Abstract
Marked racial differences exist in dietary patterns and obesity, as well as cancer mortality. This study aims to assess whether dietary patterns are associated with cancer mortality overall and by race. We identified 22,041 participants from the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. Dietary patterns were categorized into: Convenience (Chinese and Mexican foods, pasta, pizza), Plant-based (fruits, vegetables), Southern (added fats, fried foods, sugar-sweetened beverages), Sweets/Fats (sugary foods) and Alcohol/Salads (alcohol, green-leafy vegetables, salad dressing). Using Cox regression, we examined the association between quartiles of dietary patterns and cancer mortality, adjusted for potential confounders, overall among all participants and stratified by race. A total of 873 cancer deaths were observed over the 10-year observation period: 582 (66.7%) in Whites and 291 (33.3%) in Blacks. Greater adherence to the Southern dietary pattern was associated with an increased risk of cancer mortality (4th vs. 1st quartile HR: 1.67; 95% CI: 1.32–2.10) overall, especially among Whites (4th vs. 1st quartile HR: 1.59; 95% CI: 1.22–2.08). The convenience (HR: 0.73; 95% CI: 0.56–0.94) and Plant-based (HR: 0.72; 95% CI: 0.55–0.93) dietary patterns were associated with up to a 28% reduced risk of cancer mortality, but only among Whites. Greater adherence to the Southern dietary pattern increased the risk of cancer mortality, while greater adherence to the convenience and Plant-based diets reduced the risk of cancer mortality among Whites. Racial differences were observed in the association between dietary patterns and cancer mortality, but warrant further study.
Keywords: diet, cancer, mortality, racial disparities, prospective cohort
Introduction
Significant disparities in cancer mortality have been well documented among Black and White adults in the United States (U.S.). A recent comprehensive review of health disparities in cancer outcomes among US adults between 2000 and 2010 showed that although progress has been made in reducing Black–White differences in cancer mortality rates, Black women still experience up to 14% higher cancer mortality rates compared with White women, while Black men experience 27% higher mortality rates compared with White men.1 Documented lifestyle risk factors for cancer mortality include obesity, physical inactivity, smoking and alcohol use,2–5 and recent studies suggest that dietary patterns, a major contributor to obesity, may play a role in carcinogenesis and survival after diagnosis.6
Recommendations from the World Cancer Research Fund (WCRF) encourage limiting the relative fat intake to less than 30% of total daily energy, with saturated and trans fatty acids contributing no greater than 10%.7 Detailed assessment of nutritional composition of food intake remains logistically challenging in large cohort studies, however food consumption patterns based on the frequency and quantity of major food items consumed may provide important information on the impact of specific dietary patterns on disease outcomes. A recent analysis of the large REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort identified five primary dietary patterns based on factor analysis of baseline dietary data: “Convenience,” “Plant-based,” “Sweets/Fats,” “Southern” and “Alcohol/Salads,” and observed that participants with greater adherence to the “Southern” dietary pattern (characterized by added fat, fried food, processed meats and sugar-sweetened beverages) were more likely to be Black, and about 56% more likely to experience incident acute coronary disease events.8 Several research studies have assessed the role of diet in increasing the risk of cancer mortality, especially for specific cancers such as breast, prostate and colorectal cancers; however, most of those previous studies have focused on specific dietary patterns, were limited by low sample sizes, potential recall bias or lack of racial diversity in the study cohort. 2,3,9–11 If we find that specific dietary patterns at baseline increase or decrease the risk of cancer mortality in this large, prospective cohort, this information may lead to targeted interventions to reduce racial disparities in cancer mortality.
Given the marked racial differences in diet and obesity, and the higher cancer mortality experienced by Blacks, we aim to address some of the stated limitations by examining the association between dietary patterns and cancer mortality in a large, prospective and racially diverse cohort.
