Abstract
Results from our recent studies have led to the novel hypothesis that radiation-induced coagulopathy (RIC) and associated hemorrhage occurring as part of the acute radiation syndrome (ARS) is a major cause of death resulting from radiation exposure in large mammals, including humans. This article contains information related to RIC, as well as potential strategies for the prevention and treatment of RIC. In addition, new findings are reported here on the occurrence of RIC biomarkers in humans exposed to radiation. To determine whether irradiated humans have RIC biomarkers, blood samples were obtained from radiotherapy patients who received treatment for different types of malignancies. Blood samples from allogeneic hematopoietic cell transplantation (allo-HCT) patients obtained before, during and after irradiation indicated that exposure led to prolonged clot formation times, increased levels of thrombin-antithrombin III (TAT) complex and increased circulating nucleosome/ histone (cNH) levels, which suggest potential coagulopathies in the allo-HCT patients. Since these allo-HCT patients received chemotherapy prior to radiotherapy, it is possible that the chemical agents could have influenced the observed results. Frozen plasma samples from radiotherapy patients with prostate, lung and breast cancer were also obtained for analyses of cNH levels. The results indicated that some of these patients had very high cNH blood levels. Analysis of cNH levels in plasma samples from irradiated ferrets also indicated increased cNH levels compared to preirradiation baseline levels. The results from irradiated animals and some radiotherapy patients suggest the possibility that anti-histone antibodies, which block the toxic effects of elevated cNH levels in the blood, might be useful as therapeutic agents for adverse biological radiation-induced effects. The detection of increased levels of cNH in some radiotherapy patient blood samples demonstrates its potential as a biomarker for diagnosing and/or predicting the propensity for developing coagulopathies/hemorrhage, offering possible treatment options with personalized medicine therapies for cancer patients.
INTRODUCTION/BACKGROUND INFORMATION
Review of Information Related to Radiation-Induced Coagulopathy
Overview of previous studies on RIC
The results from our previous studies have indicated that radiation-induced coagulopathy (RIC) can result in bleeding/hemorrhage/ microvascular thrombosis, organ damage and death from multi-organ failure (MOF) [from disseminated intravascular coagulation (DIC)], which can occur at doses near the LD50, with RIC-associated hemorrhage occurring well before the expected decline in peripheral platelet counts (1–6). It has also been observed in irradiated dogs (7) as well as the individuals exposed to radiation from the atomic bomb (8) that bleeding often occurs in the absence of a drop in platelet counts. It is well established that radiation exposure can lead to extensive bleeding/hemorrhage which can then lead to death in mammals. The mechanism by which hemorrhaging occurs in irradiated subjects has not been a controversial topic, since it has been widely thought to arise from radiation-induced cell-killing effects in platelets and platelet precursors, which can lead to low numbers of circulating platelets and resultant hemorrhage(s). It is now clear that other biological effects resulting from radiation exposure can lead to hemorrhaging as well. Findings from our work indicate that radiation activates the coagulation cascade and induces changes in hemostasis resulting in hemorrhage and RIC. We recently published the results of numerous experiments indicating that radiation exposure can result in bleeding and ultimate death in ferrets and minipigs at doses near the LD50; the evidence suggests that the early changes related to RIC can progress to death resulting from a coagulopathy [(1–5); reviewed in (6)]. It is expected that changes similar to those observed in ferrets and pigs also occur in other large mammals, including humans, irradiated at or near their respective LD50 levels (3, 5, 6).
This article uses two different terms to identify the changes leading to radiation-induced death (RID) from DIC; these are RIC and radiation-induced DIC (RDIC). The differences between RIC and RDIC are related to the stage of progression in the changes that lead to death from DIC. RIC is a very broad term that includes the earliest changes brought about by radiation exposure, such as the increases in blood clotting times, which may or may not be accompanied by clinically observed changes in patient symptoms. RDIC refers to a later stage of RIC development, which presumably results from the progression of blood clotting changes in patients to disease states in which the symptoms of DIC can be observed (e.g., hemorrhage).
Characteristics of DIC in Humans
Disseminated intravascular coagulation is a serious and life-threatening condition in which bleeding and clotting often occur at the same time (9, 10), and a hallmark of DIC is hemorrhage. DIC is a dreaded phrase in the clinic, as the acronym is often referred to as “death is coming”. DIC normally occurs secondary to a wide variety of underlying diseases/ processes involving systemic inflammation, which include severe infections (e.g., viral, bacterial and protozoal), neoplasia, shock, heat stroke, hemolysis, systemic inflammatory response syndrome (SIRS), trauma (severe tissue injury), sepsis, obstetrical complications, vascular disorders, reactions to toxins (e.g., drugs), immunological disorders, etc. and it is a common cause of human death (9–12). In humans and animals, DIC is often caused by microorganisms and bacterial infection. Triggers of the activation of diffuse coagulation are cell-specific membrane components of the microorganism, e.g., lipopolysaccharide (LPS)/ endotoxin, which cause a generalized inflammatory response (9). The major goal in management of DIC in the clinic is treatment of the underlying disorder (9).
Treatment of full-blown DIC can be successful, depending on the cause of DIC, however, more often than not the treatment is not successful, presumably because diagnosis occurs only after the symptoms of overt DIC have become evident, at which time it can be too late to save the patient’s life. There is now much focus in clinical medicine on early detection and diagnosis of DIC through the scoring systems that have been developed [e.g., see ref. (11)], as it is believed that treatments will be considerably more effective at early development stages rather than at later times that represent advanced stages of DIC development.
DIC involves a massive inflammatory response with the release of proteases, cytokines and hormones from multiple inflammatory and vascular cell types, which results in extensive damage to the microvascular endothelium (11). The damage to the microvascular endothelium is associated with the following phenomena: 1. Vasodilation, involving the loss of tight junctions between endothelial cells, which leads to capillary leakage; 2. Escape from regulatory control, which leads to the activation of coagulation pathways and excessive thrombin generation, with microthrombus formation, which ultimately leads to ischemia and multiple organ dysfunction; and 3. Consumption and exhaustion of platelets and coagulation factors, which leads to bleeding and hemorrhage into tissues (11). No single factor can lead to a diagnosis of human DIC; multiple factors must be considered, as described in detail elsewhere (11). Several end points are considered in the diagnosis of human DIC through the use of a scoring system [developed by the International Society on Thrombosis and Haemostasis (ISTH)]. The human scoring system utilizes the following parameters to generate an “overt” DIC score: 1. prolonged clotting time [specifically, prothrombin time (PT)]; 2. greatly reduced platelet counts; 3. elevated fibrin-related marker (e.g., soluble fibrin/fibrin degradation products, such as D-dimer); and 4. reduced fibrinogen level. If a score is ≥5, the condition of the patient is compatible with overt DIC. If a score is <5, it is suggestive (but not affirmative) for nonovert DIC (11). A low fibrinogen level is observed in only about one-third of human cases of overt DIC (13). When low fibrinogen levels are observed, this is secondary to the initial increase in the fibrinogen level from the inflammatory state that accompanies DIC. Thus, a drop in fibrinogen produced as part of overt DIC may bring the fibrinogen blood level to within the normal range, making it difficult to use as part of the diagnosis of DIC in animals as well as humans.
The global coagulation parameters/tests described above are of little value in the assessment of nonovert DIC in the clinic (11). More sensitive coagulation tests for a diagnosis of nonovert DIC, such as the levels of a thrombin generation product known as thrombin/antithrombin III (TAT) complex, are recommended as part of the ISTH scoring system (11). Thus, TAT complex is considered to be a more sensitive marker of DIC compared to the global coagulation parameters (described above and used to diagnose overt DIC in the clinic) in patients with nonovert DIC.
In patients with DIC, there are numerous changes in the levels, or activity, of the compounds that play an important role in the development of DIC. As examples, DIC patients have reduced levels, or reduced activity, of the major physiological means of anticoagulation, which are anti-thrombin III, protein C and tissue factor pathway inhibitor (TFP1). Plasma levels of antithrombin III, the most important inhibitor of thrombin, are markedly reduced as a result of the ongoing coagulation, degradation by elastase (released from activated neutrophils) and impaired synthesis of antithrombin III (9). Reductions in the levels of antithrombin are correlated with death from DIC in humans (14). There is a reduction in the activity of the protein-C system caused by a combination of impaired protein synthesis, a cytokine-mediated decrease in the activity of endothelial thrombomodulin and a decline in the level of the free fraction of protein S, which is also synthesized by endothelial cells (15, 16). Protein S is the essential cofactor of protein C. Tissue factor is inhibited by TFP1, but TFP1 does not regulate tissue factor activity sufficiently well in DIC patients. Thus, all three physiologic means of anticoagulation are impaired in patients with DIC.
Characteristics of DIC/RDIC in animal model systems and comparison to human DIC
Almost all of the coagulation tests used to diagnose DIC in humans have shown major changes related to the development of RDIC in irradiated ferrets and minipigs. We have produced scoring schemes for RDIC similar to the human DIC scoring scheme in large animals [e.g., minipigs (5)]. In our studies on the results of diagnostic tests for DIC in irradiated ferrets and pigs with apparent DIC, however, fibrinogen levels were not reduced. This finding is similar to what is observed in some human DIC patients, as not all human patients with DIC exhibit reduced fibrinogen levels (13).
Ferrets have been extensively utilized in radiation biology research as part of radiation-induced emesis studies [reviewed in ref. (17)]. The ferret model is also well established for virology/influenza studies (18), toxicology/ safety assessment studies (19) and as a hemostasis model for the study of von Willebrand factor (VWF) and factor XIII, due to the similarities of their coagulation system to that of humans (20, 21). There are numerous animal model systems that have been used to study hemostasis, including model systems involving transgenic mice. For example, studies with knockout mice for plasminogen activator (PA) inhibitor 1 (PAI-1) have been described by Abderrahmani et al. (22).
In our studies, DIC was diagnosed in irradiated ferrets from the following evidence: elevated soluble fibrin concentrations in the blood (4), longer bleeding times (2), decreased clot size, inability to generate a stable clot, lengthened initiation time of clotting, platelet clumping, ecchymosis, petechiae, hemorrhaging and, occurring at or shortly before death/euthanasia, reduced numbers of platelets (and other blood cell types) in the peripheral blood and extensive hemorrhaging (3). Activation of clotting was confirmed after death by the presence of fibrin clots in the major organs evaluated at necropsy as well as the presence of platelet and fibrin clumps in the blood, observed by blood smear analysis. Similar evidence from irradiated pigs suggesting the development of RDIC included: impairment of whole blood clotting, as evidenced by thromboelastography (TEG) alterations, elevated D-dimer concentrations in the blood, decreased platelet and white blood cell counts and/or hemorrhaging and the presence of fibrin in tissues observed during postmortem examination (3, 5).
The data from these studies indicated that radiation exposure produced stepwise changes in hemostasis. One of the first responses to radiation involves the activation of the clotting cascade, which results in cleavage of fibrinogen and the formation of fibrin clots. Activation of the coagulation cascade also results in increased prothrombin time (PT) and activated partial thromboplastin time (APTT) due to the consumption of associated factors (which included deficiencies of the following factors: II, V, VII, VIII, IX, X, XI and XII) (2). These are very early changes, occurring as early as 3 h postirradiation, with the detection of soluble fibrin in the blood as well as fibrin clots in the blood vessels of irradiated tissues (4). Other early markers of coagulopathy/DIC evaluated included the calculation of international normalized ratio (INR) values: about 70% of ferrets that received a 1 Gy dose had INR values >1.5 (2). In ferrets that received a 2 Gy dose (lethal dose), the abnormal hemostasis parameters initially observed became progressively more severe and the changes in clot formation characteristics were dramatic by 13 days postirradiation, when death occurred or euthanasia was necessary due to morbidity (3). At a lower (sublethal) radiation dose (e.g., 1 Gy), the abnormal hemostasis characteristics and coagulation biomarker values observed up to 21 days postirradiation began to return to preirradiation or baseline values by day 30. Specifically, in these ferrets, clot formation times (CFTs), clot size, platelet clumping values, etc., returned to baseline measurements. Our studies in both ferrets and pigs have shown that the early stages of DIC development and progression occur, but can regress, in these two different animal model systems in which we have developed scoring systems similar to those used in the hospital setting to diagnose DIC in humans.
The studies in ferrets indicated that the early radiation-induced changes leading to RDIC can be reversed, with some ferrets correcting the early changes observed as abnormal hemostasis parameters (and then ultimately surviving the exposure to a potentially lethal dose of radiation), while others in the same cohort died (3). Similar results have been obtained in our studies on RDIC in pigs (5).
Our studies have indicated that radiation-induced changes in hemostatic parameters become progressively more severe with time in ferrets destined to die. Furthermore, there is a major and sudden change that occurs in the last day before death or euthanasia of experimental ferrets, which is characterized by an almost complete disappearance in the circulating blood of neutrophils and platelets (among other sudden, marked changes, such as the change from a normal, healthy appearance to a moribund appearance, at which time euthanasia would occur). A similar sudden major change, resulting in almost the complete disappearance of white blood cells (including neutrophils) and platelets in the blood, has been observed at approximately 24 h before death in irradiated dogs (23). This sudden change in the irradiated animals occurs well into the time frame when overt DIC can be diagnosed (through the use of the scoring systems that have been developed).
