Abstract
Background
Binge ethanol (EtOH) intake during adolescence leads to an array of behavioral and cognitive consequences including elevated intake of EtOH during adulthood, with female mice showing greater susceptibility than males. Administration of the metabotropic glutamate receptor 5 (mGluR5) antagonist 3-((2-Methyl-1,3-thiazol-4-yl)ethynyl)pyridine (MTEP) has been shown to reduce EtOH self-administration in adult male mice, but little is known about its effect on female and adolescent mice.
Methods
MTEP (0, 10, 20 mg/kg, i.p.) was repeatedly administered to female and male, adult and adolescent C57BL/6J mice during binge sessions using the scheduled high alcohol consumption paradigm. Next, we assessed whether MTEP administration during binge altered the subsequent 24-hour EtOH intake following a period of abstinence. Finally, we investigated whether MTEP administration during binge followed by an abstinence period altered mRNA of glutamatergic genes within the nucleus accumbens of female mice.
Results
MTEP significantly decreased binge EtOH intake in all mice, but only female mice exhibited altered subsequent 24-hour EtOH intake. Interestingly, the alteration in subsequent EtOH intake in female animals was age dependent, with adolescent exposure to MTEP during binge decreasing 24-hour intake and adult exposure to MTEP during binge increasing 24-hour intake. Finally, while there were no effects of MTEP pretreatment on the genes examined, there was a robust age effect found during analysis of mGluR1 (Grm1), mGluR5 (Grm5), the NR2A subunit of the NMDA receptor (Grin2a), phosphatidylinositol 3-kinase (Pik3r1), mammalian target of rapamycin (Mtor), and extracellular signal-regulated kinase (Mapk1) mRNA, with adolescent female animals having lower expression than their adult counterparts.
Conclusions
Collectively, the present findings add to existing evidence implicating the contribution of long-term effects of adolescent binge drinking to enhance alcohol abuse in adulthood, while suggesting that mGluR5 antagonism may not be the best pharmacotherapy to treat binge alcohol consumption in female and adolescent animals.
Keywords: Alcohol, MTEP, Adolescent, Adult, Glutamate
INTRODUCTION
Binge ethanol (EtOH) drinking is the most prevalent form of alcohol use disorder (Centers for Disease Control and Prevention [CDC], 2012) and was estimated to cost society $170.7 billion in 2006 (Bouchery et al., 2011); therefore, development of pharmacotherapies to reduce binge drinking is an important goal. One potential pharmacotherapeutic target is the metabotropic glutamate receptor 5 (mGluR5). Inhibition ofmGluR5 signaling using the antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reduced the rewarding effects of EtOH in animal models of EtOH drinking as well as during self-administration and reinstatement (Bäckström et al., 2004; Besheer et al., 2010; Hodge et al., 2006; Lominac et al., 2006; McMillen et al., 2005; Olive et al., 2005; Schroeder et al., 2005). When locally infused into the nucleus accumbens (NAc), MPEP significantly decreased binge EtOH consumption (Cozzoli et al., 2009, 2012). Other groups also had success using 3-((2-Methyl-1,3-thiazol-4-yl)ethynyl)pyridine (MTEP), a more selective mGluR5 antagonist with less nonspecific pharmacological effects (Anderson et al., 2003; Gass and Olive, 2008). MTEP reduced EtOH self-administration following systemic injection (Cowen et al., 2005, 2007) or infusion into the NAc shell (Gass and Olive, 2009), and infusions of MTEP into the NAc or basolateral amygdala significantly decreased cue-induced reinstatement of EtOH seeking in males (Sinclair et al., 2012). Additionally, when locally infused into the central nucleus of the amygdala, MTEP significantly decreased binge EtOH consumption (Cozzoli et al., 2013). However, no data to date have investigated the impact of MTEP administration during EtOH consumption in female animals, nor has there been an evaluation of the consequences of mGluR5 inhibition in adolescents. Considering that binge drinking places females and adolescents at an increased risk for developing health problems (CDC, 2013; U.S. Department of Health and Human Services, National Institute on Alcohol Abuse and Alcoholism [USDHHS/NIAAA], 2006), studies designed to specifically examine these groups are important.
We have developed a murine model of binge drinking (scheduled high alcohol consumption [SHAC]) that consistently produces high blood EtOH concentrations (BECs > 100 mg/dl or 1.0 mg/ml; Finn et al., 2005; Strong et al., 2010) and exceeds the NIAAA definition of binge (BECs > 80 mg/dl; USDHHS/NIAAA, 2004). Using the SHAC model, previous data from our laboratory reported that 7 intermittent binge exposures during adolescence (postnatal Days 28 to 42; Spear, 2000) significantly increased EtOH intake during adulthood and that female mice were more vulnerable to this effect than male mice (Strong et al., 2010). The results of this study complement the growing body of literature that suggest adolescent EtOH exposure increases EtOH intake in adulthood in a variety of rodent models (Broadwater et al., 2013; Carrara-Nascimento et al., 2013; Moore et al., 2010; Pascual et al., 2009; Siciliano and Smith, 2001). However, it is not known whether pharmacological manipulation to decrease binge EtOH drinking in adolescence is capable of augmenting subsequent EtOH intake in adulthood.
To gain further understanding into the sex- and age-dependent characteristics of binge EtOH drinking, the current work determined whether male and female adolescent and adult mice were differentially sensitive to mGluR5 antagonism with MTEP to significantly decrease binge drinking and whether MTEP administration would alter subsequent EtOH intake after a period of abstinence. Because EtOH intake after the period of abstinence was altered only in female mice, a study was conducted in adolescent and adult female mice to determine whether the ability of MTEP to alter binge drinking and subsequent EtOH intake was associated with alterations in expression of various glutamatergic genes in the NAc, including mGluR1 (Grm1) and mGluR5 (Grm5) and selected downstream signaling molecules.
