Abstract
Background
Data on gastrointestinal (GI) side effects of bariatric surgery are limited due to incomplete reporting, cross sectional samples, and non-standardized assessments.
Objective
To report on GI side effects over the first 6 months following Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric banding (LAGB).
Setting
Academic Medical Center, United States.
Methods
One hundred forty-four patients completed a standardized clinical interview 6 months after operation, including questions on the occurrence and frequency of episodes of dumping syndrome, vomiting, and plugging for each of the past 6 months; monthly rates were stable, so results were averaged over the entire period. Although data were collected as part of a randomized controlled trial, randomization group and the interaction of group by surgical procedure were not related to GI side effects. Thus, results are reported by procedure only (RYGB; n = 87, LAGB; n= 56).
Results
RYGB patients had a higher preoperative Body Mass Index (BMI) than LAGB patients (46.8 ± 6.8 vs. 43.5 ± 4.8 kg/m2, respectively, p = 0.001), were more likely to report dumping (45.7% vs. 4.7%, p < 0.0001) and were less likely to report plugging (45.7% vs. 79.1%, p = 0.0005). Vomiting did not differ significantly by procedure (68.6% vs. 65.1%, p = 0.7). Most patients experienced each GI side effect less than once per week.
Conclusions
Although self-reported GI side effects were common over the first 6 months after operation, the frequency of episodes was relatively low. Longer-term follow-up is needed to determine whether symptoms worsen or improve over time.
Keywords: Bariatric surgery, gastric bypass, laparoscopic adjustable gastric banding, gastrointestinal complications
Introduction
Although bariatric surgery is recommended for treatment of severe obesity, the potential benefits of surgically induced weight loss must be weighed against the risks. Most postoperative complications are related to the type of procedure performed and length of follow-up.[1] However, systematic data are limited even for some of the most common postoperative gastrointestinal (GI) side effects.
In a review of 62 studies involving 3,626 combination [e.g., Roux-en-Y gastric bypass (RYGB)] and 5,568 restrictive procedures [e.g. laparoscopic adjustable gastric banding (LAGB)], Monteforte and Turkelson[2] summarized complications by type of surgical procedure. Dumping syndrome, a group of symptoms associated with food emptying too quickly into the small intestine, was the most common complication for combination procedures (14.64%), but was uncommon following restrictive procedures (0.28%). Vomiting was the most common symptom associated with restrictive procedures (8.49%) and was also reported after combination procedures (2.56%). However, these rates do not take into account the duration of follow-up, and may be underestimated due to a lack of standardized assessment and reporting procedures.
Studies that have utilized standardized assessments suggest higher rates of GI side effects. For example, Sigstad’s Clinical Index,[3] has been used to document symptoms associated with dumping syndrome in several studies of RYGB. It was administered to 50 patients one month after RYGB, and 42% met criteria for dumping syndrome (Sigstad’s Index score > 7).[4] In a different sample of patients followed up 18 to 24 months after operation,[5] 76.9% were classified as dumpers.[5] Additionally, in a detailed interview assessment of eating behavior 18 to 35 months after RYGP, 50.5% of patients reported dumping, 62.7% of patients reported vomiting associated with epigastric discomfort, 11.9% reported self-induced vomiting to influence body weight, and 76.3% reported plugging, defined as the feeling that food has become stuck in the upper digestive track or pouch.[4]
In summary, results of previous studies on GI side effects after bariatric surgery are highly variable and limited by incomplete reporting of symptoms, cross sectional samples, and use of nonstandardized instruments. This makes it difficult for providers to inform patients about the potential for common GI symptoms by procedure at different time points after operation. In this report, we document the prevalence of dumping syndrome, vomiting, and plugging over the first 6 months following RYGB and LAGB using a semi-structured clinical interview that incorporated Sigstad’s Clinical Index.
Materials and Methods
Study Design and Participants
The present investigation includes participants enrolled in a randomized, controlled trial of a 6-month preoperative behavioral lifestyle intervention relative to usual care prior to bariatric surgery.[6] All patients were at least 18 years of age at enrollment. Exclusion criteria included: 1) Intellectual disability or psychosis; 2) Previously diagnosed genetic obesity syndrome; 3) Participation in a weight management program in the 6 months prior to study enrollment; 4) Uncontrolled psychiatric symptomatology sufficiently severe to require immediate treatment; 5) Pregnant or lactating in the previous 6 months; 6) Taking a medication known to affect body weight in the previous 6 months; 8) Any previous weight loss surgery; 9) Medical condition requiring a specialized preoperative regimen; and 10) Participation in a conflicting research protocol. The study was approved by the local Institutional Review Board and registered at ClinicalTrials.gov (identifier NCT00623792).