Methods
Study design and data source
We analyzed data obtained from the prospective and population-based REGARDS cohort. REGARDS is one of the largest ongoing national longitudinal cohorts of community-dwelling adults in the United States.12 Designed to evaluate the origins of racial and geographic differences in stroke mortality, the REGARDS study consists of 30,239 community-dwelling adults ages ≥45 years at baseline; 45% male, 41% Black, and 69% >60 years old. Participants were recruited between January 2003 and October 2007, and detailed information about demographics, health behaviors, chronic medical conditions, diet and medications were collected.12 During the follow-up period, each participant was contacted by telephone every 6-months to identify any medical event or hospitalizations experienced since the prior contact. Further details about the REGARDS cohort are described elsewhere.12
Diet assessment
Detailed dietary data were collected from REGARDS participants at baseline using the Block 98 food frequency questionnaire (FFQ), a semi-quantitative, 110-item FFQ assessing usual diet in the past year, including frequency of consumption (average times per day, week or month) and portion size for specific foods or beverages (e.g., 1/2 cup of carrots, 2 slices of bacon).13–15 FFQs were completed at baseline, mailed to the REGARDS Operations Center, checked for completeness and scanned. Scanned FFQ files were forwarded to NutritionQuest for processing and analysis. Details about the food frequency questionnaire are described elsewhere.13–15 The primary exposures of interest were empirically derived dietary pattern scores from factor analysis.13,15 Using 56 investigator-defined individual food groups, Judd et al. performed an exploratory and confirmatory factor analysis to derive diet patterns and factor loadings for each of the 56 individual food groups.13 The retained patterns (“Convenience,” “Plant-based,” “Sweets/Fats,” “Southern,” “Alcohol/Salads”) were named according to the highest food group loadings within each factor. In general, the “Convenience” pattern was characterized by high factor loadings for Chinese and Mexican food, pasta dishes, pizza, soup and other mixed dishes including frozen or take-out meals; the “Plant-based” pattern by fruits, vegetables, cereal, beans, poultry and fish; the “Sweets/Fats” pattern by added sugars, desserts, chocolate, candy and sweetened breakfast foods; the “Southern” pattern by added fats, eggs and egg dishes, organ meats, fried foods, processed meats, sugar-sweetened beverages and greens typical of southern cuisines; and the “Alcohol/Salads” pattern by alcohol, green leafy vegetables, tomatoes and salad dressing.13,15 A factor score for each of the patterns was calculated for each study participant by summing observed intake of component food groups weighted by their respective factor loadings.13,15 Scores were further divided into quartiles for analysis. Factor analysis differs from cluster analysis in that individuals may adhere to more than one dietary pattern identified in this analysis.16
Cancer mortality outcomes
The primary outcome of this study was cancer mortality, regardless of cancer type. Cancer mortality was assessed through semi-annual telephone follow-up, death information from participant proxies, linkages with the Social Security Death Index (SSDI) as well as the National Death Index (NDI). Date of death was confirmed using death certificates, SSDI and/or NDI, and cause of death was adjudicated by a committee of experts using all available information as recommended by national guidelines.17 Follow-up data for this analysis were available through December 31, 2012. We calculated follow-up time for each participant using the date of the in-home visit to the date of death, cancer death or last telephone follow-up through December 31, 2012.
Covariates of interest
Participant sociodemographic characteristics assessed included self-reported age, sex, income and education. Health behaviors included tobacco use, alcohol use and physical activity. Smoking status included current, past and never. We defined alcohol use as none, moderate or heavy based on the National Institute on Alcohol Abuse and Alcoholism classification.18 Physical activity was assessed through a single question: “How many times per week do you engage in intense physical activity, enough to work up a sweat?” Geographic region was defined as residence within the Stroke Belt (defined as residence in the states of Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina and Tennessee) or Non-Stroke Belt (all other states) based on home address.19 Baseline chronic medical conditions assessed included chronic lung disease, coronary artery disease, diabetes, dyslipidemia, hypertension, myocardial infarction, obesity, peripheral artery disease and stroke. Obesity was defined based on body mass index (BMI) of ≥30 kg/m2.20
Ethics and consent statement
The Institutional Review Board at all participating institutions approved this study. We obtained informed consent from all participants of the study during baseline visit.