MATERIALS AND METHODS
Subjects
Male and female C57BL/6J (B6) mice were utilized. Adult mice were either obtained from a breeding colony maintained by the Portland Alcohol Research Center in the Department of Veterans Affairs Veterinary Medical Unit (Portland, OR) or from Jackson Laboratory—West (Davis, CA) at 7 weeks of age. Equal numbers of adult animals were used from the internal breeding colony and Jackson Laboratory, and animals were counterbalanced in each experimental condition to control for possible differences. All adolescent mice were obtained postweaning (3 weeks ± 3 days) from Jackson Laboratory. Mice were maintained on a 12-hour light–dark cycle (lights on 0600) in a temperature (22 ± 2°C) and humidity controlled environment. Rodent chow (Labdiet 5001 rodent diet; PMI International, Richmond, IN) and water were available ad libitum, except for the SHAC procedure where fluid availability was scheduled. All rodent cages were changed once per week. One week prior to testing, mice were individually housed in clear polycarbonate cages (28 × 18 × 13 cm) on Ecofresh bedding and allowed to acclimate to the procedure room. Therefore, at the start of SHAC procedures, adult and adolescent animals were at least 8 and 4 weeks old, respectively. All procedures complied with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the local Institutional Animal Care and Use Committee.
SHAC Procedure
The procedure has been described elsewhere (Finn et al., 2005; Strong et al., 2010). Briefly, mild fluid restriction is used to condition mice to drink their daily fluid requirement on a schedule during the light cycle. Animals have varying amounts of total fluid access per day, ranging from 4 to 10 hours, with 30-minute access to a 5% v/v EtOH solution (5E) in water every third day. Following the EtOH access period, water is provided for the remainder of the period of fluid availability. Water is the only fluid available on the intervening days. This 3-day cycle of fluid access is repeated so that animals receive a total of seven 30-minute binge EtOH sessions (21 days total). Animals are weighed daily.
In this study, groups were balanced for fluid intake over Days 1 and 2 of the procedure, then separate groups of mice were administered MTEP (10 or 20 mg/kg in saline, 0.01 ml/gm body weight, intraperitoneally [i.p.]; Tocris Bioscience, Bristol, UK) or saline in a between subjects design at 30 minutes prior to each binge session. The MTEP doses have been shown to maintain receptor occupancy in mice for 40 minutes postinjection (Anderson et al., 2003, p. 39) and to alter behavior in murine models (Anderson et al., 2003; Cowen et al., 2007).
Blood EtOH Concentration
BEC was measured after the final binge EtOH session (Day 21). A 20-μl blood sample was taken from the retro-orbital sinus immediately following the session, and the blood sample was analyzed for EtOH content via headspace gas chromatography (Finn et al., 2007).
Unlimited Access Procedure
During this procedure, water and food were freely available. After 1 month of abstinence, a subset of animals was introduced to a 24-hour access procedure. The paradigm was a modification of that described by Yoneyama and colleagues (2008), with mice having 24-hour access to 2 bottles; 1 contained an EtOH solution and 1 contained water. On consecutive weeks, mice had 24-hour access to 5E for 4 days, 10% v/v EtOH (10E) for 4 days, and 5E again for 4 days, with 72-hour abstinence over the weekends. We retested the 5E solution, as the initial group differences in 5E intake disappeared during exposure to 10E (in the second week). At each concentration change, body weights were measured.
NAc Dissection and Sample Preparation
A subset of the female animals was decapitated after the 1-month period of abstinence. The brain was extracted, chilled on ice, and sectioned (freehand) on a plate over ice. Coronal sections (1 to 2 mm) were cut, and the NAc was micropunched using a 16-gauge hollow needle, based on established anatomical coordinates from the mouse brain atlas (Paxinos and Franklin, 2001). All samples were placed in microcentrifuge tubes (1.5 ml), frozen immediately and then stored at −80°C until total RNA isolation. Total RNA isolation and purification was performed according to the Absolutely RNA Microprep Kit (Agilent Technologies, Santa Clara, CA). The purity of the DNase-treated RNA was confirmed by UV 260/280 ratios of 2.0. RNA integrity was confirmed using denaturing agarose gel electrophoresis followed by staining with SYBR Gold (Life Technologies, Grand Island, NY).
Real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed with the iCycler IQ Real Time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA) using a 1-step QuantiTect SYBR Green RT-PCR kit (Qiagen, Valencia, CA) on DNase-treated total RNA (Hashimoto et al., 2004, 2011). The qRT-PCR reactions were carried out in 25 μl with 20 ng of total RNA.
Steady-state mRNA levels were determined using qRT-PCR analysis. Real-time qRT-PCR efficiency was determined for each primer set using a 5-fold dilution series of total RNA and did not differ significantly from 100%. Possible contamination of the PCR reaction was characterized with melt curve analysis. Relative expression of the RT-PCR product was determined using the comparative ΔΔCt method, after normalizing expression to total RNA measured with RiboGreen (Molecular Probes, Eugene, OR; Hashimoto et al., 2004). Primers were purchased predesigned from Qiagen.
Data Analysis
For the SHAC procedure, the dependent variables were BEC, volume (ml) of EtOH or water consumed, dose of EtOH consumed (g/kg) and body weight. The effect of the period of fluid access as well as the drug treatment, age, and sex of the animal on these dependent measures was assessed by analysis of variance (ANOVA). When appropriate, simple main effect analysis and post hoc comparisons were conducted.
