Abstract
Background
Pneumococcal adherence to the nasopharyngeal epithelium is a critical step in colonisation and disease. The probiotic bacterium, Streptococcus salivarius, can inhibit pneumococcal adherence to epithelial cells in vitro. We investigated the mechanism(s) of inhibition using a human pharyngeal epithelial cell line (Detroit 562) following pre-administration of two different strains of S. salivarius.
Results
Whilst the bacteriocin-encoding megaplasmids of S. salivarius strains K12 and M18 were essential to prevent pneumococcal growth on solid media, they were not required to inhibit pneumococcal adherence. Experiments testing S. salivarius K12 and two pneumococcal isolates (serotypes 19F and 6A) showed that inhibition of 19F may involve S. salivarius-mediated blocking of pneumococcal binding sites: a negative correlation was observed between adherence of K12 and 19F, and no inhibition occurred when K12 was prevented from contacting epithelial cells. K12-mediated inhibition of adherence by 6A may involve additional mechanisms, since no correlation was observed between adherence of K12 and 6A, and K12 could inhibit 6A adherence in the absence of cell contact.
Conclusions
These results suggest that S. salivarius employs several mechanisms, including blocking pneumococcal binding sites, to reduce pneumococcal adherence to pharyngeal epithelial cells. These findings extend our understanding of how probiotics may inhibit pneumococcal adherence and could assist with the development of novel strategies to prevent pneumococcal colonisation in the future.
Electronic supplementary material
The online version of this article (doi:10.1186/s12866-016-0843-z) contains supplementary material, which is available to authorized users.
Keywords: Probiotics, Probiotic mechanisms, Respiratory tract, Streptococcus salivarius, Streptococcus pneumoniae, Pneumococcus, Colonisation, Adherence
Background
Streptococcus pneumoniae (the pneumococcus) commonly colonises the nasopharynx of healthy humans, especially young children. Carriage is considered a prerequisite for pneumococcal disease and facilitates the transmission of pneumococci throughout communities [1]. Dissemination of pneumococci from the nasopharynx to other body sites can give rise to diseases such as meningitis, sepsis, pneumonia, and otitis media. An estimated 800,000 children under the age of five die from pneumococcal infections each year, with most deaths occurring in low-income countries where carriage rates are especially high [2]. Strategies targeting the reduction of pneumococcal colonisation could potentially reduce this burden of disease.
Current pneumococcal conjugate vaccines (PCVs) induce protection against 10–13 of the most common disease-causing serotypes via the induction of anti-capsular antibodies. Although PCVs have successfully reduced carriage and disease caused by vaccine serotypes, they are expensive to produce and have led to an increase in colonisation by non-vaccine serotypes (serotype replacement) [3]. In recent years, there has been increased interest in the use of probiotics, which are defined as live microorganisms that can confer a health benefit to the host, to reduce pathogen colonisation and respiratory tract infections [4]. Proposed mechanisms of probiotic action include inhibition of pathogen colonisation via competition for binding, direct inhibition due to the activity of secreted antimicrobial molecules and the induction of immunomodulatory effects in the host [5–9].
Streptococcus salivarius is a member of the respiratory tract microbiota and has been commercially available as an oral probiotic for more than a decade [10]. Small clinical trials have shown that administration of S. salivarius strains K12 and M18 can reduce the occurrence of tonsillitis and otitis media [11] and reduce dental plaque levels in children [12], as well as treat halitosis in adults [13]. Several in vitro studies have found that S. salivarius can prevent the growth of a range of respiratory pathogens, including the pneumococcus, through production of megaplasmid-encoded bacteriocins and bacteriocin-like inhibitory substances (BLIS) [7, 14–16]. However, the mechanisms by which they inhibit pathogen adherence in vivo are unknown. We have previously shown that S. salivarius K12 can inhibit pneumococcal adherence to a human epithelial cell line (CCL-23) [17]. Here, we demonstrate that the same phenomenon is observed in Detroit 562 pharyngeal epithelial cells and investigate the inhibitory mechanisms involved, including the role of the S. salivarius megaplasmid. Our results suggest that S. salivarius K12 inhibits pneumococcal adherence by blocking pneumococcal binding sites, although other mechanisms such as direct interference through the action of secreted molecules may also contribute.
Methods
Bacterial strains, cell lines and culture conditions
Bacterial isolates are described in Tables 1 and 2. Pneumococcal isolates were selected to represent a range of serotypes and based on their ability to adhere to human epithelial cells. S. salivarius isolates were sourced from Blis Technologies Ltd, New Zealand. All isolates were cultured at 37 °C in 5 % CO2 on horse blood agar (HBA; Thermo Fisher Scientific) plates, in Todd-Hewitt broth (THB; Oxoid), or THB supplemented with 0.5 % (w/v) yeast extract (THY; Becton Dickinson). Deferred antagonism testing was carried out on BaCa (Columbia agar base; Life Technologies Ltd.) plates supplemented with human blood (5 %, v/v) and CaCO3 (0.1 %, w/v) except where noted.
Table 1.
Streptococcus salivarius isolates used in this study
| S. salivarius isolate | Known bacteriocins |
|---|---|
| K12 | SalA, SalB |
| K12 mp− | – |
| M18 | SalA, Sal9, SalM |
| M18 mp− | – |
| A234 | SalA |
| NR | SalB |
| T18A | SalA, SalB |
| T30A | Unknown |
| Min5 | SalA, SalB |
| 20P3 | SalA |
Table 2.