A total of 144 patients who were enrolled in the parent study and randomized to preoperative lifestyle intervention (n = 72) or usual care (n = 72) underwent bariatric surgery. All patients received a single session preoperative nutrition consultation and psychological evaluation as part of the routine preoperative approval process. Of these, 87 had RYGB, 56 had LAGB; one patient had sleeve gastrectomy and was excluded. The present analysis includes 113 patients who completed a standardized clinical interview 6 months after operation, including questions on the occurrence and frequency of episodes of dumping, vomiting, and plugging for each of the past 6 months.
Measures
An investigator-designed questionnaire was used to collect demographic data including sex, age, race/ethnicity, education, employment status, income and marital status at study entry. Height was measured at study entry using a mounted stadiometer, and weight was measured using a digital scale. Participants were weighed and height measured in street clothes, without shoes. Body Mass Index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
Patients completed questionnaires and semi-structured clinical interviews at each assessment. The Eating Disorder Examination (EDE) [7] was adapted to document dumping syndrome, vomiting, and plugging and administered 6 months after surgery.[4] The adaptation included collection of data utilizing Sigstad’s Clinical Diagnostic Index. [3] A score of > 7 on Sigstad’s Index indicates dumping, and scores of 5 to 7 are suggestive of dumping. Vomiting included episodes that were spontaneous or self-induced, and was also rated as to whether or not the episode was associated with concerns about body weight or shape. Plugging was operationalized as “problems with the small opening in your stomach becoming plugged, or food becoming stuck in the small opening of your stomach,” and participants were asked to identify which foods were associated with plugging. All GI side effects (dumping, vomiting, and plugging) were rated retrospectively for their presence and monthly frequency for each of the 6 months preceding the interview.
Analytic Plan
Descriptive statistics were used to summarize demographic characteristics and GI side effects of interest (dumping, vomiting, and plugging) among study participants. Two-sample t tests (or Wilcoxon tests) and chi-square analyses (or Fisher’s exact tests) were performed for continuous and categorical variables, respectively, to test for differences between surgical procedures. We also compared baseline characteristics of participants who did and did not complete interviews 6 months after operation.
We employed logistic regression to examine the relationship of randomization group, type of surgical procedure (RYGB, LAGB), and the interaction of group by procedure to the GI side effects (dumping, vomiting, and plugging). There was no significant effect for group or group by procedure, therefore the analysis was performed by procedure (RYGB, LAGB), controlling for randomization group. We also investigated whether age and sex were related to GI side effects. The results were similar after controlling for age and sex, so unadjusted values are reported here.
We classified patients as to whether they did or did not report each of the GI side effects (dumping, vomiting, and plugging) in the past 6 months, and tested whether patients with RYGB or LAGB differed in self-report of each of the GI side effects using chi-square analyses or Fisher’s exact tests. Among those reporting each side effect, we compared the number of episodes reported per month over each of the previous 6 months using a repeated measure mixed-effect model. The effect for time was not significant for plugging [F(5,325) = 1.78, p = 0.12], dumping [F(5,165) = 0.84, p = 0.52], or vomiting [F(5, 375) = 1.89, p = 0.10]. Therefore, for each side effect, episodes per month were averaged over the entire 6 month period.
Statistical significance was set at p < .05, and all tests were two-tailed. All analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, North Carolina, USA).
Results
Participant Characteristics
Patient demographics and preoperative BMI are presented in Table 1. Patients who underwent RYGB were significantly more obese than those who had LAGB, but did not differ in demographic characteristics.
Table 1.
Participant characteristics
| RYGB (n = 87) | LAGB (n =56) | p- value | |
|---|---|---|---|
| Age (years, M ± SD) | 44.8 ± 10.9 | 45.0 ± 11.1 | 0.94 |
| Preoperative BMI (kg/m2, M ± SD) | 46.8 ± 6.8 | 43.5 ± 4.8 | 0.001 |
| Female (%) | 92.0 | 87.5 | 0.38 |
| Caucasian (%) | 89.7 | 82.1 | 0.20 |
| married (%) | 56.3 | 51.8 | 0.59 |
| income ≤ $30,000 (%) | 28.1 | 25.0 | 0.69 |
| employed full time (%) | 58.6 | 71.4 | 0.12 |
| high school or less (%) | 17.2 | 12.5 | 0.44 |
Legend.