For the preference drinking procedures, the dependent variables were volume of EtOH or water consumed, g/kg of EtOH consumed, body weight, and preference ratio (calculated by dividing volume of EtOH consumed by the total fluid consumed). ANOVA assessed sex and drug exposure effects on the dependent variables. Significant interactions were pursued by 1-way ANOVAs and post hoc evaluations. Due to our a priori hypothesis that there would be more robust differences in the drinking patterns of female, rather than male mice, planned comparisons were conducted in female mice in the absence of a significant interaction.
For the qRT-PCR data, all data were first normalized to Ribo-Green. These ratios were then normalized to the mean ratio for each gene of the saline adult for each qPCR reaction. ANOVAs on the data were then conducted with age and treatment as factors.
In all cases, data are presented as the mean ± SEM. The level of significance was set as p ≤ 0.05. Statistical analyses were conducted with SYSTAT (version 11; SYSTAT Software, Inc., Richmond, CA).
RESULTS
Effect of MTEP Pretreatment on Binge EtOH Intake
MTEP administration significantly decreased binge EtOH consumption in male and female, adult and adolescent B6 mice. Data for EtOH consumed (g/kg) for all cohorts of mice are depicted in Fig. 1. Adolescent and adult mice were tested in separate experimental passes, so data for each age were analyzed separately.
Fig. 1.
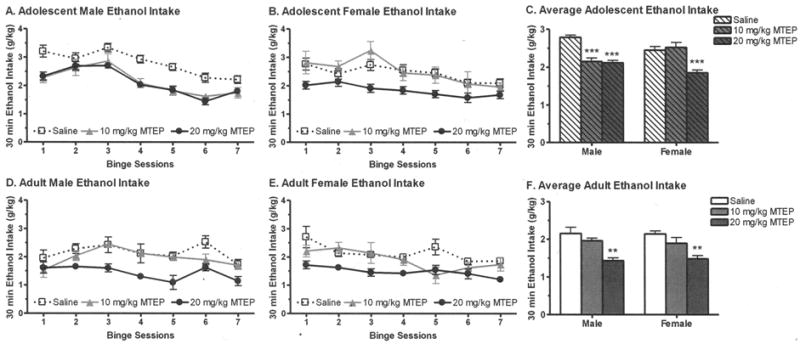
Systemic administration of MTEP significantly decreased binge drinking in (A–C) adolescent and (D–F) adult mice. Both MTEP doses decreased binge ethanol (EtOH) intake by 23 to 24% in adolescent males, while the highest dose decreased binge intake by 24% in adolescent females. In adult mice, the highest MTEP dose decreased binge EtOH intake by 31% (females) or 33% (males). Values are the mean ± SEM for 6 (saline) or 7 (per MTEP dose). **p < 0.01, ***p ≤ 0.001 versus respective saline group.
In adolescent mice (Fig. 1A–C), binge EtOH intake was significantly reduced by MTEP pretreatment, F(2, 66) = 35.50, p < 0.001. The significant interaction between sex and MTEP, F(2, 66) = 7.86, p < 0.01, suggested that the efficacy of MTEP to reduce binge EtOH intake differed in the male and female mice. While the effect of MTEP was significant in both male, F(2, 31) = 34.47, p < 0.001, and female, F(2, 35) = 16.73, p < 0.001, mice, deconstruction of the interaction revealed a significant difference between males and females at every dose (saline: males > females; 10 mg/kg MTEP: males < females; 20 mg/kg MTEP: males > females; ps < 0.05). While both MTEP doses significantly decreased EtOH intake by 23 to 24% in male mice, only the 20 mg/kg MTEP dose significantly decreased EtOH intake by 24% in the female mice.
In adult mice (Fig. 1D–F), binge EtOH intake was similar in male and female saline-injected mice and was also significantly reduced by MTEP pretreatment, F(2, 52) = 21.82, p < 0.001. However, the interaction between sex and MTEP treatment was not significant, indicating a similar efficacy of MTEP to reduce binge EtOH intake in male and female mice. Post hoc tests indicated that the 20 mg/kg MTEP dose significantly decreased EtOH intake by 33 or 31% in the male and female mice, respectively.
To verify that the effective dose of MTEP not only reduced EtOH intake but also BECs, the BECs were assessed directly following the seventh binge EtOH session on Day 21 in a subset of animals (Table 1). In general, the MTEP-induced decrease in EtOH intake corresponded to a significant decrease in BEC and g/kg EtOH intake on Day 21. In adolescent male and female mice, BEC and g/kg EtOH intake on Day 21 were significantly decreased by 20 mg/kg MTEP pretreatment, BEC: F(1, 28) = 22.09, p < 0.001; g/kg: F(1, 53) = 10.079, p < 0.01. Similar results were found in adult male and female mice, with 20 mg/kg MTEP significantly reducing BEC and g/kg EtOH intake on Day 21, BEC: F(1, 40) = 31.64, p < 0.001; g/kg: F(1, 39) = 21.82, p < 0.001. Overall, MTEP significantly decreased Day 21 BEC and binge EtOH intake in adolescent and adult male and female mice, confirming the results depicted in Fig. 1 when data were averaged across all binge sessions.
Table 1.
