Abstract
A prerequisite for vaccine-mediated induction of CD8+ T-cell responses is the targeting of dendritic cell (DC) subsets specifically capable of cross-presenting antigen epitopes to CD8+ T cells. Administration of a number of cationic adjuvants via the intraperitoneal (i.p.) route has been shown to result in strong CD8+ T-cell responses, whereas immunization via e.g. the intramuscular (i.m.) or subcutaneous (s.c.) routes often stimulate weak CD8+ T-cell responses. The hypothesis for this is that self-drainage of the adjuvant/antigen to the lymphoid organs, which takes place upon i.p. immunization, is required for the subsequent activation of cross-presenting lymphoid organ-resident CD8α+ DCs. In contrast, s.c. or i.m. immunization usually results in the formation of a depot at the site of injection (SOI), which hinders the self-drainage and targeting of the vaccine to cross-presenting CD8α+ DCs. We investigated this hypothesis by correlating the biodistribution pattern and the adjuvanticity of the strong CD8+ T-cell inducing liposomal cationic adjuvant formulation 09 (CAF09), which is composed of dimethyldioctadecylammonium bromide/monomycoloyl glycerol liposomes with polyinosinic:polycytidylic acid electrostatically adsorbed to the surface. Biodistribution studies with radiolabeled CAF09 and a surface-adsorbed model antigen [ovalbumin (OVA)] showed that a significantly larger fraction of the vaccine dose localized in the draining lymph nodes (dLNs) and the spleen 6 h after i.p. immunization, as compared to after i.m. immunization. Studies with fluorescently labelled OVA + CAF09 demonstrated a preferential association of OVA + CAF09 to DCs/monocytes, as compared to macrophages and B cells, following i.p. immunization. Administration of OVA + CAF09 via the i.p. route did also result in DC activation, whereas no DC activation could be measured within the same period with unadjuvanted OVA and OVA + CAF09 administered via the s.c. or i.m. routes. In the dLNs, the highest level of activated, cross-presenting CD8α+ DCs was detected at 24 h post immunization, whereas an influx of activated, migrating and cross-presenting CD103+ DCs to the dLNs could be measured after 48 h. This suggests that the CD8α+ DCs are activated by self-draining OVA + CAF09 in the lymphoid organs, whereas the CD103+ DCs are stimulated by the OVA + CAF09 at the SOI. These results support the hypothesis that the self-drainage of OVA + CAF09 to the draining LNs is required for the activation of CD8α+ DCs, while the migratory CD103+ DCs may play a role in sustaining the subsequent induction of strong CD8+ T-cell responses.
Keywords: Liposome, Subunit vaccine, Biodistribution, Cross-presentation, Adjuvant, Nanomedicine
Graphical abstract
1. Introduction
Vaccination against infectious diseases is one of the most successful and cost-effective medical inventions [1]. Many licensed vaccines induce robust antibody responses, which are sufficient for the protection against a number of different pathogens [2]. However, there is an unmet medical need for novel vaccines that concomitantly induce strong cell-mediated immunity (CMI) and cytotoxic T-lymphocyte (CTL) responses, in particular against certain intracellular pathogens, e.g. HIV and Mycobacterium tuberculosis [3]. One subunit vaccine technology exploits pathogen-specific and highly purified, synthetic peptides or recombinant proteins as antigens, in combination with an adjuvant. This enables the safe control of the specific type of immune response induced [2], [4], [5]. Vaccine delivery systems, e.g. liposomes, emulsions and virus-like particles (VLPs), have appeared very useful for the induction of strong antigen-specific immunity when combined with one or several immunostimulating compounds [6]. This allows for the design of vaccine adjuvants inducing highly customized immune responses through careful selection and optimization of the delivery system, the immunostimulator(s), and the administration route [4], [5], [7].
The CTL-inducing cationic adjuvant formulation (CAF) 09 (Statens Serum Institut, Denmark) is a promising novel adjuvant [8]. It is composed of the Toll-like receptor (TLR)-3 ligand polyinosinic:polycytidylic acid [poly(I:C)] electrostatically adsorbed to dimethyldioctadecylammonium (DDA) bromide/monomycoloyl glycerol (MMG) liposomes. This adjuvant has been shown to induce robust antigen-specific CD8+ T-cell responses for a number of different surface-adsorbed antigens, and it has been shown to be efficacious as a vaccine adjuvant for cancer vaccines in a number of preclinical animal models [8]. However, the induction of CD8+ T-cell responses appears to be highly dependent on the administration route, as also reported for the comparable adjuvant CAF05, which is composed of poly(I:C) adsorbed to liposomes comprised of DDA and the glycolipid trehalose 6,6′-dibehenate (TDB): Induction of strong CD8+ T-cell responses is only observed upon intraperitoneal (i.p.) or nasal immunization for both adjuvants [8], [9], [10], whereas subcutaneous (s.c.) and intramuscular (i.m.) administration elicit weak CD8+ T-cell responses [8].
Antigen-presenting cells (APCs), e.g. dendritic cells (DCs), link the innate and adaptive immune system by presenting pathogen-specific antigens and providing activation signals to naïve T cells [11]. The activation of CD8+ T cells and their subsequent differentiation into effector CTLs requires the presentation of antigen epitopes on major histocompatibility complex class I (MHC-I) molecules, which usually present endogenously derived peptide epitopes [12]. However, specialized DC subsets are capable of processing antigens and presenting epitopes from exogenously derived peptides and proteins on MHC-I via a process referred to as cross-presentation [12], [13], [14]. It is well established that both lymph node (LN)-resident CD8α+ DCs and epithelium-resident CD103+ DCs play a role in cross-presentation of protein antigens to CD8+ T cells. The CD8α+ and the CD103+ DCs are developmentally related [15], but there are conflicting data in the literature with respect to whether both subsets are capable of cross-presenting antigens in the dLNs [16], [17], or if one of the subsets is the predominant inducer of CD8+ T-cell responses [18], [19]. The site of pathogen infection may likely have an influence on this [17], [19], [20]. In mice, the LN-resident CD8α+ DCs effectively cross-present antigens derived from particles or cell debris capable of self-drainage to the LNs [21], [22], and depletion of the CD8α+ DC population has been shown to abrogate CD8+ T-cell responses in mice [23]. On the other hand, after migration to the LNs, the CD103+ DCs have been proposed to either cross-present exogenous antigen or pass it on to CD8α+ DCs for cross-presentation [14], [21].
Self-drainage of vaccines from the site of injection (SOI) to the draining LNs (dLNs) is hypothesized to be the main requirement for targeting the LN-resident CD8α+ DCs [24]. However, targeting of CD103+ DCs at the SOI might also play a role for the induction of CD8+ T-cell responses. In the present study we show that the drainage of a subunit vaccine composed of the model antigen ovalbumin (OVA) surface-adsorbed to CAF09 following i.p. immunization far exceeds that of the drainage measured after i.m. immunization. The result is increased activation of DCs and induction of significantly stronger CD8+ T-cell responses.
2. Materials and methods
2.1. Materials
DDA (Clauson-Kaas A/S, Farum, Denmark), the synthetic analogue of the mycobacterial lipid MMG, also referred to as MMG-1 [25], poly(I:C) (Sigma-Aldrich, St. Louis, MO, USA) and endograde chicken egg ovalbumin (OVA) (Hyglos GmbH, Bernried am Starnberger See, Germany) were used for the preparation of the vaccine. All other chemicals were obtained commercially at analytical grade. Tris buffer (10 mM, pH 7.4) was used throughout the studies.
2.2. Preparation and physicochemical characterization of the vaccine formulations
The liposomes were prepared by using the thin film method combined with high shear mixing, and characterized with respect to the average intensity-weighted hydrodynamic diameter (z-average), polydispersity index (PDI) and zeta-potential (Laser-doppler electrophoresis) essentially as described by Korsholm et al. [8]. The final concentration of CAF09 was 2.5/0.5/0.5 mg/ml DDA/MMG/poly(I:C). In addition, a control dispersion was prepared consisting of DDA/MMG at 2.5/0.5 mg/ml, referred to as CAF04. CAF04 has been shown to induce mixed Th1/Th17 T-cell responses [25]. The degree of adsorption of OVA to CAF09 was determined by mixing different amounts of OVA in Tris buffer (0.10–1.5 mg/ml final concentration) with equal volumes of CAF09, and the mixtures were left to equilibrate for 30 min at room temperature (rt) followed by ultracentrifugation at 135,700 × g for 30 min. The OVA concentration in the supernatant was determined by using the bicinchoninic acid assay (BCA) analysis (Thermo Scientific, Waltham, MA, USA) according to the manufacturer's recommendations. The amount of OVA adsorbed to CAF09 was calculated as the difference between the added amount of OVA and the amount recovered in the supernatant. The morphology of CAF09 was determined by cryo-transmission electron microscopy (cryo-TEM) using a Philips CM100 BioTWIN electron microscope (Philips, Eindhoven, The Netherlands) equipped with a side-mounted Olympus Veleta camera (Olympus Soft Imaging Solutions GmbH, Münster, Germany) essentially as described elsewhere [10]. The gel-to-liquid crystalline phase transition temperature (Tm) of the undiluted liposomal dispersions was determined by using differential scanning calorimetry (DSC). Thermograms were recorded using a μDSC3evo (Setaram, Caluire, France) heating 0.8 g samples from 30 °C to 60 °C at 0.5 °C/min with Tris buffer as the reference solution.