Statistical analysis
We compared baseline demographics, health behaviors and chronic medical conditions by quartiles of dietary pattern scores using χ2 tests for categorical characteristics and analysis of variance (ANOVA) for continuous variables. We examined the survival function for cancer mortality by quartiles of each dietary pattern using the Kaplan-Meier method, overall and stratified by race, and compared the survival probability among individuals at the highest quartile of each dietary pattern. After confirming the proportionality of hazards assumption, we estimated the relative survival rates by quartile of dietary patterns, separately. Using the Wald test for significance, we examined whether race modified the association between dietary patterns and risk of cancer mortality with multiplicative interaction terms (i.e., race by dietary pattern). We specified a priori that results would be presented overall for all study participants, as well as in race-stratified models. For each dietary pattern, we estimated Hazard Ratios (HRs) using Cox proportional hazards models with time-to-cancer related death as the outcome and dietary pattern as the primary exposure of interest. We censored REGARDS participants at the time of death, or end of follow-up (December 31, 2012). We further performed sequentially adjusted Cox models. Model 1 was adjusted for sociodemographics (i.e., age, sex, education and region). Model 2 was our main analytical model, and additionally adjusted for health behaviors (i.e., smoking status, alcohol use and physical activity). Model 3 was additionally adjusted for chronic medical conditions such as chronic lung disease, coronary artery disease, diabetes, dyslipidemia, hypertension, myocardial infarction, obesity, peripheral artery disease and stroke, conditions that may be on the causal pathway between diet and mortality. The results of all models were expressed as hazard ratios (HR) and the corresponding 95% confidence intervals (CI). We used SAS version 9.4 and STATA version 13 for all analyses. We considered p values ≤0.05 statistically significant.
Results
Dietary patterns by baseline socio-demographics and health status
Of the 30,239 REGARDS participants, we excluded 355 participants due to missing follow-up data, and 7,843 participants due to missing diet information, resulting in a total of 22,041 participants; 7,317 Black and 14,724 Whites, in the analytic dataset. There were racial and socio-economic differences in the level of adherence (defined by a greater proportion of participants in the fourth quartile as compared with the first) to dietary patterns in the study population.
Participants with greater adherence to the convenience dietary pattern were more likely to be younger, male, White, with higher education and income (Table 1). Greater adherence was also associated with current smoking status, heavy alcohol use, higher prevalence of obesity, and lower prevalence of diabetes. Participants with greater adherence to the plant-based dietary pattern were more likely to be older, female, Black, with higher education and income. In addition, greater adherence to the plant-based dietary pattern was associated with lower prevalence of current smoking and heavy alcohol use, and lower prevalence of physically inactivity, although there was a higher prevalence of diabetes. Participants with greater adherence to the sweets/fat dietary pattern were more likely to be male, White, with lower education and income. Greater adherence was also associated with current smoking, lower prevalence of heavy alcohol use, lower physical activity, lower obesity and diabetes prevalence. Participants with greater adherence to the southern dietary pattern were younger, male, Black, with lower education and income. Greater adherence was associated with higher current smoking status and heavy alcohol use, lower physical activity and higher prevalence of diabetes and obesity. Participants adherent to the alcohol/salads dietary pattern were younger, male, White, with higher education and income. Greater adherence was also associated with current smoking status, heavy alcohol use, physical inactivity and lower prevalence of diabetes and obesity.
Table 1. REGARDS baseline participant characteristics by quartiles (Q) of diet pattern scores (N = 22,041).