Effect of MTEP (20 mg/kg) on EtOH Intake and BEC During the Seventh Binge EtOH Session
| Sex/Age | Treatment | EtOH intake (g/kg) | BEC (mg/ml) |
|---|---|---|---|
| Male adult | Saline | 1.74 ± 0.17 (6) | 1.30 ± 0.08 (6) |
| MTEP | 1.14 ± 0.16* (7) | 0.67 ± 0.08*** (7) | |
| Female adult | Saline | 1.86 ± 0.09 (16) | 1.09 ± 0.08 (16) |
| MTEP | 1.20 ± 0.11*** (14) | 0.67 ± 0.07** (15) | |
| Male adolescent | Saline | 2.22 ± 0.13 (13) | 1.60 ± 0.10 (7) |
| MTEP | 1.81 ± 0.10* (14) | 1.11 ± 0.10** (7) | |
| Female adolescent | Saline | 2.10 ± 0.15 (13) | 1.35 ± 0.13 (8) |
| MTEP | 1.68 ± 0.13* (17) | 0.88 ± 0.07** (10) |
Depicted are data from the final (seventh) binge ethanol (EtOH) session, which confirm that the significant decrease in binge EtOH intake following MTEP (20 mg/kg) pretreatment averaged across sessions (e.g., Fig. 1) also existed on Day 21. Analysis of blood EtOH concentrations (BECs) assessed on Day 21 confirmed that MTEP significantly decreased BECs in all animals. Values are the mean ± SEM for the number of animals shown in parentheses. Significant differences versus respective saline are depicted in bold font.
p < 0.05,
p < 0.01,
p < 0.001, post hoc test.
Analysis of total intake (in ml; Table 2), averaged over the 21 days, revealed a main effect of age (adolescents < adults), F(1, 118) = 4.78, p < 0.05, and sex (females < males), F(1, 118) = 4.70, p < 0.05. Thus, while fluid intake was lower in adolescents versus adults and lower in adult males versus females, there was no effect of MTEP.
Table 2.
Effect of MTEP (20 mg/kg) on Total Fluid Intake and Body Weight
| Sex/Age | Treatment | n/Group | Daily fluid intake (ml) | Body weight (g) |
|---|---|---|---|---|
| Male adult | Saline | 6 | 3.43 ± 0.09 | 20.79 ± 0.75 |
| MTEP | 7 | 3.73 ± 0.13 | 21.28 ± 0.63 | |
| Female adult | Saline | 16 | 3.28 ± 0.13 | 18.70 ± 0.44 |
| MTEP | 15 | 3.38 ± 0.16 | 17.69 ± 0.33 | |
| Male adolescent | Saline | 13 | 3.43 ± −0.11 | 15.44 ± 0.32 |
| MTEP | 14 | 3.31 ± 0.08 | 15.17 ± 0.37 | |
| Female adolescent | Saline | 14 | 3.11 ± 0.14 | 13.08 ± 0.27 |
| MTEP | 17 | 3.13 ± 0.12 | 13.59 ± 0.25 |
Depicted are the average daily fluid intake (ml) and body weight (g) of all animals. MTEP pretreatment did not impact total daily fluid intake or body weight.
Analysis of body weight (Table 2), averaged over the 21 days, revealed main effects of both sex (females < males), F(1, 118) = 90.63, p < 0.001, and age (adolescents < adults), F(1, 118) = 439.11, p < 0.001, as well as a significant interaction between sex and age, F(1, 118) = 4.76, p < 0.05, but no effect of MTEP pretreatment.
Effect of Prior MTEP Pretreatment During Binge EtOH Exposure on Subsequent EtOH Intake After a Period of Abstinence
After a 1-month period of abstinence, adolescent and adult male and female mice that had undergone SHAC binge exposure in conjunction with either MTEP or vehicle pretreatment were tested for EtOH preference drinking (intake of 5E, 10E, and 5E during separate weeks). Due to a lack of significant MTEP dose differences, data were collapsed across the 10 and 20 mg/kg doses. Total 24-hour intake (g/kg) across days for all animals is shown in Fig. 2. Data for 24-hour EtOH intake and preference averaged across each week are presented in Table 3 (females) and Table 4 (males). Overall, MTEP pretreatment decreased subsequent EtOH intake and preference in adolescent female mice, but increased EtOH intake and preference in adult female mice. In contrast, no changes in subsequent EtOH intake and preference were observed after MTEP pretreatment in male adolescent and adult mice.
Fig. 2.
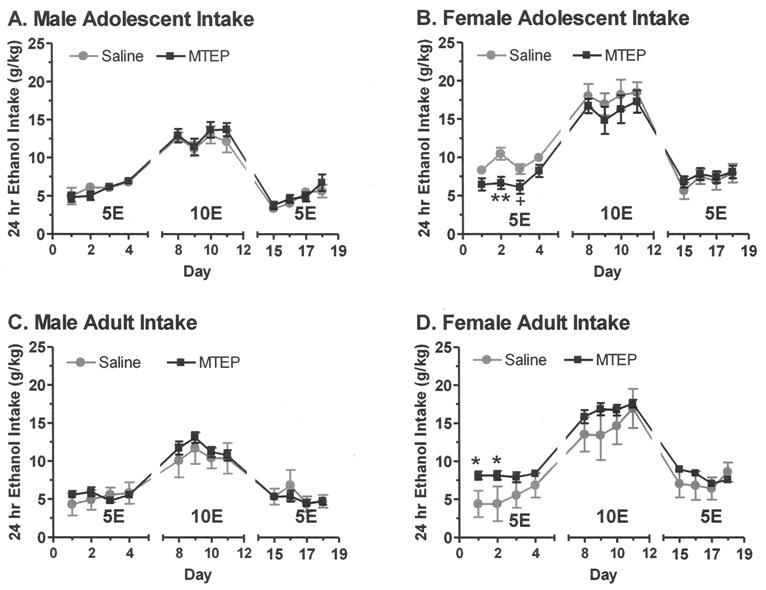
Administration of MTEP during binge ethanol (EtOH) drinking altered subsequent 24-hour EtOH intake in (B) adolescent and (D) adult female mice after a 1-month period of abstinence without altering intake in (A) adolescent and (C) adult male mice. A previous history of MTEP administration during binge EtOH sessions reduced 24-hour EtOH intake of 5% EtOH (5E) on Days 2 and 3 in adolescent females (panel B) and increased 5E intake on Days 1 and 2 in adult females (panel D). No changes occurred in adolescent (panel A) or adult (panel C) male mice. Values are the mean ± SEM for 6 (saline) or 12 to 14 (MTEP). *p < 0.05, **p < 0.01, +p < 0.10 versus respective saline group.