2.3. Immunization of mice for immunological studies
All animal experiments were conducted in accordance with the national Danish guidelines for animal experiments as approved by the Danish Council for Animal Experiments and in accordance with EU directive 2010/63/EU for animal experiments. All efforts were made to ensure maximum comfort for the animals. Female, 6–8 week old C57BL/6 mice were purchased from Harlan (Horst, The Netherlands). Mice (4–8/group) were immunized s.c. or i.p. with a dose of 10 μg unadjuvanted OVA or OVA adjuvanted with CAF09 at a dose of 250/50/50 μg DDA/MMG/poly(I:C), all in a dose-volume of 200 μl in isotonic, 9% (w/v) trehalose Tris buffer. Mice received three immunizations with two-week intervals, and the immune responses were evaluated eight days after the final immunization. The studies were repeated twice.
2.4. Antigen-specific CD8+ T-cell responses
Blood lymphocytes were separated by centrifugation using Lympholyte (Cedarlane, Burlington, Canada) at 900 × g for 20 min, followed by two washes in phosphate-buffered saline (PBS). In a V-bottomed 96-well plate, 1 × 106 lymphocytes were stained with antibody panel 1 (Supplementary data, Table S1). The data was acquired using a FACSCanto (BD, Franklin Lakes, NJ, USA) followed by analysis using the FlowJo v10 software (Tree Star Inc., Ashland, OR, USA).
2.5. Preparation of radiolabelled CAF09 and OVA
125I-OVA was prepared by mixing OVA and 3 MBq/mg OVA Na125I (Perkin Elmer, London, UK) in an Iodo-Gen pre-coated tube (Pierce Biotechnology, Rockford, IL, USA) and incubating for 1 h at rt. with intermittent mixing. Unincorporated Na125I was removed by gel filtration using a G-75 Sephadex chromatography column (GE Healthcare, Amersham, UK) with Tris elution buffer. Aliquots were collected and quantified for the protein content by BCA analysis according to the manufacturer's protocol. The 125I content was quantified by gamma counting using a Cobra CPM Auto Gamma-counter (Perkin Elmer, London, UK), and aliquots containing both protein and 125I were pooled and subsequently used in the experiments. 32P-poly(I:C) was prepared by dephosphorylation of poly(I:C) with FastAP thermosensitive alkaline phosphatase (Thermo Fisher Scientific, Hempstead, UK) and subsequent phosphorylation using γ-32P-ATP and T4 polynucleotide kinase (Thermo Fisher Scientific) according to the manufacturer's specifications. Unreacted 32P-ATP was removed using illustra ProbeQuant G-50 μ columns (GE Healthcare). The CAF09 liposomes were radiolabelled by mixing DDA and MMG dissolved in CHCl3 with trace amounts of 3H-cholesterol (Perkin Elmer), and the liposome dispersions were prepared as described above. Poly(I:C) mixed with trace amounts of 32P-labelled poly(I:C) was added as described above. Un-labelled OVA with 20% (w/w) 125I-OVA was added 30 min prior to immunization and adsorbed onto the liposome surface, facilitated by intermittent vortexing.
2.6. Biodistribution assessed by using radioactively labelled vaccine components
Biodistribution studies were conducted in accordance with the EU directive 2010/63/EU for animal experiments. Female, 6–8 week old BALB/c mice were immunized with unadjuvanted OVA or OVA adsorbed to CAF09, either i.p. or i.m. using a final dose of 250/50/50/20 μg DDA/MMG/poly(I:C)/OVA and 100/12/100 kBq/dose 3H/32P/125I in a total volume of 200 μl or 50 μl, respectively. The studies were repeated twice. At 6 h after the immunization, the mice were euthanized and the dLNs [the mediastinal lymph node (MLN) and the tracheobronchial lymph node (TLN) upon i.p. immunization and the popliteal lymph node (PLN) for i.m. immunization, respectively], the spleen and the tissue surrounding the SOI were removed. The organs were dissolved in Solvable (Perkin Elmer) for 2 h with shaking at 60 °C, and the 125I content was analyzed by using a Cobra CPM Auto Gamma-counter (Perkin Elmer). Subsequently, the samples were bleached with H2O2 for 15 min at 60 °C followed by addition of Ultima Gold (Perkin Elmer), and the 3H and 32P contents were quantified using a 1600TR Liquid Scintillation Counter (Perkin Elmer). Samples of the total administered dose were counted for reference purposes.
2.7. Preparation of fluorescently labelled liposomes
Fluorescently labelled liposomes were prepared as described above with the fluorescent label 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO, Thermo Scientific, Waltham, MA, USA). The final dose was 250/50/50/0.002 μg DDA/MMG/poly(I:C)/DiO in 200 μl (i.p. immunization) or 50 μl (i.m. immunization) isotonic, 9% (w/v) trehalose Tris buffer and 20 μg/dose OVA-AlexaFluor (AF) 647 (Thermo Scientific).
2.8. Biodistribution assessed by fluorescent labelling of the vaccine components
Female, 6–8 week-old C57BL/6 mice from Harlan were immunized either i.m. or i.p. with either unadjuvanted OVA-AF 647 or OVA-AF 647 adsorbed to CAF09-DiO. A naïve group was also included as a negative control. The studies were repeated at least twice. Mice were euthanized 1, 6, 24, or 48 h after the immunizations, and the dLNs [the TLN and the MLN for i.p. immunization, and the inguinal lymph node (ILN) upon i.m. immunization] and the spleens were removed. Single cell suspensions of splenocytes were obtained by passing the spleens through a nylon-mesh cell-strainer. The LNs were treated with Liberase TL (Roche, Hvidovre, Denmark) to liberate the APCs from the LN collagen structure. Each LN was treated with 1.5 ml RPMI 1640 supplemented as described elsewhere [10] containing 3 μg DNAse I and 30 μg Liberase. After 15 min incubation at 37 °C the LNs were passed through a nylon-mesh cell-strainer, treated with 150 μl 100 mM EDTA for 3 min, and washed in ice-cold PBS. Hereafter, the LNs were treated as the spleens. For each spleen or LN 1 × 106 cells, or everything if the sample contained fewer cells, were transferred to a 96-well, V-bottomed plate and treated with Fc-block followed by fluorescent staining with antibody panel 2 (Supplementary data, Table S2). The data was acquired using a FACSCanto followed by analysis using the FlowJo v10 software.
2.9. Activation of DCs and T cells following immunization
The study design was comparable to the design of the biodistribution studies of the fluorescently labelled vaccines. Mice were immunized i.p. with unlabeled vaccines, and the lymphoid organs were analyzed at 1, 6, 24, 48 and 72 h. Single cell suspensions were stained with antibody panel 3 (Supplementary data, Table S3) to assess DC activation. In addition, activation of DCs following i.p. immunization with CAF04 and CAF09 was analyzed with antibody panel 4 (Supplementary data, Table 4). T-cell activation was assessed with antibody panel 5 (Supplementary data, Table 5). The data was acquired using a FACSCanto or a FACSFortessa followed by analysis using the FlowJo v10 software.