Pneumococcal isolates used in this study
| Pneumococcal isolate | Serotype | Origin |
|---|---|---|
| PMP1081 | 1 | Australia |
| PMP278 | 3 | Fiji |
| PMP241 | 4 | South Africa |
| PMP812 | 5 | Bangladesh |
| PMP6 (ATCC® 6305™) | 5 | Germany |
| PMP1043 | 6A | USA |
| PMP17 | 6A | Fiji |
| PMP434 | 6B | Fiji |
| PMP437 | 6C | Fiji |
| PMP1086 | 7F | Australia |
| PMP296 | 9V | Fiji |
| PMP130 | 14 | Fiji |
| PMP222 | 18C | South Africa |
| PMP843 | 19F | USA |
| PMP292 | 19A | Fiji |
| PMP283 | 22F | Unknown |
The Detroit 562 pharyngeal epithelial carcinoma cell line (ATCC CCL-138) was maintained in RPMI 1640 (Sigma-Aldrich) supplemented with 10 % (v/v) foetal bovine serum (FBS, Thermo Fisher Scientific). Monolayers were released by incubation with 0.25 % trypsin/EDTA (0.25 % (w/v) trypsin, 0.1 mM EDTA, Life Technologies) for 10 min at 37 °C in 5 % CO2 and seeded into 24-well trays at a concentration of 1.5 × 105 cells/well in RPMI supplemented with 5 % FBS for use in adherence assays.
Deferred antagonism testing
Testing for deferred antagonism was performed as described previously [18]. Briefly, overnight THB cultures of S. salivarius (producer strain) were used to inoculate a 1-cm-wide line down the middle of a human blood agar plate (as described above). Plates were incubated for 18 h at 37 °C in 5 % CO2, S. salivarius growth was removed, and the plate inverted over a chloroform-soaked cloth for 30 min to kill any remaining bacteria. Residual chloroform was then evaporated by exposure of the agar surface to air for 30 min. Overnight THB cultures of pneumococcal indicator strains were then streaked at right angles to the previously grown S. salivarius and incubated for a further 12–16 h. Plates were examined for growth where S. salivarius had previously grown, and prevention of pneumococcal growth recorded as + (observed) or – (not observed).
Adherence assays
Adherence assays were performed as described previously [17] with several modifications. D562 cells seeded into 24-well trays were washed twice with Hanks buffered salt solution (HBSS; Gibco®, Life Technologies) before the addition of 500 μl/well RPMI without serum. Bacteria were grown to log phase in THY (S. salivarius: 1.5 h, pneumococci: 3 h) and resuspended in 0.85 % (w/v) NaCl (Merck) to ~1.5 × 109 CFU/ml. A 10-fold dilution series of S. salivarius was prepared in 0.85 % NaCl and 10 μl aliquots of high (~1.5 × 107 CFU/well), medium (~1.5 × 106 CFU/well), and low (~1.5 × 105 CFU/well) concentrations of bacteria were administered to duplicate wells before centrifugation at 114 × g for 3 min to promote bacterial adherence to the cell monolayer. Plates were incubated at 37 °C in 5 % CO2 for 1 h. Pneumococci were prepared as above and added to all wells at a concentration of ~1.5 × 106 CFU/well (MOI of 10 pneumococci: 1 D562 cell) 1 h after S. salivarius. Centrifugation was performed as above and plates were incubated for a further 1 h. Cells (and adherent bacteria) were then washed three times with HBSS and harvested after the addition of 200 μl 0.25 % trypsin/EDTA to each well, incubation for 10 min at 37 °C in 5 % CO2, and addition of 800 μl of THY to each well with mechanical disruption. Lysates were stored at −20 °C until tested. Pneumococcal adherence (genome copies/well) was determined by quantitative real-time PCR (qPCR) targeting lytA (see below). The effect of bacterial infection on D562 cells was monitored microscopically and no significant cytopathic effects were observed during the adherence assay conditions. The effect of S. salivarius on pneumococcal adherence was calculated by normalising all treatments against wells containing pneumococci alone. The ability of the pneumococcal isolates to invade cells was measured by incubating cells with media containing 10 μg/ml penicillin and 200 μg/ml gentamicin for 15 min after incubation with pneumococci for 1 h (Additional file 1), confirming the validity of this model to investigate adherence. The adherence of S. salivarius to D562 cells at the time of pneumococcal administration was calculated following incubation of ~1.5 × 106 CFU/well bacteria for 1 h at 37 °C in 5 % CO2. Cells were washed three times with HBSS to remove non-adherent bacteria and harvested as above. CFU/well was determined by viable count on HBA plates and expressed as mean % adherence ± standard deviation compared to the inoculum added.
We performed preliminary experiments to determine whether heparin, which can inhibit pneumococcal adherence to epithelial cells in vitro by competitively binding to glycosylaminoglycans [19], could also inhibit S. salivarius adherence to D562 cells. Cells were incubated with 100 U/well of heparin (Pfizer) for 1 h before the addition of S. salivarius as above and results expressed as % adherence compared to S. salivarius without heparin. S. salivarius adherence to D562 cells over time was measured by qPCR targeting dex (see below).
We also investigated whether non-adherent S. salivarius had an effect on pneumococcal adherence. To do this, adherence assays were performed as described above, except that wells were washed three times with HBSS to remove unbound S. salivarius and fresh medium was added after S. salivarius pre-administration and before the addition of pneumococci. To determine the inhibitory effect of S. salivarius in the absence of cell contact, S. salivarius was pre-administered to D562 cells via permeable transwell inserts with a pore size of 0.4 μm (Corning, In vitro Technologies). To further investigate the mechanisms involved in the inhibition of pneumococcal adherence, preparations of K12 were: a) heat-killed by incubating at 70 °C for 30 min with their lack of viability confirmed by the absence of growth on HBA; b) enzymatically treated to remove outer surface proteins and carbohydrates of S. salivarius K12 by (i) resuspending K12 inocula in a final concentration of 5 mg/ml pronase E [20], or (ii) by the addition of 5 μl of 1 mg/ml sodium periodate [21] to K12 inocula prepared in 0.85 % NaCl for 15 min at 37 °C in 5 % CO2, and c) reducing K12 protein synthesis by resuspending K12 inocula in 2 mg/ml spectinomycin and incubating for 30 min at 37 °C in 5 % CO2. Following all treatments, K12 inocula were washed twice in 0.85 % NaCl prior to use in adherence assays.