The time frames for RID in animal model systems versus humans at doses of radiation near LD50
The time frame for RID at doses near LD50 level varies for different species (24). The time frame for rodent death is measured over a 30-day period (LD50/30), with a peak incidence of death at 10–15 days postirradiation, and deaths are complete by 30 days postirradiation. The time frame over which the LD50 is measured in humans is 60 days (LD50/60), with the peak incidence of death at about day 30, with deaths occurring up to day 60 (24). The time frame for the maximal reduction of neutrophils in the blood is also different depending on the species irradiated. In ferrets that have survived a potentially lethal dose of radiation (~1.5 Gy), the maximal reduction in peripheral neutrophil levels is observed at day 10 postirradiation. Ferrets that do not survive a potentially lethal radiation dose perish by approximately day 13 postirradiation (3). The time frame for the death of irradiated minipigs (at approximately LD50) is at 14–16 days postirradiation, with the nadir in peripheral neutrophil counts being shortly before this time (5). In humans exposed to accidental large doses, the greatest reduction in peripheral neutrophils occurs at approximately 25–35 days postirradiation (24), and in those exposed to the Hiroshima A-bomb (who had 0–10% mortality from the ARS, groups III and IV), the white blood cell counts (with blood normally containing ~50–80% neutrophils) reached a nadir at day 25 postirradiation (25). It is noteworthy that in irradiated mammals, death occurs at >1 Gy doses, when cell killing effects become significant. The time period over which the majority of deaths occurred in atomic bomb casualties receiving doses near the human LD50 (in the range of 2–4.5 Gy; group II) was 20–40 days, with deaths in group II greatly diminishing after that time (25). The time frames described above suggesting that the height of neutrophil death (i.e., the nadir in peripheral blood counts) occurs just prior to death in irradiated populations has led to our hypothesis that neutrophil death is mechanistically linked to death from exposure to radiation. The fact that the timing of death in the different species listed above is so precise from a given dose of radiation to an irradiated population suggests that a biochemical change is likely to be involved in precipitating death, rather than a phenomenon that can occur at random times in a population, such as death from a bacterial infection. Examples of hypotheses related to certain biochemical changes that could result in RID from RDIC are given below.
Examples of biochemical changes in irradiated subjects that could lead to RID; death of neutrophils resulting in neutrophil extracellular traps (NETs) and/or nucleosome release or increased fibrin levels in irradiated subjects
One hypothesis is that the change in appearance of irradiated animals (described above) occurs at the time of the greatest radiation-induced killing of neutrophils, which is correlated with the height of formation of NETs and/or nucleosome release, and it is the accumulation of NETs and/or nucleosomes to a critical level that actually precipitates death.
The radiation-induced cell killing effects in neutrophils can result in the production of NETs, which can lead to DIC (26–28). NETs are fibrous structures, containing fibers made of chromatin (histones and DNA) and granule-derived antimicrobial proteins (e.g., neutrophil elastase and myeloperoxidase), which are released from activated neutrophils or dying neutrophils as part of an active cell death program called NETosis (29–33). The main components of NETs are the core histones (H2A, H2B, H3, H4), which together account for approximately 70% of the protein mass (34). NETs play a major role in the immune defense system, with strong antimicrobial activity (33), but they can have adverse effects in mammalian systems. NETs provide a scaffold for platelet binding and aggregation and a stimulus for thrombus formation (35, 36). Thus, NETs have a major role in blood clotting (35, 36), with the ability to promote coagulation and thrombus growth in vivo (37). Activated and dying neutrophils also release nucleosomes (DNA-histone complexes), which serve as a platform for intravascular fibrin generation; NETs produce many of the same effects observed for nucleosomes (37).
Another hypothesis concerning the mechanism for the cause of RID is related to the accumulation of fibrin in tissues and organs during the development of RDIC. Fibrin-related markers in the blood are particularly important for a diagnosis of overt DIC in both humans and animals. The observation of fibrin deposition in organs is essential for a diagnosis of DIC from histopathologic examination of organs obtained at autopsy/necropsy in both animals and humans. Fibrin deposition in organs, which leads to thrombosis, ischemia and organ dysfunction, is thought to play a major role in MOF; fibrin deposition is thought to be a major contributor to death from DIC [e.g. see ref. (9)]. Fibrin deposition, retention and clearance in organs are primarily controlled by the levels of proteolytic enzymes and their inhibitors, the major ones being plasminogen activators (PAs) [e.g., tissue plasminogen activator (tPA), urokinase, etc.] and PAI-1. In animal models of DIC, and in human patients with DIC, the fibrinolytic system is almost completely suppressed at the time of maximal activation of coagulation, primarily due to the increase in the plasma level of PAI-1 [e.g. see refs. (38, 39)]. These findings have been confirmed in human DIC patients; the suppression of fibrinolysis in humans is mediated by PAI-1, and although some fibrinolytic activity occurs in response to the formation of fibrin in patients with DIC, it is not close to being sufficient to counteract the systemic deposition of fibrin occurring in these patients (40–42). Thus, in DIC patients, the high levels of PAI-1 inhibit PA activity, thereby reducing the rate of plasmin formation, which results in a reduced ability to degrade fibrin and clear organs of fibrin deposits. Fibrinolysis is greatly reduced or absent in patients with overt DIC. The combination of increased fibrin formation and an inadequate ability to remove fibrin deposits results in intravascular thrombosis, which contributes to the subsequent changes leading to MOF and death from DIC. Thus, another hypothesis concerning the mechanism of death from RDIC is that it is caused by the accumulation of fibrin deposits until they reach a critical level in irradiated subjects, and it is this biochemical change that precipitates death.
The role of platelets in RID from RDIC
There is no question that platelets play a very important role in the terminal stages of overt DIC and RDIC. It is unclear, however, whether platelets play an important role in the early bleeding changes that are thought to lead to RDIC.
The current paradigm in radiation biology is that the nadir of platelet counts in humans destined to die from radiation exposure is responsible for bleeding and ultimately death in some of those exposed to LD50 doses of radiation. In fact, in irradiated humans, the nadir of platelet counts occurs at approximately the same time as the nadir in neutrophil counts (24). In our work on RDIC, we are focusing on changes occurring in coagulation parameters before the platelet numbers drop to levels that can no longer control bleeding problems. In the ferret studies, the platelet counts remained well within the normal range, while the initial coagulopathy, and sequential blood clotting and bleeding changes, occurred and became progressively more severe with time in the ferrets that were destined to die [e.g. see ref. (3)]. The platelet counts did become significantly reduced at times of overt or terminal DIC in ferrets, but that was well after diagnosis of the coagulopathy could be made. The generally held view is that platelet loss is due to radiation-induced cytotoxicity. Our data suggest that a mild change in platelet numbers, which would not affect the ability to control bleeding, may occur early after irradiation, before the time when overt or terminal coagulopathy results in large reductions in platelet counts; the large reductions in platelet counts are expected to reduce the ability of platelets to control bleeding (3). A similar phenomenon occurs in irradiated dogs, as bleeding abnormalities are observed when preterminal platelet counts are at normal levels; however, in irradiated dogs that developed terminal coagulopathy, platelet counts are reduced (7). Similar findings have also been reported in humans exposed to radiation at doses near the LD50. Widespread hemorrhages were observed in humans exposed to the atomic bomb at times when their platelet counts were not at the reduced levels expected for hemorrhaging to occur (8). These results suggest that radiation-induced hemorrhage may not be due solely to the cell killing effects of radiation leading to low numbers of circulating platelets. In addition, while the loss of platelets clearly plays an important role in death from terminal DIC, it is not as clear that platelets play a crucial role in the bleeding episodes that occur relatively soon after irradiation, which lead to petechiae, ecchymosis and other bleeding abnormalities.
Is radiation exposure a form of trauma leading to trauma-induced coagulopathy (TIC)
Trauma-induced coagulopathy is a disorder involving coagulation dysregulation, which arises secondary to injury; the causation and progression of TIC are driven by the interplay of platelet dysfunction, endothelial activation, endogenous anticoagulation, oxidative damage and hyperfibrinolysis (43). RIC shares many similarities with TIC, which is a relatively new area of research that is considered a subset of DIC research (43, 44). One of the prominent characteristics of TIC is that platelet levels are within the normal range while bleeding is occurring; it is known that a large fraction of the platelets in TIC patients are dysfunctional [reviewed in ref. (43)]. Another finding from the TIC research field that could be applicable to the radiation research field is that factor V has major beneficial effects in TIC patients. One common treatment for trauma patients has involved platelet transfusions, which do have beneficial effects in this population. The guidelines for the use of platelet transfusions in patients have been described (45). It has been discovered that the primary active component in platelet transfusions is factor V (44) for TIC patients, not the platelets. Factor V, once activated to factor Va, is a cofactor for coagulation that is synthesized in the liver, circulates in plasma and is found within platelets, which release factor V at times of injury. The use of clotting factors in the treatment of coagulopathy is discussed elsewhere (12).
While radiation exposure has not yet been named as a causative agent for TIC, its ability to cause oxidative damage is well established. It is known that sudden and substantial increases in circulating reactive oxygen species (ROS) can have a major effect on hemostatic potential in vivo, and many coagulation proteins (e.g., PAI-1, protein C and thrombomodulin) are susceptible to oxidative regulation (43). While TIC and DIC have some common characteristics, there are differences between these conditions (46), as summarized here. Early TIC is a result of heightened activity during the initiation phase of plasma coagulation, with many sites of endothelial disruption; it is marked by early onset, prolonged PT and APPT and a relative sparing of platelets and fibrinogen (46). One major difference between TIC and DIC is that there are large reductions in platelet counts in DIC (11) that are not observed in TIC. Although there are many similarities between TIC and DIC, the initiators, underlying mechanisms and management of these conditions/diseases are different (46). There are some characteristics of TIC that are not part of radiation exposure, the most prominent of which is that hemorrhaging often occurs as part of the initial injury in TIC patients due to physical trauma. In humans, observable radiation-induced bleeding (e.g., petechiae, purpura) at doses near the LD50 level (2–4.5 Gy) does not usually begin until 2–3 weeks postirradiation (8, 24, 25). While some trauma patients die within the first few hours after injury, others live for significantly longer periods of time, however, TIC patients can deteriorate in days to weeks after injury (e.g., death at day 28) (47). The timing of death from late mortality in TIC patients corresponds approximately to that of the atomic bomb casualties dying from doses near the LD50 level [i.e., group 2, patients suffering severe symptoms or dying in the third, fourth, fifth and sixth weeks, with death (if it occurred) often occurring at approximately one month postirradiation] (25). Many of the individuals exposed to higher doses of radiation from the atomic bomb died at considerably earlier times postirradiation (group 1, patients dying in the first and second weeks) (8, 48).
Recently, pigs have become the major animal model for trauma research (49). It is known that pigs and humans have similar coagulation systems (50), and both exhibit coagulopathic bleeding and dysfunctional platelets after injury. In patients with TIC, there is prolonged coagulopathic bleeding (despite normal platelet counts); coagulopathic bleeding is characterized by uncontrolled systemic diffuse hemorrhage and not simply bleeding from injury sites (43). In fact, there is dramatic and persistent platelet dysfunction in TIC patients, which is associated with both early and late mortality (51). It is clear that platelet dysfunction is commonly observed in pigs, beginning as early as 15 min after injury (52).
Biomarkers for TIC that could be useful for the diagnosis of RDIC
At sufficient doses, radiation is known to have major cell killing effects in many different types of cells. Such cytotoxic effects may be viewed as trauma resulting in severe tissue injury and tissue necrosis. It is highly likely that circulating cell debris from dying cells (i.e., those killed by radiation) contributes to RDIC. Circulating intracellular contents such as histones, which are known to be released from injured cells/tissue, are associated with TIC (53). High levels of circulating nucleosomes/histones (cNHs) in the blood rise dramatically in human trauma and the levels correlate with the development of coagulopathy. In these studies, there was a higher level of circulating histones in observed patients with higher anatomical injury severity, and the increased histone concentrations were present in trauma patients with abnormal coagulation tests, increased markers of fibrinolysis and elevated activated protein C (aPC) levels (54). It has been reported that there is a significant increase in extracellular DNA levels in the plasma of trauma patients, and that the patterns of elevated histones and extracellular DNA derived from activated neutrophils followed the reduction in leukocyte counts and were associated with MOF and death (55). Extracellular histones are known to be cytotoxic for endothelial cells in vitro and are lethal in mice; anti-histone antibodies have served as potential countermeasures for the toxicity of high levels of cNHs in the blood in mouse studies (56). Circulating histones are also highly toxic in humans and are thought to contribute to numerous pathophysiological processes and progression of human diseases, with particularly strong toxicity for the liver and kidney, as recently reported by Chen et al. (57). Circulating histones are thought to play an important role in MOF (58). In intensive care patients with sepsis-induced DIC, cNH levels were increased in non-survivors compared to survivors, indicating that high cNH levels were also associated with mortality in these patients (14). Circulating levels of microparticles also increase in patients with sepsis and DIC (59, 60). The role of microparticles in sepsis induced DIC has recently been reviewed (61).
