3a-CM (Supplementary Figure S5A). Conversely, knockdown of Merlin by siRNA further upregulated LRP6 phosphorylation as well as the active form of β-catenin in HEK293T cells treated with Wnt3a-CM for 60 min (Figure 4b). Phosphorylation of LRP6 is required for interaction with the Axin complex at the plasma membrane, which is necessary for activation of downstream Wnt/β-catenin signaling.36 Thus, we hypothesized that Merlin inhibits Wnt/β-catenin signaling by blocking the interaction between LRP6 and Axin. Consistent with the hypothesis, the interaction between LRP6 and Axin was clearly inhibited by ectopic expression of Merlin (Figure 4c). Taken together, these results suggest that Merlin inhibits Wnt/β-catenin signaling by impeding association of LRP6 with Axin, resulting in inhibition of Wnt/β-catenin signaling.
Figure 4.
Wnt3a-mediated phosphorylation of LRP6 is inhibited by Merlin. (a) HEK293T cells were transfected with Flag-Merlin and treated with Wnt3a-CM for the indicated time before being harvested and subjected to SDS-PAGE, followed by western blotting with the indicated antibodies. (b) siGFP- or siMerlin-transfected HEK293T cells were treated with or without Wnt3a-CM for 60 min. Each sample was lysed, subjected to SDS-PAGE, and immunoblotted with the indicated antibodies. The relative level of phopho-LRP6 using the Image J program was shown below the phosphor-LRP6 blot. (c) Plasmids encoding VSVG-LRP6, Myc-Axin, and HA-GSK3β were transfected with or without Flag-Merlin into HEK293T cells. After lysis, each sample was immunoprecipitated with anti-VSVG antibody and immunoblotted with the indicated antibodies. All western blotting and immunoprecipitation experiments were performed more than three times, and these data are representative
Wnt3a activates PAK and PAK phosphorylates Merlin in a PIP2-dependent manner
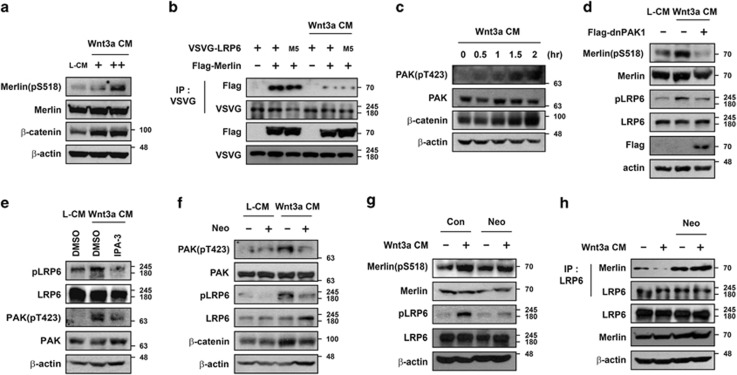
As the interaction between Merlin and LRP6 was shown to be inhibited by Wnt3a-CM treatment and the phospho-mimetic form of Merlin (Merlin-SD) did not bind to LRP6 (Figure 1), we reasoned that the phosphorylation status of Ser518 in Merlin might be enhanced by treatment with Wnt3a-CM. Phosphorylation of Ser518 in Merlin was enhanced in a dose- and time-dependent manner in HEK293T cells incubated with Wnt3a-CM (Figure 5a and Supplementary Figure S5B). As phosphorylation of LRP6 is also enhanced by Wnt3a,37 we tested whether or not the phosphorylation status of LRP6 influences the interaction with Merlin. Mutated LRP6, in which all five serines in the PPPSP motif are changed to alanine (VSVG-LRP6-M5),12 interacted with Merlin in the absence of Wnt3a. This interaction was inhibited by treatment with Wnt3a-CM, which suggests that Wnt3a-mediated disruption of the interaction between LRP6 and Merlin could be attributed to phosphorylation of Merlin and not LRP6 phosphorylation (Figure 5b).
Figure 5.