2.10. Statistics
Statistical analysis of the in vivo studies was performed using one-way or two-way ANOVA at a 0.05 significance level followed by Tukey's multiple comparisons test using Prism v. 6.05 (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Preparation and physicochemical characterization of the CAF09-adjuvanted vaccine formulation
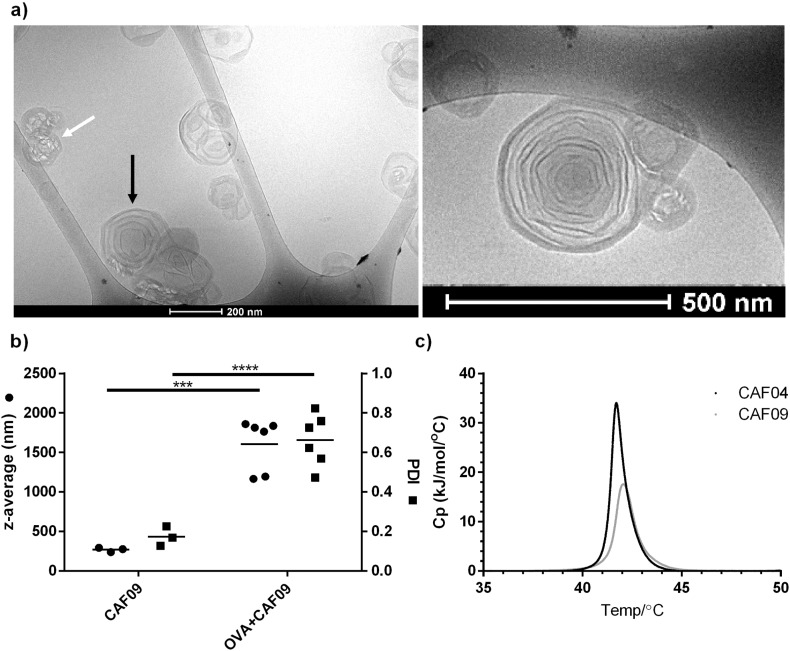
The CAF09 adjuvant was prepared by using the thin film method combined with high shear mixing, as previously described [8]. The resulting multilamellar liposomes (Fig. 1a) had an average hydrodynamic diameter of 311 ± 108 nm (Fig. 1b), a PDI of 0.18 ± 0.08 (Fig. 1b), and a zeta-potential of + 48 ± 2 mV (n = 3), which are well in accordance with previously reported values for CAF09 and CAF05 [8], [10]. The Tm was 42.0 ± 0.3 °C (Fig. 1c), with membranes in the gel state at normal human body temperature, as observed for CAF05 [10], while DDA:MMG-liposomes without poly(I:C) (CAF04) had a Tm of 41.6 ± 0.1 °C (Fig. 1c) as previously reported [25]. The model antigen OVA was 100% adsorbed to the surface of CAF09 (results not shown). The isoelectric point (pI) of OVA is approx. 4.5 [26], which suggests that attractive electrostatic interaction is an important adsorption mechanism of the net anionic protein to the cationic CAF09 liposomes, as observed for CAF01 [26]. CAF01 is liposomes comprised of DDA and TDB [27]. The hydrodynamic diameter and the PDI of CAF09 was significantly increased following OVA adsorption (Fig. 1b), probably due to flocculation of the dispersion upon addition of OVA. This is in contrast to adsorption of OVA to CAF01, where minimal flocculation was observed in the same dose range [26].
Fig. 1.
a) Representative CryoTEM images of CAF09 reveal multilamellar liposomes (black arrow). The focus beam melted some liposomes due to the high electron density (white arrow). b) Addition of OVA to CAF09 causes an increase in the average intensity-weighted hydrodynamic particle diameter (circles, left axis) and PDI (squares, right axis), respectively. Data points represent individual measurements (circles, squares) and mean values (horizontal lines), n = 3–6. ***p ≤ 0.001, ****p ≤ 0.0001. c) Representative thermograms of CAF04 and CAF09, 3 mg lipids/ml.
3.2. I.p. immunization with CAF09-adjuvanted OVA induces strong antigen-specific CD8+ T-cell responses in vivo
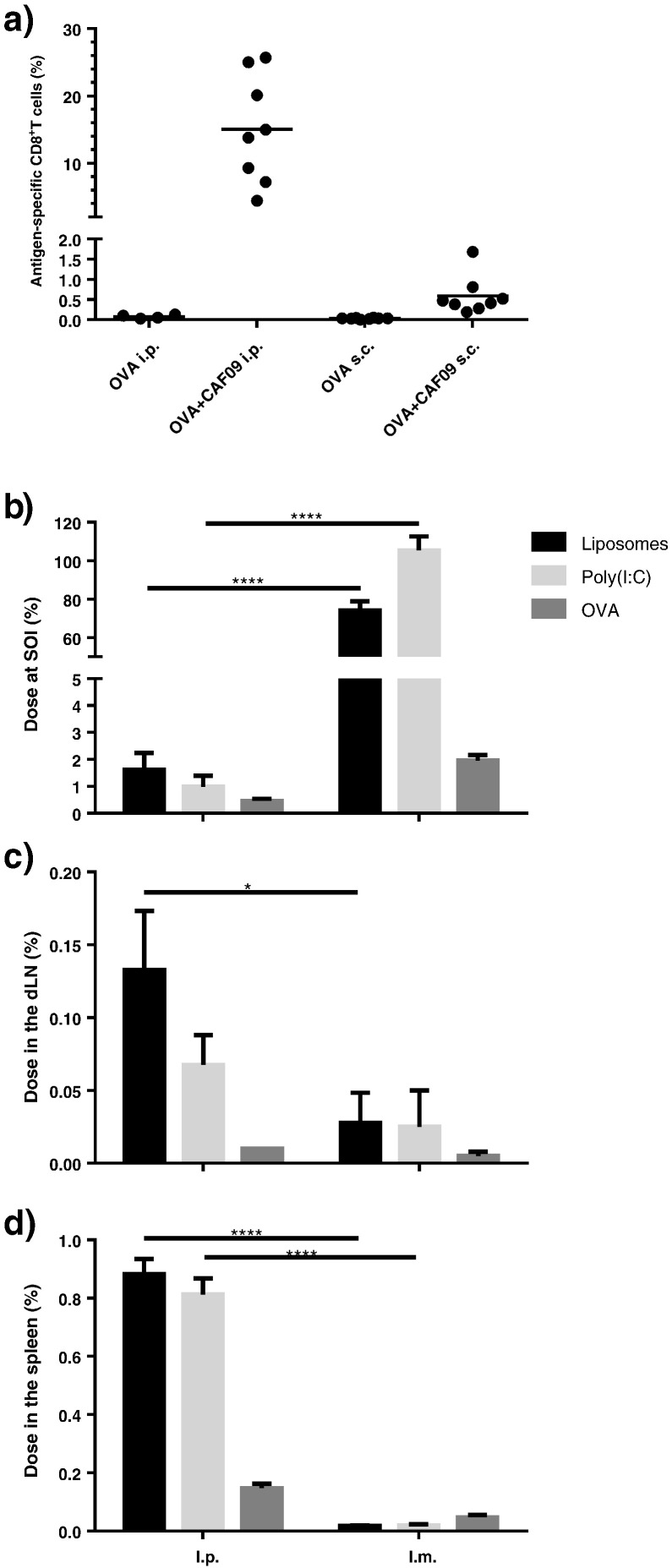
I.p. immunization was compared to the s.c. administration route. Either unadjuvanted (unadj.) OVA (10 μg/dose) or OVA adjuvanted with CAF09 (OVA + CAF09) was administered. Immunization with OVA + CAF09 via the i.p. route resulted in induction of very strong CD8+ T-cell responses (Fig. 2a), whereas the responses were very weak when OVA + CAF09 was administered s.c. These results are in accordance with results from previous studies using CAF09 or CAF05 as adjuvants [8], [9], [10], which also showed that neither the Th1/Th17-inducing adjuvant CAF01 nor poly(I:C) alone induce CD8+ T-cell responses [10]. Robust CD8+ T-cell responses were achieved upon vaccination with a number of different recombinant synthetic peptide and protein antigens surface-adsorbed to CAF09; the M. tuberculosis antigens TB10.3-P1 and H56, the human papilloma virus antigen HPV16-E7, and the HIV antigen Gag p24 [8]. Immunization with OVA + CAF09 did not stimulate CD4+ T-cell responses (results not shown), which might be explained by the weak MHC-II epitopes of OVA, as CAF09 has previously been reported to induce CD4+ T-cell responses with other antigens [8].
Fig. 2.
a) Immune responses in mice immunized s.c. or i.p. with OVA + CAF09 three times with two-week intervals, assessed eight days after the final immunization. Percentage of antigen-specific CD8+ T cells in the blood identified as SIINFEKL+ CD44hi CD8+ T-cells, as described in Supplementary Data Fig. S1. Data points represent individual mice and mean values, n = 4–8. b–d) Recovery of radiolabelled vaccine components [3H-liposomes, 32P-poly(I:C) and 125I-OVA] as the fraction of the administered dose at the SOI (b), the dLN (c) and the spleen (d) 6 h after administration. Data points represent mean values + SEM (n = 4). *p ≤ 0.05, ***p ≤ 0.001, ****p ≤ 0.0001.
