Abstract
Background
Studies of the impact of pre-surgery weight loss and lifestyle preparation on outcomes following bariatric surgery are needed.
Objective
To evaluate whether a pre-surgery behavioral lifestyle intervention improves weight loss through 24-months post-surgery.
Setting
Bariatric Center of Excellence at a large, urban medical center.
Methods
Candidates for bariatric surgery were randomized to a 6-month behavioral lifestyle intervention or to 6 months of usual pre-surgical care. The lifestyle intervention consisted of 8 weekly face-to-face sessions followed by 16 weeks of face-to-face and telephone sessions prior to surgery; the intervention also included 3 monthly telephone contacts after surgery. Assessments were conducted at 6-, 12- and 24-months post-surgery.
Results
Participants who underwent surgery (n = 143) were 90.2% female and 86.7% White. Average age was 44.9 years, and average BMI was 47.5 kg/m2 at study enrollment. At follow-up, 131 (91.6%), 126 (88.1%), 117 (81.8%) patients participated in the 6-, 12- and 24 month assessments, respectively. Percent weight loss from study enrollment to 6- and 12-months post-surgery was comparable for both groups, but at 24-months post-surgery, the lifestyle group had significantly smaller percent weight loss than the usual care group (26.5% vs. 29.5%, respectively, p = 0.02).
Conclusions
Pre-surgery lifestyle intervention did not improve weight loss at 24 months post-surgery. Findings raise questions about the utility and timing of adjunctive lifestyle interventions for bariatric surgery patients.
Keywords: bariatric surgery, preoperative intervention, behavioral weight control
Introduction
There has been debate as to whether patients should be required to complete pre-surgery diet and lifestyle modification programs immediately prior to bariatric surgery. Although pre-bariatric surgery weight loss has been associated with fewer perioperative complications, shorter operative time, less blood loss, and shorter hospital stays,(1) questions persist about the post-surgery benefits. To date, there has been little evidence to support the positive impact of pre-surgery weight loss on post-surgery outcomes.(2–4) If pre-surgery weight loss does not enhance longer-term post-surgery outcomes, mandatory supervised dieting may pose an unnecessary barrier to surgery.(5) However, if pre-surgery weight loss is associated with improved post-surgery outcomes, implementation and addtional evaluation of pre-surgical diet and lifestyle modification programs is indicated.
We are aware of only one prospective, randomized trial evaluating the impact of pre-surgery weight loss on post-surgery weight loss.(6) In that trial, patients instructed to lose at least 10% of their excess body weight prior to surgery had greater weight loss three months after Roux-en-Y gastric bypass (RYGP) than patients who did not receive this instruction, but differences in weight loss by group were not sustained at 6 months6 or 1 year after surgery.(7) However, the trial did not utilize a standardized behavioral lifestyle intervention, and was restricted to one surgical procedure, which limits generalizability of findings.
Behavioral lifestyle programs have been studied extensively in nonsurgical samples and lead to loss of 5 to 10% of initial body weight among extremely obese individuals,(8,9) suggesting their potential utility as a pre-surgery intervention. Thus, we conducted a randomized, controlled trial to evaluate the efficacy of a pre-surgery behavioral lifestyle program in promoting post-surgery weight loss and minimizing complications in comparison to usual care, which consisted of participation in any physician-supervised diet program. Initial results supported the hypothesis that the behavioral lifestyle intervention would be associated with greater weight loss among patients relative to those receiving usual care prior to surgery.(10) The intervention group lost more weight [8.3 ± 7.8 kg vs. 3.3 ± 5.5 kg, p < 0.0001] and was more likely to lose at least 5% of initial body weight than the usual care group (OR = 4.98, p < 0.0001).
In this report of longer-term results from the randomized, controlled trial, we examined the impact of the pre-surgery lifestyle intervention on weight loss at 6-, 12- and 24-months post-surgery, as well as its impact on 30-day major adverse events among patients undergoing RYGB or laparoscopic adjustable gastric banding (LAGB). We also explored whether pre-surgical weight loss, independent of intervention, was associated with post-surgery outcomes.