The role of endothelial cells in the development of RDIC
The evidence leading to the conclusion that changes in endothelial cells drive the development of DIC has been recently reviewed (62). The major functions of endothelial cells (62, 63), as well as the effects of radiation on endothelial cells (64–67), have also been reviewed. As part of vascular hemostasis, endothelial cells regulate many different processes, which include fluidity (anticoagulants/ procoagulants) and inflammation (pre-inflammatory/anti-inflammatory); changes in fluidity and inflammation are both important in the development of DIC. Endothelial cells are known to be the most radiosensitive among the fixed elements of the mesenchyme (64). Depending on the dose, exposure to radiation can produce lethal or sublethal injury; radiation becomes lethal to endothelial cells, specifically, human umbilical vein endothelial cells (HUVECs), bovine aortic endothelial cells (BAECs) or capillary endothelial cells [e.g., human dermal microvascular endothelial cells (HDMECs)], evaluated in in vitro systems, when it reaches D0 values of 1–2 Gy in clonogenic survival curves (64); the D0 value is the dose required to reduce the fraction of surviving cells to 37% of its previous value. Endothelial cells may undergo mitotic death or apoptosis, which occurs through a pathway that probably involves the formation of ceramide (68).
It is well established that radiation induces endothelial cell dysfunction (69–72). Notably, endothelial dysfunction is also known as endothelial activation, a term that is more frequently used now to refer to other characteristics, in addition to the well-established changes in vascular tone, that describe an altered state of endothelial cells. These characteristics include loss of vascular integrity, expression of leucocyte adhesion molecules, change in phenotype from antithrombotic to prothrombotic and cytokine production and upregulation of human leukocyte antigen (HLA) molecules, all of which interact to cause local inflammation (73). Endothelial cell activation includes loss of the surface anticoagulant molecules (thrombomodulin and heparin sulphate), reduced fibrinolytic potential due to enhanced PAI-1 release, loss of platelet anti-aggregatory effects of ecto-ADPases and prostacyclin and production of platelet-activating factor, nitric oxide and expression of tissue factor (73, 74). Endothelial dysfunction has been defined as an imbalance between vasodilating and vasoconstricting substances produced by (or acting on) the endothelium. A decrease in the levels of nitric oxide (NO) is considered to be the hallmark of endothelial dysfunction, and occurs even at relatively low radiation doses (e.g., 2 Gy) (70). In their study evaluating the mechanisms of endothelial dysfunction in rabbits exposed to gamma radiation, Soloviev et al. (70) reported that endothelium-dependent relaxation of rabbit aorta evoked by acetylcholine or A23187 was impaired in a dose-dependent manner by ≥2 Gy irradiation. The intimal surface is normally both anticoagulant and antithrombotic. Vessel damage (or exposure to certain cytokines or pro-inflammatory stimuli) shifts the balance towards a procoagulant/prothrombotic phenotype of endothelial cells (75). Notably, endothelial dysfunction/activation is one of the early signs of systemic inflammation and is considered a trigger for the initiation of organ failure in sepsis-induced DIC (76).
As endothelial cells regulate both coagulation and inflammation, it is not surprising that their activities are intimately involved in the development of, and death from, DIC. Endothelial cell products are known to regulate the amount of fibrin deposition, as well as retention, in organs [reviewed in refs. (62, 63)]. Fibrin is deposited in organs as a result of activation of the coagulation cascade. Thrombin is known as the main “effector” protease of the coagulation cascade. Thrombin converts prothrombin to active thrombin, which converts fibrinogen to fibrin; fibrin then becomes the fibrous matrix of a blood clot. A main trigger of the coagulation cascade is tissue factor. When exposed to an activating stimulus such as thrombin, endothelial cells synthesize and express tissue factor at their surface, which leads to widespread intravascular deposition of fibrin and ultimately to thrombosis of small and midsize vessels. Fibrin deposition in organs is removed through the process of fibrinolysis, and again, endothelial cell products are the major substances involved in the removal of fibrin deposits. While many compounds are involved in the regulation of fibrinolysis, regulators that are of particularly importance include the endothelial cell products, PAs (e.g., urokinase), with the primary PA being tPA, and PA inhibitors such as PAI-1 [as reviewed in refs. (9, 62, 63)]. tPA initiates and accelerates the conversion of plasminogen to the active enzyme plasmin, which then hydrolyzes cross-linked fibrin, resulting in the release of a variety of complexes made up of fibrin degradation products, and PAI-1 specifically regulates the initial phase of fibrinolysis by inhibiting PAs. In addition to the production of PAs and PAI-1, endothelial cells produce numerous other substances regulating coagulation parameters. Endothelial cells regulate the adhesion and aggregation of platelets through VWF, nitric oxide and other products. Different products are inducible with specific stimuli and are produced at high levels in different tissues (resulting in tissue-specific pathologic responses). Some other endothelial cell products affecting coagulation include: procoagulants (e.g., tissue factor and factor V), anticoagulants (e.g., thrombomodulin, TFPI), and ADAMTS metalloproteases (e.g., ADAMTS13). In addition to the direct production of these substances involved in coagulation parameters, endothelial cells also produce substances that activate compounds made by other cell types in the body that affect coagulation. One example is the endothelial cell product known as endothelial protein C receptor; this substance activates protein C, which is produced in the liver. Blocking the protein C receptor promotes thrombosis (77). It has been shown that the activity of the protein-C system is particularly important in radiation-induced adverse effects, including death (78).
von Willebrand factor is one of the most important endothelial cell products playing a role in coagulation. VWF is an acute-phase protein that is elevated in plasma during systemic inflammation. When released from endothelial cells, the native multimeric glycoprotein is mostly present in an ultra-large form, which is known as ultra-large von Willebrand factor (ULVWF). The multimers undergo proteolysis by a specific plasma metalloprotease known as ADAMTS-13, which is a disintegrin and metalloprotease also known as VWF-cleaving protease. In healthy individuals, there is almost no circulating ULVWF. In pro-inflammatory conditions, ADAMTS13 activity decreases due to various mechanisms (including downregulation on a transcriptional level, proteolytic degradation and consumption due to the high substance level), resulting in substantial amounts of plasma ULVWF. Increased levels of circulating ULVWF are negatively correlated with platelet count and positively correlated with the degree of inflammation and organ failure (79). Previous studies have demonstrated that radiation induces VWF secretion from HUVECs irradiated in vitro (80) and that VWF mRNA levels are increased when either human or bovine endothelial cells are irradiated with electrons (81). It has been observed that 2 Gy irradiation in pigs leads to increased levels of circulating ULVWF in pig blood that lasted as long as 30 days, which represented the end of the pig observation period in this experiment.2
Does radiation exposure lead to RDIC in humans?
There are no known studies in which it has been concluded that human populations exposed to significant doses of radiation develop DIC, but markers for the presence of DIC (including the hallmark of DIC, hemorrhage) have been observed and reported in irradiated humans, as discussed elsewhere (1, 3, 5, 6). There is extensive evidence that widespread hemorrhages occurred in the Hiroshima and Nagasaki A-bomb casualties, even in those exposed to the lowest doses (25). For those exposed to doses near the LD50 level, there was moderate prolongation of whole blood coagulation time, concomitant with the onset of hemorrhagic diatheses, as has been reviewed elsewhere (25). It was suspected that patients exposed to radiation from the atomic bomb had DIC, but this was not confirmed (25). The LD50 of the atomic bomb casualties has been estimated to be approximately 2.5 Gy (82, 83). The percentage of those dying from exposure to atomic bomb radiation with evidence of hemorrhage can be estimated from the atomic bomb casualty data, which has been reviewed elsewhere (8). The casualty dose estimates are given in a report by the U.S. Atomic Energy Commission (84). From the data on the casualties, it is estimated that 60–100% of the atomic bomb casualties receiving radiation doses near the LD50 had evidence of hemorrhaging at death (6). There is now extensive evidence that bleeding/hemorrhage is a major problem in many different irradiated populations, as recently reviewed (85).
Notably, bleeding at doses near the LD50 is a characteristic of humans and large animals, e.g., dogs and pigs, as recently exhibited photographically by Moroni et al. (86), but this is not a characteristic of small animals, such as mice, as has been reviewed elsewhere(1). The fact that the bleeding characteristics and LD50 doses are so much lower in humans compared to mice has suggested that different processes operate in the causation of death at doses near the LD50 in humans and mice. There is evidence that the occurrence of bleeding at death (and potentially, the development of RDIC) is directly related to the LD50 of various species, as has been reviewed elsewhere (5).
Treatments for DIC and potential agents for the prevention or treatment of RDIC
There are numerous treatments that have been used for DIC, as reviewed elsewhere (9). The most effective treatment involves the removal of the underlying disorder (9), which would not be possible for radiation accidents or mass casualty events involving exposure to radiation. The standard clinical treatment of DIC can include the use of whole blood transfusions (or platelets, clotting factors, plasma, etc.) and/ or anticoagulants (e.g., heparin). DIC is known to be a consumptive coagulopathy in humans, and we have shown that RDIC is also a consumptive coagulopathy in ferrets and pigs in which many clotting factors and platelets are consumed (2, 3, 5, 6). Currently, the consumption of coagulation constituents in humans can be corrected by treatment with fresh frozen plasma, platelets or cryoprecipitated antihemophilic factor (AHF), also known as Cryo or cryoprecipitate (which is a portion of plasma rich in clotting factors that includes factor VIII and fibrinogen). The shelf life of each of these human-derived products is short. Plasma or Cryo can be kept frozen, with a shelf life of 1 year. Platelets are kept at room temperature, with constant agitation to prevent clumping, and they are only useful for a period of five days. The shelf life of platelets is extremely short, and there are frequent platelet shortages in hospitals under normal operating conditions. Hospitals have very limited supplies of these products readily available for emergencies. Thus, if a large population of military personnel and/or civilians were exposed to significant radiation doses, such as during a mass casualty event, even U.S. hospitals would not be sufficiently equipped to provide treatment for a consumptive coagulopathy such as RDIC. However, the use of coagulation factors may be helpful when treating a smaller number of irradiated persons at potentially lethal doses.
It is possible that clotting factors will be particularly useful for the earliest stages of RDIC and play a preventive role in the development of a later stage of RDIC. Our studies have indicated that early radiation effects that lead to prolonged clotting times can be prevented by treatment with a clotting factor (Benefix®, clotting factor IX) (4), although studies have not been performed to determine whether this effect on a TEG parameter can prevent death from RDIC. Another potential countermeasure for the blood clotting alterations produced by exposure was phytonadione, however, studies with phytonadione indicated that this potential countermeasure for the radiation-induced lengthened clotting times was not effective (4).
The role of antioxidants in prevention and treatment of DIC
Oxidative stress is thought to play an important role in the development of DIC. As just one example, there is extensive evidence that radiation reduces NO levels in endothelial cells, as described above. It has been concluded that gamma radiation selectively impairs the nitric oxide pathway as a consequence of oxidative stress (70). We have reported extensively on the ability of antioxidants to prevent or mitigate radiation-induced oxidative stress and its consequences, and these studies have been recently reviewed elsewhere (6, 87). Numerous antioxidants frequently used in radiation biology research have been shown to prevent or suppress the adverse effects of radiation in endothelial cells; these agents include WR-2721, WR-1065, superoxide dismutase (SOD), N-acetylcysteine (NAC), selenium, lazaroids (synthetic compounds such as vitamin E), glutathione and other agents interacting with ROS (88–90). It is tempting to speculate that the beneficial effects of antioxidants that lead to reduced mortality from radiation exposure are a result of effects on endothelial cells that reverse or suppress the changes in these cells produced by radiation, ultimately resulting in the development and progression of RDIC.
Potential agents for the treatment of later stages of RDIC
Current treatments for late-stage DIC have been reported elsewhere (9). Anti-histone antibodies have been used as countermeasures for DIC in animal studies (56), and they could represent one potential countermeasure against the progression of late-stage RDIC. Anti-histone antibodies are expected to block the toxic effects of products released by dead/dying cells (such as histones and nucleosomes) that can lead to MOF and death (56, 58). Agents that affect the later stages of RIC development would be particularly helpful as mitigating agents for RDIC, as discussed below.
Evidence of, and treatments for, RIC in radiotherapy patients
There is extensive information indicating that many of the late effects of radiation exposure result from radiation-induced vascular injury (64–67, 91–94) and that coagulopathy is a well-documented manifestation of radiation-induced vascular injury, particularly in the liver and lung (90). Radiation hepatitis is a form of venocclusive disease (VOD) in which the lumen of central veins becomes blocked by fibrillar material. It is expected that radiation injury to sinusoidal endothelial cells and central vein endothelium initiates activation of the coagulation cascade, leading to accumulation of fibrin and fibrin clots in the central veins and hepatic sinusoids (95). The fibrin then serves as a scaffold for the deposition of reticulin and collagen, which eventually occludes the vessel. Treatment of VOD includes: 1. tPA, with or without anticoagulation therapy using heparin (96); and 2. defibrotide, which has fibrinolytic and antithrombotic properties (97, 98).