These data thus confirm that the CD8+ T-cell responses induced by CAF09 are largely dependent on the administration route. We therefore hypothesized that this difference may be a consequence of distinct biodistribution patterns of the vaccine administered via different routes. Following s.c. or i.m. immunization, the vaccine is expected to form a depot at the SOI as a result of the net positive surface charge and/or the particle size of the liposomes, as reported for CAF01 [28], [29]. Particles with a positive surface charge tend to aggregate in the interstitial fluid [28], and the formation of larger aggregates prevents their self-drainage via the relatively narrow lymphatic vessels to the local LNs [30]. In contrast, self-drainage of OVA + CAF09 to the local LNs is expected following i.p. immunization [31]. To confirm this, we performed a biodistribution study for a quantitative pharmacokinetic evaluation. The liposomes were labelled with trace amounts of 3H-cholesterol incorporated in the membrane bilayer, 32P-labelled poly(I:C) and 125I-labelled OVA. Mice were immunized i.p. or i.m. with either unadj. OVA or OVA + CAF09. The radioactivity in the dLNs was determined at 6 h as the percentage of the administered dose. I.m. immunization was chosen for the biodistribution studies to enable the recovery of the SOI and evaluate the depot-forming ability of the adjuvant. Data showed that the adjuvant, but not the antigen, remained at the SOI 6 h after i.m. immunization with a recovery of approx. 80% of the initial liposome dose (Fig. 2b). In contrast, the vaccine was rapidly cleared following i.p. immunization with approx. 2% of the administered liposome dose recovered in the peritoneum after 6 h (Fig. 2b).
In accordance with the hypothesis, only a small fraction of the administered liposome dose was recovered in the spleen (approx. 0.05%) and the dLN (approx. 0.05%) following i.m. immunization (Fig. 2c and d). In contrast, approx. 44 and 7 times higher levels of the adjuvant, respectively, were recovered in the spleen and the TLN following i.p. immunization (Fig. 2c and d). The vaccine did also drain to the MLN following i.p. immunization (data not shown since the drainage kinetics to the MLN was similar to the drainage kinetics to the TLN). These results support the hypothesis that the adjuvant forms a depot at the SOI following i.m. immunization, but not upon i.p. immunization.
3.3. OVA co-administered with CAF09 is preferentially associated with DCs/monocytes
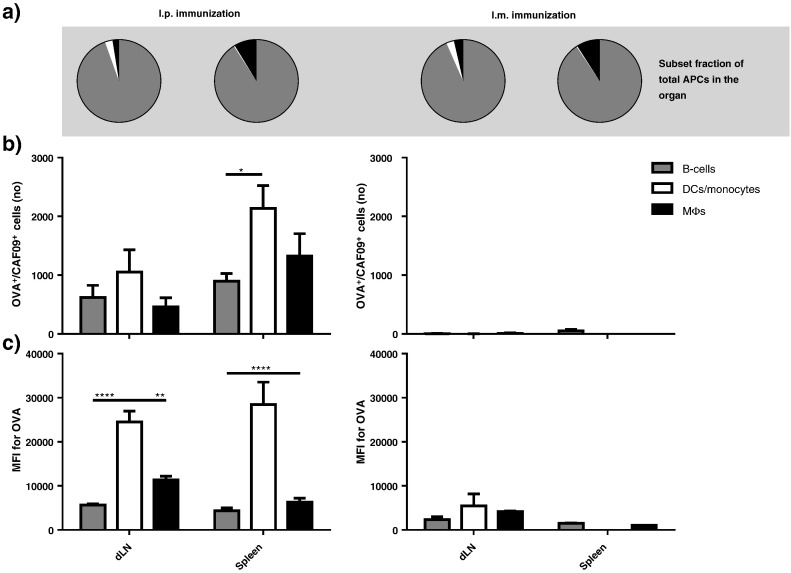
DCs are expected to be the main cell type responsible for driving the induction of CD8+ T-cell responses [11], [14]. Therefore, it was of particular interest to analyze the association of this APC subset with the vaccine components. Mice were immunized once i.m. or i.p. with fluorescently labelled OVA and CAF09. At 6 h post immunization, single cell suspensions from the spleen and the dLNs were stained with a simplified antibody panel directed against major APC markers; DC/monocytes (CD11c+, F4/80−, CD19−), B cells (CD19+, CD11c−, F4/80−) and macrophages (MΦs; F4/80+, CD11c−, CD19−) (Supplementary data, Table S2). Boolean gating was chosen for the separation of the individual APC subsets to weight the different subsets equally during analysis. Within each subset, vaccine association was defined as OVA+ or OVA+/CAF09+ cells (Supplementary data, Fig. S2).
The total fraction of each APC subset in the spleen and dLNs, respectively (Fig. 3a), as well as the total number of vaccine-associated cells, were assessed (Fig. 3b). The higher dose-fraction of the adjuvant recovered following i.p. immunization, as compared to i.m. immunization, in the biodistribution studies (Fig. 2b and c) correlated well with a higher number of OVA+/CAF09+ cells in both the dLNs and the spleen (Fig. 3b). The B cell population was the most numerous APC subset in both the spleen and the dLNs (Fig. 3a). However, this was not reflected in the vaccine association pattern following i.p. immunization; OVA+/CAF09+ DCs/monocytes were more numerous than both B cells and MΦs (Fig. 3b, left). Furthermore, the mean fluorescence intensity (MFI) values for the antigen (i.e. an estimation of the amount of OVA associated to each cell), showed that significantly higher levels of OVA were associated with DCs/monocytes than with B cells and MΦs. This confirms the biodistribution results (Fig. 2 c and d), since only insignificant numbers of OVA+/CAF09+ APCs were measured in the dLN and the spleen 6 h after i.m. immunization (Fig. 3c) and the MFI levels of the APCs were low as compared to after i.p. immunization (Fig. 3d).
Fig. 3.
a) Fractions of different APCs in the examined organs. b) Number of OVA+/CAF09+ associated APCs in the dLN (ILN for i.m. or TLN for i.p. immunization) and the spleen upon i.p. or i.m. immunization with OVA + CAF09 6 h after immunization. Data represent mean values + SEM (n = 9–13). c) MFI values for OVA as represented in b). Data represent mean values + SEM (n = 2–10). **p ≤ 0.01, ****p ≤ 0.0001.
3.4. CAF09 retains OVA in the draining lymph nodes and the spleen upon i.p. administration
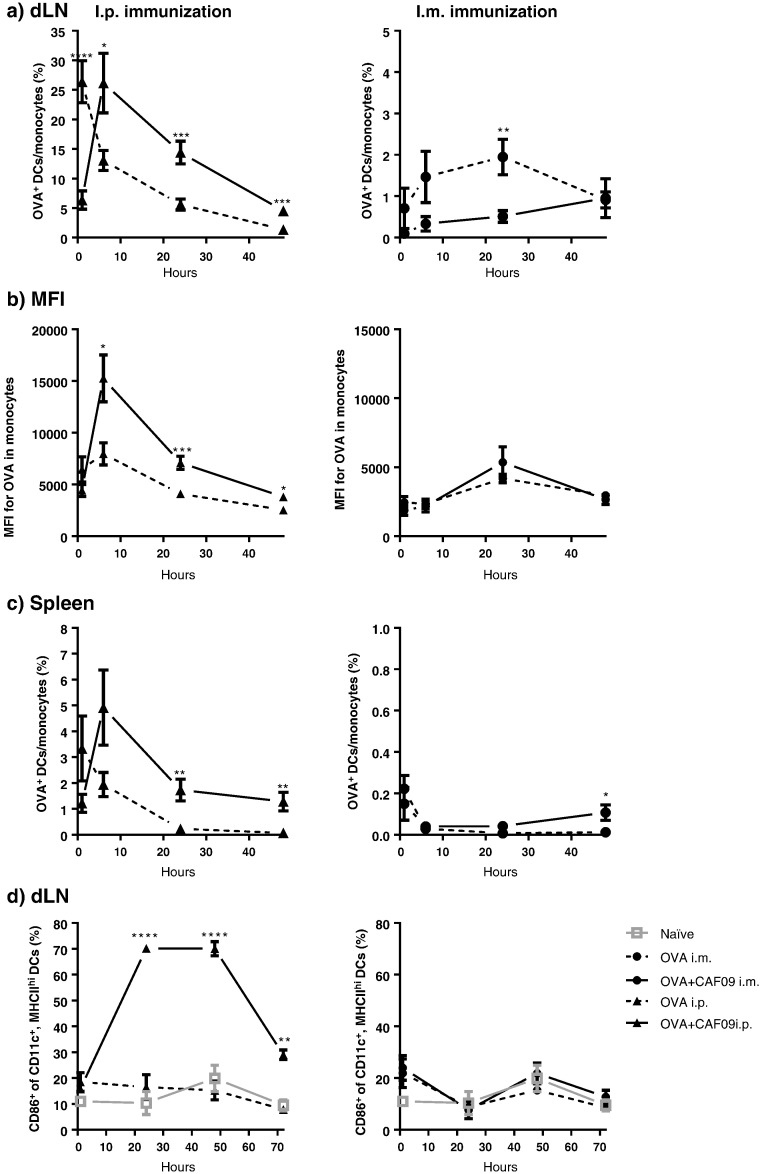
The association kinetics of OVA to DCs/monocytes in the dLNs were subsequently evaluated for 48 h, with samples collected at 1, 6, 24 and 48 h (Fig. 4a). Unadj. OVA drained rapidly upon i.p. immunization, evident from the high association degree to DCs/monocytes (~ 26% OVA+ DCs/monocytes) 1 h post immunization with a steady decline over the 48 h study period. The profile for mice immunized i.p. with OVA + CAF09 showed a delayed drainage, with the highest OVA association to DCs/monocytes measured 6 h post immunization (Fig. 4a) and with significantly (p < 0.001) elevated levels of OVA+ monocytes detected for the remaining study period as compared to immunization with unadj. OVA. Furthermore, the MFI values for the unadj. OVA group were significantly lower than the values for the OVA + CAF09 group (Fig. 4b). The observed OVA association to DCs/monocytes and MFI peaks coincided 6 h post immunization, indicating that the drainage of the majority of the vaccine took place within the first 6 h following i.p. administration. The association kinetics between OVA and DCs/monocytes in the spleen was comparable to that observed in the dLNs following i.p. administration (Fig. 4c). Only very low levels of OVA+ DCs/monocytes were measured in the dLNs and the spleen within the 48 h study period following i.m. immunization with both unadj. OVA and OVA + CAF09. These observations are in accordance with results obtained with the closely related adjuvant CAF01, which has been shown to form a depot at the SOI, with only minute amounts of vaccine+ DCs detected in the dLNs [29].