Materials and Methods
Design, participants, and setting
We conducted a randomized, controlled trial between January 2008 and March 2013. The study was approved by the local Institutional Review Board (IRB) and registered at ClinicalTrials.gov (identifier: NCT00623792). Initial results have been reported previously,10 and longer-term outcomes are reported here.
Participants were at least 18 years of age and seeking surgery through a Bariatric Center of Excellence at a large, urban medical center. Exclusion criteria included: 1) Intellectual disability or psychosis; 2) Previously diagnosed genetic obesity syndrome; 3) Participation in a weight management program in the 6 months prior to study enrollment; 4) Uncontrolled psychiatric symptomatology sufficiently severe upon screening to require immediate treatment; 5) Pregnant or lactating in the previous 6 months; 6) Taking a medication known to affect body weight (e.g., second generation antipsychotics) during the previous 6 months; 8) Previous weight loss surgery; 9) Medical condition requiring a specialized preoperative regimen (e.g., nonambulatory individuals, chronic obstructive pulmonary disease requiring oxygen, BMI over 70 kg/m2 requiring a low energy liquid diet as per the Center’s practice algorithm); and 10) Participation in a conflicting research protocol.
After completing a baseline assessment, 240 surgery candidates were randomized to study conditions (1:1) by permuted block randomization with a block size of 4, stratified by BMI (< or > 50 kg/m2); the allocation sequence was generated by the study statistician. Participants were assigned by study staff to a 6-month behavioral lifestyle intervention or usual pre-surgery care (completion of a 6-month physician-supervised diet and activity program as required for approval for surgery).
Behavioral lifestyle intervention and assessments
The pre-surgery intervention has been previously described in detail.(11) In brief, the intervention included state-of-the art information on diet, physical activity and bariatric surgery, incorporating behavioral strategies such as self-monitoring and goal setting. The intervention consisted of weekly contacts. For the first 2 months, participants received 8 weekly face-to-face sessions. Over the next 4 months, participants completed one face-to-face session and 3 telephone phone sessions per month. In total, the program was comprised of 24 weekly contacts including 12 face-to-face and 12 telephone sessions. On average, participants completed 80.3% of the scheduled contacts (19.3/24). With respect to the 12 face to face sessions, 4 participants completed fewer than 6; 32 participants completed 6 to 11; and 25 participants completed all 12 sessions.
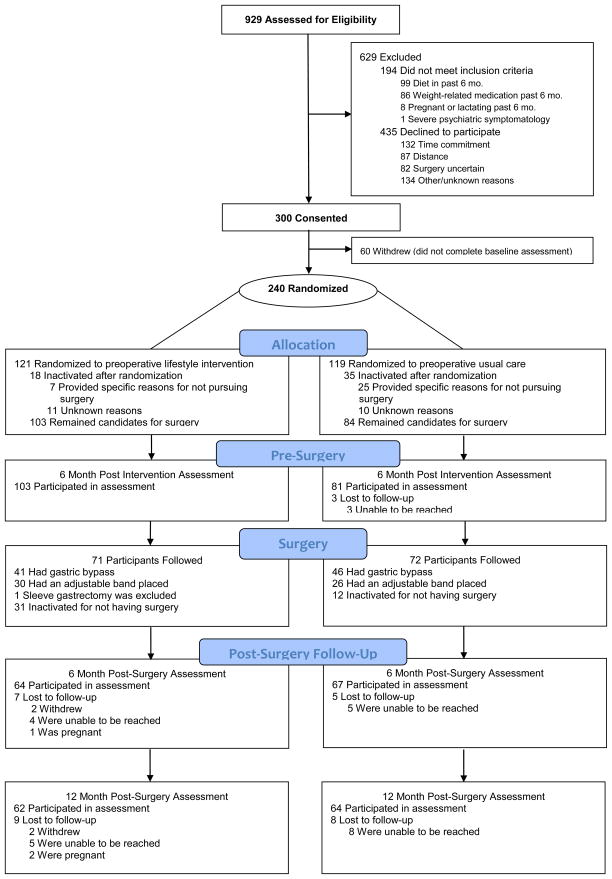
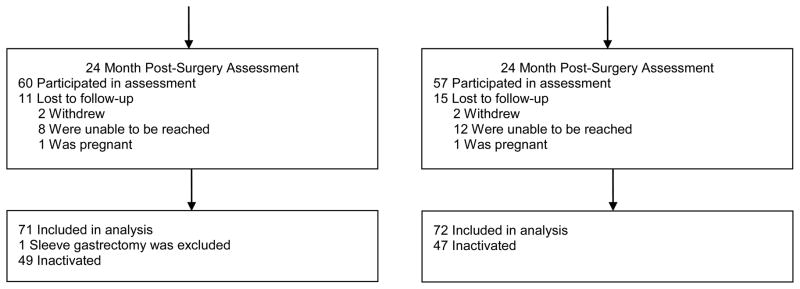
After surgery, participants in the lifestyle intervention group received 3 monthly telephone contacts; the usual care group did not receive any additional contact. Assessments were conducted at 6-, 12- and 24-months after surgery. Patient recruitment and flow are shown in Figure 1.