Phosphorylation of Merlin upon Wnt3a treatment is mediated by PAK in a PIP2-dependent manner. (a) Treatment with Wnt3a-CM induced phosphorylation of Merlin. HEK293T cells were treated with L-CM or Wnt3a-CM (+, 2-fold diluted CM; ++, original CM) overnight and harvested, followed by western blotting with indicated antibodies. (b) Reduced interaction between LRP6 and Merlin upon Wnt3a-CM treatment is irrelevant to the phosphorylation status of LRP6. HEK293T cells transfected with VSVG-LRP6, VSVG-LRP6-M5, or Flag-Merlin were treated with Wnt3a-CM overnight and subjected to immunoprecipitation with anti-VSVG antibody. (c) Phosphorylation of PAK increased upon Wnt3a-CM treatment. HEK293T cells were treated with Wnt3a-CM at the indicated time and harvested, followed by western blotting with indicated antibodies. (d) Ectopic expression of the dominant-negative form of PAK1 (dnPAK1) inhibited Wnt3a-mediated phosphorylation of LRP6 and Merlin. After empty vector or dnPAK1 was transfected, cells were treated with L-CM or Wnt3a-CM for 2 h before being harvested, followed by western blotting with indicated antibodies. (e) Inhibition of PAK activity blocked Wnt3a-mediated phosphorylation of LRP6. Cells were pretreated with DMSO or IPA-3 (30 μM) and incubated with L-CM or Wnt3a-CM for an additional 2 h before being harvested. (f and g) Blocking of PIP2 formation inhibited Wnt3a-mediated phosphorylation of PAK (f), Merlin (g), and LRP6. HEK293T cells were treated with 10 mM neomycin for 30 min before incubation with Wnt3a-CM and neomycin for an additional 2 h. (h) Blocking of PIP2 formation inhibited Wnt3a-mediated dissociation of Merlin from LRP6. HEK293T cells were treated with 10 mM neomycin for 30 min before incubation with Wnt3a-CM and neomycin for an additional 2 h, subjected to immunoprecipitation with anti-LRP6 antibody, and immunoblotted with the indicated antibodies. All western blotting and immunoprecipitation experiments were performed more than three times, and these data are representative
PAK (p21 activated kinase) is known to phosphorylate Ser518 in Merlin, resulting in formation of an open, inactive form.38 In addition, two previous studies have indicated that Wnt activates Rac1-JNK or PAK for the phosphorylation of β-catenin and its accumulation in the nucleus.39, 40 We confirmed that treatment with Wnt3a-CM caused phosphorylation/activation of PAK, whereas ectopic expression of PAK1 enhanced phosphorylation of Merlin at Ser518 (Figure 5c and Supplementary Figure S5C). Thus, we hypothesized that Wnt induces phosphorylation of Merlin via activation of PAK1. To test this, we checked whether or not Wnt3a-mediated phosphorylation of Merlin could be inhibited by overexpression of the dominant-negative form of PAK1 (dnPAK1). Ectopic expression of dnPAK1 completely blocked phosphorylation of Merlin even in the presence of Wnt3a-CM (Figure 5d). Moreover, when PAK1 was inhibited by either ectopic expression of dnPAK1 or treatment with IPA-3, a chemical inhibitor of PAK1, Wnt3a-CM did not induce LRP6 phosphorylation (Figures 5d and e). However, when Merlin was knocked down, IPA-3 treatment did not block Wnt-mediated phosphorylation of LRP6 (Supplementary Figure S5D). These data suggest that Wnt3a-induced phosphorylation of Merlin at Ser518 by PAK1 may lead to detachment of Merlin from LRP6, which in turn allows LRP6 phosphorylation.
Expression of PIP2 is induced by Wnt3a and has a critical role in phosphorylation of LRP6.9, 37 Recently, it was reported that PIP2 along with rac1 or cdc42 is necessary for activation of PAK1 by blocking the autoinhibitory domain of PAK1 both in vitro and in vivo.41 Thus, Wnt3a-mediated PIP2 production might not only be necessary for phosphorylation and aggregation of LRP6 but also for activation of PAK to phosphorylate Merlin. To determine the role of PIP2 in Wnt-mediated Merlin phosphorylation, HEK293T cells were treated with neomycin, a PIP2 scavenger,42, 43 with or without Wnt3a-CM. Interestingly, treatment with neomycin reduced Wnt3a-mediated elevation of phospho-PAK, phospho-LRP6, and β-catenin levels (Figure 5f). In addition, neomycin treatment inhibited Wnt3a-CM-mediated phosphorylation of Merlin at Ser518 (Figure 5g, compare lanes 2 and 4). Removal of PIP2 by neomycin treatment did not cause dissociation of Merlin from LRP6 even in the presence of Wnt3a-CM (Figure 5h). Overall, these data suggest that Wnt3a activates PAK1, which phosphorylates Merlin in a PIP2-dependent manner. Further, this event led to the dissociation of Merlin from LRP6 and phosphorylation of LRP6 for the activation of downstream Wnt/β-catenin signaling (Figure 7).