While the liver and lung are particularly sensitive to RIC, other irradiated tissues are also sensitive; clinically relevant radiation doses induce defects in fibrinolysis in many tissues (90). Some effective modifiers of radiation-induced defects in fibrinolysis have been identified. Drugs able to affect the suppression of fibrinolysis include actinomycin D, dextran sulfate (a fibrinolytic stimulator) and angiotensin converting enzyme (ACE) inhibitors, as reviewed by Ward (90). There is reduced PA activity, leading to depressed fibrinolysis, in blood vessels (99, 100), liver (101) and lung (102–104). There is radiographic evidence of the beneficial effects of dextran sulfate in the treatment of human pulmonary radiation injury (105).
Numerous radiation-induced coagulation defects have been identified previously in radiotherapy patients (90). Notably, radiation abolishes some functions related to coagulation at clinically relevant doses (e.g., fibrinolysis), while it enhances other functions playing important roles in the development of DIC (e.g., permeability, soluble coagulation, platelet adhesion and aggregation, etc.) (64–66). The fact that clinical radiation therapy abolishes fibrinolysis suggests that fibrin deposits in radiotherapy patient organs may rise to highly elevated levels like those known to exist in patients with DIC, as described above. Various modifiers of radiation-induced coagulation defects have also been identified, including heparin, dicumarol, diuretics, steroids, dexamethasone, warfarin and antibodies to von Willebrand factor (90). Some of these modifiers have been shown to be effective in radiotherapy patients. For example, heparin and warfarin, used as “full coagulation”, have been successfully utilized for the treatment of nervous system injuries in cancer patients; the use of these drugs resulted in improvement in symptoms and function (106).
Many patients with different types of malignancies receive radiation therapy as all or part of their cancer therapy. Most of the cancer patients receive partial-body radiation therapy. A relatively large number of radiotherapy patients who are total-body irradiated (TBI) are allogeneic hematopoietic cell transplantation (allo-HCT) patients. Allo-HCT is often used either in the initial management or second-line management of leukemias and lymphomas, specifically acute myeloid leukemia, acute lymphoblastic lymphoma, chronic lymphocytic leukemia and chronic myeloid leukemia. TBI is one of two methods used in the preparation of patients for allo-HCT; the other method utilizes chemotherapy-based regimens. TBI is often combined with chemotherapy agents as well, such as cyclophosphamide (Cytoxan®) (Cy), etoposide, fludarabine, etc. (107). The main principle for the use of TBI in these patients is that it results in a cytopenia that is long lasting and lethal unless hematopoietic cells are infused within a certain time frame postirradiation (as is the case in allo-HCT). There have been multiple studies in which the efficacy of chemotherapy-alone regimens such as busulfan (Bu)/Cy have been compared to that resulting from a TBI-containing regimen such as Cy/TBI; in these studies, it has been concluded that both regimens are equivalent in terms of treatment-related morbidity, risk of graft-versus-host disease, leukemia-free survival or overall survival (108, 109).
Overview of the Studies Reported as Part of this Article
Our current research is focused on identifying and establishing RDIC biomarkers in irradiated subjects that could indicate which subjects might benefit from treatment with potential RDIC-mitigating agents, and on the identification of agents that prevent or suppress the development of RDIC in humans and animals. In this review, we present the results of our studies measuring cNH levels, as biomarkers of RDIC, in humans and animals before and after irradiation, with the expectation that these results could give some indication of whether anti-histone antibodies might be helpful as mitigating agents for the acute biological effects of radiation exposure that can lead to RDIC.
cNH levels in blood samples from irradiated animals and humans were measured using the Cell Death Detection ELISA kit (Roche Diagnostics, Indianapolis, IN). The Roche Cell Detection assay kit is now used by many investigators in the area of TIC research for basic research studies in cells and experimental animals, as well as clinical studies designed to determine the histone/nucleosome levels in trauma patients (54). Given its current use by many investigators in immunology as well as TIC research to detect changes in cNH levels, the Roche Cell Death assay system was used here for our analyses.
To ascertain whether cNH blood levels would be useful biomarkers for the development of RIC, we determined the cNH blood levels in animals and humans at pre- and postirradiation times. The irradiated humans were cancer patients at the University of Pennsylvania Perelman School of Medicine. Blood samples from TBI/allo-HCT patients were evaluated in the studies reported here to help determine whether changes in the characteristics of several different RIC markers, such as prolonged CFT and elevated levels of TAT complex and cNH levels, could be detected in these patients.
Blood samples from radiotherapy patients with other types of cancer (specifically, prostate, breast or lung cancer) were also evaluated in these studies. The studies in these patients were limited to determination of the cNH levels in patient blood samples before, during and after the radiation therapy period.
As part of the research program in our laboratory, we have performed numerous experiments in ferrets and Yucatan minipigs recently that involved the evaluation of various adverse effects of different types of radiation exposure; the results of these studies (reviewed in reference 6) have led to our concepts on RIC in large animals that are described in this report. The rationales, aims and experimental designs for each group of studies involved in a particular area of research are described in individual published articles. As part of the work in each area of investigation in those published studies, plasma samples from the control and irradiated animals at pre- and postirradiation time points were frozen and then stored as archival samples for use in future investigations. Many of these frozen archival samples from experimental animals have been evaluated as part of the work of this report. Frozen archival samples from radiotherapy patients, from the National Institutes of Health (NIH), were also evaluated as part of the studies in this report. The archival samples from both the experimental animals and radiotherapy patients received from the NIH were frozen, and stored as part of studies performed previously in which the overall experimental designs and aims were different from those in the studies performed here related to the measurements of cNH levels in these populations. The frozen archival samples could not be used to evaluate changes in TEG parameters (e.g., CFT).
MATERIALS AND METHODS
All animal care and treatment procedures, including irradiation conditions and details, were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. For the studies in experimental ferrets described in this report, the details of the experimental procedures used for these studies have been previously reported (3). The ferrets used in our studies were obtained from Marshall Farms (North Rose, NY).
In the ferrets, serial whole blood samples were obtained from the superior vena cava under light anesthesia. Whole blood was collected in the presence of 3.8% sodium citrate (9:1, blood:sodium citrate). Ferret blood samples were collected prior to irradiation and at 4 h, 12 h, 48 h, 7 days and 13 days postirradiation. An aliquot of citrated blood samples was centrifuged at 3,000g for 15 min at 4°C to isolate plasma and if not used immediately, was stored at −80°C until further use.
The radiotherapy patient samples described in this report were collected as part of the University of Pennsylvania Radiation Oncology Registry and Biosample Repository (RBR), a joint program between the University of Pennsylvania Department of Radiation Oncology and the NIH. The RBR collects and banks specimens from patients who are treated in University of Pennsylvania Department of Radiation Oncology at preirradiation, during (“on”) and after radiation therapy. The protocol for the use of the human samples from the RBR for the studies described here was approved by the RBR Protocol Review Committee as well as the University of Pennsylvania Institutional Review Board. All patients participating in the RBR program gave written informed consent to collect/store specimens for analyses to predict clinical outcome.
The University of Pennsylvania radiotherapy patients included those with prostate, breast and lung cancer and who received radiation therapy for their cancer. Analyses on these samples, referred to as legacy samples by the RBR, were limited to the cNH assay. These patient samples (i.e., the prostate, breast and lung cancer patient blood samples) were taken from patients at the times indicated above during their radiation therapy periods. After the patient blood draws, the samples were immediately frozen and shipped to NIH; they were then shipped back to University of Pennsylvania for the frozen sample analyses described in this report.
A separate cohort of samples collected as part of the RBR included fresh blood samples from TBI/allo-HCT patients. The studies of radiotherapy patients were designed to evaluate whether there were other expected biomarkers (in addition to cNH levels) of RIC in these patients, such as prolonged CFT and/or elevated levels of TAT complex. Normally, patients undergoing conditioning (e.g., Cy/TBI) for a myeloablative allo-HCT receive six fractions of 2 Gy (twice a day for 3 days), immediately after 2 days of Cy treatment. Patients undergoing a non-myeloablative conditioning regimen (“mini-transplant”) received a 2 Gy TBI dose several days after chemotherapy. Three radiotherapy TBI/allo-HCT patients participated in these studies, and were diagnosed with either chronic myelogenous leukemia (patient 1) or acute myeloid leukemia (patients 2 and 3). For these patients, there were no treatments with other medications (e.g., tacrolimus and methotrexate to prevent graft vs. host disease) during the radiation therapy period, and the dosing characteristics of the chemical agents administered to these patients were kept constant to facilitate the identification of changes caused by radiation exposure.
It was initially planned that blood samples would be collected at the following times from the allo-HCT patients: preirradiation; at 3 h after the second dose (4 Gy cumulative dose); and at 3 h after the last dose (12 Gy cumulative dose). Only one of these patients (patient 2) received the full 12 Gy TBI dose as part of a myeloablative regimen; the other two patients received a “mini” transplant, in which they received a single 2 Gy dose as part of the conditioning regimen. The blood samples gathered from the allo-HCT patients were 15 ml each. The timing of the blood collections for the allo-HCT patients is shown in Table 1, along with other characteristics of each of the allo-HCT patients.
TABLE 1.
Patient Data
| Patient | Treatment | Preirradiation | During irradiation | Postirradiation | Units |
|---|---|---|---|---|---|
| Patient 1 (CML) | Fludarabine/ cyclophosphamide; TBI, 2 Gy × 1 fraction | Neutrophils 2.27, lymphocytes 2.44, platelets 47 | ~15 h after initial radiation dose (2 Gy cumulative dose); neutrophils 0.67, lymphocytes 0, platelets 35 | 4 days after initial radiation dose (2 Gy cumulative dose); neutrophils 0.28, lymphocytes 0.08, platelets 21 | Neutrophils, lymphocytes and platelet counts: thousand cells/μl |
| Patient 2 (AML) | Cyclophosphamide; TBI, 2 Gy × 6 fractions | Neutrophils 9.65, lymphocytes 0.57, platelets 455 | ~24 h after initial radiation dose (6 Gy cumulative dose); neutrophils 3.45, lymphocytes 0.11, platelets 207 | 13 days after initial radiation dose (12 Gy cumulative dose); neutrophils 0, lymphocytes <0.1, platelets 30 | |
| Patient 3 (AML) | Fludarabine/ cyclophosphamide; TBI, 2 Gy × 1 fraction | Neutrophils 0.85, lymphocytes 0.517, platelets 107 | ~7 h after initial radiation dose (2 Gy cumulative dose); neutrophils 0.36, lymphocytes 0, platelets 42 | ~30 h after initial radiation dose (2 Gy cumulative dose); neutrophils 0.18, lymphocytes 0, platelets 43 | |
| Lung cancer | Surgery; gemcitabine/ cisplatin; local irradiation 18 Gy × 28 fractions | CBC N/A | 36 days after initial radiation dose (45 Gy cumulative dose); CBC N/A | 66 days after initial radiation dose (50.4 Gy cumulative dose); CBC N/A | |
| Breast cancer | Surgery; local irradiation 3.4 Gy × 10 fractions (MammoSite) | CBC N/A | ~2 days after initial radiation dose (30.6 Gy cumulative dose); CBC N/A | 27 days after initial radiation dose (34 Gy cumulative dose); CBC N/A | |
| Prostate cancer patients | |||||
| Patient 1 | Surgery; androgen deprivation; local irradiation 1.8 Gy × 39 fractions | CBC N/A | 37 days after initial radiation dose (61.2 Gy cumulative dose); CBC N/A | 152 days after initial radiation dose (70.2 Gy cumulative dose); CBC N/A | |
| Patient 2 | Local irradiation 1.8 Gy × 44 fractions | CBC N/A | 37 days after initial radiation dose (50.4 Gy cumulative dose); CBC N/A | 158 days after initial radiation dose (79.2 Gy cumulative dose); CBC N/A | |
| Patient 3 | Local irradiation 1.8 Gy × 44 fractions | CBC N/A | 24 days after initial radiation dose (27 Gy cumulative dose); CBC N/A | 151 days after initial radiation dose (79.2 Gy cumulative dose); CBC N/A | |
The levels of cNHs were measured in plasma samples from experimental ferrets (before and after irradiation at various time points) and radiotherapy patients [before, during (on) and after radiotherapy] using the Cell Death Detection ELISA assay (Roche Diagnostics), following the vendor’s instructions. Two monoclonal antibodies bind histones and DNA. Whereas the anti-histone antibody binds to histones H2, H2A, H3 and H4, the other antibody binds to DNA, catching the nucleosomes, specifically, with absorbance measured at wavelength 405 nm. For the TBI/allo-HCT cohort of radiotherapy patients, additional analyses were performed within 1 h of blood collection; whole blood clotting was analyzed using a rotational thromboelastogram system (ROTEM®, Tem Intl., Munich, Germany), as previously described by Krigsfeld et al. (3). Briefly, whole blood was added to a heated cuvette at 37°C. The system inserts a pin connected to an optical detector in the cuvette. Upon initiation, the pin rotates and begins detection of blood clot formation (CFT) as fibrin strands form. Blood clotting was measured over 90 min and CFT data were recorded. Also in the TBI/allo-HCT patient plasma samples, TAT complex concentrations were determined following the vendor’s instructions (Enzygnost® TAT micro; Siemens Healthcare, Forchheim, Germany). This assay is a photometric sandwich enzyme immunoassay for the determination of TAT complex levels in human plasma, with absorbance measured at wavelength 492 nm.