Figure 1.
Participant recruitment and flow.
Patients self-reported sociodemographic characteristics including sex, age, race/ethnicity, education, employment status, income and marital status. Racial/ethnic categories were American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, and White. Participants also self-reported whether or not they were Hispanic or Latino.
Height was measured prior to randomization using a mounted stadiometer. Participant weight was measured at enrollment and follow-up assessments using a digital scale (Scale-Tronix 5002, Carol Stream IL) at the study office. Participants were weighed and height measured in street clothes, without shoes. BMI was calculated as weight in kilograms divided by the square of height in meters. Weight change is reported as the total percent change from study enrollment (initial weight minus weight at follow-up, divided by initial weight). At all follow-up assessments, weight was collected from the electronic medical record for participants who were unable to complete the in-person study assessment. If neither a measured weight nor a weight from the medical record was available, self-reported weight was used (1 participant self-reported weight at 6 months, 3 at 12 months, and 8 at 24 months). Self-reported weight following bariatric surgery has been shown to be close to measured weight and therefore appropriate for use in studies of surgically induced weight change.(12) Weights of participants who were pregnant at follow-up were not included in analyses (see Figure 1).
We collected information on death and other adverse events within 30 days of discharge using an adapted version of Post-Operative Evaluation Form utilized in the Longitudinal Assessment of Bariatric Surgery (LABS) study.(13) We created a composite endpoint based on occurrence of any one of the following events: death, venous thrombosis, pulmonary embolism, tracheal reintubation, endoscopy, reoperation, and nondischarge at 30 days.
Power and analytic plan
An a priori power calculation based on a two-sample t-test for the difference in outcome measures from pre- to post intervention and across the two groups was computed. The computations assumed a two-sided significance level of 0.05 and an initial sample size of 100 subjects in each of the two groups with 80% retention. Based on these assumptions, the study had a power of 0.80 to detect a moderate effect size between the two groups at post-intervention, with excellent power to evaluate outcomes through 12 months, and sufficient power to model post-surgery weight loss at 24 months. Recognizing that not all study participants would proceed to surgery, 240 individuals were randomized.
The present report includes outcomes through 24 months after surgery among 143 patients who enrolled in the trial and subsequently underwent RYGB or LAGB. Two-sample t tests (or Wilcoxon tests) and chi-square analyses (or Fisher’s exact tests) were performed to test for differences in demographics and baseline variables between groups as well as between participants who were and were not lost at 6- 12- and 24-month post-surgery follow-up.
Mixed-effect models were used to evaluate percent weight loss over time (enrollment to post-intervention, as well as 6, 12- and 24-months post-surgery). Fixed terms included intervention group, surgery type, group by surgery type interaction, time, group by time, procedure by time, and the three-way interactions, as well as BMI at enrollment. Participant was included as a random term to account for dependence among repeated measures from the same participant. Contrasts were computed to compare the two groups on percent weight loss at 6-, 12- and 24-months post-surgery among all surgery patients and separately for RYGB and LAGB patients. Next, the mixed models were re-run with the covariates of sex, race, age and income level. Race and age were significant, with White or older patients losing more weight in both groups [F(1,137) = 5.2, p = 0.02 for race and F(1, 139) = 6.4, p = 0.01 for age]. Results were similar after adjusting for race and age, and thus unadjusted results are presented here.