Merlin inhibits Wnt/β-catenin signaling in Xenopus embryos
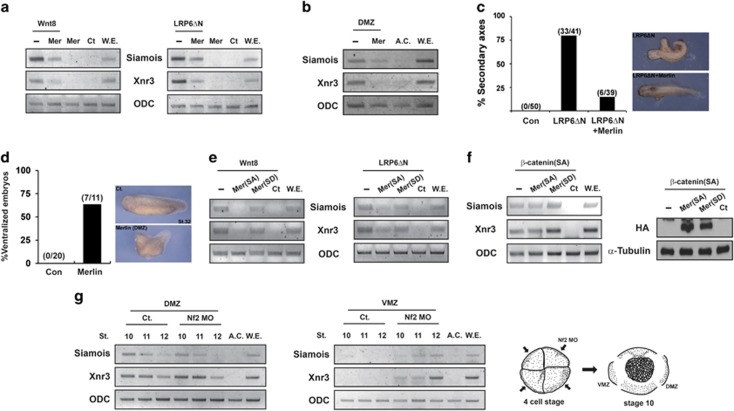
To examine the inhibitory role of Merlin in Wnt signaling in a more physiological context, we employed Xenopus embryos, which are a well-known model system to evaluate the role of candidate proteins in Wnt signaling.44 As shown in Figure 6a, co-injection of Merlin with xWnt8 or LRP6ΔN in animal cap explants significantly blocked xWnt8 or LRP6ΔN-mediated induced expression of Wnt target genes such as siamois and Xnr3. Moreover, injection of Merlin mRNA alone into the dorsal marginal zone (DMZ), where Wnt signaling activity is high, significantly reduced expression of Wnt target genes (Figure 6b). Formation of a secondary axis, which was induced by injection of LRP6ΔN into the ventral side of embryos, was significantly blocked by co-injection of Merlin mRNA (Figure 6c). In addition, injection of Merlin mRNA into the dorsal side of embryos caused ventralization of embryos (Figure 6d). Consistent with the data shown in Figure 3b, co-injection of Merlin-SA but not Merlin-SD blocked enhanced expression of siamois and Xnr3 induced by Wnt8 or LRP6ΔN (Figure 6e). However, co-injection of Merlin-SA or Merlin-SD into animal cap explants did not inhibit enhanced expression of siamois and Xnr3 induced by ectopic expression of β-catenin S37A (Figure 6f). Overall, these data obtained by ectopic injection into Xenopus embryos strongly support the idea that the unphosphorylated active form of Merlin inhibits Wnt/β-catenin signaling at the level of LRP6.
Figure 6.
Merlin inhibits Wnt/β-catenin signaling in Xenopus embryos. (a) Animal caps isolated from embryos were injected with 2 pg of Wnt8 (or 10 pg of LRP6ΔN) at one cell stage with or without 500 pg of each Merlin mRNA. Expression levels of Wnt target genes (Siamois, Xnr3) and others were examined by RT-PCR at stage 10.5. ODC (Ornithine decarboxylase 1), a loading control; Ct (Control), animal cap samples obtained from non-injected embryos; WE, whole embryo as a positive control. (b) Merlin mRNA was injected at one cell stage, and total RNA was extracted from the dorsal marginal zone (DMZ) at stage 10.5 to synthesize cDNA for RT-PCR analysis. (c) Formation of secondary embryonic axis by injection of 1 pg of LRP6ΔN mRNA into the ventral side of two blastomeres from four-cell stage embryos was severely reduced by co-injection of 200 pg of Merlin mRNA. (d) Injection of Merlin mRNA into the dorsal side of embryos blocked formation of the embryonic axis (ventralization). (e and f) Animal caps isolated from embryos were injected with 2 pg of Wnt8, 10 pg of LRP6ΔN, or 100 pg of β-catenin S37A at one cell stage with or without 500 pg of each Merlin (SA/SD) mRNA. Expression of Wnt target genes and ODC was examined by RT-PCR at stage 10.5 (left panel). Ectopic expression of Flag-Merlin was examined by western blotting (right panel). (g) Nf2 morpholino (Nf2 MO) was injected into the dorsal or ventral side of two blastomeres from four-cell stage embryos (right panel), and total RNAs were extracted from the DMZ (left panel) or VMZ (middle panel) at indicated stages for RT-PCR. Ct (Control), DMZ, or VMZ samples were obtained from non-injected embryos; AC, animal cap as a negative control for Wnt target gene expression; WE, whole embryo as a positive control. All experiments were performed more than three times, and these data are representative
To elucidate the endogenous role of Merlin, we used a morpholino antisense oligonucleotide targeting xMerlin (Nf2 MO). Expression of xMerlin peaked between stages 8 and 10 in both dorsal and ventral marginal zones (Supplementary Figure S6). Interestingly, injection of Nf2 MO into the ventral marginal zone (VMZ) but not dorsal marginal zone enhanced expression of Wnt target genes (Figure 6g). These results suggest that Merlin represses Wnt/β-catenin signaling in the ventral marginal zone (see Discussion).