Table 3.
Twenty-Four-Hour Ethanol (EtOH) Intake and Preference After a Period of Abstinence in Female Mice
| Week 1: 5% EtOH Days 1–4
|
Week 2: 10% EtOH Days 8–11
|
Week 3: 5% EtOH Days 15–18
|
||||
|---|---|---|---|---|---|---|
| Age/Prior treatment | Intake (g/kg) | Preference ratio | Intake (g/kg) | Preference ratio | Intake (g/kg) | Preference ratio |
| Adult saline | 6.58 ± 1.55 | 0.59 ± 0.11 | 15.47 ± 2.65 | 0.75 ± 0.07 | 8.13 ± 1.34 | 0.83 ± 0.06 |
| Adult MTEP | 8.12 ± 0.43ˆ | 0.80 ± 0.02* | 16.83 ± 0.59 | 0.82 ± 0.03 | 7.99 ± 0.35 | 0.83 ± 0.03 |
| Adolescent saline | 9.30 ± 0.41 | 0.86 ± 0.02 | 17.90 ± 1.28 | 0.75 ± 0.05 | 6.97 ± 1.05 | 0.65 ± 0.10 |
| Adolescent MTEP | 6.85 ± 0.65* | 0.68 ± 0.06+ | 16.15 ± 1.39 | 0.70 ± 0.06 | 7.52 ± 0.67 | 0.71 ± 0.06 |
Mice were pretreated with MTEP or saline prior to each binge EtOH session (total of 7 binge sessions), and EtOH preference drinking was measured after a 1-month period of abstinence. Prior MTEP administration exerted a differential effect on subsequent 24-hour EtOH intake and preference in adolescent and adult female mice. As data did not differ between the 2 MTEP doses, the MTEP groups were combined and analyzed versus saline. Prior MTEP treatment decreased EtOH intake and preference in the adolescent mice and had the opposite effect in the adult mice. Values are the mean ± SEM for 14 (MTEP) or 6 (saline) mice. Differences versus respective saline are depicted in bold font.
p < 0.01, post hoc test;
p = 0.08, post hoc test;
p < 0.05 on Day 1 and 2 only.
Table 4.
Twenty-Four-Hour Ethanol (EtOH) Intake and Preference After a Period of Abstinence in Male Mice
| Week 1: 5% EtOH Days 1–4
|
Week 2: 10% EtOH Days 8–11
|
Week 3: 5% EtOH Days 15–18
|
||||
|---|---|---|---|---|---|---|
| Age/Prior Treatment | Intake (g/kg) | Preference ratio | Intake (g/kg) | Preference ratio | Intake (g/kg) | Preference ratio |
| Adult saline | 5.14 ± 1.18 | 0.63 ± 0.14 | 10.19 ± 1.96 | 0.67 ± 0.13 | 4.75 ± 0.90 | 0.68 ± 0.12 |
| Adult MTEP | 5.56 ± 0.44 | 0.74 ± 0.05 | 11.74 ± 0.51 | 0.75 ± 0.03 | 4.99 ± 0.41 | 0.69 ± 0.05 |
| Adolescent saline | 5.98 ± 0.55 | 0.73 ± 0.04 | 12.25 ± 0.84 | 0.69 ± 0.05 | 4.61 ± 0.25 | 0.64 ± 0.04 |
| Adolescent MTEP | 5.73 ± 0.32 | 0.69 ± 0.03 | 12.88 ± 0.82 | 0.70 ± 0.03 | 5.00 ± 0.41 | 0.64 ± 0.03 |
Mice were pretreated with MTEP or saline prior to each binge EtOH session (total of 7 binge sessions), and EtOH preference drinking was measured after a 1-month period of abstinence. Prior MTEP administration did not significantly alter subsequent 24-hour EtOH intake or preference in adolescent and adult male mice. As data did not differ between the 2 MTEP doses, the MTEP groups were combined and analyzed versus saline. Values are the mean ± SEM for 14 (MTEP) or 6 (saline) mice.
In adolescent mice, there was a significant reduction in Week 1 EtOH intake (5E, g/kg) by MTEP pretreatment, F(1, 36) = 5.01, p < 0.05, and 5E intake was significantly higher in female versus male mice, F(1, 36) = 13.65, p = 0.001. Although the interaction failed to reach significance (p = 0.075), planned comparisons examined the effect of MTEP pretreatment by sex based on our a priori hypothesis that females would be differentially sensitive to prior MTEP treatment. Analysis confirmed that MTEP pretreatment did not alter averaged (Table 4) or daily (Fig. 2A) 5E intake in adolescent males. However, in female adolescent mice, MTEP pretreatment significantly decreased average 5E intake (Table 3), significantly decreased 5E intake on Day 2, and tended to decrease 5E intake on Day 3 (Fig. 2B). Analysis of Week 2 (10E, g/kg) and Week 3 (5E, g/kg) revealed a significant increase in 24-hour EtOH intake in females when compared with males, Week 2: F(1, 36) = 11.03, p < 0.01; Week 3: F(1, 36) = 12.265, p = 0.001, but no effect of MTEP pretreatment.