| Dietary pattern | Quartile of dietary pattern | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p Values1 | ||
| Convenience | Age, mean (SD) | 67.4 (9.2) | 65.6 (9.1) | 64.6 (9.1) | 62.4 (8.9) | <0.001 |
| Male (%) | 35.4 | 39.2 | 47.9 | 54.6 | <0.001 | |
| Black (%) | 45.6 | 36.9 | 27.4 | 22.9 | <0.001 | |
| ≤High school (%) | 14.4 | 9.7 | 7.5 | 7.8 | <0.001 | |
| ≤$20,000 Annual family income (%) | 20.9 | 16.4 | 13.6 | 13.3 | <0.001 | |
| Residence in the Stroke Belt (%) | 61.6 | 57.4 | 55.5 | 50.7 | <0.001 | |
| Current smoker (%) | 13.5 | 13.9 | 12.8 | 14.7 | <0.001 | |
| Heavy alcohol use (%) | 2.5 | 4.1 | 5.1 | 5.9 | <0.001 | |
| No physical activity (%) | 34.1 | 33.2 | 31.9 | 33.6 | <0.001 | |
| Diabetes (%) | 23.1 | 21.2 | 19.0 | 19.1 | <0.001 | |
| Obesity (%) | 52.3 | 51.4 | 50.6 | 53.3 | 0.03 | |
| Plant based | Age, mean (SD) | 62.8 (9.0) | 65.3 (9.4) | 65.9 (9.1) | 65.9 (9.2) | <0.001 |
| Male (%) | 53.2 | 45.4 | 41.4 | 37.2 | <0.001 | |
| Black (%) | 27.2 | 33.8 | 34.3 | 37.5 | <0.001 | |
| ≤High school (%) | 10.6 | 10.6 | 9.7 | 8.5 | <0.001 | |
| ≤$20,000 Annual family income (%) | 16.4 | 15.9 | 16.0 | 15.8 | 0.02 | |
| Residence in the Stroke Belt (%) | 55.6 | 58.4 | 56.0 | 55.2 | 0.004 | |
| Current smoker (%) | 23.3 | 14.2 | 9.9 | 7.6 | <0.001 | |
| Heavy alcohol use (%) | 8.4 | 3.6 | 3.2 | 2.5 | <0.001 | |
| No physical activity (%) | 40.5 | 35.0 | 30.6 | 26.8 | <0.001 | |
| Diabetes (%) | 17.7 | 21.1 | 21.7 | 21.8 | <0.001 | |
| Obesity (%) | 52.1 | 53.1 | 51.0 | 51.4 | 0.1 | |
| Sweets/fats | Age, mean (SD) | 64.4 (8.9) | 65.5 (9.3) | 65.4 (9.4) | 64.6 (9.4) | <0.001 |
| Male (%) | 37.9 | 41.8 | 47.4 | 50.1 | <0.001 | |
| Black (%) | 44.4 | 32.7 | 28.1 | 27.5 | <0.001 | |
| ≤High school (%) | 9.9 | 9.3 | 8.8 | 11.3 | <0.001 | |
| ≤$20,000 Annual family income (%) | 16.3 | 15.1 | 15.2 | 17.6 | <0.001 | |
| Residence in the Stroke Belt (%) | 53.2 | 54.9 | 56.1 | 60.9 | <0.001 | |
| Current smoker (%) | 12.5 | 12.0 | 13.5 | 17.0 | <0.001 | |
| Heavy alcohol use (%) | 9.0 | 3.8 | 3.0 | 1.9 | <0.001 | |
| No physical activity (%) | 31.0 | 32.2 | 33.1 | 36.5 | <0.001 | |
| Diabetes (%) | 23.7 | 22.1 | 19.6 | 17.0 | <0.001 | |
| Obesity (%) | 54.4 | 51.9 | 50.6 | 50.8 | <0.001 | |