Discussion
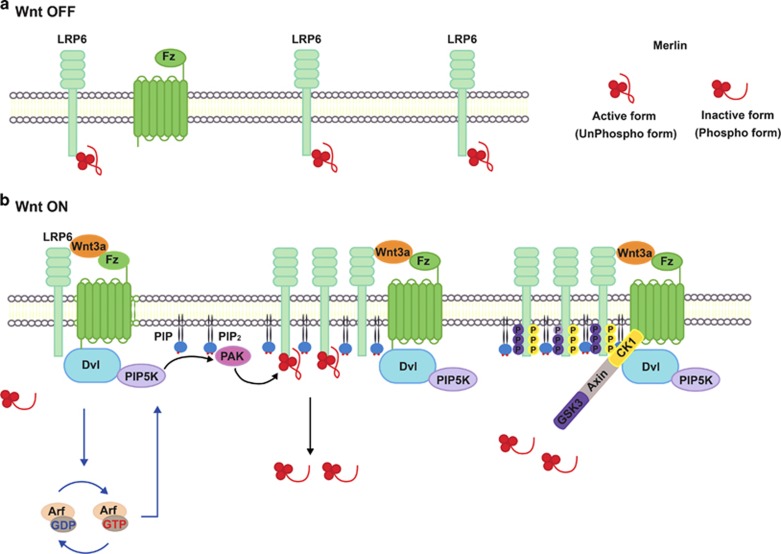
The findings presented here demonstrate the unexpected role of Merlin, a key regulator of the Hippo signaling pathway, in the regulation of Wnt/β-catenin signaling as well as the biochemical significance of elevated PIP2 during the early steps of this signaling pathway (Figure 7 for a model). In the absence of Wnt or below a certain threshold of Wnt, the unphosphorylated form of Merlin (see Discussion below) binds to LRP6 and blocks initiation of Wnt/β-catenin signaling, which may allow cells to selectively respond to moderate levels of Wnt (Figure 7a). However, in the presence of Wnt, the level of PIP2 is increased, which may serve as a docking site for PAK1. Activated PAK1 upon Wnt binding to Fz phosphorylates Ser518 in Merlin, resulting in dissociation of Merlin from LRP6. Phosphorylated LRP6 serves as a docking site for Axin, leading to formation of the signalosome and in turn initiating downstream signal transduction (Figure 7b).
Figure 7.
Schematic model. (a) Wnt-off conditions. In the absence of Wnt or below a certain threshold of Wnt, the unphosphorylated form of Merlin binds to LRP6 and blocks initiation of Wnt/β-catenin signaling. (b) Wnt-on conditions. Binding of Wnt to Fz and LRP6 increases expression of PIP2 in a Dvl- and Arf1/6-dependent manner, which may serve as a docking site for PAK1. Activated PAK1 phosphorylates Ser518 in Merlin, resulting in dissociation of Merlin from LRP6. Phosphorylated LRP6 serves as a docking site for Axin, leading to formation of the signalosome and in turn initiating downstream signal transduction
Increased PIP2 is necessary for Wnt-induced aggregation and phosphorylation of LRP5/6, although the detailed biochemical mechanism remains obscure.9 More recently, it was shown that Amer1/WTX, previously known as a negative regulator of Wnt/β-catenin signaling, has a positive role in LRP5/6 phosphorylation by recruiting the Axin/GSK3β complex to the plasma membrane in a PIP2-dependent manner.45 Here, we present evidence that elevation of the PIP2 level is required for activation of PAK1, which subsequently phosphorylates Merlin to allow phosphorylation of LRP6 (Figures 5 and 7). Considering that Merlin interacts with multiple proteins in various signaling pathways, the mechanism for phosphorylation of Merlin specifically bound to LRP6 may be essential for the selective activation of Wnt/β-catenin signaling.