qPCR
DNA extraction and pneumococcal qPCR assays were performed as described previously [17]. Primers (Sigma Aldrich) and dual-labelled probes (Eurogentec) were designed to detect chromosomal (dextranase gene, dex) and megaplasmid (mp) target sequences in S. salivarius. Primer/probe sequences and final concentrations were as follows: dexF2: TGAAGCAGATAACTTGGTGGTG (300 nM); dexR2: CTCTCTGCTGGCACAGCTT (300 nM); dex probe2: HEX-AGAAGTAGGTCCATCATCTGCC-3′-BHQ-1 (75 nM); mpF: AAGCCTTGTGCATCGACTCT (200nM); mpR: AACCAAGACGCGACTGTTGA (200 nM); mp probe: FAM-TGACCCTTTTTGTTGGTCGT-3′-BHQ-1 (300 nM). Specificity of both assays was confirmed by sequencing the amplified product and testing against a panel of closely related streptococcal species (Additional file 2: Table S1). qPCR reactions were carried out in duplicate using Agilent Brilliant III master mix (Agilent Technologies, Integrated Sciences), containing 1 μl of template DNA in 25 μl final volume using a Stratagene Mx3005P qPCR machine (Agilent Technologies). The cycling conditions were: 1 × 3 min at 95 °C, 40 × 20 s at 95 °C followed by 20 s at 60 °C. Pneumococcal and S. salivarius DNA was quantified using standard curves of reference strain isolates (ATCC 6305 and K12, respectively) and bacterial loads were calculated as described previously [22], based on 1 pg of genomic DNA being equivalent to 447.4 pneumococcal cells and 422.1 S. salivarius cells (assuming one genome per cell, and one copy of the target gene per genome).
Statistical analysis
Data were analysed using Prism 6.0d (GraphPad Software, Inc.) and Excel (Microsoft). Student’s t-test was used to compare pneumococcal adherence in all treatment groups. Spearman’s rank test was used to correlate pneumococcal and S. salivarius K12 adherence in adherence assays. A p value of < 0.05 was considered statistically significant for all assays. For all experiments n ≥ 3 unless otherwise stated.
Results
S. salivarius reduces pneumococcal adherence to pharyngeal epithelial cells
Previous studies in our laboratory demonstrated that S. salivarius K12 inhibits pneumococcal adherence to CCL-23 human epithelial cells in vitro, with the strongest effect seen when K12 was added before pneumococci (pre-administration). In this study, we investigated the mechanisms underlying this inhibition using a cell line more relevant to the pneumococcal ecological niche of the nasopharynx, Detroit 562 human pharyngeal epithelial cells (D562), and two isolates representing serotypes that commonly colonise (6A [PMP1043] and 19F [PMP843]). Pneumococcal adherence was measured following pre-administration of commercial probiotic S. salivarius strains K12 or M18 at high, medium and low doses.
All doses of K12 significantly inhibited 6A and 19F adherence (p < 0.001, Fig. 1). All doses of M18 inhibited 6A adherence (high: p < 0.001, medium: p < 0.001, low: p = 0.023, Fig. 1a), but only the high dose inhibited 19F adherence (p < 0.001, Fig. 1b). K12 adherence to D562 cells was higher than that of M18 (532.3 ± 86.49 % vs. 12.5 ± 8.14 %, p < 0.001). Heparin, which is known to inhibit pneumococcal adherence to epithelial cells by competing for binding to cell surface glycosylaminoglycans [19], did not affect S. salivarius K12 adherence to D562 cells (115.8 ± 27.81 %, p = 0.380, Student's t-test).
Fig. 1.
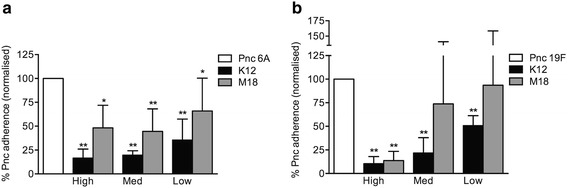
Pneumococcal adherence of serotypes 6A (a) and 19F (b) to pharyngeal epithelial cells following pre-administration of S. salivarius. Approximately 1.4 × 106 CFU pneumococci were added to D562 monolayers at an MOI of 11:1. Pneumococcal adherence was determined when incubated with pneumococci alone (Pnc, normalised to 100 %), or pre-incubated for 1 h with S. salivarius K12 or M18 at high (~1.6 × 107 CFU), medium (~1.6 × 106 CFU), or low (~1.6 × 105 CFU) doses. Data are mean + SD; n ≥ 3. * indicates p < 0.05, ** indicates p < 0.001 when compared to Pnc alone (Student’s t-test)
K12 adherence showed significant negative correlation with 19F adherence but not with 6A adherence (19F: r = −0.68, p = 0.002; 6A: r = −0.12, p = 0.66; Fig. 2). Significant inhibition of pneumococcal adherence was also observed following removal of non-adherent K12 by washing prior to the addition of pneumococci (Direct + wash, Table 3: all doses p < 0.001).
Fig. 2.