| Southern | Age, mean (SD) | 65.1 (9.4) | 65.3 (9.3) | 65.6 (9.3) | 63.9 (9.0) | <0.001 |
| Male (%) | 37.4 | 39.3 | 45.9 | 54.6 | <0.001 | |
| Black (%) | 9.4 | 23.9 | 39.9 | 59.6 | <0.001 | |
| ≤High school (%) | 4.4 | 6.7 | 10.7 | 17.4 | <0.001 | |
| ≤$20,000 Annual family income (%) | 9.1 | 12.8 | 17.8 | 24.4 | <0.001 | |
| Residence in the Stroke Belt (%) | 48.4 | 53.7 | 59.1 | 64.1 | <0.001 | |
| Current smoker (%) | 8.3 | 11.0 | 14.8 | 20.9 | <0.001 | |
| Heavy alcohol use (%) | 3.9 | 3.9 | 4.3 | 5.5 | <0.001 | |
| No physical activity (%) | 28.1 | 33.4 | 34.7 | 36.6 | <0.001 | |
| Diabetes (%) | 13.3 | 18.7 | 22.3 | 28.1 | <0.001 | |
| Obesity (%) | 40.8 | 50.1 | 55.1 | 61.6 | <0.001 | |
| Alcohol/salads | Age, mean (SD) | 66.4 (9.6) | 65.3 (9.3) | 64.7 (9.1) | 63.6 (8.9) | <0.001 |
| Male (%) | 36.2 | 40.4 | 47.7 | 52.9 | <0.001 | |
| Black (%) | 49.8 | 37.6 | 26.2 | 19.1 | <0.001 | |
| ≤High school (%) | 16.0 | 10.9 | 7.3 | 5.1 | <0.001 | |
| ≤$20,000 Annual family income (%) | 25.8 | 17.4 | 12.9 | 8.1 | <0.001 | |
| Residence in the Stroke Belt (%) | 58.9 | 59.2 | 56.3 | 50.8 | <0.001 | |
| Current smoker (%) | 11.5 | 13.3 | 15.0 | 15.2 | <0.001 | |
| Heavy alcohol use (%) | 0.5 | 1.4 | 3.4 | 12.2 | <0.001 | |
| No physical activity (%) | 36.9 | 34.6 | 32.2 | 29.1 | <0.001 | |
| Diabetes (%) | 22.6 | 21.7 | 20.1 | 18.0 | <0.001 | |
| Obesity (%) | 53.3 | 52.6 | 50.6 | 51.1 | 0.02 | |
Significance determined using χ2 test or ANOVA. SD, standard deviation; %, presented as column percentages.
Dietary patterns and cancer mortality
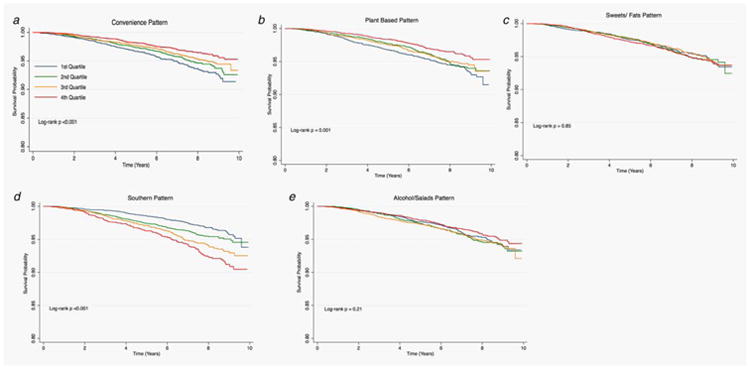
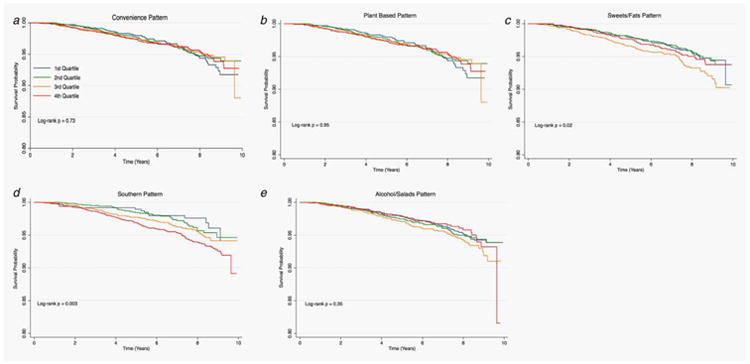
Figures 1–3 provide the survival distribution among study participants overall and stratified by race in relation to dietary patterns. Among all participants, greater adherence to the southern dietary pattern was associated with the lowest average survival after the 10-year observation period (Fig. 1). Among Black participants, those with greater adherence to the “Alcohols/Salads” diet had lower survival (Fig. 2), while White participants with greater adherence to the southern dietary pattern had the lowest survival (Fig. 3). Adherence to the Southern dietary pattern was associated with the lowest survival over time compared with the other dietary patterns (Fig. 4)
Figure 1.