BMI = Body Mass Index; RYGB = Roux-en-Y Gastric Bypass; LAGB = Laparoscopic Adjustable Gastric Banding
Those who completed the EDE interview 6 months after operation (n = 113) and those who had surgery but failed to complete the interview (n =30) did not differ in preoperative BMI, gender, race, income, employment status or education level. However, completers were more likely to be married (67/113; 59.3% vs. 11/30; 36.7%, p=0.03) and Caucasian (113/113; 100% vs. 28/30; 93.3%, p = 0.04) than non-completers. Completers were also older (113; 46.1 ± 11 vs. 30; 40.6 ± 9.8 years, p = 0.01) than non-completers.
See Table 2 for data on frequency of episodes of dumping, vomiting, and plugging over the first 6 months after operation.
Table 2.
Frequency of gastrointestinal side effects over the past 6 months
| Side Effect | Frequency | RYGB N = 70 |
LAGB N = 43 |
|---|---|---|---|
| Dumping* | None | 54% (n=38) |
95% (n=41) |
| < one episode per month | 27% (n=19) |
2% (n=1) |
|
| 1–3 episodes per month | 11% (n=8) |
0% (n=0) |
|
| > once per week | 7% (n=5) |
2% (n=1) |
|
| Vomiting | None | 31% (n=22) |
35% (n=15) |
| < one episode per month | 36% (n=25) |
35% (n=15) |
|
| 1–3 episodes per month | 26% (n=18) |
19% (n=8) |
|
| > once per week | 7% (n=5) |
12% (n=5) |
|
| Plugging | None | 54% (n=38) |
21% (n=9) |
| < one episode per month | 23% (n=16) |
28% (n=12) |
|
| 1–3 episodes per month | 17% (n=12) |
30% (n=13) |
|
| > once per week | 6% (n=4) |
21% (n=9) |
Sigstad’s Clincal Index > 5
Legend.
RYGB = Roux-en-Y Gastric Bypass; LAGB = Laparoscopic Adjustable Gastric Banding
Dumping Syndrome
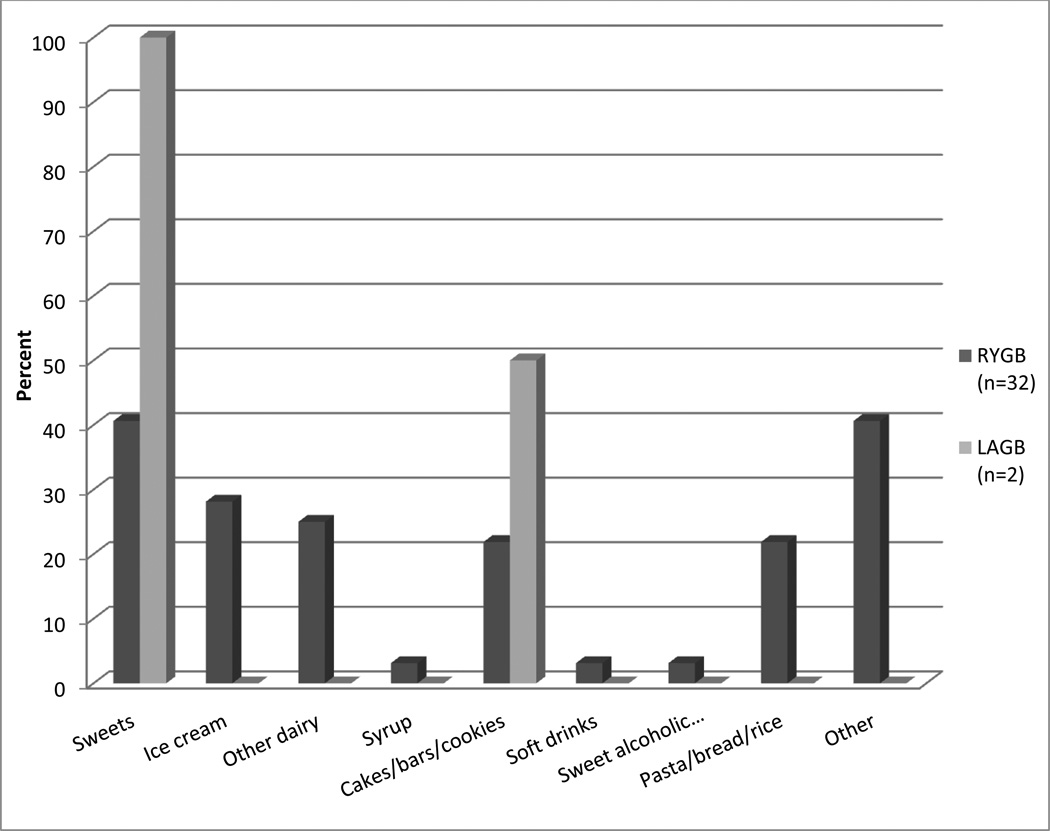