Weight losses of > 5% in lifestyle intervention programs have been shown to be associated with significant improvements in cardiometabolic risk factors(14,15) Thus, in an exploratory analysis, we examined the impact of > 5 % pre-surgery weight loss, independent of group assignment, on post-surgery weight loss. Mixed-effect models were applied to percent weight loss at 6-, 12- and 24-month post-surgery follow-up. Fixed terms were the same as in previous analysis, in addition to percent weight loss post-intervention (pre-surgery), which was dichotomized at < or > 5 percent. We also examined the relationship within the RYGB and LAGB subgroups.
Results
Participant characteristics are summarized in Table 1. Patients who were lost to follow-up were younger than those who completed the assessments at 6-months (38.5 y vs. 45.5 y, p = 0.03), 12-months (36.5 y vs. 46 y, p = 0.0006) and 24-months (39.3 y vs. 46.2 y, p = 0.004). Those lost to follow-up at 12-months were more likely to have had LAGB (64.7% vs. 35.7%, p = 0.02) than those who completed the 12-month assessment. Those lost to follow-up at 24-months lost less weight during the pre-surgery intervention period (2.5% vs. 4.5%, p = 0.05), compared to those who completed the final assessment.
Table 1.
Characteristics of study participants by randomization group
| Total Sample | Lifestyle intervention (n=71) | Usual care (n=72) | p-value | |
|---|---|---|---|---|
| Age (years) | 44.9±11 | 43.9±10.3 | 45.9±11.6 | 0.26 |
| Baseline BMI (kg/m2) | 47.5±6.4 | 47.4±6.2 | 47.6±6.5 | 0.88 |
| Presurgery BMI (kg/m2) | 45.5±6.3 | 44.6±5.7 | 46.4±6.7 | 0.10 |
| % female | 90.2 | 90.1 | 90.3 | 0.98 |
| % White | 86.7 | 78.9 | 94.4 | 0.007 |
| % Hispanic or Latino | 1.4 | 2.8 | 0 | 0.24 |
| % married | 54.6 | 56.3 | 52.8 | 0.67 |
| % income > $30,000 | 73.2 | 72.9 | 73.5 | 0.93 |
| % above high school | 84.6 | 90.1 | 79.2 | 0.07 |
| % RYGB | 60.8 | 57.8 | 63.9 | 0.45 |