The ferret blood cell count data were obtained as previously described (3). Briefly, blood samples were collected from each animal at designated time points in collection tubes containing anticoagulant, dipotassium ethylenediaminetetraacetic acid. Within 24 h of blood collections, samples were transported to Antech Diagnostic Laboratories where complete blood count with differential analysis was performed using a Bayer ADVIA® 120 model hematology analyzer (Antech® Diagnostics, Lake Success, NY), resulting in absolute counts for neutrophil and lymphocyte cells (as well as other blood cell types not reported here). The preirradiation counts represent the “control” or baseline value, and data were normalized to preirradiation counts and are reported as a fraction of control.
Comparisons of the blood cell count data at designated time points with preirradiation values were performed using the Student’s t test (GraphPad Prism, version 5.0; Graphpad Software Inc., LaJolla, CA). Comparisons of the cNH levels at the diverse time points were made by the Wilcoxon Mann-Whitney test. A P value of <0.05 was considered statistically significant. Results are presented as the mean ± SEM. Lastly, linear regression analysis was performed to determine whether the change in cNH levels and the differences between the pre-and postirradiation blood cell counts at various time points after irradiation were statistically significant.
RESULTS
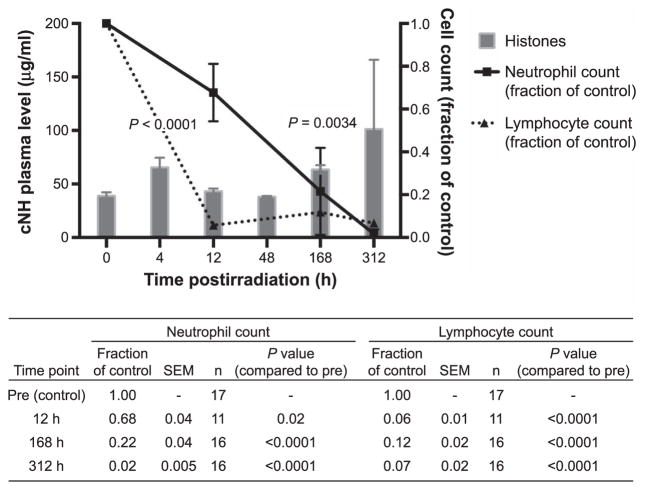
Our results include data from ferret experiments that were initially designed to determine changes in blood cell counts and coagulation parameters resulting from animal irradiations with various doses (3). Data derived from frozen plasma samples from TBI (2 Gy) ferrets are shown in Fig. 1. The effects of 2 Gy irradiation on blood cell counts in ferrets have been previously reported in detail (3, 110) and are included here for a cohort of animals (Fig. 1). The time course suggests that increased levels of DNA-histone complexes (also referred to as cNH levels in this article) at the statistically significant 4 h and 7 day time points correlate with the observed statistically significant reduction in blood lymphocytes and neutrophils. A 2 Gy dose of either gamma rays or protons is known to result in death in 100% of the ferrets at ~day 13 (3). Linear regression analysis was performed to fit straight lines through the data (cNH levels, neutrophil counts and lymphocyte counts over time after irradiation) and slopes were compared (data not shown). The differences between the slopes for the radiation-induced loss of either neutrophils or lymphocytes and the slope of the line indicating the increasing trend in cNH levels after irradiation were statistically significant.
FIG. 1.
Plasma levels of cNH concentrations are elevated in 2 Gy irradiated ferrets at two time points prior to the time of death (which occurs in ferrets at ~13 days or 312 h). Neutrophil and lymphocyte counts are significantly reduced at all time points shown. When cNH levels were compared, statistically significant differences among treatment groups (vs. the preirradiation group) were determined by the Wilcoxon Mann-Whitney test; sample sizes for each treatment group are as follows: preirradiation =17, 4 h =14, 12 h =13, 48 h = 15, 168 h = 16 and 312 h = 32 (the assay was performed once). When blood cell counts were compared, statistically significant differences among treatment groups were determined by the Student’s t test. Sample sizes for each group are reported in the table shown below the figure.
The blood cell count data (for lymphocytes, neutrophils and platelets) at the preirradiation, on-irradiation and postirradiation time points for the allo-HCT patients participating in these studies are shown in Table 1. The blood cell count data corresponding to the radiation treatment times (preirradiation, on irradiation and postirradiation) were not available from the RBR for the prostate, lung and breast cancer patients.
The data related to the changes in cNH blood levels, TAT complex levels and CFTs of the allo-HCT patients are shown in Figs. 2–4, and the data from prostate, lung and breast cancer patients on cNH blood levels are shown in Fig. 5. It should be noted that some of the preirradiation hematologic parameters in the allo-HCT TBI patients were below normal levels; for example, in patient 1 the platelet count was 47,000, and for patients 2 and 3, the lymphocyte counts were 570 and 517, respectively. These low baseline counts reflect the fact that these patients had leukemia and had already been heavily pretreated with chemotherapy long before they made it to transplant. In these transplant patients there was a further reduction in some parameters during and after irradiation. For example, the platelet count for patient 2 went from 455,000 preirradiation to 207,000 (during irradiation) and then 30,000 (postirradiation). These reductions represent a myelosuppressive effect of the conditioning regimen, both the chemotherapy and the TBI that immediately followed.
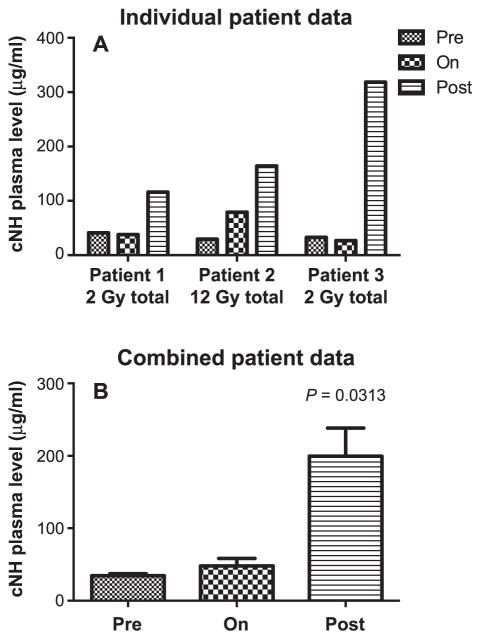
FIG. 2.
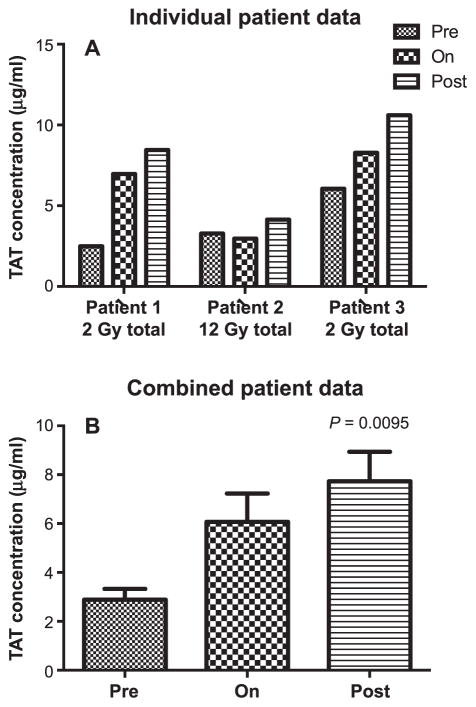
Detection of cNH levels in human plasma taken from allo-HCT patients at different time points prior to (pre), during (on) and after (post) irradiation. Panel A: Individual patient data. Panel B: Grouped patient data (n =3). Statistically significant differences were determined by the Wilcoxon Mann-Whitney test. Samples were assayed in duplicate.
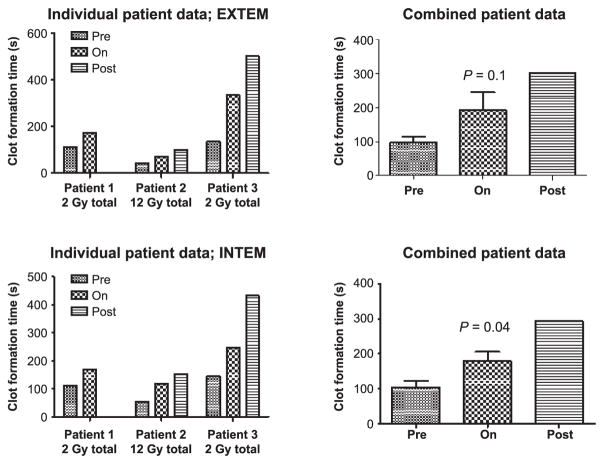
FIG. 4.
Clot formation time (CFT) in allo-HCT patients, measured by ROTEM. Both extrinsic (EXTEM) and intrinsic (INTEM) pathways are assessed as a global analysis of coagulation. Statistically significant differences were determined by Wilcoxon Mann-Whitney test, comparing time points prior to (pre) with during irradiation (n = 3). Statistical analysis was not performed for postirradiation (n = 2).
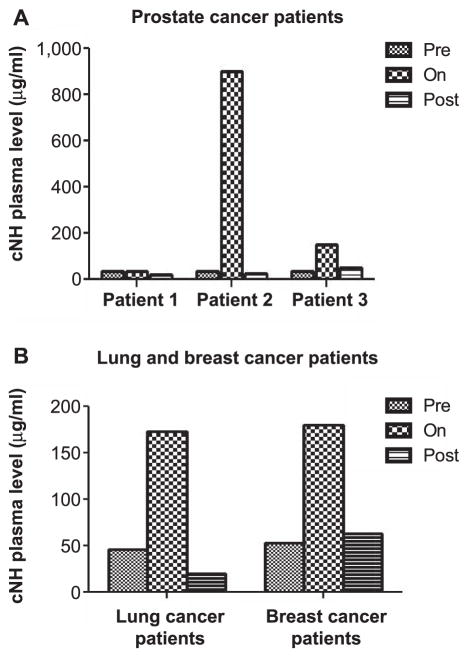
FIG. 5.
Detection of cNH levels at different time points prior to (pre), during (on) and after (post) irradiation in the plasma of prostate cancer patients (panel A) and lung and breast cancer patients (panel B).
There was a statistically significant increase in cNH levels in the allo-HCT patients at the time points after irradiation, but not during irradiation (Fig. 2A and B). There were markedly increased levels of TAT complex observed in the blood samples of the allo-HCT patients irradiated with a total dose of 2 Gy, and a statistically significant increase in the TAT complex levels was observed when all three patients were considered together (Fig. 3). The TAT complex levels in the plasma of the allo-HCT patients were relatively high at the preirradiation time point and were increased to higher levels postirradiation. Figure 4 shows that the CFT in the allo-HCT patient samples is significantly increased during irradiation, as measured by TEG measurements. The differences between the preirradiation and on-irradiation CFTs in the allo-HCT patients were statistically significant. While a test of statistical significance for the CFT results in the allo-HCT patients at the postirradiation time point was not performed (as data were available from only two patients in this treatment group), the CFT increased with time at the postirradiation time points for which patient data were available. The plasma samples from the prostate, lung and breast cancer patients indicated that several of these patients had elevated cNH levels, as shown in Fig. 5. Samples from three prostate cancer patients, five lung and five breast cancer patients were evaluated. Two out of three prostate cancer patient samples exhibited increased cNH levels in the plasma during radiotherapy. The cNH levels were elevated 24-fold in patient 2 and fourfold in patient 3 during radiotherapy, compared to the average preirradiation baseline level (Fig. 5A). One lung and one breast cancer patient exhibited an approximate 3.5-fold increase in cNH levels during radiotherapy, compared to their respective preirradiation values (Fig. 5B). For the other patient samples taken from the breast or lung cancer patients, four out of five of both the breast and lung cancer patients exhibited cNH levels that did not show major changes in the cNH levels observed during or after radiotherapy (data not shown). These results indicate that in radiotherapy patients receiving radiation for various types of malignant diseases, there are increased cNH levels in the blood samples of some of the patients.
FIG. 3.
Detection of TAT complex in human plasma from allo-HCT patients at different time points prior to (pre), during (on) and after (post) irradiation. Panel A: Individual patient data. Panel B: Grouped data (n = 3). Statistically significant differences were determined by the Wilcoxon Mann-Whitney test. Samples were assayed in duplicate.