Sigstad’s Clinical Index averaged 5.9 + 7.5 (range 0 to 29) for RYGB and 0.8 + 3.7 (range 0 to 21) for LAGB patients. RYGB patients were significantly more likely than LAGB patients to have scores > 5 (32/70; 45.7% vs. 2/43; 4.7%, p < .0001) or > 7 (25/70; 35.7% vs. 2/43; 4.7%, p < .0001). Among patients with scores > 5 on Sigstad’s Index, dumping symptoms are shown in Table 3, and foods associated with dumping are shown in Figure 1.
Table 3.
Dumping symptoms self-reported by patients with score of > 5 on Sigstad’s Index
| Symptom (weight) | RYGB (n=32) |
LAGB (n=2) |
|---|---|---|
| Pre-shock, shock (+5) | 0% (n=0) |
0% (n=0) |
| “Almost fainting,” syncope, unconsciousness (+4) | 15.6% (n=5) |
50% (n=1) |
| Desire to lie or sit down (+4) | 81.3% (n=26) |
100% (n=2) |
| Breathlessness, dyspnea (+3) | 21.9% (n=7) |
0% (n=0) |
| Weakness, exhaustion (+3) | 65.6% (n=21) |
100% (n=2) |
| Sleepiness, drowsiness, yawning, apathy, falling asleep (+3) | 50% (n=16) |
100% (n=2) |
| Palpitation (rapid heart rate) (+3) | 46.9% (n=15) |
0% (n=0) |
| Restlessness (+2) | 37.5% (n=12) |
50% (n=1) |
| Dizziness (+2) | 46.9% (n=15) |
50% (n=1) |
| Headache (+1) | 25% (n=8) |
50% (n=1) |
| Feeling of warmth, sweating, pallor, clammy skin (+1) | 84.4% (n=27) |
100% (n=2) |
| Nausea (+1) | 81.3% (n=26) |
100% (n=2) |
| Fullness in the abdomen, meteorismus (bloating) (+1) | 68.8% (n=22) |
50% (n=1) |
| Borborygmia (stomach noises) (+1) | 46.9% (n=15) |
50% (n=1) |
| Eructation (belching) (−1) | 31.3% (n=10) |
50% (n=1) |
| Vomiting (−4) | 25% (n=8) |
0% (n=0) |
Figure 1. Foods associated with dumping in patients with Sigstad’s Index > 5.
RYGB = Roux-en-Y Gastric Bypass; LAGB = Laparoscopic Adjustable Gastric Banding
Vomiting
About two-thirds of RYGB (48/70; 68.6%) and LAGB (28/43; 65.1%) patients reported vomiting over the past 6 months (p = 0.7). However, only one patient reported self-induced vomiting associated with concerns about body shape or weight.