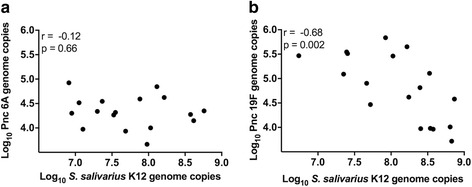
Correlation of adherence of S. salivarius K12 and pneumococcal serotype 6A (a) and 19F (b) adherence to pharyngeal epithelial cells. Adherence of S. salivarius and pneumococci to D562 monolayers was determined following pre-incubation for 1 h with high (~1.6 × 107 CFU), medium (~1.6 × 106 CFU), or low (~1.6 × 105 CFU) doses of S. salivarius K12 and subsequent incubation with ~1.4 × 106 CFU pneumococci for 1 h. r = Spearman rank correlation coefficient
Table 3.
Pneumococcal adherence to pharyngeal epithelial cells following pre-administration of S. salivarius K12 by various methods
| Pneumococcal adherencea | |||||||
|---|---|---|---|---|---|---|---|
| Direct | Transwell | Direct + wash | |||||
| Pneumococcal serotype | K12 Dosec | % (95 % CI) | p valueb | % (95 % CI) | p valueb | % (95 % CI) | p valueb |
| 6A | High | 16.6 (6.8, 26.5) | <0.001 | 90.5 (23.9, 157.1) | 0.698 | 25 (12.5, 37.4) | <0.001 |
| Medium | 19.6 (14.7, 24.4) | <0.001 | 61.5 (18.4, 104.5) | 0.049 | 45.2 (21.9, 68.5) | <0.001 | |
| Low | 35.4 (12.3, 58.5) | <0.001 | 44.1 (22.8, 65.5) | <0.001 | 45.1 (29.1, 61.1) | <0.001 | |
| 19F | High | 10.4 (2.5, 18.4) | <0.001 | 84.7 (33.6, 135.7) | 0.338 | 41.8 (17.6, 66.4) | <0.001 |
| Medium | 21.8 (1.7, 41.8) | <0.001 | 121.3 (16.5, 226.2) | 0.581 | 31.4 (13.2, 49.6) | <0.001 | |
| Low | 50.7 (33.8, 67.5) | <0.001 | 102.2 (33.7, 170.5) | 0.933 | 45.3 (16.7, 74.0) | <0.001 | |
aDirect: pre-administration of K12 directly onto D562 monolayer; Transwells: pre-administration of K12 via transwell inserts; Direct + wash: pre-administration of K12 directly onto D562 monolayers followed by a washing step to remove non-adherent K12
b p values calculated from pneumococcal adherence alone compared to pneumococcal adherence following different methods of K12 pre-administration (Student’s t test, *p < 0.05)
cHigh: ~1.5 × 107 CFU/ml; Medium: ~1.5 × 106 CFU/ml and Low: ~1.5 × 105 CFU/ml
The K12 megaplasmid is essential to prevent pneumococcal growth but not adherence
The megaplasmids of S. salivarius K12 and M18 encode bacteriocins that prevent the growth of a range of bacterial species on solid media [14, 16, 23, 24], but their roles in inhibition of pneumococcal growth or adherence are unknown. We compared the ability of K12, M18, their megaplasmid-negative derivatives, and a panel of S. salivarius strains to inhibit the growth of 15 pneumococcal isolates by deferred antagonism. S. salivarius strains K12, M18, A234, NR, T18A and Min5 inhibited all of the pneumococcal isolates tested (Table 4). Neither megaplasmid-negative strain (K12mp− or M18mp−) or strain T30A prevented the growth of any of the pneumococcal isolates tested (Table 4).
Table 4.
Deferred antagonism of S. salivarius strains against a panel of pneumococcal isolates
| S. salivarius producer strain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pneumococcal test strain | Serotype | K12 | K12 mp− | M18 | M18 mp− | A234 | NR | T18A | T30A | Min5 | 20P3 |
| PMP1081 | 1 | + | − | + | − | + | + | + | − | + | + |
| PMP278 | 3 | + | − | + | − | + | + | + | − | + | + |
| PMP241 | 4 | + | − | + | − | + | + | + | − | + | − |
| PMP812 | 5 | + | − | + | − | + | + | + | − | + | + |
| PMP1043 | 6A | + | − | + | − | + | + | + | − | + | − |
| PMP17 | 6A | + | − | + | − | + | + | + | − | + | + |
| PMP434 | 6B | + | − | + | − | + | + | + | − | + | + |
| PMP437 | 6C | + | − | + | − | + | + | + | − | + | + |
| PMP1086 | 7F | + | − | + | − | + | + | + | − | + | + |
| PMP296 | 9V | + | − | + | − | + | + | + | − | + | + |
| PMP130 | 14 | + | − | + | − | + | + | + | − | + | − |
| PMP222 | 18C | + | − | + | − | + | + | + | − | + | + |
| PMP292 | 19A | + | − | + | − | + | + | + | − | + | − |
| PMP843a | 19F | + | − | + | – | NT | NT | NT | NT | NT | NT |
| PMP283 | 22F | + | − | + | − | + | + | + | − | + | + |
“+” indicates inhibition of pneumococcal growth and “−" indicates no inhibition, n = 2
NT not tested
aperformed on horse blood agar plates
Since the S. salivarius megaplasmids were essential to prevent the growth of pneumococci, and K12 caused a greater reduction in pneumococcal adherence than M18 (Fig. 1), we next compared the ability of K12 and K12 mp− strains to inhibit pneumococcal adherence to D562 cells. Although the exact copy number of the megaplasmid is unknown, preliminary qPCR analysis confirmed that the ratio of K12 chromosomal DNA to megaplasmid DNA remained stable during bacterial growth (1.43, n = 1) and throughout assay conditions (1.17 and 1.20 after 1 and 2 h incubation with D562 monolayer, respectively, n = 1). All doses of K12 mp− significantly inhibited 6A adherence (high: p < 0.001, medium: p < 0.001, low: p = 0.016, Fig. 3a). When compared to K12, there was some evidence that the K12 mp− strain resulted in less inhibition than the K12 strain, but this difference was only statistically significant at the high dose (p = 0.044, Fig. 3a). For the 19F strain, only the high and medium doses of K12 mp− significantly inhibited pneumococcal adherence. We found no differences in the ability of K12 and K12 mp− to adhere to D562 cells (532 ± 86.49 % vs. 511 ± 6.92 %, p = 0.695) or in their adherence to D562 cells over time (Fig. 4, p > 0.05 for all time points).