Fig. 4.
Qualitative association of fluorescently labelled vaccine components with DCs/monocytes was evaluated as described in Supplementary data, Fig. S2. a) Mice were immunized with unadj. OVA or OVA + CAF09, respectively, either i.p. (left) or i.m. (right), and the percentage of OVA+ monocytes in the dLNs was evaluated. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 for each time point. Data points represent mean ± SEM (n = 8–12). b) MFI values determined for OVA+ monocytes in the dLNs. *p ≤ 0.05, ***p ≤ 0.001, within each time point. Data points represent mean ± SEM (n = 6–12). c) The percentage of OVA+ monocytes in the spleen following i.p. (left) and i.m. (right) immunization. *p ≤ 0.05, **p ≤ 0.01 for each time point. Data points represent mean ± SEM (n = 8–12). d) Maturation of DCs (CD86+) in the dLNs as response to immunization with unadj. OVA and OVA + CAF09, respectively, either i.p. (left) or i.m. (right). **p ≤ 0.01, ****p ≤ 0.0001, of the OVA + CAF09 group compared to all other groups for that time point. No markers: No significance. Data points represent mean ± SEM (n = 4). The data are also presented in Fig. S5 as the number of cells.
3.5. Activation of DCs is dependent on the presence of the adjuvant in the lymphoid organs
We further characterized the immunostimulatory capacity of CAF09 by assessing the activation of DCs (CD11c+ MHC-IIhi) in the dLNs via their expression of the maturation marker CD86. I.m. and i.p. immunization with unadj. OVA did not activate DCs, as compared to unimmunized mice, and approx. 10% of the DCs were CD86+ in both groups at 24 h (Fig. 4d). In contrast, i.p. immunization with OVA + CAF09 resulted in a significant increase in the frequency of CD86+ DCs, and approx. 70% of the total DC population expressed CD86 at 24 h (p ≤ 0.001) (Fig. 4d). The highest measured level of DC activation was at 24 h and 48 h post i.p. immunization, and the activation was thus delayed, as compared to the observed highest level of OVA+/CAF09+ DCs/monocytes (Fig. 4a). The ability of CAF09 to induce CD8+ T-cell responses thus correlates well with its propensity to drain rapidly to the LNs.
3.6. Only i.p. immunization with OVA + CAF09 induces CD86+ DCs in the spleen
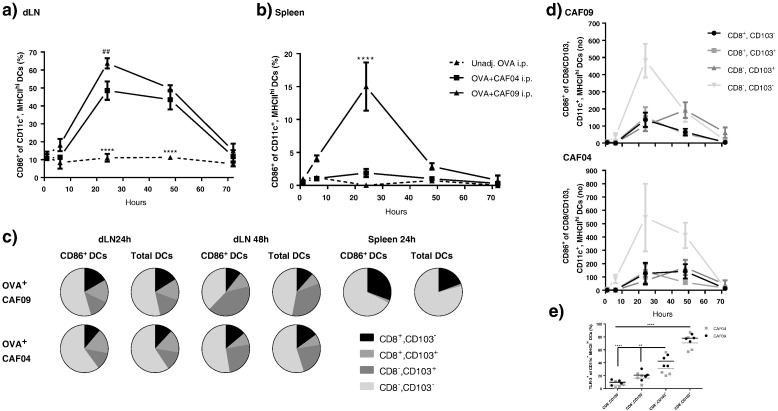
The investigations described above do not explain why poly(I:C) is required for the induction of CD8+ T-cell responses with CAF09 [8], since the draining kinetics observed for CAF09-adjuvanted vaccines following i.p. and i.m. immunization are largely expected to be dependent on the physicochemical properties of the delivery system. We therefore compared the ability of CAF09 to activate DCs with its poly(I:C)-free counterpart CAF04 [32].
I.p. immunization with OVA + CAF04 and OVA + CAF09 both resulted in increased frequencies of CD86+ DCs of the total DC population in the dLNs at 24 and 48 h, as compared to OVA alone (p ≤ 0.001) (Fig. 5a). At 24 h the frequency of activated DCs was significantly higher (p ≤ 0.01) for the OVA + CAF09 immunized group (63% CD86+ DCs) than for the OVA + CAF04 immunized group (49% CD86+ DCs) (Fig. 5a). In the spleen, only immunization with CAF09 activated the DCs (p ≤ 0.0001), though at a lower frequency than in the dLNs (15% CD86+ DCs at 24 h) (Fig. 5b). Division of the CD86+ DCs in the dLN into subsets according to their expression of CD8α and CD103 revealed that the phenotype distribution of the activated DCs resembled that of the total DC population at 24 and 48 h (Fig. 5c). However, the CD8α expression in the spleen was more prevalent on the activated CD86+ DCs (~ 30%) than on the total DC population (~ 20%) (Fig. 5c). As expected, no CD103+ DCs were observed in the spleen, as this organ has no direct connection to the lymph ducts (Fig. 5c). The highest number of the proposed migratory CD86+ CD8α−/CD103+ DCs was measured at 48 h post immunization in the dLNs for the OVA + CAF09 immunized mice, whereas the highest number of CD86+ CD8α+/CD103− DCs was measured at 24 h (Fig. 5d top). The measured maximum of the amount of CD8α−/CD103+ DCs was delayed by 24 h, as compared to the other DC subsets in the dLNs of OVA + CAF09 immunized mice. This suggests that this particular subset migrates from the peritoneum in response to the local activation by CAF09. An influx of CD86+ CD8α−/CD103+ DCs was also observed in the OVA + CAF04 immunized group, though the effect was less pronounced in relation to the other DC subsets, as compared to the OVA + CAF09 group (Fig. 5d bottom).
Fig. 5.
a) Activation of DCs in the dLNs following i.p. immunization with unadj. OVA, OVA + CAF04, and OVA + CAF09. Data represent mean ± SEM (n = 3–4). ##p ≤ 0.01, of the OVA + CAF09 group as compared to OVA + CAF04 at 24 h, and ****p ≤ 0.0001, of the unadj. OVA group as compared to all other groups for that time point. No marker: No significance. b) Activation of DCs in the spleen following i.p. immunization with unadj. OVA, OVA + CAF04, and OVA + CAF09. Data represent mean ± SEM (n = 3–4). ****p ≤ 0.0001, of the OVA + CAF09 group as compared to all other groups at 24 h. No marker: No significance. c) Fractions of CD8α+, CD103−; CD8α+, CD103+; CD8α−, CD103+; and CD8α−, CD103− in CD86+ DCs and total DCs in the dLNs and spleen at 24 h and 48 h following immunization with OVA + CAF09, OVA + CAF04, and unadj. OVA. n = 3–4. d) Number of CD86+ CD8α+ CD103−; CD8α+ CD103+; CD8α− CD103+; and CD8α− CD103− DCs in the dLNs following i.p. immunization with OVA + CAF09 (top) or OVA + CAF04 (bottom). Data represents mean ± SEM after background subtraction of naïve mice with negative values set to 0, n = 3–4. e) Expression of TLR3 on CD8α+, CD103−; CD8α+, CD103+; CD8α−, CD103+; and CD8α−, CD103− DCs. Data represent mean of mice immunized with OVA + CAF09 at 24 h in the dLNs, n = 4. The gating strategies used for flow cytometric analyses are shown in Supplementary data, Fig. S3. The data are also presented in Fig. S6 as the number of cells.