Plugging
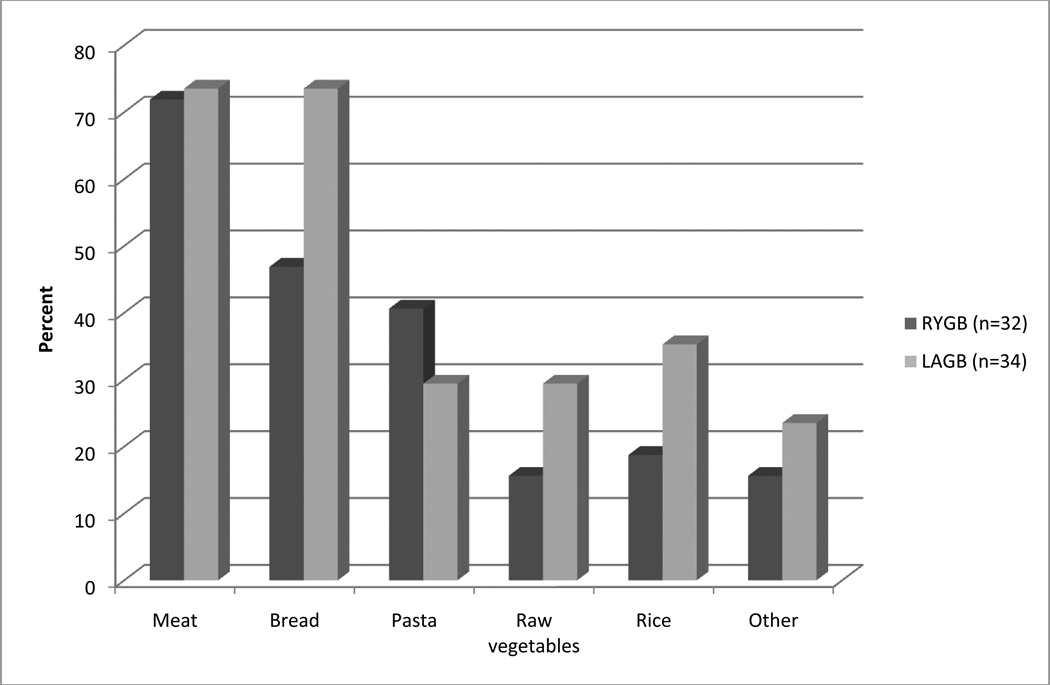
A greater proportion of LAGB (34/43; 79.1%) than RYGB (32/70; 45.7%) patients reported plugging in the past 6 months (p = .0005). The top two situations associated with plugging were eating too rapidly (25/34; 73.5% of LAGB vs. 25/32; 78.1% of RYGB patients, p = .66) and not chewing well (29/34; 85.3% and 24/32; 75.0%, respectively, p = .29); all other situations were reported by a minority of patients. See Figure 2 for foods associated with plugging.
Figure 2. Foods associated with self-reported plugging.
RYGB = Roux-en-Y Gastric Bypass; LAGB = Laparoscopic Adjustable Gastric Banding
Discussion
Data obtained from standardized clinical interviews document that although a substantial proportion of bariatric surgery patients self-reported GI side effects, the frequency of monthly episodes was relatively low and stable on average over the first 6 months after RYGB and LAGB. Consistent with previous reports,[2, 5] RYGB patients were much more likely than LAGB patients to report dumping syndrome. However, LAGB patients were more likely than RYGB to report plugging, or the sense of food becoming stuck in the upper digestive tract. Vomiting did not vary by type of procedure over this time period.
Dumping syndrome, defined as Sigstad’s Clinical Index > 7, was reported by 25 of 70 (35.7%) RYGB patients. This rate is lower than those observed in previous studies utilizing Sigstad’s Index, which ranged from 42% to 76.9%. [4, 5] Prior studies administered the assessment at varying points in time ranging from one month through 36 months after operation, and it appears that samples assessed at a longer interval of postoperative follow-up found a higher prevalence of patients with dumping syndrome. Prospective assessment, including laboratory assessments, is needed to determine if symptoms of dumping increase over time as patients reintroduce foods into their diet and adapt to the postoperative regimen. Although dumping syndrome is not expected as a side effect after LAGB, 2 LAGB patients self-reported symptoms, indicating the perception of dumping symptoms in a small number of patients and highlighting the limitations of self-report. Some recent studies have incorporated clinical provocation of dumping symptoms using an oral glucose challenge.[8] Including laboratory assessments may provide more objective information in future studies and lead to an improved understanding of the pathophysiology of both early dumping, which tends to occur soon after eating, and late dumping syndrome, a type of reactive hypoglycemia, occurring 1 to 3 hours afterward.
Although approximately two-thirds of patients self-reported vomiting in the past six months, those individuals reported less than one episode per week on average. Of note, only one patient acknowledged self-induced vomiting to counteract the effects of eating on body weight or shape, a disordered eating behavior. In the only other examination of this behavior, deZwaan and colleagues found that 7 participants (approximately 12%) in their sample reported weight- or shape-related vomiting 18 to 25 months after RYGB.[4] The discrepancy between studies raises the possibility that disordered eating behaviors may increase over time.