Fig. 3.
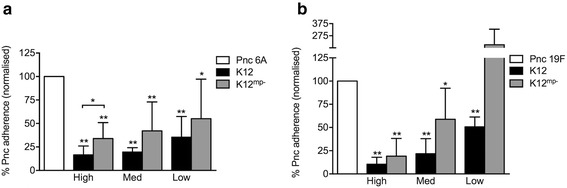
Adherence of pneumococcal serotypes 6A (a) and 19F (b) to pharyngeal epithelial cells following pre-administration of megaplasmid positive (K12) and negative (K12 mp−) S. salivarius K12 strains. Approximately 1.5 × 106 CFU pneumococci were added to D562 monolayers at an MOI of 10:1. Pneumococcal adherence was determined when incubated with pneumococci alone (Pnc, normalised to 100 %), or pre-incubated for 1 h with S. salivarius K12 or S. salivarius K12mp - at high (~2 × 107 CFU); medium (~2 × 106 CFU, med); or low (~2 × 105 CFU) doses. Data are mean + SD; n ≥ 6. * indicates p < 0.05, ** indicates p < 0.001 when compared to Pnc alone (Student’s t test)
Fig. 4.
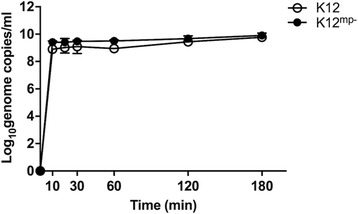
Time course of S. salivarius adherence to D562 cells. Cells were inoculated with either ~1.1 × 105 CFU of S. salivarius K12 or ~1.9 × 105 CFU of S. salivarius K12mp- and the number of adherent bacteria measured over three hours. Median ± IQR for both S. salivarius isolates are depicted (n ≥ 2)
Cell contact is required for K12 to inhibit adherence by serotype 19F, but not serotype 6A
To determine whether contact of S. salivarius with D562 cells was required to inhibit pneumococcal adherence, we performed adherence assays in which K12 was administered using transwell inserts, preventing direct contact with epithelial cells but allowing the passage of secreted molecules. Using this transwell system, no inhibition of 19F adherence was observed at any dose, while 6A adherence was inhibited at the medium (p = 0.049) and low (p < 0.001) doses (Table 3). Preliminary experiments showed that culture supernatants did not inhibit pneumococcal growth in the deferred antagonism test.
Protein synthesis may be required for K12 to inhibit 6A adherence
The above experiments indicated the involvement of multiple mechanisms for S. salivarius inhibiting pneumococcal adherence. We further investigated the molecular mechanism(s) of S. salivarius K12 inhibition of 6A adherence by performing different treatments on the K12 inoculum prior to its use in adherence assays. Denaturing proteins and killing K12 cells (heat-treatment), interrupting protein synthesis (spectinomycin treatment), and removing outer surface carbohydrates (sodium periodate treatment) and proteins (pronase E treatment) all reduced inhibition of 6A adherence compared with untreated K12 (Fig. 5). However, only spectinomycin-treated K12 could no longer significantly inhibit 6A adherence (p = 0.402, Fig. 5).
Fig. 5.
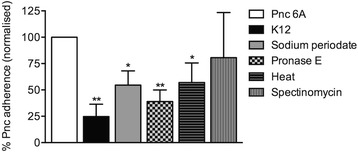
Adherence of pneumococcal serotype 6A to pharyngeal epithelial cells following pre-administration of treated S. salivarius K12. Approximately 1.5 × 106 CFU pneumococci were added to D562 monolayers at an MOI of 10:1. Pneumococcal adherence was determined when incubated with pneumococci alone (Pnc, normalised to 100 %), or pre-incubated for 1 h with approximately 1.5 × 107 CFU S. salivarius K12 (untreated), or treated with sodium periodate, Pronase E, heat-killed (heat), or spectinomycin. Data are mean + SD; n ≥ 3. * indicates p < 0.05, ** indicates p < 0.001 when compared to Pnc alone (Student’s t test)
Discussion
In this study, we found that various bacteriocin-producing strains of S. salivarius can prevent pneumococcal growth on solid media and that the commercial probiotic strains, K12 and M18, inhibit pneumococcal 6A and 19F adherence to a pharyngeal epithelial cell line. Studies investigating the probiotic mechanisms of S. salivarius have largely focused on the role of bacteriocins and BLIS. The ~190 kb megaplasmid of S. salivarius K12 harbours genes encoding the bacteriocins salivaricin A2 and B, which prevent the growth of some bacterial pathogens, including Streptococcus pyogenes, in vitro [7, 14, 15]. Our results showed that the K12 megaplasmid is required to prevent pneumococcal growth in vitro, but is not essential to inhibit pneumococcal adherence. Therefore the primary mechanism of inhibition of pneumococcal adherence in this model does not appear to be mediated by megaplasmid-encoded bacteriocins; although we note that lack of inhibition of 19F adherence by a low dose of K12mp- indicates a possible role for the megaplasmid at this concentration. Instead, our findings indicated that S. salivarius K12 can inhibit pneumococcal adherence by blocking pneumococcal binding sites on D562 cells. We observed a correlation between S. salivarius K12 adherence to D562 cells and inhibition of adherence by pneumococcal isolate PMP843 (19F). We also found that K12 contact with cells was essential for this inhibition. K12 binding to D562 cells did not require megaplasmid-encoded molecules, since a megaplasmid-negative strain of K12 displayed equal adherence. We observed differences in the inhibition of pneumococcal adherence depending on both the strain of S. salivarius used and the pneumococcal isolate tested. This was not unexpected, given that S. salivarius strains vary in their capacity to adhere to epithelial cells and inhibit other pathogens [7, 15, 16]. The pneumococcus is a diverse pathogen, with different strains and serotypes known to behave differently in vitro and in vivo [25, 26]. Overall, S. salivarius K12 displayed stronger inhibition of pneumococcal adherence than the M18 strain, possibly due to its increased capacity to adhere to D562 cells. Interestingly, K12 adherence to D562 cells did not correlate with 6A adherence, suggesting that mechanisms other than S. salivarius-mediated blocking of pneumococcal binding sites on epithelial cells may be involved in the inhibition of 6A adherence. This was further supported