Poly(I:C) is recognized by TLR-3, which mediates the induction of CD8+ T-cell responses upon activation [33]. In this study, TLR-3 was expressed by approx. 80% of the CD103+ DCs, but only on approx. 20% of the CD8α+ DCs (Fig. 5e). Within the TLR-3+ DC population there was no significant difference in the expression level (MFI) between the CD8α+ and the CD103+ subsets (results not shown). Furthermore, no significant difference in the expression (frequency and MFI), was observed between mice immunized with OVA, OVA + CAF04 or OVA + CAF09, respectively, suggesting that the expression of TLR-3 is subset-, rather than activation-dependent (results not shown).
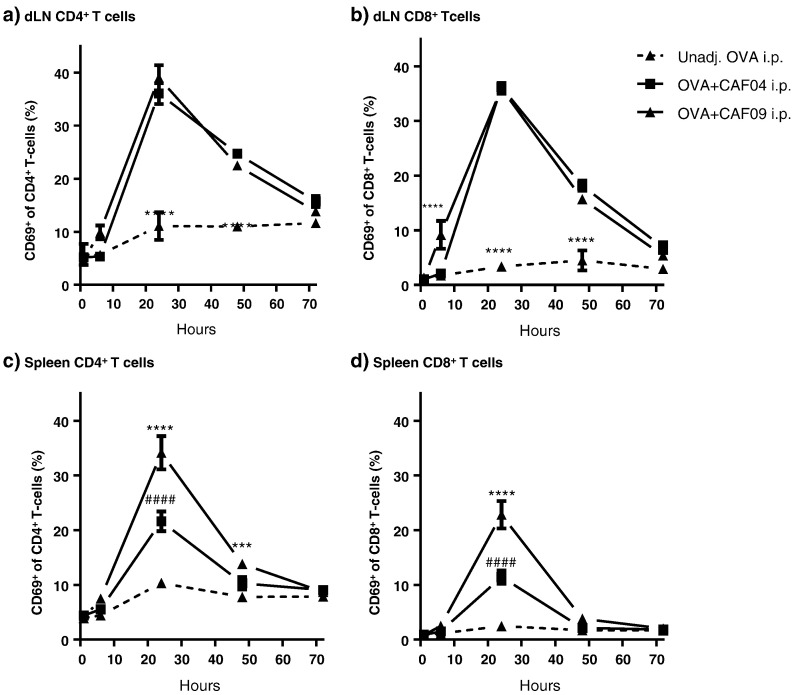
3.7. Unspecific activation of T cells in the spleen is highest following OVA + CAF09 immunization
Immunization with OVA + CAF04 and OVA + CAF09, respectively, induced the expression of CD69 on T cells in the dLNs and spleen with the highest level measured at 24 h post immunization (Fig. 6). The expression of CD69 by T cells is not necessarily antigen specific, because other noncognate stimuli can induce CD69 [34], [35]. When CD69 is expressed by T cells, migration out of the lymphoid tissue is prevented due to downregulation of the sphingosine 1-phosophate receptor-1 [34]. No significant differences in the CD69 expression levels were observed between OVA + CAF04 and OVA + CAF09 in the dLNs (Fig. 6a-b). However, immunization with OVA + CAF09 resulted in a significantly stronger activation of both CD4+ and CD8+ T-cells in the spleen (p ≤ 0.0001) (Fig. 6c–d), suggesting that poly(I:C) is partly responsible for the unspecific T cell activation in the spleen.
Fig. 6.
Activation of T cells in the dLNs and the spleen following i.p. immunization with unadj. OVA, OVA + CAF04, and OVA + CAF09. a) Fraction of CD69+ CD4+ T-cells in the dLNs following immunization. Data represent mean ± SEM (n = 3–4). ****p ≤ 0.0001, of the unadj. OVA group compared to all other groups within that time point. No marker: No significance. b) Fraction of CD69+, CD8+ T cells in the dLNs following immunization. Data represent mean ± SEM (n = 3–4). ****p ≤ 0.001, of the OVA + CAF09 group, as compared to all other groups at 6 h, and ****p ≤ 0.0001, of the unadj. OVA group compared to all other groups within that time point. No marker: No significance. c) Fractions of CD69+ CD4+ T-cells in the spleen following immunization. Data represent mean ± SEM (n = 3–4). ####p ≤ 0.0001, of the OVA + CAF04 group, as compared to unadj. OVA at 24 h, ****p ≤ 0.0001, of the OVA + CAF09 group, as compared to all other groups at 24 h, and ***p ≤ 0.001, of the OVA + CAF09 group, as compared to all other groups at 48 h. No marker: No significance. d) Fractions of CD69+ CD8+ T-cells in the spleen following immunization. Data represent mean ± SEM (n = 3–4). Left: ####p ≤ 0.0001, of the OVA + CAF04 group, as compared to unadj. OVA at 24 h, ****p ≤ 0.0001, of the OVA + CAF09 group, as compared to all other groups at 24 h. The gating strategies used for the flow cytometric analyses are shown in Supplementary data, Fig. S4. The data are also shown in Fig. S7, where the number of cells are presented.
4. Discussion
In the present study, we confirm that the stimulation of a CD8+ T-cell response upon CAF09-adjuvanted immunization is highly dependent on the administration route. We furthermore show that i.p. immunization results in a fast drainage to the local LNs, whereas i.m. immunization leads to the formation of a depot at the SOI, which might prevent the self-drainage of the vaccine to the dLNs and the spleen at a sufficient level to pass the threshold for CD8+ T-cell activation.
The recovery of a major fraction of the vaccine components in the dLNs and the spleen following i.p. immunization correlated closely with a high frequency of vaccine+ APCs in the respective organs (Fig. 3). Furthermore, adsorption of OVA to CAF09 caused a significant retention of the antigen in the dLNs and the spleen, as compared to administration of unadj. OVA. The influx of a large dose fraction of OVA + CAF09 in the draining lymphoid organs and the high prevalence of vaccine+ APCs thus correlate with the ability of CAF09 to induce a strong CD8+ T-cell response upon i.p. administration.
This suggests that i.p. immunization facilitate the concomitant delivery of the OVA antigen and the CAF09 adjuvant to professional cross-presenting CD8α+ DCs in the lymphoid tissues. In agreement with previous studies with cationic liposomes [29], [36], we observed that OVA adsorbed to CAF09 was preferentially associated with DCs/monocytes, as compared to B cells and MΦs, both with respect to the total number of cells and the amount of OVA delivered to each cell (Figs. 3b–c and 4a–c). Furthermore, CAF09 facilitated the activation of DCs within the first 24 h after i.p. but not i.m. immunization.
In accordance with the results of the present study, other biodistribution studies of vaccine adjuvants upon immunization of mice via different administration routes generally show that there is a preferential uptake of the antigen and/or the vaccine adjuvant by DCs and macrophages residing in the dLNs [36], [37], [38]. One example are cationic liposome-antigen-nucleic acid complexes that are mainly associated with CD11b+ cells in the dLN 4 h after i.p. immunization [36]. Similarly, s.c. immunization with OVA-conjugated nanoparticles in the footpad and the non-conjugated nanoparticles in the tail showed a preferential uptake of OVA and the nanoparticles by DCs in the dLNs [37], [38]. I.p. injection of the dye Indian ink showed that the MLN is the main dLN as compared to the jejunal, gastric and maxillary LNs; the spleen and the liver were also identified as target organs [39]. In accordance with this, Alum was found to have the strongest adjuvant activity in the MLN following i.p. immunization, and i.p. immunization with Alum resulted in recruitment of innate immune cells to the peritoneum [40].