Analysis of Week 1 preference revealed a significant reduction in EtOH preference by MTEP pretreatment, F(1, 36) = 3.93, p < 0.05, in adolescent mice. Post hoc tests revealed no effect of MTEP pretreatment on adolescent males (Table 4) but a trend toward a decrease in adolescent females (Table 3). Analysis of Week 2 and 3 failed to reveal any effect of MTEP pretreatment or sex on 24-hour EtOH preference.
In adult mice, 24-hour EtOH intake (g/kg) during each week was significantly higher in females than in males, Week 1: F(1, 35) = 6.905, p = 0.01; Week 2: F(1, 35) = 20.10, p < 0.0001; Week 3: F(1, 35) = 24.82, p < 0.0001. Planned comparisons were conducted and (as shown in Fig. 2D) MTEP pretreatment significantly increased 5E intake on Days 1 and 2 in adult female mice, but there was no effect of MTEP pretreatment on daily 5E intake in adult male mice (Fig. 2C).
Week 1 EtOH preference was significantly increased by MTEP pretreatment, F(1, 35) = 4.92, p < 0.05, in adult mice. Post hoc tests revealed no effect of MTEP pretreatment on preference in adult males (Table 4) but a significant increase in preference in adult females (Table 3). Deconstruction of this effect by day revealed a significant increase in EtOH preference in the adult females on Day 1 (p < 0.01) and Day 2 (p < 0.05) as well as a slight trend on Day 3 (p = 0.10; data not shown). Analysis of Week 2 and 3 failed to reveal any effect of MTEP pretreatment, although females showed an increase in EtOH preference when compared with males during Week 3, F(1, 35) = 5.75, p < 0.05.
Effect of Prior MTEP Pretreatment During Binge Drinking on mRNA of Select Glutamatergic Genes Following a Period of Abstinence
To establish whether mRNA expression of glutamatergic genes and downstream signaling molecules were altered following MTEP pretreatment during binge EtOH experience, qRT-PCR analysis was performed. Analysis was performed at the same time point that alterations in unlimited EtOH intake were seen in female mice on tissue isolated from the NAc of a subset of the female mice. As changes in drinking after abstinence were not observed in male mice, only female mice were examined in this study.
As shown in Fig. 3, there was no effect of prior MTEP treatment on glutamatergic mRNA levels within the NAc expressed relative to adult saline control groups. However, for most of the genes examined, levels were significantly lower in adolescent versus adult mice. Expression of Grm1, F(1, 24) = 16.16, p < 0.01, and Grm5, F(1, 24) = 47.72, p < 0.01, was significantly lower by 35 and 46% in the adolescent versus adult mice, respectively. Additional qRT-PCR was conducted on the mRNA encoding NMDA receptor subunits, Homer2, and signaling molecules downstream of Group 1 mGluRs. Within the NAc, expression of the gene encoding the NR2A subunit of the NMDA receptor (Grin2a) was significantly lower (71%) in adolescent versus adult mice, F(1, 24) = 250.42, p < 0.001. This age-dependent alteration carried over to several of the signaling molecules investigated. Specifically, expression of genes encoding phosphatidylinositol 3-kinase (Pik3r1), F(1, 24) = 59.21, p < 0.001, mammalian target of rapamycin (Mtor), F(1,24) = 129.43, p < 0.001, and extracellular signal-regulated kinase (Mapk1), F(1, 24) = 49.50, p < 0.001, were significantly lower in adolescent versus adult mice by 36.5, 47, and 48%, respectively. Additional investigation of Grm1, Grm5, and Grin2a levels in NAc of a separate cohort of female mice that were only administered water throughout the SHAC procedure showed no difference between adult and adolescent animals (Table 5), confirming that the age effect was the direct result of binge experience.
Fig. 3.
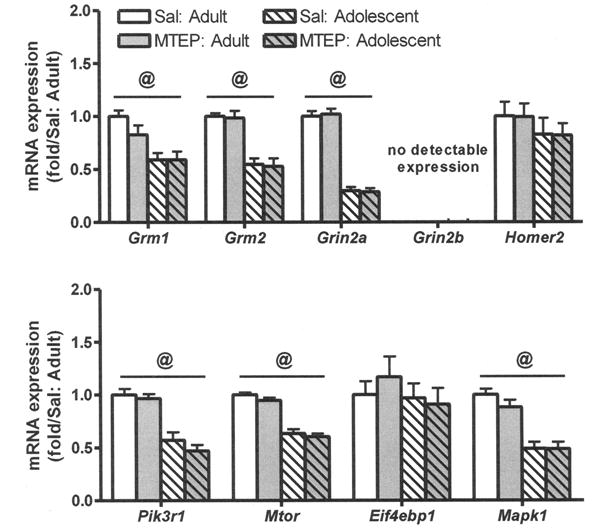
Effect of MTEP (20 mg/kg) administration during binge ethanol exposure, followed by a 1-month period of abstinence, on mRNA in the nucleus accumbens (NAc) of adolescent and adult female mice. With the exception of Eif4ebp1 and Homer2, mRNA expression of all genes detected in the NAc was lower in adolescent versus adult female mice but was not altered by prior MTEP pretreatment. Fold regulation was determined by normalizing all values to the mean of the relative expression for the adult (saline [SAL]) control group. Values represent the mean ± SEM for 7 per group. @p < 0.05 for main effect of age.