Impact of pre-surgery lifestyle intervention on post-surgery weight loss
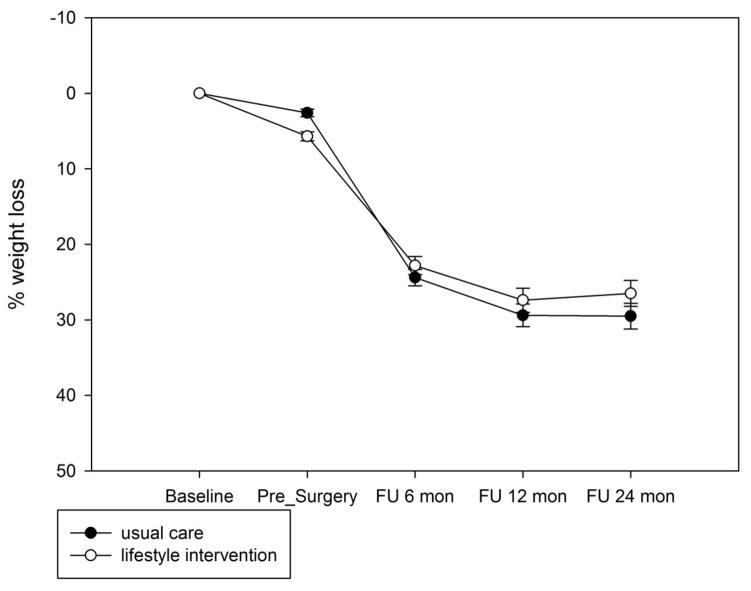
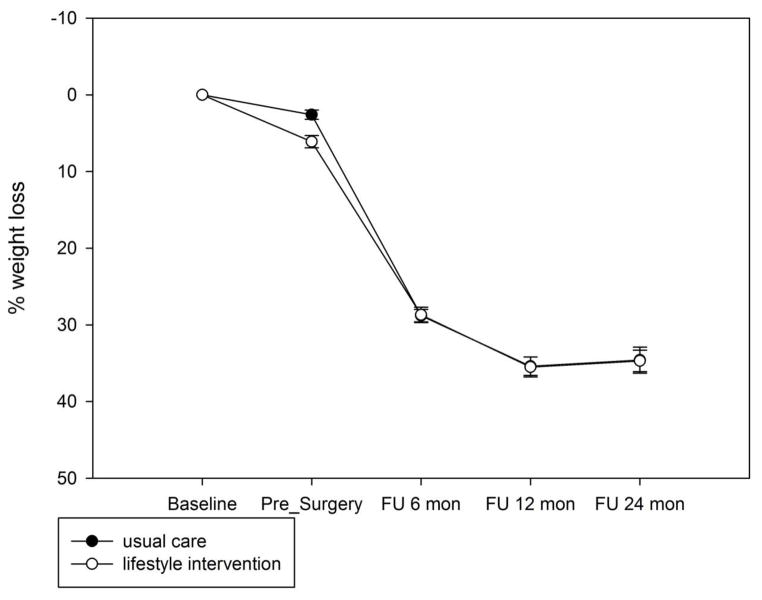
There was a significant interaction between group and time [F(3,133) = 6.49, p = 0.0004]. Contrasts indicated that although the lifestyle intervention group lost a greater percentage of weight from enrollment to post-intervention (5.7% for lifestyle intervention vs. 2.6% for usual care, t = 4.12, p < 0.0001), percent weight loss was comparable at 6-month (22.8% for the lifestyle group vs. 24.4% for the usual care group, t = −1.58, p = 0.12) and 12-month (27.4% vs. 29.4, t = −1.56, p = 0.12) follow-up. The lifestyle group lost less than the usual care group at 24-month follow-up (26.5% vs. 29.5%, t = −2.4, p = 0.02), which corresponded to a modest effect size of 0.23. Weight loss by group is shown in Figure 2.
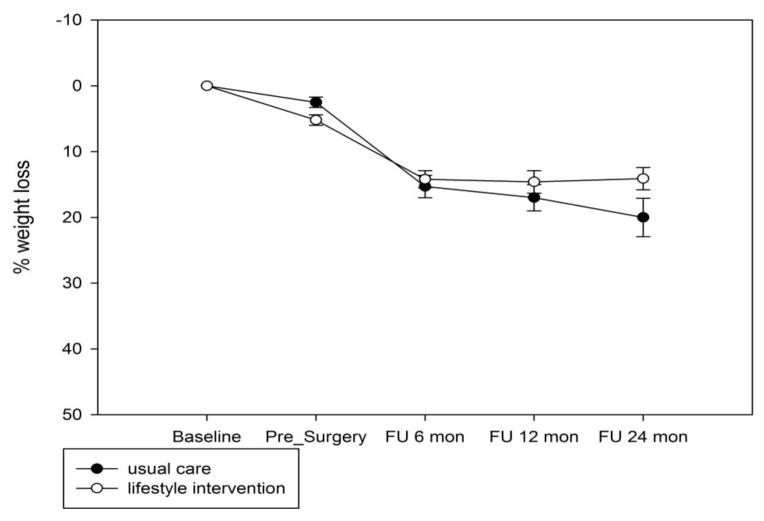
Figure 2.
Percent weight loss by group.
Figure 2a. Percent weight loss by group, adjustable gastric banding only.
Figure 2b. Percent weight loss by group, gastric bypass only.
As expected, there was a significant main effect of surgery type [F(1,139) = 161, p < 0.0001] and a significant interaction of procedure by time such that the patients who had RYGB lost more weight over time than those who had LAGB [F(3,133) = 61, p < 0.0001]. The interaction between group and procedure and the three-way interaction with time were not significant; the results were similar after we controlled for the two significant factors of race and age. Weight loss by group is shown for LAGB and RYGB in Figures 2a and 2b, respectively. On average patients who received LAGB lost 16.8% of weight from enrollment to 24 months (14.1% for the lifestyle group vs. 20% for the usual care group, p = 0.04). For RYGB, the two groups lost comparable amount of weight at 24 months (34.7% for lifestyle vs. 34.5% for usual care, p = 0.4).