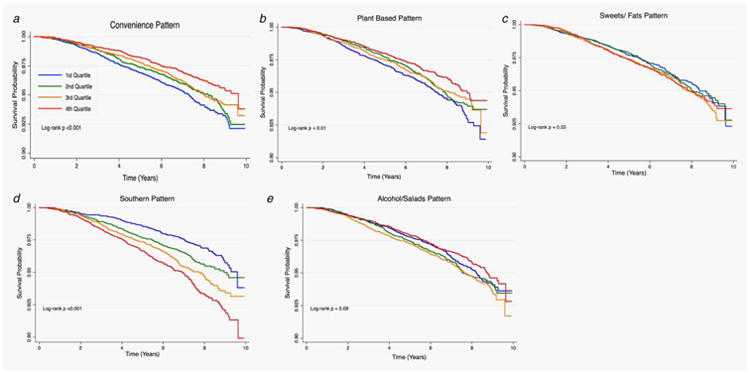
Cancer survival by quartile of dietary patterns among all participants in REGARDS (N = 22,041).
Figure 3.
Cancer survival by quartile of dietary patterns among Whites in REGARDS (N = 14,724).
Figure 2.
Cancer survival by quartile of dietary patterns among Blacks in REGARDS (N = 7,317).
Figure 4.
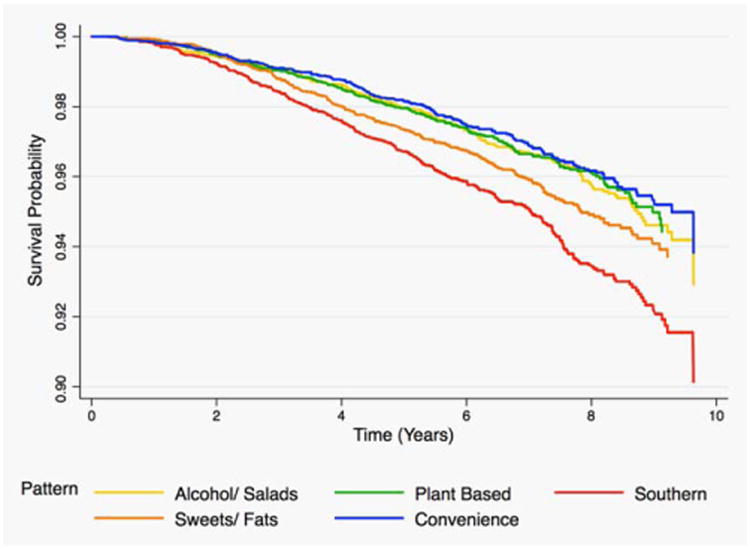
Cancer survival in the highest quartile of each dietary pattern among all participants in REGARDS (n = 22,041).
There were a total of 873 cancer deaths among 22,041 REGARDS participants included in the analysis (Table 2). In unadjusted analysis, greater adherence to the Convenience (4th quartile vs. 1st quartile HR: 0.66; 95% CI: 0.54–0.79) and Plant-based (4th quartile vs. 1st quartile HR: 0.72; 95% CI: 0.59–0.86) dietary patterns reduced the risk of cancer mortality over the observation period. However, greater adherence to the Southern dietary pattern (4th quartile vs. 1st quartile HR: 2.10; 95% CI: 1.71–2.55) was associated with significantly increased risk of cancer mortality. After adjustment for socio-demographics (i.e., age, sex, race, education, income and region) in Model 1, the inverse association between adherence to the Plant-based diet became stronger (4th quartile vs. 1st quartile HR: 0.65; 95% CI: 0.53–0.79), and the Southern dietary pattern became attenuated but still significant (4th quartile vs. 1st quartile HR: 1.88; 95% CI: 1.51–2.35), while a positive association of Alcohol/Salad dietary pattern was observed (4th quartile vs. 1st quartile HR: 1.26; 95% CI: 1.03–1.55). Upon adjusting for baseline health behaviors (smoking, alcohol and physical activity) in Model 2, the Southern dietary pattern became further attenuated but still significant (4th quartile vs. 1st quartile HR: 1.67; 95% CI: 1.32–2.10). In the final model accounting for chronic medical conditions, the Convenience (4th quartile vs. 1st quartile HR: 0.79; 95% CI: 0.64–0.98) and Plant-based (4th quartile vs. 1st quartile HR: 0.80; 95% CI: 0.65–0.99) dietary patterns were associated with a lower risk of cancer mortality by 20–21%, while the Southern dietary pattern (4th quartile vs. 1st quartile HR: 1.75; 95% CI: 1.38–2.23) was associated with a higher risk of cancer mortality by almost twofold.