Table 5.
mRNA in the Nucleus Accumbens of Water Drinking Adolescent and Adult Female Mice
| Gene | Age | n/Group | mRNA levels |
|---|---|---|---|
| Grm1 | Adults | 7 | 1.0 ± 0.124 |
| Adolescents | 7 | 1.036 ± 0.091 | |
| Grm5 | Adults | 7 | 1.0 ± 0.089 |
| Adolescents | 7 | 1.121 ± 0.102 | |
| Grin2a | Adults | 7 | 1.0 ± 0.095 |
| Adolescents | 7 | 0.793 ± 0.096 |
A separate cohort of adolescent and adult female mice served as naïve controls (i.e., no MTEP or binge ethanol [EtOH]). Mice were introduced to the scheduled high alcohol consumption procedure, but on EtOH drinking days, these animals received only water. Once the 21 days of drinking were concluded, mice were euthanized 24 hours later, and tissue was collected for subsequent quantitative reverse transcriptase polymerase chain reaction analysis. Fold regulation was determined by normalizing all values to the mean relative expression of the adult group. Values are the mean ± SEM.
DISCUSSION
Evidence indicates that mGluR5 antagonism decreased binge drinking in adult male rodents, but the effects of mGluR5 antagonists have not been examined in adult female or in adolescent male and female mice. We report here that MTEP (20 mg/kg) significantly decreased binge EtOH intake in adult and adolescent, male and female mice, although the decrease was greater in adult (↓ 31 to 33%) versus adolescent (↓ 23 to 24%) mice. Analysis of each binge session revealed a similar and significant decrease in EtOH intake following 20 mg/kg MTEP in adult and adolescent mice (Fig. 1). Importantly, MTEP significantly decreased BEC in all animals (Table 1), but it decreased BEC from binge to nonbinge levels only in adult mice. These results suggest that MTEP was more efficacious in adult versus adolescent mice.
In our prior work (Strong et al., 2010), binge EtOH experience in adolescent mice significantly increased EtOH intake during adulthood after a period of abstinence, when compared with adult mice with prior binge exposure, and female mice were particularly vulnerable to this effect. Therefore, we also determined whether the mGluR5-induced reduction in binge drinking would differentially alter the subsequent EtOH intake that occurred after a period of abstinence. An interesting finding was that only the female mice pretreated with MTEP during binge EtOH exposure exhibited altered EtOH intake after a period of abstinence, with opposite changes in adolescent versus adult females. Notably, 5E intake following abstinence was reduced in mice that received MTEP treatment as adolescents and was increased in mice that received MTEP treatment as adults, when compared to respective saline-treated counterparts. This change in 5E intake only was evident during the first week of re-exposure to EtOH, suggesting that MTEP may have interacted with binge EtOH experience to transiently influence subsequent EtOH intake. In the work presented here, mice receiving MTEP versus saline had different EtOH exposures, making it difficult to distinguish whether MTEP administration or reduced binge EtOH experience or an interaction of the 2 factors was contributing to EtOH preference drinking after abstinence. Additionally, the interaction between these effects may differ between males and females or between adults and adolescents, given the sex- and age-related differences in the effect of MTEP pretreatment on subsequent EtOH preference drinking. It is possible that the lower efficacy in the adolescent animals was due to the development of tolerance with repeated administration or that age-related differences in absorption or metabolism of MTEP produced lower receptor occupancy in the adolescent animals. Anderson and colleagues (2003, p. 39) reported that the ED50 value for MTEP occupancy at 60 minutes postinjection in adult male C57BL/6J mice was 17.3 mg/kg and that a 16 mg/kg dose produced full receptor occupancy for up to 40 minutes postinjection. Based on this evidence, we presume that the 20 mg/kg MTEP dose produced full receptor occupancy in adult male mice during the first half of the binge EtOH sessions. However, further studies are needed to more fully address the temporal relationship between receptor occupancy and behavior as well as tolerance development following repeated MTEP treatment in adolescent versus adult mice. Nonetheless, the novel finding that an MTEP-induced decrease in binge drinking differentially altered subsequent EtOH intake, depending on sex and age of the animal, could have important clinical implications with regard to possible limitations in the therapeutic potential of short-term mGluR5 antagonism for the treatment of alcohol abuse.
Previous work in adult male rats found that repeated MTEP injections produced a nonsignificant decrease in the mRNA for the gene encoding mGluR5 in the NAc (Cowen et al., 2005) and frontal cortex (Gass and Olive, 2008), but similar data are not available in female rodents, and the interaction with binge EtOH intake has never been examined. The present qPCR study was conducted in adolescent and adult female mice at the time point when 24-hour drinking was altered, to determine whether adaptations in the glutamatergic system following mGluR5 antagonism of binge drinking and a period of abstinence contributed to the changes in subsequent EtOH intake. In the present work, MTEP treatment did not alter mRNA expression of glutamatergic genes in the NAc compared with values in saline injected female animals with binge exposure, suggesting that high EtOH intake exerted a greater effect on gene expression than MTEP pretreatment. Consistent with this idea, adolescent binge EtOH treatment (5 g/kg/d intragastric EtOH) potentiated the developmental reduction in gene expression in whole brain, measured via microarray hybridization (Coleman et al., 2011). So, it is possible that binge drinking experience (~2 g/kg/30 min) contributed to the consistently lower NAc expression of most of the glutamatergic genes examined in adolescent versus adult animals. Additionally, mGluR5s regulate the activity of multiple DNA transcription factors (Gass and Olive, 2008), implying that repeated blockade of mGluRs with MTEP could change the expression of multiple genes beyond those implicated in glutamatergic signaling. For instance, some of the biological processes altered by repeated MTEP injections in EtOH na€ve male rat frontal cortex were related to ATP synthesis, hydrolase activity, and mitogen-activated protein kinase signaling pathways (Gass and Olive, 2008). Although beyond the scope of the present studies, future work can identify novel signaling pathways and determine common and distinct neuroadaptive networks regulated by binge drinking and mGluR5 antagonism in male and female adolescent and adult mice.