Impact of intervention on 30-day major adverse events
All study participants in both groups were discharged within 30 days after surgery. Only one patient experienced an adverse event meeting study criteria, which was a reoperation in the intervention group. Thus, the groups did not differ in the composite endpoint, which was too infrequent for analysis.
Exploratory analysis
There was a significant interaction between time and pre-surgery weight loss [F(2,127) = 4.04, p = 0.02]. At 6 months after surgery, participants with ≥ 5% pre-surgery weight loss lost significantly more weight as compared to those with < 5% pre-surgery weight loss (25.7% vs. 22.3%, t = 3.5, p = 0.0006). However, post surgery weight loss did not differ as a function of 5% pre-surgery weight loss at 12-months (28.5% vs. 28.3%, t = 0.98, p = 0.33) or 24-months (28.1% vs. 27.8%, t = 0.9, p = 0.37), independent of group and surgery type. Similar results were observed in the RYGB and LAGB subgroups, where at 6 months post-surgery, participants with ≥ 5% pre-surgery weight loss lost significantly more weight than those with < 5% pre-surgery weight loss for RYGB (31.1% vs. 27.3%, t = 3.38, p = 0.001); the same pattern was observed in LAGB, but the differences were not significant (16.5% vs. 13.5%, t = 1.6, p = 0.1). The two pre-surgery weight loss groups did not differ in post-surgery weight loss at 12- or 24-months for either surgical subgroup (all p’s ≥ 0.2).
Discussion
The present investigation addresses a timely and important question as to whether a lifestyle preparation program delivered during the pre-surgery period improves longer-term weight loss after surgery. We previously documented that patients randomized to an evidence-based pre-surgery lifestyle intervention lost significantly more body weight prior to surgery and were more likely to achieve at least 5% weight loss than those receiving usual care.(10) In a subsequent report, we documented that the intervention condition was unrelated to self-reported gastrointestinal symptoms at 6 months after surgery.(16) This longer term follow-up documents that the pre-surgery intervention did not favorably impact weight loss over the first two years following surgery or rates of major 30-day post-operative complications. Collectively, these reports suggest that the benefits of pre-surgery lifestyle intervention do not extend to the post-surgery period.
Contrary to expectation, the lifestyle intervention group had significantly lower percent weight loss at 24 months than the comparison group, but the overall between-group difference was modest (26.5% vs. 29.5%). This unexpected result must be interpreted with caution, especially considering the well documented variability in weight loss by procedure and across individuals.(17) It appears that the weight loss differential between the groups was driven by weight loss among the subgroup of patients who underwent laparoscopic adjustable gastric banding. That is, patients undergoing adjustable gastric banding in the usual care group achieved 20% weight loss at 24 months, whereas gastric banding patients in the lifestyle intervention group lost just 14.1%. It is challenging to speculate why completion of a 6-month physician-supervised diet and activity program as required for approval for surgery in usual care would slightly improve weight loss at 24 months among banding patients. Moreover, it is important to keep in mind that patients were not randomized to surgical procedure, and self-selection of procedure may introduce bias, complicating interpretation of results. Nonetheless, this study provides no evidence that the study intervention improved weight loss following gastric bypass or adjustable gastric banding.
The groups in the present study did not differ in 30-day major adverse events. This finding also must be interpreted with caution, as only one patient out of 143 experienced an event (.006%). By comparison, a rate of 4.3% was documented in the LABS study, an observational cohort study which included a much larger sample of 3,412 patients.(18) The difference in rates between studies may be related to characteristics of patients enrolled in this university-based clinical trial, such as the exclusion of patients with a BMI over 70 kg/m2 or high risk surgical profiles. It is also possible that the low rate of adverse events could be an artifact of the small sample size. Thus, this investigation did not detect any benefits of pre-surgery intervention on 30-day adverse events.