It has long been considered that the unphosphorylated closed form of Merlin is active.22 However, a recent biochemical study suggested that phosphorylation converts Merlin into a less active and more closed form.24 Our model shown in Figure 7 can be modified according to whether a closed or open form of Merlin interacts with LRP6 in the absence of Wnt.
Enhanced expression of Wnt target genes upon injection of Nf2 MO into the ventral but not dorsal marginal zone is consistent with our model (Figure 6g). Merlin may be dissociated already from LRP6 on the dorsal side, which has a higher level of Wnt signaling. Therefore, knockdown of Nf2 might not have any effect on expression of Wnt target genes. However, it is important to note that induction of target gene expression was delayed and peaked at stage 12 upon injection of Nf2 MO into the ventral marginal zone as compared with stage 10 in the dorsal marginal zone (Figure 6g). This may explain why injection of Nf2 MO into the ventral side did not induce ectopic embryonic axis (data not shown).
It is interesting to note that except for Merlin, germline or somatic mutations in core components of the Hippo pathway are very rare in human cancers.19 This may be due to the fact that Merlin inhibits cell proliferation by blocking the EGF, VEGF, IGF, mTOR, and Wnt/β-catenin pathways as well as by activating the Hippo pathway.14, 46 Although the number of cases of NF2 disease was low in our study, the level of β-catenin significantly increased while elevation of YAP was not significant in NF2 patients (Supplementary Figure S3). This result suggests that schwannomas in these patients are caused not by loss of Hippo signaling but by activation of Wnt/β-catenin and possibly other signaling pathways regulated by Merlin.
Taken together, we suggest that Merlin is a bona fide regulator of Wnt/β-catenin signaling. Our data from NF2 patients (Supplementary Figure S3) suggest that release of Merlin from LRP5/6 may enhance sensitivity to Wnt and increase downstream signaling. Small molecules capable of blocking the interaction between Merlin and LRP5/6 could be developed into specific drugs to treat diseases caused by low-level Wnt/β-catenin signaling such as osteoporosis or neuro-degeneration. Conversely, inhibition of PIP2 upregulation or usage of PAK1 inhibitors such as FRAX59747, 48 may be a valuable therapeutic strategy to cure diseases such as cancer caused by increased Wnt/β-catenin signaling.
Materials and Methods
Cell culture and transient transfection
HEK293T, MDCK, HeLa, and RT4-D6P2T cells were cultured with Dulbecco's modified Eagle's Medium (DMEM, Lonza, Basel, Switzerland) supplemented with 10% FBS and 1% antibiotics. For transient transfection, cells were transfected with each plasmid by the calcium phosphate precipitation method as described previously.49 For RT4-D6P2T rat schwannoma cells, plasmids were transfected with 10 mM polyethyleimine (PEI, Sigma, St. Louis, MO, USA).
Western blotting and immunoprecipitation
For western blotting, cells were suspended with lysis buffer (20 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EGTA, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF, and 1 μg/ml Leupeptin) for 30 min on ice and centrifuged at 12 500 r.p.m. for 30 min. Supernatants were collected for protein assay.
For immunoprecipitation, 800–1000 μg of lysates was incubated with proper antibodies, anti-LRP6 (Cell Signaling, Danvers, MA, USA), VSVG (Sigma), and IgG (Stratagene, Santa Clara, CA, USA), overnight at 4 °C and incubated with protein agarose G (Millipore, Darmstadt, Germany) for an additional 2 h. After washing with lysis buffer five times, pellets were boiled with SDS sample buffer and subjected to PAGE and western blotting.
Antibodies
To detect appropriate proteins, we used antibodies specific for anti-Merlin, YAP, PAK, EGFP (Santa Cruz, Santa Cruz, CA, USA), phosphor-YAP, LRP6, Phospho-LRP6 (Cell Signaling), Phospho-Merlin (Ser518), Phospho-PAK (Ser423) (Rockland, Limerich, PA, USA), β-actin, VSVG (Sigma), Flag (Stratagene), Myc (Abm, Richmond, BC, Canada), β-catenin (for histochemistry, ZyMed (South San Francisco, CA, USA); for western blot, BD Bioscience, Franklin Lakes, NJ, USA), E-cadherin (BD Bioscience), and active-β-catenin (Millipore).