In conclusion, the current findings provide evidence that MTEP reduced binge EtOH intake in male and female adolescent and adult mice, with lower efficacy in adolescent mice following repeated MTEP administration (as BECs remained above binge levels on Day 21 in these animals). Because age at time of binge EtOH experience can impact later drinking behavior, we present important novel information that an MTEP-induced decrease in binge drinking differentially altered subsequent drinking, depending on the sex and age of the animal. Finally, adolescent binge drinking experience produced a persistent decrease in the expression of several glutamatergic genes and signaling molecules downstream of mGluR5 in females that was evident following a period of abstinence when the animals were adults. Collectively, the present findings add to existing evidence implicating the contribution of long-term effects of adolescent binge drinking to enhanced alcohol abuse in adulthood. These results suggest that mGluR5 antagonism may not be the best pharmacotherapy to treat binge alcohol consumption.
Acknowledgments
This research was supported by grants from the Department of Veterans Affairs (DAF, KMW). DKC was supported by T32 AA007468. Additionally, this material is the result of work supported with resources and the use of facilities at the Portland VA Medical Center (DAF, KMW). We thank the Portland Alcohol Research Center Animal Core for providing some of the C57BL/6J mice used in the present studies and Chris Snelling for conducting the BEC analyses.
References
- Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford NDP, Varney MA. In vivo receptor occupancy of mGluR5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridine-2-ylethynyl)pyridine) Eur J Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- Bäckström P, Bachteler D, Koch S, Hyytiä P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the US, 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Effects of voluntary access to sweetened ethanol during adolescence on intake in adulthood. Alcohol Clin Exp Res. 2013;37:1048–1055. doi: 10.1111/acer.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara-Nascimento PF, Lopez MF, Becker HC, Olive MF, Camarini R. Similar ethanol drinking in adolescent and adult C57BL/6J mice after chronic ethanol exposure and withdrawal. Alcohol Clin Exp Res. 2013;37:961–968. doi: 10.1111/acer.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Vital signs: binge drinking prevalence, frequency, and intensity among adults- United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:14–19. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Vital signs: binge drinking among women and high school girls- United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62:9–13. [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Jr, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315:590–600. doi: 10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Krstew E, Lawrence AJ. Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology. 2007;190:21–29. doi: 10.1007/s00213-006-0583-0. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, Wroten MG, Zhang PW, Xiao B, Hu JH, Klugmann M, Metten P, Worley PF, Crabbe JC, Szumlinski KK. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin Exp Res. 2012;36:1623–1633. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK. Binge alcohol drinking by mice requires intact Group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.214. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu J-H, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC. A procedure to produce high alcohol intake in mice. Psychopharmacology. 2005;178:471–480. doi: 10.1007/s00213-004-2039-8. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Transcriptional profiling of the rat frontal cortex following administration of the mGluR5 receptor antagonists MPEP and MTEP. Eur J Pharmacol. 2008;584:253–262. doi: 10.1016/j.ejphar.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Role of protein kinase C epsilon (PKCε) in the reduction of ethanol reinforcement due to MGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology. 2009;204:587–597. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto JG, Beadles-Bohling AS, Wiren KM. Comparison of RiboGreen and 18S rRNA quantitation for normalizing real-time RT-PCR expression analysis. Biotechniques. 2004;36:58–60. doi: 10.2144/04361BM06. [DOI] [PubMed] [Google Scholar]
- Hashimoto JG, Forquer MR, Tanchuck MA, Finn DA, Wiren KM. Importance of genetic background for risk of relapse shown in altered prefrontal cortex gene expression during abstinence following chronic alcohol intoxication. Neuroscience. 2011;173:57–75. doi: 10.1016/j.neuroscience.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL6/6J mice. Psychopharmacology. 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their antiaddictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Crawford MS, Kulers CM, Williams HL. Effects of a metabotropic, mGlu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol Alcohol. 2005;40:494–497. doi: 10.1093/alcalc/agh200. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Lisenbardt DN, Melon LC, Boehm SL. Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl) pyridine decreases ethanol consumption via a protein kinase Cε-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2nd. Academic Press; San Diego, CA: 2001. [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology. 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano D, Smith RF. Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiol Behav. 2001;74:637–643. doi: 10.1016/s0031-9384(01)00623-0. [DOI] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol Biochem Behav. 2012;101:329–335. doi: 10.1016/j.pbb.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm Behav. 2010;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, National Institute on Alcohol Abuse and Alcoholism. NIAAANewsletter (No. 3) National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 2004. [Google Scholar]
- U.S. Department of Health and Human Services, National Institute on Alcohol Abuse and Alcoholism. Underage Drinking: Why do Adolescents Drink, What are the Risks, and How Can Underage Drinking Be Prevented? (Alcohol Alert No. 67) National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 2006. [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