We evaluated the relationship of sociodemographic factors, including sex, race, age and income level, to weight loss. Race and ethnicity were included in the examination because they have been related to obesity(19) and weight loss in previous investigations.(20) As expected, older and White patients lost more in both intervention groups, but neither age nor race/ethnicity interacted with group. Similarly, Roux-en-Y gastric bypass patients lost more weight than laparoscopic adjustable gastric banding patients, but surgery type did not interact with group. Thus, based on the results of this study, there does not appear to be a sociodemographic characteristic or surgical procedure for which pre-surgery lifestyle intervention improves post-surgical weight loss.
Weight loss of >5% is achievable even among extremely obese individuals,(8,9) and has been associated with cardiometabolic health benefits.(14,15) Thus, we explored whether > 5% weight loss immediately prior to surgery confers benefits after bariatric surgery. In the present study, achieving > 5% weight loss, independent of group assignment, was not associated with post-surgery weight loss overall, nor for the RYGB and LAGB subgroups. Interestingly, however, those lost to follow-up at 24-months lost significantly less weight during the pre-surgery intervention period (2.5% vs. 4.5%), compared to those who were retained. This suggests that pre-surgery weight loss may serve to improve engagement with the treatment team over time, a possibility which would require additional evaluation. Any potential benefits of pre-surgery weight loss must be weighed against the lack of improved post-surgery weight loss, as well as concerns that the requirement for documentation of prolonged physician supervised weight loss efforts before approval of bariatric surgery could delay or deny access to treatment.(21)
To our knowledge this is the first prospective evaluation of a pre-surgery lifestyle intervention with patients followed over two years post-surgery. Study strengths include its randomized, controlled design, standardized data collection, evidence-based intervention, and good retention of patients. Of 143 patients who underwent gastric banding or gastric bypass, 117 (82%) participated in the 24 month assessment. Limitations include the relatively large proportion of patients who declined or were ineligible upon screening. In particular, patients with a medical condition requiring a specialized preoperative regimen were ineligible. Thus, individuals with the greatest severity of obesity and concomitant health problems were not enrolled in the trial. Additionally, many participants were inactivated after enrollment in the trial because they decided not to pursue surgery. We did not systematically collect information from patients randomized to receive usual care, and thus cannot describe their pre-surgical weight loss programs. Consistent with the bariatric surgery patient population, there was limited representation of men as well as racial and ethnic minority groups. Finally, with gastric banding having decreased in popularity, results may not generalize to the most common types of surgery currently performed. It should be noted that sleeve gastrectomy has increased in popularity accounting for 42.1% of 179,000 procedures in 2013, followed by gastric bypass (34.2 %) and gastric banding (14.0 %).(22)
Conclusions
In summary, this study provides no evidence that pre-surgery lifestyle intervention improves weight loss following bariatric surgery. Contrary to expectation, the intervention group lost significantly less weight at 2 years after operation, although the difference between groups was modest. Due to study inclusion criteria, the results may not generalize to individuals with more severe obesity, medically compromised patients, or other subgroups who could potentially achieve health benefits from pre-surgery lifestyle change. We recommend that any future studies of pre-surgery intervention be tailored to the needs of vulnerable subgroups undergoing different types of bariatric surgery. Additionally, the development of post-surgery lifestyle interventions is clearly warranted, particularly in the first two years after bariatric surgery when patients appear to reach their weight nadir and begin a pattern of gradual regain.(17) Post-surgery behavioral, pharmacological or surgical interventions initiated during this critical period may help to maximize post-surgery weight loss and sustain the health benefits of bariatric surgery.
Acknowledgments
We appreciate the contribution of the many patients and staff at the University of Pittsburgh for their assistance with recruitment, assessments, intervention delivery, and data management. Gina Sweeny, BS, MS, provided project coordination and oversight of data collection.