by the inhibition of 6A adherence by all doses of M18 despite its low adherence capability. Although a high dose of megaplasmid-negative K12 could still inhibit 6A adherence, the effect was significantly less than that observed with megaplasmid-positive K12, indicating a possible contribution to inhibition by the K12 megaplasmid in this assay. Transwell experiments demonstrated that for 19F, contact of S. salivarius K12 with D562 cells was required to inhibit pneumococcal adherence, consistent with a previous report showing that contact with epithelial cells was needed for S. salivarius K12 to inhibit adherence by S. pyogenes to HEp-2 cells [27]. However, low doses of K12 administered in the absence of cell contact could inhibit adherence of 6A. This could be due to bacteriocins or other secreted products, which may not necessarily be encoded on the megaplasmid. The differences observed using varying doses of probiotic suggest that quorum sensing may play a role in the expression of S. salivarius genes relevant to pneumococcal inhibition.
Further transwell experiments that test the K12 megaplasmid-negative strain may determine whether the molecules involved are chromosomally or plasmid encoded. Preliminary attempts to detect if bacteriocins were secreted through transwell membranes by deferred antagonism were unsuccessful, however this approach may have been insufficiently sensitive.
Overall, the data obtained in this study suggest that the primary mechanism by which S. salivarius K12 inhibits pneumococcal adherence to D562 cells is by blocking pneumococcal attachment to the epithelial cell surface. A number of pneumococcal surface molecules have been shown to play a role in adherence, such as the adhesion molecule PsaA, which binds to cell surface carbohydrates [28]. For S. salivarius, various cell surface molecules including fibrils and fimbriae have been implicated in adherence [29]. As heparin does not block S. salivarius adherence to D562 cells, glycosylaminoglycans do not seem to be a shared cell surface receptor. Since airway epithelial cells derived from carcinomas, such as D562 cells, can display altered expression of surface molecules compared to primary human epithelial cells [30], further investigation of common receptors used by these species should include testing of primary cells. Although inhibition of pneumococcal adherence could be due to competition for receptor-mediated binding, it could also result through a less specific mechanism, such as steric hindrance, or the ability of S. salivarius to mask pneumococcal binding sites. Other potential mechanisms by which S. salivarius may inhibit pneumococcal adherence, such as immune modulation of epithelial cells [31], were not examined in this study. Additionally, the in vitro model lacks the complexity of the nasopharynx, which can harbour a wide range of colonising bacterial species that interact with each other and with epithelial cells.
Whilst megaplasmid-encoded bacteriocins are required to inhibit the growth of pneumococci on solid media, our data suggest that it is the adherence of S. salivarius to epithelial cells that primarily blocks pneumococcal adherence in vitro. Therefore, strategies to use S. salivarius as a probiotic in the respiratory tract should optimise the ability of this species to colonise the target tissue. In vitro studies performed in our laboratory, and by others, have shown that the addition of probiotic bacteria to epithelial cells prior to pathogen administration is more effective than attempting to disrupt established pathogen colonisation [17, 27]. In clinical studies, high doses of M18 achieve greater colonisation rates and density in the oral cavity compared to lower doses [15]. Clinical studies of S. salivarius K12 as an oral probiotic have involved daily administration of high doses of the bacterium in an effort to achieve long-term colonisation of the oral cavity [32–34]. S. salivarius may be better suited as a probiotic for the oral cavity, where it predominates, than for the nasopharynx, the preferred niche of the pneumococcus. Strategies that facilitate S. salivarius adherence to the nasopharyngeal epithelium, such as the nasal spray recently employed by Santagati and colleagues [35], would likely be needed for this probiotic to inhibit pneumococcal colonisation in vivo.
Conclusions
Bacteriocin-encoding megaplasmids of S. salivarius strains K12 and M18 were essential to prevent pneumococcal growth on solid media but were not required to inhibit pneumococcal adherence to pharyngeal epithelial cells. Our results suggest that S. salivarius K12 employs several mechanisms, including blocking pneumococcal binding sites, to reduce pneumococcal adherence to pharyngeal epithelial cells. Further research is needed to identify the specific molecules involved. These findings contribute to our understanding of how probiotics may inhibit pneumococcal adherence and could assist with the development of novel strategies to prevent pneumococcal colonisation in the future.
Acknowledgements
We thank Casey Pell, Monica Nation, Jenna Smyth and Laura Boelsen for expert technical assistance, and Dr Paul Licciardi for critical review of the manuscript. For the provision of isolates we thank A/Prof Fiona Russell and the Fiji pneumococcal project team; Prof Kate O’Brien, Johns Hopkins Bloomberg School of Public Health; Prof Susan Hollingshead, University of Birmingham; Prof Samir Saha and the staff of the Child Health Research Foundation, Dhaka Shishu Hospital, Prof Shabir Madhi, University of Witwatersand and A/Prof Jorge Vidal, Rollins School of Public Health, Emory University.