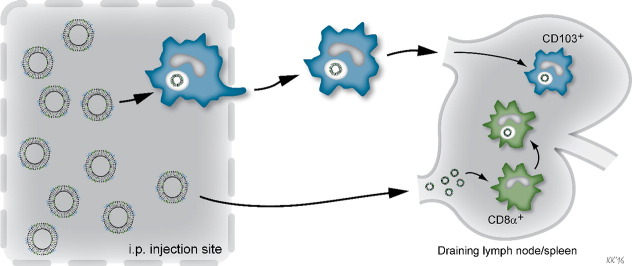
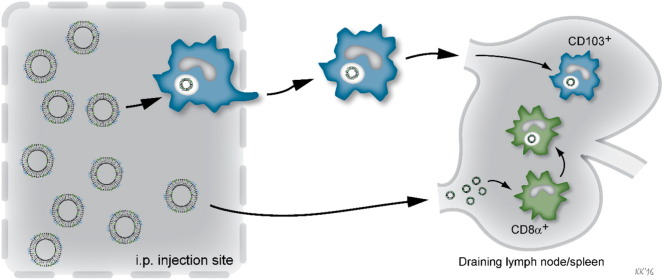
The distinct differences previously observed in the capability of CAF04 and CAF09 to induce CD8+ T-cell responses [8] could not be readily explained by the activation patterns of DCs in the dLNs, which were very similar for the two adjuvants. In contrast, we observed that only CAF09 was capable of activating DCs in the spleen, suggesting that poly(I:C) plays a role in the activation of DCs in the spleen. Interestingly, both CAF04 and CAF09 facilitated an unspecific activation (CD69 expression) of both CD4+ and CD8+ T cells in the dLNs and the spleen. This activation coincided with the activation of CD8α+ DCs peaking at 24 h, whereas the influx of CD103+ DCs was observed at 48 h, when the unspecific activation of the T cells had declined. Even though CAF04 did not induce significant DC activation in the spleen, it still facilitated unspecific activation of T cells, although this activation was significantly lower than the activation induced by CAF09. These results indicate that the self-draining OVA + CAF09 and the activation of CD8α+ DCs in the spleen may play important roles for the induction of CD8+ T-cell responses. On the other hand, the migratory CD103+ DCs may contribute to sustaining the CD8+ T-cell response in the dLNs in the CAF09 immunized mice (Fig. 7).
Fig. 7.
The hypothesis is that CAF09 can self-drain to the dLNs upon i.p. immunization enabling direct interaction with CD8α+ DCs in the dLNs, which have the ability to cross-present antigen. Simultaneously, CD103+ DCs at the SOI take up the vaccine particles by phagocytosis and subsequently migrate to the dLNs.
The differences between CAF04 and CAF09 in their ability to induce CD8+ T-cell responses might be explained by the pro-inflammatory effects of the poly(I:C) component of CAF09. The cross-presentation of antigen on MHC-I is not sufficient for the induction of CD8+ T-cell responses. Cross-licensing, which activates the CD8+ T-cells, does also require type I IFN signaling, which is induced by activation of e.g. TLR-3 [41]. However, the further quantification of the induction of type I IFNs in response to immunization with the adjuvants was considered beyond the scope of the present study.
The liposome-based CAF09 is a potent new adjuvant with the ability to induce strong CD8+ T-cell responses against several peptide- and protein-based antigens [8]. However, it has become evident that efficacious induction of CD8+ T cells in particular with CAF09 and related adjuvants is highly dependent on the administration route, and that s.c. or i.m. immunizations do not facilitate CD8+ T-cell induction, whereas particularly airway and i.p. immunization do [8], [42]. The choice of administration route thus seems to play a pivotal role for the quality and the magnitude of the CD8+ T-cell response. Most particulate vaccine delivery systems, including different types of nanoparticles, liposomes, and VLPs, which have been shown to stimulate CD8+ T-cell responses through cross-presentation, were also administered via the i.p. route [10], [36], [43], [44], [45], or via the nasal routes [44], by footpad [37], [38], [46], [47], or intradermal administration [48].
Engineering the physicochemical characteristics of the adjuvant particles might be used to optimize the targeting of LN-resident DCs by enhancing the self-drainage from the SOI, e.g. by reduction of the particle size and shielding of the net positive surface charge. Thorough knowledge of the biodistribution profile of the adjuvants following immunization may thus facilitate a more rational design of novel adjuvants.
5. Conclusion
The results of the current studies show that optimal delivery to the required immune cell subsets is necessary for the induction of a sufficient immune response. When aiming at inducing CD8+ T-cell responses, the delivery is further complicated because the target DCs are located in the secondary lymphoid organs, which are difficult to target via the conventional administration routes. However, some studies have shown that it is in fact possible to design delivery systems that induce CD8+ T-cell responses after s.c. or i.m. immunization [36], [37], [48], [49]. These delivery systems were of different types (cationic liposomes, microspheres, and nanoparticles), particle size and surface charge, thus illustrating that the specific characteristics required for the efficacious induction of CD8+ T-cell responses remain to be fully defined. Therefore, care must be taken when designing adjuvants and vaccines intended for the induction of CD8+ T-cell responses. Furthermore, alternative administration routes, such as the pulmonary, nasal, and intradermal routes, may be suitable alternatives to the conventional parenteral routes. Therefore, it is important to consider the engineering of the delivery system (chemical composition and physicochemical properties) in the context of the administration route at an early stage of the development process when designing novel subunit vaccines.
Conflict of interests
Karen Smith Korsholm, Peter Andersen and Dennis Christensen are employed by Statens Serum Institut, a nonprofit government research facility, which holds patents on the cationic liposomal adjuvants (CAF).
Acknowledgements
The work was funded by University of Copenhagen (STS) and Statens Serum Institut. Additional funding was provided by Innovation Fund Denmark (grant number 069-2011-1), Centre for Nano-vaccine (grant number 09-067052), NIH (R01 AI105422) and the European Commission through contract FP7-HEALTH-2011.1.4-4-280873 (ADITEC). The funding sources had no involvement in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; nor in the decision to submit the paper for publication.
We wish to thank C. B. Roces, Aston University, Birmingham, UK, for technical assistance. We also wish to thank the staff of the adjuvant group at Department of Infectious Disease Immunology, Statens Serum Institut, in particular Rune Fledelius Jensen, and the Department for Biological Services, Statens Serum Institut.
Footnotes
Supplementary data associated with this article can be found in the online version, at http://dx.doi.org/10.1016/j.jconrel.2016.08.034.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Rappuoli R., Miller H.I., Falkow S. The intangible value of vaccination. Science. 2002;297:937–939. doi: 10.1126/science.1075173. [DOI] [PubMed] [Google Scholar]
- 2.Zepp F. Principles of vaccine design — lessons from nature. Vaccine. 2010;28(Suppl. 3):C14–C24. doi: 10.1016/j.vaccine.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Foged C., Hansen J., Agger E.M. License to kill: formulation requirements for optimal priming of CD8+ CTL responses with particulate vaccine delivery systems. Eur. J. Pharm. Sci. 2012;45:482–491. doi: 10.1016/j.ejps.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Guy B. The perfect mix: recent progress in adjuvant research. Nat. Rev. Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 5.Petrovsky N., Aguilar J.C. Vaccine adjuvants: current state and future trends. Immunol. Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 6.O'Hagan D.T., Valiante N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2003;2:727–735. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrie Y., Mohammed A.R., Kirby D.J., McNeil S.E., Bramwell V.W. Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int. J. Pharm. 2008;364:272–280. doi: 10.1016/j.ijpharm.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Korsholm K.S., Hansen J., Karlsen K., Filskov J., Mikkelsen M., Lindenstrøm T., Schmidt S.T., Andersen P., Christensen D. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. Vaccine. 2014;32:3927–3935. doi: 10.1016/j.vaccine.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 9.Hansen J., Lindenstrøm T., Lindberg-Levin J., Aagaard C., Andersen P., Agger E. CAF05: cationic liposomes that incorporate synthetic cord factor and poly(I:C) induce CTL immunity and reduce tumor burden in mice. Cancer Immunol. Immunother. 2011;1-11 doi: 10.1007/s00262-011-1156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordly P., Rose F., Christensen D., Nielsen H.M., Andersen P., Agger E.M., Foged C. Immunity by formulation design: induction of high CD8+ T-cell responses by poly(I:C) incorporated into the CAF01 adjuvant via a double emulsion method. J. Control. Release. 2011;150:307–317. doi: 10.1016/j.jconrel.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Masson F., Mount A.M., Wilson N.S., Belz G.T. Dendritic cells: driving the differentiation programme of T cells in viral infections. Immunol. Cell Biol. 2008;86:333–342. doi: 10.1038/icb.2008.15. [DOI] [PubMed] [Google Scholar]