Immunohistochemistry
Immunohistochemical stains for β-catenin, YAP, and phosphor-YAP from paraffin-embedded specimens of four cases of NF2 patients, three cases of NF1 patients, and normal nerve tissue, which was used as a control sample, were taken (Table 1). Serial sections (4 μm thick) of formalin-fixed, paraffin-embedded samples were cut and mounted on glass slides. Sections were dewaxed by passage through xylene and then rehydrated in graded alcohol (100, 95, and 75%). Endogenous peroxidase activity was blocked by incubating sections in 3% H2O2 for 10 min. Slides were then rehydrated with 0.01 M citrate buffer (pH 6) and microwaved three times, without boiling, for antigen retrieval. After rinsing with 0.01 M phosphate-buffered saline (pH 7.4) non-specific antibody binding was reduced by incubating sections with pre-diluted blocking serum (normal horse serum) for 10 min. After decanting excess serum, sections were incubated overnight at 4 °C with anti-β-catenin antibody (1 : 200, ZyMed), YAP (1 : 100, Santa Cruz), and phosphor-YAP (1 : 200, Cell Signaling). After washing thoroughly with phosphate-buffered saline, sections were incubated with biotinylated universal secondary antibody (iView DAB Detection kit, Ventana Medical Systems, Inc., Tucson, AZ, USA) for 10 min. Slides were developed with diaminobenzidine tetrahydrochloride for 10 min and counter-stained with hematoxylin.
Cell proliferation assay
RT4-D6P2T rat schwannoma cells (1.46 × 104) were seeded in triplicate on 24-well plates containing DMSO or Wnt signaling inhibitor, ICG-001 (10 μM) or IWP-2 (5 μM). Every other day, cells were trypsinized and counted.
Transwell migration assay
Migration assays were performed according to the manufacturer's instructions. Cells (1 × 105) in 0.3 ml of serum-free medium were seeded onto the upper chamber of cell culture inserts (SPL, 8 μm membrane Pore Size), and 0.7 ml of complete growth medium containing 10% FBS was added to the lower chamber. Following incubation for 24 h, non-migrated cells were removed from the upper chamber, and the migrated cells in the lower chamber were fixed with methanol, stained with 0.5% crystal violet and then photographed under a light microscope.
siRNA-mediated knockdown
siRNA specific for human Merlin (sense: 5′-GGACAAGAAGGUACUGGAUCAUGAU-3′ antisense: 5′-AUCAUGAUCCAGUACCUUCUUGUCC-3′), siRNA specific for human LRP6 (sense: 5′-ACAUUGUUCUGCAGUUAGA-3′ antisense: 5′-UCUAACUGCAGAACAAUGU-3′), siRNA specific for human β-catenin (sense: 5′-CCAAGAAGCAGAGAUGGCCCAGAAU-3′ antisense: 5′-AUUCUGGGCCAUCUCUGCUUCUUGG-3′), and GFP (sense: 5′-GUUCAGCGUGUCCGGCGAG-3′ antisense: 5′-CUCGCCGGACACGCUGAAC-3′) were transfected into HEK293T cells for 48 h and harvested for experiments. Knockdown efficiencies were checked by western blotting.
Dual luciferase assay
HEK293T cells were seeded in triplicate on 12-well plates and transfected with pSuperTop (0.5 μg), pRL-TK (0.05 μg), and the indicated plasmids: 100 ng each of VSVG-LRP6ΔN, Flag-mDvl, β-cat-HA, and β-cat(S37A)-HA along with 0.5 μg each of Flag-Merlin, Flag-Merlin-SA, Flag-Merlin-SD, Flag-Merlin-L64P, and Flag-Merlin-K79E. After 24 h of transfection, luciferase activities were measured by a dual luciferase assay kit (Promega, Madison, WI, USA).
Embryo injection and explant culture
Xenopus laevis embryos were obtained by artificial fertilization.50 Developmental stages were designated according to Nieuwkoop and Faber. Vitelline membranes were removed by immersing embryos in the animal pole with mRNA or DNA as described in the figure legends. Animal caps were dissected from the injected embryos at stages 8–9 and cultured stage at 10.5 in 67% Leibovitzs L-15 medium (GIBCO/BRL, Waltham, MA, USA) with BSA (1 mg/ml), 7 mM Tris-HCl (pH 7.5), and gentamicin (50 μg/ml). Cultured explants were incubated in RAN at 4 °C before harvesting.