Funding
This study was funded by the Murdoch Childrens Research Institute and the Victorian Government’s Operational Infrastructure Support program. Blis Technologies provided the S. salivarius isolates. JM is supported by an Australian Postgraduate Award, Australian Government. CS is supported by a Career Development Fellowship, National Health and Medical Research Council, Australia. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Authors’ contributions
All authors contributed to conception and design of the study and critical revision of the manuscript. JM performed deferred antagonism testing and adherence assays. JM, PAW and JDFH analysed and interpreted deferred antagonism results. JM, EMD and CS analysed and interpreted adherence assay results. JM, EMD and CS drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
JDF H is a current employee, PAW is a former employee and JRT is an advisor of the company Blis Technologies Ltd. This does not alter the authors’ adherence to the Good Publication Practice guidelines for pharmaceutical companies (GPP3).
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- ATCC
American type culture collection
- BLIS
Bacteriocin-Like Inhibitory Substance
- CFU
Colony forming unit
- DNA
deoxyribonucleic acid
- EDTA
Ethylenediaminetetraacetic acid
- FBS
Foetal bovine serum
- HBA
Horse blood agar
- HBSS
Hanks buffered salt solution
- MOI
Multiplicity of infection
- PCR
Polymerase chain reaction
- THB
Todd Hewitt broth
- THY
Todd Hewitt broth supplemented with yeast extract
Additional files
Supporting Information S1. Raw data used to generate figures and tables. (XLSX 52 kb)
Specificity of dex and mp gene targets to detect Streptococcus salivarius. DNA was extracted from pure cultures grown on HBA agar and 0.1 ng DNA of each isolate listed was used as a template for the dex and mp qPCR reactions, as described in the methods section. Mean Ct from duplicate wells is shown. (DOCX 15 kb)
Contributor Information
Jayne Manning, Email: jayne.manning@mcri.edu.au.
Eileen M. Dunne, Email: eileen.dunne@mcri.edu.au
Philip A. Wescombe, Email: philip.wescombe@oceaniadairy.co.nz
John D. F. Hale, Email: john.hale@blis.co.nz
E. Kim Mulholland, Email: Kim.Mulholland@lshtm.ac.uk.
John R. Tagg, Email: john.tagg@otago.ac.nz
Roy M. Robins-Browne, Email: r.browne@unimelb.edu.au
Catherine Satzke, Email: catherine.satzke@mcri.edu.au.
References
- 1.Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O'Brien KL, et al. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11:841–55. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Licciardi PV, Toh QZ, Dunne EM, Wong SS, Mulholland EK, Tang M, et al. Protecting against Pneumococcal Disease: Critical Interactions between Probiotics and the Airway Microbiome. PLoS Pathog. 2012 doi: 10.1371/journal.ppat.1002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prince T, McBain AJ, O'Neill CA. Lactobacillus reuteri protects epidermal keratinocytes from Staphylococcus aureus-induced cell death by competitive exclusion. Appl Environ Microbiol. 2012;78:5119–26. doi: 10.1128/AEM.00595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–7. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 7.Santagati M, Scillato M, Patane F, Aiello C, Stefani S. Bacteriocin-producing oral streptococci and inhibition of respiratory pathogens. FEMS Immunol Med Microbiol. 2012;65:31. doi: 10.1111/j.1574-695X.2012.00928.x. [DOI] [PubMed] [Google Scholar]
- 8.Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, et al. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun. 2008;76:4163–75. doi: 10.1128/IAI.00188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park MK, Vu N, Kwon YM, Lee Y-T, Yoo S, Cho YH, et al. Lactobacillus plantarum DK119 as a probiotic confers protection against Influenza virus by modulating innate immunity. PLoS One. 2013 doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wescombe PA, Hale JD, Heng NC, Tagg JR. Developing oral probiotics from Streptococcus salivarius. Future Microbiol. 2012;7:1355–71. doi: 10.2217/fmb.12.113. [DOI] [PubMed] [Google Scholar]
- 11.Di Pierro F, Donato G, Fomia F, Adami T, Careddu D, Cassandro C, et al. Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. Int J Gen Med. 2012;5:991–7. doi: 10.2147/IJGM.S38859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton JP, Drummond BK, Chilcott CN, Tagg JR, Thomson WM, Hale JD, et al. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J Med Microbiol. 2013;62:875–84. doi: 10.1099/jmm.0.056663-0. [DOI] [PubMed] [Google Scholar]
- 13.Burton JP, Moore CJ, Speiser G, Tagg JR. A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J Appl Microbiol. 2006;100:754–64. doi: 10.1111/j.1365-2672.2006.02837.x. [DOI] [PubMed] [Google Scholar]
- 14.Wescombe PA, Burton JP, Cadieux PA, Klesse NA, Hyink O, Heng NCK, et al. Megaplasmids encode differing combinations of lantibiotics in Streptococcus salivarius. Anton Leeuw Int J G. 2006;90:269–280. doi: 10.1007/s10482-006-9081-y. [DOI] [PubMed] [Google Scholar]