- 12.Heath W.R., Belz G.T., Behrens G.M.N., Smith C.M., Forehan S.P., Parish I.A., Davey G.M., Wilson N.S., Carbone F.R., Villadangos J.A. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 13.Kurts C., Robinson B.W.S., Knolle P.A. Cross-priming in health and disease. Nat. Rev. Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 14.Joffre O.P., Segura E., Savina A., Amigorena S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 15.Edelson B.T., Kc W., Juang R., Kohyama M., Benoit L.A., Klekotka P.A., Moon C., Albring J.C., Ise W., Michael D.G., Bhattacharya D., Stappenbeck T.S., Holtzman M.J., Sung S.-S.J., Murphy T.L., Hildner K., Murphy K.M. Peripheral CD103(+) dendritic cells form a unified subset developmentally related to CD8α(+) conventional dendritic cells. J. Exp. Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedoui S., Whitney P.G., Waithman J., Eidsmo L., Wakim L., Caminschi I., Allan R.S., Wojtasiak M., Shortman K., Carbone F.R., Brooks A.G., Heath W.R. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 17.Lee H.K., Zamora M., Linehan M.M., Iijima N., Gonzalez D., Haberman A., Iwasaki A. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J. Exp. Med. 2009;206:359–370. doi: 10.1084/jem.20080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerovic V., Houston S.A., Westlund J., Utriainen L., Davison E.S., Scott C.L., Bain C.C., Joeris T., Agace W.W., Kroczek R.A., Mowat A.M., Yrlid U., Milling S.W. Lymph-borne CD8[alpha] + dendritic cells are uniquely able to cross-prime CD8+ T cells with antigen acquired from intestinal epithelial cells. Mucosal Immunol. 2015;8:38–48. doi: 10.1038/mi.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belz G.T., Smith C.M., Eichner D., Shortman K., Karupiah G., Carbone F.R., Heath W.R. Cutting edge: conventional CD8α+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 20.Belz G.T., Smith C.M., Kleinert L., Reading P., Brooks A., Shortman K., Carbone F.R., Heath W.R. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal I., Ramsey J.D. The role of the lymphatic system in vaccine trafficking and immune response. Adv. Drug Deliv. Rev. 2011;63:909–922. doi: 10.1016/j.addr.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 22.den Haan J.M.M., Lehar S.M., Bevan M.J. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildner K., Edelson B.T., Purtha W.E., Diamond M., Matsushita H., Kohyama M., Calderon B., Schraml B.U., Unanue E.R., Diamond M.S., Schreiber R.D., Murphy T.L., Murphy K.M. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachmann M.F., Jennings G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 25.Nordly P., Korsholm K.S., Pedersen E.A., Khilji T.S., Franzyk H., Jorgensen L., Nielsen H.M., Agger E.M., Foged C. Incorporation of a synthetic mycobacterial monomycoloyl glycerol analogue stabilizes dimethyldioctadecylammonium liposomes and potentiates their adjuvant effect in vivo. Eur. J. Pharm. Biopharm. 2011;77:89–98. doi: 10.1016/j.ejpb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Hamborg M., Jorgensen L., Bojsen A., Christensen D., Foged C. Protein antigen adsorption to the DDA/TDB liposomal adjuvant: effect on protein structure, stability, and liposome physicochemical characteristics. Pharm. Res. 2013;30:140–155. doi: 10.1007/s11095-012-0856-8. [DOI] [PubMed] [Google Scholar]
- 27.Davidsen J., Rosenkrands I., Christensen D., Vangala A., Kirby D., Perrie Y., Agger E.M., Andersen P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate) — a novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta. 2005;1718:22–31. doi: 10.1016/j.bbamem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Henriksen-Lacey M., Bramwell V.W., Christensen D., Agger E.M., Andersen P., Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J. Control. Release. 2010;142:180–186. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Kamath A.T., Rochat A.-F., Christensen D., Agger E.M., Andersen P., Lambert P.-H., Siegrist C.-A. A liposome-based mycobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swartz M.A. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 31.Allen T.M., Hansen C.B., Guo L.S.S. Subcutaneous administration of liposomes: a comparison with the intravenous and intraperitoneal routes of injection. Biochim. Biophys. Acta. 1993;1150:9–16. doi: 10.1016/0005-2736(93)90115-g. [DOI] [PubMed] [Google Scholar]
- 32.Nordly P., Agger E., Andersen P., Nielsen H., Foged C. Incorporation of the TLR4 agonist monophosphoryl lipid A into the bilayer of DDA/TDB liposomes: Physico-chemical characterization and induction of CD8+ T-cell responses in vivo. Pharm. Res. 2011;28:553–562. doi: 10.1007/s11095-010-0301-9. [DOI] [PubMed] [Google Scholar]
- 33.Jelinek I., Leonard J.N., Price G.E., Brown K.N., Meyer-Manlapat A., Goldsmith P.K., Wang Y., Venzon D., Epstein S.L., Segal D.M. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J. Immunol. 2011;186:2422–2429. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackay L.K., Braun A., Macleod B.L., Collins N., Tebartz C., Bedoui S., Carbone F.R., Gebhardt T. Cutting edge: CD69 interference with Sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 35.Sancho D., Gómez M., Sánchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Zaks K., Jordan M., Guth A., Sellins K., Kedl R., Izzo A., Bosio C., Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J. Immunol. 2006;176:7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 37.Keller S., Wilson J.T., Patilea G.I., Kern H.B., Convertine A.J., Stayton P.S. Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8+ T cell responses. J. Control. Release. 2014;191:24–33. doi: 10.1016/j.jconrel.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy S.T., Rehor A., Schmoekel H.G., Hubbell J.A., Swartz M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Marco A.J., Domingo M., Ruberte J., Carretero A., Briones V., Dominguez L. Lymphatic drainage of Listeria inonocytogenes and Indian ink inoculated in the peritoneal cavity of the mouse. Lab. Anim. 1992;26:200–205. doi: 10.1258/002367792780740549. [DOI] [PubMed] [Google Scholar]
- 40.Kool M., Soullié T., van Nimwegen M., Willart M.A.M., Muskens F., Jung S., Hoogsteden H.C., Hammad H., Lambrecht B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Bon A., Tough D.F. Type I interferon as a stimulus for cross-priming. Cytokine Growth Factor Rev. 2008;19:33–40. doi: 10.1016/j.cytogfr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Lindenstrøm T., Aagaard C., Christensen D., Agger E.M., Andersen P. High-frequency vaccine-induced CD8+ T cells specific for an epitope naturally processed during infection with M. tuberculosis do not confer protection. Eur. J. Immunol. 2014;44:1699–1709. doi: 10.1002/eji.201344358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox R.G., Erickson J.J., Hastings A.K., Becker J.C., Johnson M., Craven R.E., Tollefson S.J., Boyd K.L., Williams J.V. Human metapneumovirus virus-like particles induce protective B and T cell responses in a mouse model. J. Virol. 2014;88:6368–6379. doi: 10.1128/JVI.00332-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knuschke T., Sokolova V., Rotan O., Wadwa M., Tenbusch M., Hansen W., Staeheli P., Epple M., Buer J., Westendorf A.M. Immunization with biodegradable nanoparticles efficiently induces cellular immunity and protects against influenza virus infection. J. Immunol. 2013;190:6221–6229. doi: 10.4049/jimmunol.1202654. [DOI] [PubMed] [Google Scholar]
- 45.Luo Z., Li P., Deng J., Gao N., Zhang Y., Pan H., Liu L., Wang C., Cai L., Ma Y. Cationic polypeptide micelle-based antigen delivery system: a simple and robust adjuvant to improve vaccine efficacy. J. Control. Release. 2013;170:259–267. doi: 10.1016/j.jconrel.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 46.LoBue A.D., Thompson J.M., Lindesmith L., Johnston R.E., Baric R.S. Alphavirus-adjuvanted norovirus-like particle vaccines: heterologous, humoral, and mucosal immune responses protect against murine norovirus challenge. J. Virol. 2009;83:3212–3227. doi: 10.1128/JVI.01650-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stano A., Scott E.A., Dane K.Y., Swartz M.A., Hubbell J.A. Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials. 2013;34:4339–4346. doi: 10.1016/j.biomaterials.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 48.Evans J.T., Ward J.R., Kern J., Johnson M.E. A single vaccination with protein-microspheres elicits a strong CD8 T-cell-mediated immune response against mycobacterium tuberculosis antigen Mtb8.4. Vaccine. 2004;22:1964–1972. doi: 10.1016/j.vaccine.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 49.Zhou W., Moguche A.O., Chiu D., Murali-Krishna K., Baneyx F. Just-in-time vaccines: Biomineralized calcium phosphate core-immunogen shell nanoparticles induce long-lasting CD8+ T cell responses in mice. Nanomedicine. 2014;10:571–578. doi: 10.1016/j.nano.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.