DISCUSSION
The data from animal studies described in this report indicate that 2 Gy irradiated ferrets had cNH blood levels that were increased in a statistically significant manner during the observation time period. A single 2 Gy dose of radiation is lethal in ferrets, and all ferrets receiving this dose would be expected to die by day 14 postirradiation (3). The effects of 2 Gy irradiation on blood cell counts in ferrets have been described in detail elsewhere (3). The time course suggests that increased levels of DNA-histone complexes (also referred to as cNH levels in this article) at the 4 h time point correlate with the observed statistically significant reduction in blood lymphocytes and the 7- and 13-day time period correlate with the observed statistically significant reduction in blood neutrophils, as shown in Fig. 1. A small-scale study in Yucatan minipigs (n = 3) irradiated with a nonlethal dose (2 Gy) indicated increases in cNH blood levels over a 30-day time period; one of these minipigs exhibited particularly high cNH blood levels, with the highest level observed at 30 days postirradiation, representing an eightfold increase over the baseline (preirradiation) level (data not shown; the procedures for irradiating these minipigs are described in detail elsewhere (111)]. These data correspond to the radiation-induced reduction of total white blood cell counts reported in minipigs, which persisted through the 30-day time period (111). Increases in cNH levels were observed in some radiotherapy patients, as described below. The results presented here indicate that irradiated animals, and some radiotherapy patients, have cNH plasma levels that are within the range of cNH levels observed in lipopolysaccharide (LPS)-challenged mice in which coagulopathy contributes to death as described in ref. (56), as well as within the range of cNH levels observed in other disease models in animals and humans in which coagulopathy contributes to death.3 LPS-challenged mice develop and can die from DIC associated with sepsis (56). Examples of cNH levels observed in trauma patients (54, 58), and in animals and humans with a variety of pathophysiological processes and diseases (14, 57), have been reported elsewhere.
Numerous changes related to coagulopathy (i.e., the changes in the cNH and TAT complex levels, as well as the CFTs) were observed in radiotherapy patient samples. For all three of the allo-HCT patients, the during radiotherapy time period for blood sample collection was <24 h postirradiation and analysis of their blood samples did not result in statistically significant elevations of cNH levels. However, elevated cNH levels were observed in these patients at the postirradiation time periods. In the three allo-HCT patients, the differences between the cNH values from the pre- and postirradiation time periods were statistically significant and ranged from 5.8 to 25-fold. Therefore, our human TBI patient data confirm the ferret data, indicating that cNH levels show a rise when measured several days after irradiation. It is believed that the postirradiation time points in these patients reflect both lymphocyte and neutrophil death (as well as the death of other cell types).
There were markedly elevated TAT complex levels in the two allo-HCT patients irradiated with a single 2 Gy dose; the data considering all three allo-HCT patients indicated that the differences between the TAT complex levels at the pre- and postirradiation times were statistically significant. It is noteworthy that the baseline levels of TAT complexes in the allo-HCT patients were relatively high.
The high level of TAT complex in the allo-HCT patients is probably due to the fact that a very high percentage of cancer patients have what has been referred to as early-stage DIC. There is evidence that overt or subclinical intravascular coagulation is very high in cancer patients (112–114), and that some cancer therapy agents can promote coagulation parameters (115, 116). It has been reported that up to 92% of cancer patients have abnormalities in routine blood coagulation tests that are compatible with activation of intravascular coagulation (114). In a study of cancer patients diagnosed with DIC at Memorial Sloan Kettering Cancer Center, it was reported that 80% of these patients died within one to over 30 days of DIC onset (117). The results of these studies indicate that DIC commonly occurs in association with a wide variety of malignancies and that its occurrence causes morbidity and mortality in a significant number of patients. Thus, the high TAT complex baseline levels in the allo-HCT patients observed in these studies could be due to an early stage of DIC development in this population.
The data from radiotherapy patients indicated that there were particularly marked changes for the coagulation parameters in the allo-HCT patient blood samples. In these studies, coagulation was assessed using both the EXTEM and INTEM reagents, which probe the extrinsic (FVIIa/ tissue factor) and intrinsic (FVIIIa/FIXa) pathways, respectively. These are essentially analogous to the clinical APTT-and PT-based clotting assays. All three allo-HCT patients had prolonged CFTs, as well as elevated cNH levels, and the two allo-HCT patients irradiated with a single 2 Gy dose exhibited high levels of TAT complex, associated with radiotherapy. The changes observed in the allo-HCT patient blood samples were most likely caused primarily by the radiation exposure, as the blood samples for the CFT, TAT and cNH level assays were taken from these patients directly before and after the time periods for patient irradiation (as described in Materials and Methods), with no concomitant chemotherapy treatments administered during this time period. It is acknowledged, however, that other drugs used for the allo-HCT patients before irradiation (as described in the Introduction) could have contributed to our results. It is also possible that the allo-HCT procedures contributed to the results on the coagulation parameters observed in these patients by producing low platelet counts, which are expected to occur in these patients from the treatment. A prolonged CFT is usually caused by poor platelet function, thrombocytopenia, altered fibrin polymerization or fibrinogen deficiency. One of the allo-HCT patients exhibited a platelet count that would be considered low (compared to normal values of healthy individuals) at the time of preirradiation blood sampling, two of the allo-HCT patients exhibited low platelet counts during the on-irradiation time points, and all three allo-HCT patients exhibited low platelet counts at the postirradiation time points. Although we did note reductions in platelet counts relative to baseline (preirradiation) levels in the allo-HCT patients, this alone cannot completely account for the changes in the cNH levels during irradiation. For example, in patient 3, the during irradiation and postirradiation platelet counts were essentially the same (42,000 and 43,000); however, the cNH level was normal at the during irradiation time period and did not rise until postirradiation (Fig. 2). Conversely, for patient 2, the during irradiation cNH level was already on the rise whereas the platelet count at this time point was 207,000, which is well within normal range. These results suggest that the altered hemostatic parameters observed in these patients at the times measured were not likely to be caused completely by decreased platelet counts and that there are underlying mechanisms (i.e., not just thrombocytopenia) that are impacting the coagulation/hemostatic system.
A total of 16 cancer patients participated in the studies reported here (3 allo-HCT patients, 3 prostate, 5 breast and 5 lung cancer patients). All three allo-HCT/TBI patients, two of three prostate patients, one of five of the breast and one of five lung cancer patients exhibited changes related to coagulopathy. The one prostate cancer patient (3 total) who did not exhibit elevated cNH levels in response to radiotherapy was also treated with surgery and androgen deprivation prior to radiotherapy. It is tempting to speculate that the total dose of radiation, dose distribution (TBI vs. partial-body irradiation with the upper vs. lower body receiving most of the dose) and other treatments (e.g., androgen deprivation) may have influenced the results observed for the coagulation parameters evaluated in these studies. However, conclusions from the patient data relevant to the parameters described above will not be stated here, given the very small amount of patient data presented in this report, which strongly limits any firm conclusions.
Several patients in these studies who received radiotherapy for breast, lung and prostate cancer had high cNH blood levels, while others did not exhibit elevated cNH levels. As described above, all of the allo-HCT patient blood samples indicated high cNH levels. One reason that a lower percentage of the lung, breast and prostate cancer patients’ blood samples exhibited elevated levels of cNHs is that these patients were not total-body irradiated, while the allo-HCT patients were. In addition, the different levels of cNHs in these patient blood samples could be due to a sampling issue. Histones are known to be extremely toxic and they are rapidly cleared from the blood. Hemodialysis experiments have indicated that half of injected mononucleosomes are removed after 4 min (118) and the calculated half-life of histones in spiked plasma was 4.6 min (119). This could mean that multiple samples of the same patient’s blood could indicate considerable variations in cNH levels at different times. In cases in which elevated cNH levels are observed, it is reasonable to expect that adverse effects from these toxic products might have been experienced in these subjects. It is expected that the elevated cNH levels observed in some of the subjects evaluated in this report would cause an inflammatory response due to activation of toll-like receptors 2, 4 and 9, which would include a calcium flux in the endothelium, possibly causing vasodilation, the activation of platelets, inhibition of protein-C activation, augmentation of thrombin generation and potentially, compromise of the vascular barrier function leading to edema.3 The concepts listed above are described in (56, 120–124).
It is expected that different countermeasure agents are likely to have beneficial effects on different stages of RDIC development and progression. We expect that clotting factors will be able to reverse or suppress the early stages of RDIC development (within the first few days to weeks postirradiation), as a clotting factor (Benefix) has already been shown to serve as an effective countermeasure for prolonged CFT in ferrets (4), as described above. Antioxidants are also likely to affect the early stages of RDIC, but they may also have effects on later stages as well, given their known effects on many phenomena related to coagulopathy. We expect the anti-histone antibodies to have beneficial effects at a very early stage of RIC development (within the first few weeks postirradiation, when lymphocyte death is at its highest level) or at a late stage of RIC development, when neutrophil death is at its highest level. A countermeasure working at a late stage of RDIC development is likely to be the most appropriate countermeasure for both military personnel and civilians exposed to significant doses of radiation, since chaos is likely to accompany exposure to a nuclear weapon (like that after the atomic bomb explosions in Japan). It is expected that many individuals exposed to potentially lethal doses of radiation may not receive medical care in the period of hours to a few days postirradiation, and appropriate medical treatment may not occur until many days to weeks postirradiation. Thus, a treatment known to be effective at many days to weeks postirradiation could have a major effect on survival in populations exposed to significant radiation doses. Products affecting the later stages of RDIC would include anti-histone antibodies, since the time frame at which cNH levels are high in the blood of irradiated humans includes relatively late times postirradiation. As described in the Introduction, the time when neutrophil death is at its highest level is at ~20–40 days in atomic bomb casualties receiving doses near the human LD50 (in the range of 2–4.5 Gy; group II), with deaths in group II occurring primarily from ~20–40 days postirradiation and then diminishing after that time (25). Since the neutrophil-derived NETs are such potent promoters of coagulation, as described above, neutrophil death may have considerably more important consequences for the progression of coagulopathy than the consequences caused by lymphocyte death. It is also possible that the death of neutrophils is of greater consequence to the organism (than is lymphocyte death) since their death occurs at a time when RDIC has already progressed to a more serious stage of development.
From the results of studies performed here to determine the cNH levels in irradiated subjects, it is clear that some irradiated animals and some irradiated cancer patients have highly elevated cNH levels that would make them good candidates to evaluate whether anti-histone antibodies might be countermeasures for RDIC. All of the allo-HCT patients had highly elevated cNH levels, which could have been influenced by the fact that they were exposed to chemotherapeutic agents before irradiation. They appear to be the particularly appropriate candidates for treatment with anti-histone antibodies, however, as many of these patients have symptoms and biological effects that are likely to be due to severe coagulopathies, such as VOD, as described elsewhere (125). In addition to high cNH levels, the blood samples from the allo-HCT patients also had prolonged CFTs and high TAT complex levels in their blood, indicating coagulopathies.
Some of the radiotherapy patients with prostate, breast and lung cancer also had very high blood cNH levels. The detection of increased cNH levels in some radiotherapy patient blood samples may serve as a biomarker to diagnose and/or predict the propensity for the development of radiation-induced coagulopathies/hemorrhage, offering a potential means to treat these patients via personalized medicine therapies. While anti-histone antibodies are at high blood levels in patients with numerous diseases/ conditions (57), they have not yet been developed into drugs that can be used for clinical trials at this time.
In this article, we hypothesize that RIC may share some of the mechanisms underlying TIC. It has already been reported that irradiated animals destined to die from DIC share some features with severely injured TIC patients, which include the presence of blood clotting abnormalities (coagulopathies) associated with death (3, 5), and hemorrhage occurring in the presence of normal platelet levels in the blood (i.e., that should be able to control bleeding/ hemorrhaging) (3). Another feature of patients with TIC is that they have high levels of circulating nucleosomes/ histones (cNHs) in the blood, as described above. In this article, we have presented data indicating that irradiated animals and some radiotherapy patients also have elevated cNH levels in the blood. Several findings from the TIC research field may have relevance to the diagnosis and treatment of irradiated patients who are at risk of developing DIC; thus, it is important to continue studies on RIC so that novel countermeasures can be developed for the prevention and/or mitigation of the adverse biological effects resulting from radiation exposure that can lead to death from RDIC.
Acknowledgments
The experiments with ferrets and pigs described in this report were funded by the National Space Biomedical Research Institute (NSBRI), which is funded through NASA NCC 9-58. We are grateful to the Radiation Oncology Registry and Biosample Repository for providing legacy frozen plasma samples from patients with lung, breast and prostate cancer, as well as blood samples from the allo-HCT patients. We thank Susan Mazzoni for her help in acquisition of the allo-HCT patient blood samples, and Dr. Abigail Berman for helpful discussions about the cancer therapy protocols for allo-HCT patients. We thank Dr. Rodney Camire for help with the studies related to the allo-HCT TEG data shown here, as well as helpful discussions about the significance of the results of the CFT and the platelet blood cell count data in the allo-HCT patients.
Footnotes
Personal communication, Dr. X. Long Zheng.