More LAGB (79.1%) than RYGB (45.7%) patients reported plugging, typically as a result of eating too rapidly or not chewing well. Bread and meat were the foods most often associated with plugging, defined as the subjective feeling of “food becoming stuck in the small opening of your stomach.” When foods back up into the esophagus, patients may experience dysphagia, or difficulty swallowing. Additionally, after LAGB patients may experience dysphasia after band adjustments. Clinical interventions may focus on eating slowly and chewing well, as well as identifying and limiting foods associated with GI side effects. Additionally, future work is needed to explore the potential role of postoperative band adjustments in reducing dysphagia as well as vomiting after LAGB. Future investigations should include objective documentation band obstruction or gastrojejunal stricture with Upper GI contrast or endoscopy.
Strengths of the present study include use of a semi-structured clinical interview administered 6 months after operation that carefully operationalized GI side effects. However, limitations include the lack of preoperative assessment of GI symptoms and the potential for bias associated with retrospective self-report. Additionally, it should be noted that the data were collected in the context of a randomized, controlled trial that did not include patients with a medical condition requiring a specialized preoperative regimen. Therefore, individuals with the greatest severity of obesity and high risk health problems were not included in the sample, and different results may have been obtained in an unconstrained sample. Moreover, it remains possible that patients enrolled in a clinical trial differ in other important ways that may be related to postoperative GI symptoms in full population of bariatric surgery patients, such as their level of education, motivation, and/or compliance. Nonetheless, these data may represent the best available information on dumping syndrome, vomiting, and plugging over the first 6 months after two of the most common types of bariatric surgery.
Conclusion
Patients and providers may benefit from knowing that although self-report of GI side effects is relatively common, episodes of dumping, vomiting, and plugging are relatively infrequent over the first 6 months after LAGB and RYGB. Whereas some patients may experience reductions in symptoms over time, others may experience increases as they incorporate a broader range of foods into their diet. Therefore, longer-term follow-up is needed to determine how GI symptoms change over time and whether symptoms affect longer-term weight loss or nutritional status following different types of surgical procedures. Future work should also include objective assessments when possible in order to inform the development of new diagnostic and therapeutic approaches, both behavioral and pharmacological.
Acknowledgments
Research supported by R01DK077102 from the National Institute of Diabetes and Digestive and Kidney Diseases (PI: Melissa A. Kalarchian). ClinicalTrials.gov identifier: NCT00623792
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abell TL, Minocha A. Gastrointestinal complications of bariatric surgery: diagnosis and therapy. Am J Med Sci. 2006;331:214–218. doi: 10.1097/00000441-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Monteforte MJ, Turkelson CM. Bariatric surgery for morbid obesity. Obes Surg. 2000;10:391–401. doi: 10.1381/096089200321594246. [DOI] [PubMed] [Google Scholar]
- 3.Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med Scand. 1970;188:479–486. [PubMed] [Google Scholar]
- 4.de Zwaan M, Hilbert A, Swan-Kremeier L, Simonich H, Lancaster K, Howell LM, et al. Comprehensive interview assessment of eating behavior 18–l35 months after gastric bypass surgery for morbid obesity. Surg Obes Relat Dis. 2010;6:79–85. doi: 10.1016/j.soard.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Mallory GN, Macgregor AM, Rand CS. The Influence of Dumping on Weight Loss After Gastric Restrictive Surgery for Morbid Obesity. Obes Surg. 1996;6:474–478. doi: 10.1381/096089296765556368. [DOI] [PubMed] [Google Scholar]
- 6.Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, Levine MD. Preoperative lifestyle intervention in bariatric surgery: initial results from a randomized, controlled trial. Obesity (Silver Spring) 2013;21:254–260. doi: 10.1002/oby.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, assessment, and treatment. 12th ed. New York: Guilford; 1993. pp. 317–360. [Google Scholar]
- 8.Papamargaritis D, Koukoulis G, Sioka E, Zachari E, Bargiota A, Zacharoulis D, et al. Dumping symptoms and incidence of hypoglycaemia after provocation test at 6 and 12 months after laparoscopic sleeve gastrectomy. Obes Surg. 2012;22:1600–1606. doi: 10.1007/s11695-012-0711-3. [DOI] [PubMed] [Google Scholar]