RNA isolation and real-time PCR
Total RNA was isolated using TRIzoL reagent (Sigma) according to the manufacturer's instructions. cDNA was synthesized from total RNA using Improm-II Reverse Transcriptase (Promega) with random primer. For quantitative real-time PCR, the experiment was performed as follows. cDNA from each experimental group was analyzed by an ABI prism 7000 Sequence detector (Applied Biosystem, Waltham, MA, USA) with SYBR green PCR master mix (Applied Biosystem). All PCR products showed a unique dissociation curve. Amplification was performed under the following conditions: 95 °C (10 min), followed by 40 cycles at 95 °C (30 s) and 60 °C (1 min). The threshold cycle (Ct) value for each gene was normalized to the Ct value for β-actin. Relative mRNA expression was calculated using the ΔΔCt method.
Western blot analysis for Xenopus embryos
Animal cap explants were homogenized in PhosphoSafe Extraction Buffer (Novagen, Madison, WI, USA), and supernatants were suspended in an equal volume of 1,1,2-trichloro-1,2,2-trifluoroethane (Sigma-Aldrich, St. Louis, MO, USA) to remove lipids in cell lyates. Lysates were separated by 10% SDS-PAGE (Hoefer, Holliston, MA, USA). HA-tagged proteins were visualized after western blotting using rabbit polyclonal anti-HA (1 : 1000; Cell Signaling). Loading control protein was visualized after western blotting using α-tubulin antibody (1 : 1000; Cell Signaling). Proteins were visualized using ECL Western blotting detection reagents (Amersham, Arlington Heights, IL, USA).
Acknowledgments
This research was supported by NRF grants funded by the MSIP (Ministry of Science, ICT and Future Planning; NRF-2011-0019353) and the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1420060) to E-HJ. JK was supported by NRF- 2013R1A1A2008541.
Glossary
- APC
adenomatous polyposis coli
- PIP2
phosphatidylinositol 4,5 bisphosphate
- NF2
Neurofibromatosis type II
- ERM
Ezrin, Radixin, and Moesin
- WT
wild-type
- SA
S518A
- SD
S518D
- Wnt3a-CM
Wnt3a-conditioned media
- PAK
p21 activated kinase
- dnPAK1
dominant-negative form of PAK1
- DMZ
dorsal marginal zone
- VMZ
ventral marginal zone
- PFA
paraformaldehyde
- ODC
Ornithine decarboxylase 1
- Ct
Control
- W.E
whole embryo
- Nf2 MO
Nf2 morpholino
- A.C.
animal cap
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by JP Medema
Supplementary Material
References
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell 2012; 149: 1192–1205. [DOI] [PubMed] [Google Scholar]
- Kim W, Kim M, Jho EH. Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J 2013; 450: 9–21. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009; 17: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res 2009; 19: 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Li C. Convergence between Wnt-beta-catenin and EGFR signaling in cancer. Mol Cancer 2010; 9: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collu GM, Hidalgo-Sastre A, Brennan K. Wnt-Notch signalling crosstalk in development and disease. Cell Mol Life Sci 2014; 71: 3553–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 2013; 19: 179–192. [DOI] [PubMed] [Google Scholar]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013; 13: 11–26. [DOI] [PubMed] [Google Scholar]
- Pan W, Choi SC, Wang H, Qin Y, Volpicelli-Daley L, Swan L et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science 2008; 321: 1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Kim SY, Kim T, Kim M, Bae DJ, Choi HI et al. ADP-ribosylation factors 1 and 6 regulate Wnt/beta-catenin signaling via control of LRP6 phosphorylation. Oncogene 2012; 32: 3390–3396. [DOI] [PubMed] [Google Scholar]
- Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell 2012; 149: 1245–1256. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z et al. A mechanism for Wnt coreceptor activation. Mol Cell 2004; 13: 149–156. [DOI] [PubMed] [Google Scholar]
- Hadfield KD, Smith MJ, Urquhart JE, Wallace AJ, Bowers NL, King AT et al. Rates of loss of heterozygosity and mitotic recombination in NF2 schwannomas, sporadic vestibular schwannomas and schwannomatosis schwannomas. Oncogene 2010; 29: 6216–6221. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol 2000; 16: 113–143. [DOI] [PubMed] [Google Scholar]
- Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell 2014; 26: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell 2010; 140: 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol 2006; 16: 702–709. [DOI] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell 2010; 19: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer 2013; 13: 246–257. [DOI] [PubMed] [Google Scholar]