Personal communication, Dr. Charles T. Esmon.
References
- 1.Krigsfeld GS, Kennedy AR. Is disseminated intravascular coagulation the major cause of mortality from radiation at relatively low whole body doses? Radiat Res. 2013;180:231–4. doi: 10.1667/RR3321.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krigsfeld GS, Sanzari JK, Kennedy AR. The effects of proton radiation on the prothrombin and partial thromboplastin times of irradiated ferrets. Int J Radiat Biol. 2012;88:327–34. doi: 10.3109/09553002.2012.652727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krigsfeld GS, Savage AR, Billings PC, Lin L, Kennedy AR. Evidence for radiation-induced disseminated intravascular coagulation as a major cause of radiation-induced death in ferrets. Int J Radiat Oncol Biol Phys. 2014;88:940–6. doi: 10.1016/j.ijrobp.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krigsfeld GS, Savage AR, Sanzari JK, Wroe AJ, Gridley DS, Kennedy AR. Mechanism of hypocoagulability in proton-irradiated ferrets. Int J Radiat Biol. 2013;89:823–31. doi: 10.3109/09553002.2013.802394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krigsfeld GS, Shah JB, Sanzari JK, Lin L, Kennedy AR. Evidence of disseminated intravascular coagulation in a porcine model following radiation exposure. Life Sci Space Res (Amst) 2014;3:1–9. doi: 10.1016/j.lssr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy AR. Biological effects of space radiation and development of effective countermeasures. Life Sci Space Res (Amst) 2014;1:10–43. doi: 10.1016/j.lssr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winchell HS, Anderson AC, Pollycove M. Radiation-induced hemorrhagic diathesis in dogs unassociated with thrombocytopenia: association with an intravascular protein-polysaccharide particle. Blood. 1964;23:186–92. [PubMed] [Google Scholar]
- 8.Liebow AA, Warren S, De CE. Pathology of atomic bomb casualties. Am J Pathol. 1949;25:853–1027. [PMC free article] [PubMed] [Google Scholar]
- 9.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–92. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 10.Gando S, Sawamura A, Hayakawa M. Trauma, shock, and disseminated intravascular coagulation: lessons from the classical literature. Ann Surg. 2011;254:10–9. doi: 10.1097/SLA.0b013e31821221b1. [DOI] [PubMed] [Google Scholar]
- 11.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M Scientific Subcommittee on Disseminated Intravascular Coagulation of the International Society onThrombosis and Haemostasis (ISTH) Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–30. [PubMed] [Google Scholar]
- 12.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 13.Levi M, de Jonge E, Meijers J. The diagnosis of disseminated intravascular coagulation. Blood Rev. 2002;16:217–23. doi: 10.1016/s0268-960x(02)00032-2. [DOI] [PubMed] [Google Scholar]
- 14.Wildhagen KC, Wiewel MA, Schultz MJ, Horn J, Schrijver R, Reutelingsperger CP, et al. Extracellular histone H3 levels are inversely correlated with antithrombin levels and platelet counts and are associated with mortality in sepsis patients. Thromb Res. 2015;136:542–7. doi: 10.1016/j.thromres.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 15.Stern DM, Esposito C, Gerlach H, Gerlach M, Ryan J, Handley D, et al. Endothelium and regulation of coagulation. Diabetes Care. 1991;14:160–6. doi: 10.2337/diacare.14.2.160. [DOI] [PubMed] [Google Scholar]
- 16.Esmon CT. The endothelial cell protein C receptor. Thromb Haemost. 2000;83:639–43. [PubMed] [Google Scholar]
- 17.King GL. Characterization of radiation-induced emesis in the ferret. Radiat Res. 1988;114:599–612. [PubMed] [Google Scholar]
- 18.Matsuoka Y, Lamirande EW, Subbarao K. The ferret model for influenza. Curr Protoc Microbiol. 2009;Chapter 15(Unit 15G):2. doi: 10.1002/9780471729259.mc15g02s13. [DOI] [PubMed] [Google Scholar]
- 19.Gad SC. Pigs and ferrets as models in toxicology and biological safety assessment. Int J Toxicol. 2000;19:149–68. [Google Scholar]
- 20.Reed GL, Houng AK. The contribution of activated factor XIII to fibrinolytic resistance in experimental pulmonary embolism. Circulation. 1999;99:299–304. doi: 10.1161/01.cir.99.2.299. [DOI] [PubMed] [Google Scholar]
- 21.Hoogstraten-Miller S, Bellinger D, Reddick R, Read M, Sigman J, Madden V. von Willebrand factor in plasma, platelets, and selected tissues of ferrets. Lab Anim Sci. 1995;45:151–9. [PubMed] [Google Scholar]
- 22.Abderrahmani R, Francois A, Buard V, Tarlet G, Blirando K, Hneino M, et al. PAI-1-dependent endothelial cell death determines severity of radiation-induced intestinal injury. PLoS One. 2012;7:e35740. doi: 10.1371/journal.pone.0035740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shouse SS, Warren SL, Whipple GH., II Aplasia of marrow and fatal intoxication in dogs produced by roentgen radiation of all bones. J Exp Med. 1931;53:421–35. doi: 10.1084/jem.53.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall EJ, Giaccia AJ. Acute effects of total-body irradiation. In: Hall EJ, Giaccia AJ, editors. Radiobiology for the radiologist. 7. Philadelphia: Lippincott, Williams & Wilkins; 2012. pp. 117–28. [Google Scholar]
- 25.Okita T. Review of thirty years study of Hiroshima and Nagasaki atomic bomb survivors. II. Biological effects. A Acute effects. J Radiat Res. 1975;16(Suppl):49–66. doi: 10.1269/jrr.16.supplement_49. [DOI] [PubMed] [Google Scholar]
- 26.Kambas K, Mitroulis I, Apostolidou E, Girod A, Chrysanthopoulou A, Pneumatikos I, et al. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS One. 2012;7:e45427. doi: 10.1371/journal.pone.0045427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6:415–20. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 28.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–33. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–25. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 30.Zawrotniak M, Rapala-Kozik M. Neutrophil extracellular traps (NETs) - formation and implications. Acta Biochim Pol. 2013;60:277–84. [PubMed] [Google Scholar]
- 31.Palmer LJ, Cooper PR, Ling MR, Wright HJ, Huissoon A, Chapple IL. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin Exp Immunol. 2012;167:261–8. doi: 10.1111/j.1365-2249.2011.04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkmann V, Goosmann C, Kuhn LI, Zychlinsky A. Automatic quantification of in vitro NET formation. Front Immunol. 2012;3:413. doi: 10.3389/fimmu.2012.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–35. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–96. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 38.Levi M, ten Cate H, Bauer KA, van der Poll T, Edgington TS, Buller HR, et al. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest. 1994;93:114–20. doi: 10.1172/JCI116934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suffredini AF, Harpel PC, Parrillo JE. Promotion and subsequent inhibition of plasminogen activation after administration of intravenous endotoxin to normal subjects. N Engl J Med. 1989;320:1165–72. doi: 10.1056/NEJM198905043201802. [DOI] [PubMed] [Google Scholar]
- 40.Gando S, Kameue T, Nanzaki S, Nakanishi Y. Disseminated intravascular coagulation is a frequent complication of systemic inflammatory response syndrome. Thromb Haemost. 1996;75:224–8. [PubMed] [Google Scholar]
- 41.Gando S, Nakanishi Y, Tedo I. Cytokines and plasminogen activator inhibitor-1 in posttrauma disseminated intravascular coagulation: relationship to multiple organ dysfunction syndrome. Crit Care Med. 1995;23:1835–42. doi: 10.1097/00003246-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Nossel HL. Relative proteolysis of the fibrinogen B beta chain by thrombin and plasmin as a determinant of thrombosis. Nature. 1981;291:165–7. doi: 10.1038/291165a0. [DOI] [PubMed] [Google Scholar]
- 43.Cardenas JC, Wade CE, Holcomb JB. Mechanisms of trauma-induced coagulopathy. Curr Opin Hematol. 2014;21:404–9. doi: 10.1097/MOH.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 44.Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, et al. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma. 2011;71:S427–34. doi: 10.1097/TA.0b013e318232e5ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–13. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 46.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 47.Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10:1342–51. doi: 10.1111/j.1538-7836.2012.04752.x. [DOI] [PubMed] [Google Scholar]
- 48.Outerson A, LeRoy GV, Liebow AA, Hammon EC, Bernett HL, Rosenbaum JD, et al. Medical effects of atomic bombs; the report of the Joint Commission for the Investigation of the Atomic Bomb in Japan, Office of the Air Surgeon. Oak Ridge, TN: U.S. Atomic Energy Commission; 1951. [Google Scholar]
- 49.Sondeen JL, Prince MD, Kheirabadi BS, Wade CE, Polykratis IA, de Guzman R, et al. Initial resuscitation with plasma and other blood components reduced bleeding compared to hetastarch in anesthetized swine with uncontrolled splenic hemorrhage. Transfusion. 2011;51:779–92. doi: 10.1111/j.1537-2995.2010.02928.x. [DOI] [PubMed] [Google Scholar]
- 50.Sondeen JL, de Guzman R, Amy Polykratis I, Dale Prince M, Hernandez O, Cap AP, et al. Comparison between human and porcine thromboelastograph parameters in response to ex-vivo changes to platelets, plasma, and red blood cells. Blood Coagul Fibrinolysis. 2013;24:818–29. doi: 10.1097/MBC.0b013e3283646600. [DOI] [PubMed] [Google Scholar]
- 51.Solomon C, Traintinger S, Ziegler B, Hanke A, Rahe-Meyer N, Voelckel W, et al. Platelet function following trauma. A multiple electrode aggregometry study. Thromb Haemost. 2012;106:322–30. doi: 10.1160/TH11-03-0175. [DOI] [PubMed] [Google Scholar]
- 52.Sillesen M, Johansson PI, Rasmussen LS, Jin G, Jepsen CH, Imam AM, et al. Platelet activation and dysfunction in a large-animal model of traumatic brain injury and hemorrhage. J Trauma Acute Care Surg. 2013;74:1252–9. doi: 10.1097/TA.0b013e31828c7a6b. [DOI] [PubMed] [Google Scholar]
- 53.White NJ. Mechanisms of trauma-induced coagulopathy. Hematology Am Soc Hematol Educ Program. 2013;2013:660–3. doi: 10.1182/asheducation-2013.1.660. [DOI] [PubMed] [Google Scholar]
- 54.Kutcher ME, Xu J, Vilardi RF, Ho C, Esmon CT, Cohen MJ. Extracellular histone release in response to traumatic injury: implications for a compensatory role of activated protein C. J Trauma Acute Care Surg. 2012;73:1389–94. doi: 10.1097/TA.0b013e318270d595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lam NY, Rainer TH, Chan LY, Joynt GM, Lo YM. Time course of early and late changes in plasma DNA in trauma patients. Clin Chem. 2003;49:1286–91. doi: 10.1373/49.8.1286. [DOI] [PubMed] [Google Scholar]
- 56.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen R, Kang R, Fan XG, Tang D. Release and activity of histone in diseases. Cell Death Dis. 2014;5:e1370. doi: 10.1038/cddis.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187:160–9. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–45. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 60.Joop K, Berckmans RJ, Nieuwland R, Berkhout J, Romijn FP, Hack CE, et al. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost. 2001;85:810–20. [PubMed] [Google Scholar]
- 61.Iba T, Miki T, Hashiguchi N, Tabe Y, Nagaoka I. Is the neutrophil a ‘prima donna’ in the procoagulant process during sepsis? Crit Care. 2014;18:230. doi: 10.1186/cc13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madoiwa S. Recent advances in disseminated intravascular coagulation: endothelial cells and fibrinolysis in sepsis-induced DIC. J Intensive Care. 2015;3 doi: 10.1186/s40560-015-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196:430–43. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 64.Fajardo LF. Ionizing radiation and the endothelium – a brief review. In: Rubin P, Constine LS, Marks LB, Okunieff P, editors. Late effects of cancer treatment on normal tissues. Berlin Heidelberg: Springer-Verlag; 2008. pp. 19–22. [Google Scholar]
- 65.Fajardo LF. The unique physiology of endothelial cells and its implications in radiobiology. Front Radiat Ther Oncol. 1989;23:96–112. doi: 10.1159/000416574. [DOI] [PubMed] [Google Scholar]
- 66.Fajardo LF. The endothelial cell is a unique target of radiation: an overview. In: Rubin DB, editor. The radiation biology of the vascular endothelium. Boca Raton: CRC Press; 1998. pp. 1–12. [Google Scholar]