Research supported by R01DK077102 from the National Institute of Diabetes and Digestive and Kidney Diseases (PI: Melissa A. Kalarchian). ClinicalTrials.gov identifier: NCT00623792
Footnotes
Role of the Sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Conflict of Interest: Dr. Kalarchian reports receiving funding for research in bariatric surgery from NIH/NIDDK, The Obesity Society (TOS)/Nutrisystem, and the American Society for Metabolic and Bariatric Surgery (ASMBS). Dr. Courcoulas reports grants from Nutrisystem, grants from EndoGastric Solutions, and other from J&J Ethicon. Drs. Cheng, Levine and Marcus have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tarnoff M, Kaplan LM, Shikora S. An evidenced-based assessment of preoperative weight loss in bariatric surgery. Obes Surg. 2008;18(9):1059–1061. doi: 10.1007/s11695-008-9603-y. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn GL, Hutter MM, Harvey AM, et al. Expert panel on weight loss surgery: Executive report update. Obesity. 2009;17(5):842–862. doi: 10.1038/oby.2008.578. [DOI] [PubMed] [Google Scholar]
- 3.Ochner CN, Dambkowski CL, Yeomans BL, Teixeira J, Xavier Pi-Sunyer F. Pre-bariatric surgery weight loss requirements and the effect of preoperative weight loss on postoperative outcome. Int J Obesity. 2012;36(11):1380–1387. doi: 10.1038/ijo.2012.60. [DOI] [PubMed] [Google Scholar]
- 4.Cassie S, Menezes C, Birch DW, Shi X, Karmali S. Effect of preoperative weight loss in bariatric surgical patients: A systematic review. Surg Obes Relat Dis. 2011;7(6):760–767. doi: 10.1016/j.soard.2011.08.011. discussion 767. [DOI] [PubMed] [Google Scholar]
- 5.Jamal MK, DeMaria EJ, Johnson JM, et al. Insurance-mandated preoperative dietary counseling does not improve outcome and increases dropout rates in patients considering gastric bypass surgery for morbid obesity. Surg Obes Relat Dis. 2006;2(2):122–127. doi: 10.1016/j.soard.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Alami RS, Morton JM, Schuster R, et al. Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg Obes Relat Dis. 2007;3(2):141–145. doi: 10.1016/j.soard.2006.11.006. discussion 145–146. [DOI] [PubMed] [Google Scholar]
- 7.Solomon H, Liu GY, Alami R, Morton J, Curet MJ. Benefits to patients choosing preoperative weight loss in gastric bypass surgery: New results of a randomized trial. J Am Coll Surg. 2009;208(2):241–245. doi: 10.1016/j.jamcollsurg.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA. 2010;304(16):1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unick JL, Beavers D, Jakicic JM, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: Results from the Look AHEAD trial. Diabetes care. 2011;34(10):2152–2157. doi: 10.2337/dc11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, Levine MD. Preoperative lifestyle intervention in bariatric surgery: Initial results from a randomized, controlled trial. Obesity. 2013;21(2):254–260. doi: 10.1002/oby.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalarchian MA, Marcus MD. Preoperative lifestyle intervention. In: Mitchell JE, de Zwaan M, et al., editors. Psychosocial Assessment and Treatment of Bariatric Surgery Patients. New York: Routledge; 2012. pp. 209–220. [Google Scholar]
- 12.Christian NJ, King WC, Yanovski SZ, Courcoulas AP, Belle SH. Validity of self-reported weights following bariatric surgery. JAMA. 2013;310(22):2454–2456. doi: 10.1001/jama.2013.281043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal assessment of bariatric surgery. Surg Obes Relat Dis. 2007;3(2):116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Part B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, Levine MD. Self-report of gastrointestinal side effects after bariatric surgery. Surg Obes Relat Dis. 2014;10(6):1202–1207. doi: 10.1016/j.soard.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flum D, Belle S, Berk P, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong RJ, Chou C, Ahmed A. Long term trends and racial/ethnic disparities in the prevalence of obesity. J Community Health. 2014;39(6):1150–1160. doi: 10.1007/s10900-014-9870-6. [DOI] [PubMed] [Google Scholar]
- 20.Coleman KJ, Brookey J. Gender and racial/ethnic background predict weight loss after Roux-en-Y gastric bypass independent of health and lifestyle behaviors. Obes Surg. 2014;24(10):1729–1736. doi: 10.1007/s11695-014-1268-0. [DOI] [PubMed] [Google Scholar]
- 21.Brethauer S. ASMBS position statement on preoperative supervised weight loss requirements. Surg Obes Relat Dis. 2011;7(3):257–260. doi: 10.1016/j.soard.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 22. [Accessed May 2014];New procedure estimates for bariatric surgery: What the numbers reveal. 2014 http://connect.asmbs.org/may-2014-bariatric-surgery-growth.html.