- Bosco EE, Nakai Y, Hennigan RF, Ratner N, Zheng Y. NF2-deficient cells depend on the Rac1-canonical Wnt signaling pathway to promote the loss of contact inhibition of proliferation. Oncogene 2010; 29: 2540–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ercolano E, Ammoun S, Schmid MC, Barczyk MA, Hanemann CO. Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/beta-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia 2011; 13: 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey AI, Fehon RG. Merlin and the ERM proteins—regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol 2009; 19: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surace EI, Haipek CA, Gutmann DH. Effect of merlin phosphorylation on neurofibromatosis 2 (NF2) gene function. Oncogene 2004; 23: 580–587. [DOI] [PubMed] [Google Scholar]
- Sher I, Hanemann CO, Karplus PA, Bretscher A. The tumor suppressor merlin controls growth in its open state, and phosphorylation converts it to a less-active more-closed state. Dev Cell 2012; 22: 703–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahronowitz I, Xin W, Kiely R, Sims K, MacCollin M, Nunes FP. Mutational spectrum of the NF2 gene: a meta-analysis of 12 years of research and diagnostic laboratory findings. Hum Mutat 2007; 28: 1–12. [DOI] [PubMed] [Google Scholar]
- Gutmann DH, Hirbe AC, Haipek CA. Functional analysis of neurofibromatosis 2 (NF2) missense mutations. Hum Mol Genet 2001; 10: 1519–1529. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev 2003; 17: 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, You L, Giancotti FG. Shedding light on Merlin's wizardry. Trends Cell Biol 2007; 17: 222–229. [DOI] [PubMed] [Google Scholar]
- Abramowicz A, Gos M. Neurofibromin in neurofibromatosis type 1 - mutations in NF1gene as a cause of disease. Dev Period Med 2014; 18: 297–306. [PubMed] [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev 2013; 27: 355–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene 2004; 23: 8447–8454. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 2009; 5: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci USA 2004; 101: 12682–12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Hwang SJ, Kim HR, Shin CH, Choi KH, Joung JG et al. Neurofibromatosis 2 (NF2) controls the invasiveness of glioblastoma through YAP-dependent expression of CYR61/CCN1 and miR-296-3p. Biochim Biophys Acta 2016; 1859: 599–611. [DOI] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009; 461: 614–620. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 2005; 438: 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regimbald-Dumas Y, He X. Wnt signalling: What The X@# is WTX? EMBO J 2011; 30: 1415–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell 2001; 1: 63–72. [DOI] [PubMed] [Google Scholar]
- Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 2008; 133: 340–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Wang Y, Huang B, Liang J, Ding Y, Xu et al. A Rac1/PAK1 cascade controls beta-catenin activation in colon cancer cells. Oncogene 2012; 31: 1001–1012. [DOI] [PubMed] [Google Scholar]
- Strochlic TI, Viaud J, Rennefahrt UE, Anastassiadis T, Peterson JR. Phosphoinositides are essential coactivators for p21-activated kinase 1. Mol Cell 2010; 40: 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabev E, Kasianowicz J, Abbott T, McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2). Biochim Biophys Acta 1989; 979: 105–112. [DOI] [PubMed] [Google Scholar]
- Pilot F, Philippe JM, Lemmers C, Lecuit T. Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein Btsz. Nature 2006; 442: 580–584. [DOI] [PubMed] [Google Scholar]
- Heasman J. Patterning the early Xenopus embryo. Development 2006; 133: 1205–1217. [DOI] [PubMed] [Google Scholar]
- Tanneberger K, Pfister AS, Brauburger K, Schneikert J, Hadjihannas MV, Kriz V et al. Amer1/WTX couples Wnt-induced formation of PtdIns(4,5)P2 to LRP6 phosphorylation. EMBO J 2011; 30: 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand D, Manent J, Couvelard A, Watilliaux A, Siena M, Chareyre F et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene 2009; 28: 854–865. [DOI] [PubMed] [Google Scholar]
- Yeo D, He H, Patel O, Lowy AM, Baldwin GS, Nikfarjam M. FRAX597, a PAK1 inhibitor, synergistically reduces pancreatic cancer growth when combined with gemcitabine. BMC Cancer 2016; 16: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp RL, James MF, DeSouza PA, Wagh V, Zhao WN, Jordan JT et al. A high-throughput kinome screen reveals serum/glucocorticoid-regulated kinase 1 as a therapeutic target for NF2-deficient meningiomas. Oncotarget 2015; 6: 16981–16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 1987; 7: 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Slack JM. Dorsalization and neural induction: properties of the organizer in Xenopus laevis. J Embryol Exp Morphol 1983; 78: 299–317. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.