- 67.Fajardo LF, Berthrong M. Vascular lesions following radiation. Pathol Annu. 1988;23(Pt 1):297–330. [PubMed] [Google Scholar]
- 68.Haimovitz-Friedman A, Fuks Z. Signaling in the radiation response of endothelial cells. In: Rubin DB, editor. The radiation biology of the vascular endothelium. Boca Raton: CRC Press; 1998. pp. 1–127. [Google Scholar]
- 69.Sugihara T, Hattori Y, Yamamoto Y, Qi F, Ichikawa R, Sato A, et al. Preferential impairment of nitric oxide-mediated endothelium-dependent relaxation in human cervical arteries after irradiation. Circulation. 1999;100:635–41. doi: 10.1161/01.cir.100.6.635. [DOI] [PubMed] [Google Scholar]
- 70.Soloviev AI, Tishkin SM, Parshikov AV, Ivanova IV, Goncharov EV, Gurney AM. Mechanisms of endothelial dysfunction after ionized radiation: selective impairment of the nitric oxide component of endothelium-dependent vasodilation. Br J Pharmacol. 2003;138:837–44. doi: 10.1038/sj.bjp.0705079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soucy KG, Lim HK, Attarzadeh DO, Santhanam L, Kim JH, Bhunia AK, et al. Dietary inhibition of xanthine oxidase attenuates radiation-induced endothelial dysfunction in rat aorta. J Appl Physiol (1985) 2010;108:1250–8. doi: 10.1152/japplphysiol.00946.2009. [DOI] [PubMed] [Google Scholar]
- 72.Sanzari JK, Billings PC, Wilson JM, Diffenderfer ES, Arce-Esquivel AA, Thorne PK, et al. Effect of electron radiation on vasomotor function of the left anterior descending coronary artery. Life Sci Space Res (Amst) 2015;4:6–10. doi: 10.1016/j.lssr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hunt BJ, Jurd KM. Endothelial cell activation. A central pathophysiological process. BMJ. 1998;316:1328–9. doi: 10.1136/bmj.316.7141.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bach FH, Robson SC, Winkler H, Ferran C, Stuhlmeier KM, Wrighton CJ, et al. Barriers to xenotransplantation. Nat Med. 1995;1:869–73. doi: 10.1038/nm0995-869. [DOI] [PubMed] [Google Scholar]
- 75.Pearson JD. Endothelial cell function and thrombosis. Baillieres Best Pract Res Clin Haematol. 1999;12:329–41. doi: 10.1053/beha.1999.0028. [DOI] [PubMed] [Google Scholar]
- 76.Czabanka M, Peter C, Martin E, Walther A. Microcirculatory endothelial dysfunction during endotoxemia–insights into pathophysiology, pathologic mechanisms and clinical relevance. Curr Vasc Pharmacol. 2007;5:266–75. doi: 10.2174/157016107782023389. [DOI] [PubMed] [Google Scholar]
- 77.Centelles MN, Puy C, Lopez-Sagaseta J, Fukudome K, Montes R, Hermida J. Blocking endothelial protein C receptor (EPCR) accelerates thrombus development in vivo. Thromb Haemost. 2010;103:1239–44. doi: 10.1160/TH09-11-0750. [DOI] [PubMed] [Google Scholar]
- 78.Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HP, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18:1123–9. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Claus RA, Bockmeyer CL, Sossdorf M, Losche W. The balance between von-Willebrand factor and its cleaving protease ADAMTS13: biomarker in systemic inflammation and development of organ failure? Curr Mol Med. 2010;10:236–48. doi: 10.2174/156652410790963367. [DOI] [PubMed] [Google Scholar]
- 80.Sporn LA, Rubin P, Marder VJ, Wagner DD. Irradiation induces release of von Willebrand protein from endothelial cells in culture. Blood. 1984;64:567–70. [PubMed] [Google Scholar]
- 81.Jahroudi N, Ardekani AM, Greenberger JS. Ionizing irradiation increases transcription of the von Willebrand factor gene in endothelial cells. Blood. 1996;88:3801–14. [PubMed] [Google Scholar]
- 82.Lushbaugh CC. Reflections on some recent progress in human radiobiology. Adv Radiat Biol. 1969;3:277–314. [Google Scholar]
- 83.Fujita S, Kato H, Schull WJ. The LD50 associated with exposure to the atomic bombing of Hiroshima and Nagasaki. J Radiat Res. 1991;32(Suppl):154–61. doi: 10.1269/jrr.32.supplement_154. [DOI] [PubMed] [Google Scholar]
- 84.The report of the Joint Commission for the Investigation of the Effects of the Atomic Bomb in Japan. Vol. 3. Washington, D.C: Department of Energy; 1951. Medical effects of atomic bombs. [Google Scholar]
- 85.DiCarlo AL, Kaminski JM, Hatchett RJ, Maidment BW. Role of thrombocytopenia in radiation-induced mortality and review of therapeutic approaches targeting platelet regeneration after radiation exposure. J Radiat Oncol. 2015:1–14. [Google Scholar]
- 86.Moroni M, Coolbaugh TV, Lombardini E, Mitchell JM, Moccia KD, Shelton LJ, et al. Hematopoietic radiation syndrome in the Gottingen minipig. Radiat Res. 2011;176:89–101. doi: 10.1667/rr2481.1. [DOI] [PubMed] [Google Scholar]
- 87.Kennedy AR, Wan XS. Countermeasures for space radiation induced adverse biologic effects. Adv Space Res. 2011;48:1460–79. [Google Scholar]
- 88.Rubin DB, Drab EA, Blazek ER. Anitoxidant defenses and the irradiated endothelium. In: Rubin DB, editor. The radiation biology of the vascular endothelium. Boca Raton: CRC Press; 1998. pp. 65–112. [Google Scholar]
- 89.Murray JC. Inflammatory cytokines, radiation and endothelial gene products: a common role for reactive oxygen intermediates. In: Rubin DB, editor. The radiation biology of the vascular endothelium. Boca Raton: CRC Press; 1998. pp. 147–60. [Google Scholar]
- 90.Ward DC, Molteni JV, Ts’ao CH. Endothelial-oriented strategies to spare normal tissues. In: Rubin DB, editor. The radiation biology of the vascular endothelium. Boca Raton: CRC Press; 1998. pp. 185–208. [Google Scholar]
- 91.Rubin P, Constine LS, Marks LB, Okunieff P. Late effects of cancer treatment on normal tissues. Berlin Heidelberg: Springer-Verlag; 2008. [Google Scholar]
- 92.Rubin DB. The radiation biology of the vascular endothelium. Boca Raton, FL: CRC Press LLC; 1998. [Google Scholar]
- 93.Hopewell JW. Radiation effects on vascular tissue. In: Potten CS, Hendry JH, editors. Cytotoxic insult to tissue. Edinburgh: Chuchill Livingstone; 1983. pp. 228–57. [Google Scholar]
- 94.Reinhold HS, Hopewell JW. Late changes in the architecture of blood vessels of the rat brain after irradiation. Br J Radiol. 1980;53:693–6. doi: 10.1259/0007-1285-53-631-693. [DOI] [PubMed] [Google Scholar]
- 95.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–48. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 96.Bearman SI, Lee JL, Baron AE, McDonald GB. Treatment of hepatic venocclusive disease with recombinant human tissue plasminogen activator and heparin in 42 marrow transplant patients. Blood. 1997;89:1501–6. [PubMed] [Google Scholar]
- 97.Richardson PG, Murakami C, Jin Z, Warren D, Momtaz P, Hoppensteadt D, et al. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002;100:4337–43. doi: 10.1182/blood-2002-04-1216. [DOI] [PubMed] [Google Scholar]
- 98.Richardson PG, Soiffer RJ, Antin JH, Uno H, Jin Z, Kurtzberg J, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16:1005–17. doi: 10.1016/j.bbmt.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Astedt B, Bergentz SE, Svanberg L. Effect of irradiation on plasminogen activator content in rat vessels. Experientia. 1974;30:1466–67. doi: 10.1007/BF01919698. [DOI] [PubMed] [Google Scholar]
- 100.Svanberg L, Astedt B, Kullander S. Radiation-decreased fibrinolytic-activity of vessel walls. Acta Obstet Gynecol Scand. 1976;55:49–51. doi: 10.3109/00016347609156783. [DOI] [PubMed] [Google Scholar]
- 101.Henderson BW, Bicher HI, Johnson RJ. Loss of vascular fibrinolytic-activity following irradiation of the liver - an aspect of late radiation-damage. Radiat Res. 1983;95:646–52. [PubMed] [Google Scholar]
- 102.Ward WF, Kim YT, Molteni A, Solliday NH. Radiation-induced pulmonary endothelial dysfunction in rats - modification by an inhibitor of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys. 1988;15:135–40. doi: 10.1016/0360-3016(88)90357-4. [DOI] [PubMed] [Google Scholar]
- 103.Ward WF, Molteni A, Kim YT, Tsao C. Structure-function analysis of angiotensin-converting enzyme-inhibitors as modifiers of radiation-induced pulmonary endothelial dysfunction in rats. Br J Radiol. 1989;62:348–54. doi: 10.1259/0007-1285-62-736-348. [DOI] [PubMed] [Google Scholar]
- 104.Ward WF, Molteni A, Tsao CH. Radiation-induced endothelial dysfunction and fibrosis in rat lung - modification by the angiotensin converting enzyme-inhibitor Cl242817. Radiat Res. 1989;117:342–50. [PubMed] [Google Scholar]
- 105.Ohno M, Matsuura K, Nakagawa E, Ohba T, Nakata H. Effects of dextran sulfate on pulmonary radiation injury–clinical and experimental studies. Nihon Kyobu Shikkan Gakkai Zasshi. 1978;16:756–64. (author’s translation) [PubMed] [Google Scholar]
- 106.Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC., Jr Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44:2020–7. doi: 10.1212/wnl.44.11.2020. [DOI] [PubMed] [Google Scholar]
- 107.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124:344–53. doi: 10.1182/blood-2014-02-514778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Litzow MR, Perez WS, Klein JP, Bolwell BJ, Camitta B, Copelan EA, et al. Comparison of outcome following allogeneic bone marrow transplantation with cyclophosphamide-total body irradiation versus busulphan-cyclophosphamide conditioning regimens for acute myelogenous leukaemia in first remission. Br J Haematol. 2002;119:1115–24. doi: 10.1046/j.1365-2141.2002.03973.x. [DOI] [PubMed] [Google Scholar]
- 109.Hartman AR, Williams SF, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transplant. 1998;22:439–43. doi: 10.1038/sj.bmt.1701334. [DOI] [PubMed] [Google Scholar]
- 110.Sanzari JK, Wan XS, Krigsfeld GS, Wroe AJ, Gridley DS, Kennedy AR. The effects of gamma and proton radiation exposure on hematopoietic cell counts in the ferret model. Gravit Space Res. 2013;1:79–94. [PMC free article] [PubMed] [Google Scholar]
- 111.Sanzari JK, Krigsfeld GS, Shuman AL, Diener AK, Lin L, Mai W, et al. Effects of a granulocyte colony stimulating factor, Neulasta, in mini pigs exposed to total body proton irradiation. Life Sci Space Res (Amst) 2015;5:13–20. doi: 10.1016/j.lssr.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rickles FR, Edwards RL, Barb C, Cronlund M. Abnormalities of blood coagulation in patients with cancer. Fibrinopeptide A generation and tumor growth. Cancer. 1983;51:301–7. doi: 10.1002/1097-0142(19830115)51:2<301::aid-cncr2820510223>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 113.Bowie EJ, Owen CA., Jr Symposium on the diagnosis and treatment of intravascular coagulation-fibrinolysis (ICF) syndrome, with special emphasis on this syndrome in patients with cancer. Introduction Mayo Clin Proc. 1974;49:635. [PubMed] [Google Scholar]
- 114.Sun NC, Bowie EJ, Kazmier FJ, Elveback LR, Owen CA., Jr Blood coagulation studies in patients with cancer. Mayo Clin Proc. 1974;49:636–41. [PubMed] [Google Scholar]
- 115.Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau’s syndrome revisited. Blood. 1983;62:14–31. [PubMed] [Google Scholar]
- 116.Levine MN, Gent M, Hirsh J, Arnold A, Goodyear MD, Hryniuk W, et al. The thrombogenic effect of anticancer drug therapy in women with stage II breast cancer. N Engl J Med. 1988;318:404–7. doi: 10.1056/NEJM198802183180703. [DOI] [PubMed] [Google Scholar]
- 117.Al-Mondhiry H. Disseminated intravascular coagulation: experience in a major cancer center. Thromb Diath Haemorrh. 1975;34:181–93. [PubMed] [Google Scholar]
- 118.Gauthier VJ, Tyler LN, Mannik M. Blood clearance kinetics and liver uptake of mononucleosomes in mice. J Immunol. 1996;156:1151–6. [PubMed] [Google Scholar]
- 119.Ekaney ML, Otto GP, Sossdorf M, Sponholz C, Boehringer M, Loesche W, et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit Care. 2014;18:543. doi: 10.1186/s13054-014-0543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–61. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9:1795–803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 122.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–30. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 123.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;5–6:8–9. [PubMed] [Google Scholar]
- 124.Esmon CT. Molecular circuits in thrombosis and inflammation. Thromb Haemost. 2013;109:416–20. doi: 10.1160/TH12-08-0634. [DOI] [PubMed] [Google Scholar]
- 125.Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol. 2011;21:256–63. doi: 10.1016/j.semradonc.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]