- 15.Burton JP, Wescombe PA, Macklaim JM, Chai MHC, MacDonald K, Hale JDF, et al. Persistence of the oral probiotic Streptococcus salivarius M18 is dose dependent and megaplasmid transfer can augment their bacteriocin production and adhesion characteristics. PLoS One. 2013 doi: 10.1371/journal.pone.0065991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patras KA, Wescombe PA, Roesler B, Hale JD, Tagg JR, Doran KS. Streptococcus salivarius K12 Limits Group B Streptococcus Vaginal Colonization. Infect Immun. 2015;83:3438–44. doi: 10.1128/IAI.00409-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunne EM, Toh ZQ, John M, Manning J, Satzke C, Licciardi PV. Investigating the effects of probiotics on pneumococcal colonization using an in vitro adherence assay. J Vis Exp. 2014 doi: 10.3791/51069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagg JR, Bannister LV. Fingerprinting beta-hemolytic streptococci by their production of and sensitivity to bacteriocine-like inhibitors. J Med Microbiol. 1979;12:397–411. doi: 10.1099/00222615-12-4-397. [DOI] [PubMed] [Google Scholar]
- 19.Tonnaer EL, Hafmans TG, Van Kuppevelt TH, Sanders EA, Verweij PE, Curfs JH. Involvement of glycosaminoglycans in the attachment of pneumococci to nasopharyngeal epithelial cells. Microbes Infect. 2006;8:316–322. doi: 10.1016/j.micinf.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Botes M, Loos B, van Reenen CA, Dicks LMT. Adhesion of the probiotic strains Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 to Caco-2 cells under conditions simulating the intestinal tract, and in the presence of antibiotics and anti-inflammatory medicaments. Arch Microbiol. 2008;190:573–84. doi: 10.1007/s00203-008-0408-0. [DOI] [PubMed] [Google Scholar]
- 21.Rennemeier C, Hammerschmidt S, Niemann S, Inamura S, Zaehringer U, Kehrel BE. Thrombospondin-1 promotes cellular adherence of Gram-positive pathogens via recognition of peptidoglycan. FASEB J. 2007;21:3118–32. doi: 10.1096/fj.06-7992com. [DOI] [PubMed] [Google Scholar]
- 22.Smith-Vaughn H, Byan R, Nadkarni M, Jacques NA, Halpin S, et al. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord. 2006 doi: 10.1186/1472-6815-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyink O, Wescombe PA, Upton M, Ragland N, Burton JP, Tagg JR. Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl Environ Microbiol. 2007;73:1107–13. doi: 10.1128/AEM.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heng NCK, Haji-Ishak NS, Kalyan A, Wong AYC, Lovric M, Bridson JM, et al. Genome sequence of the bacteriocin-producing oral probiotic Streptococcus salivarius strain M18. J Bacteriol. 2011;193:6402–3. doi: 10.1128/JB.06001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong SS, Toh QZ, Dunne EM, Mulholland EK, Tang ML, Robins-Browne RM, et al. Inhibition of Streptococcus pneumoniae adherence to human epithelial cells in vitro by the probiotic Lactobacillus rhamnosus GG. BMC Res Notes. 2013 doi: 10.1186/1756-0500-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis. 2003;187:1424–32. doi: 10.1086/374624. [DOI] [PubMed] [Google Scholar]
- 27.Fiedler T, Riani C, Koczan D, Standar K, Kreikemeyer B, Podbielski A. Protective mechanisms of respiratory tract Streptococci against Streptococcus pyogenes biofilm formation and epithelial cell infection. Appl Environ Microbiol. 2013;79:1265–76. doi: 10.1128/AEM.03350-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogaert D, de Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–54. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 29.Nobbs AH, Lamont RJ, Jenkinson HF. Streptococcus adherence and colonization. Microbiol Mol Biol R. 2009;73:407–50. doi: 10.1128/MMBR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rydberg C, Mansson A, Uddman R, Riesbeck K, Cardell LO. Toll-like receptor agonists induce inflammation and cell death in a model of head and neck squamous cell carcinomas. Immunology. 2009;128(Suppl 1):e600–e611. doi: 10.1111/j.1365-2567.2008.03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guglielmetti S, Taverniti V, Minuzzo M, Arioli S, Stuknyte M, Karp M, et al. Oral bacteria as potential probiotics for the pharyngeal mucosa. Appl Environ Microb. 2010;76:3948–58. doi: 10.1128/AEM.00109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horz HP, Meinelt A, Houben B, Conrads G. Distribution and persistence of probiotic Streptococcus salivarius K12 in the human oral cavity as determined by real-time quantitative polymerase chain reaction. Oral Microbiol Immunol. 2007;22:126–130. doi: 10.1111/j.1399-302X.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- 33.Power DA, Burton JP, Chilcott CN, Dawes PJ, Tagg JR. Preliminary investigations of the colonisation of upper respiratory tract tissues of infants using a paediatric formulation of the oral probiotic Streptococcus salivarius K12. Euro J Clin Microbiol Infect Dis. 2008;27:1261–63. doi: 10.1007/s10096-008-0569-4. [DOI] [PubMed] [Google Scholar]
- 34.Burton JP, Cowley S, Simon RR, McKinney J, Wescombe PA, Tagg JR. Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: A randomized, placebo-controlled, double-blind study. Food Chem Toxicol. 2011;49:2356–64. doi: 10.1016/j.fct.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 35.Santigati SM, Muscaridola N, Metoldo V, La Mantia I, Stefani S. Colonization, safety, and tolerability study of the Streptococcus salivarius 24SMBc nasal spray for its application in upper respiratory tract infections. J Clin Microbiol Infect Dis. 2015 doi: 10.1007/s10096-015-2454-2. [DOI] [PubMed] [Google Scholar]
- 36.Ross KF, Ronson CW, Tagg JR. Isolation and characterization of the lantibiotic salivaricin-A and its structural gene SalA from Streptococcus salivarius 20P3. Appl Environ Microbiol. 1993;59:2014–21. doi: 10.1128/aem.59.7.2014-2021.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wescombe PA, Upton M, Renault P, Wirawan RE, Power D, Burton JP, et al. Salivaricin 9, a new lantibiotic produced by Streptococcus salivarius. Microbiology. 2011;157:1290–9. doi: 10.1099/mic.0.044719-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.