Table 2. Hazard ratios* (HRs) and associated 95% confidence intervals (CIs) for the association between diet patterns and cancer mortality.
| Dietary pattern | Quartile of dietary pattern | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p Valuetrend* | ||
| Convenience | Events | 255 | 226 | 213 | 179 | |
| Crude | – | 0.87 (0.72–1.04) | 0.80 (0.67–0.96) | 0.66 (0.54–0.79) | <0.001 | |
| Model 1 | – | 0.99 (0.83–1.19) | 0.95 (0.79–1.15) | 0.87 (0.71–1.07) | 0.5 | |
| Model 2 | – | 0.97 (0.80–1.16) | 0.93 (0.76–1.12) | 0.83 (0.68–1.02) | 0.4 | |
| Model 3 | – | 0.90 (0.74–1.10) | 0.94 (0.77–1.14) | 0.79 (0.64–0.98) | 0.2 | |
| Plant based | Events | 256 | 220 | 211 | 186 | |
| Crude | – | 0.86 (0.72–1.03) | 0.81 (0.68–0.98) | 0.72 (0.59–0.86) | 0.005 | |
| Model 1 | – | 0.75 (0.63–0.90) | 0.72 (0.60–0.87) | 0.65 (0.53–0.79) | <0.001 | |
| Model 2 | – | 0.83 (0.69–1.00) | 0.86 (0.71–1.04) | 0.82 (0.67–1.01) | 0.2 | |
| Model 3 | – | 0.81 (0.66–0.98) | 0.88 (0.72–1.08) | 0.80 (0.65–0.99) | 0.03 | |
| Sweets/fats | Events | 206 | 215 | 227 | 225 | |
| Crude | – | 1.04 (0.86–1.26) | 1.09 (0.91–1.32) | 1.11 (0.92–1.34) | 0.7 | |
| Model 1 | – | 0.95 (0.79–1.15) | 0.97 (0.80–1.17) | 0.98 (0.81–1.18) | 1.0 | |
| Model 2 | – | 1.01 (0.83–1.24) | 1.00 (0.82–1.22) | 0.98 (0.80–1.20) | 1.0 | |
| Model 3 | – | 0.99 (0.80–1.22) | 0.99 (0.81–1.22) | 0.97 (0.78–1.20) | 1.0 | |
| Southern | Events | 146 | 200 | 242 | 285 | |
| Crude | – | 1.40 (1.13–1.74) | 1.73 (1.41–2.12) | 2.10 (1.71–2.55) | <0.001 | |
| Model 1 | – | 1.35 (1.09–1.68) | 1.53 (1.23–1.89) | 1.88 (1.51–2.35) | 0.006 | |
| Model 2 | – | 1.29 (1.03–1.61) | 1.46 (1.18–1.82) | 1.67 (1.32–2.10) | 0.03 | |
| Model 3 | – | 1.35 (1.07–1.70) | 1.51 (1.20–1.90) | 1.75 (1.38–2.23) | <0.001 | |
| Alcohol/salads | Events | 206 | 232 | 239 | 196 | |
| Crude | – | 1.12 (0.92–1.35) | 1.14 (0.94–1.37) | 0.91 (0.75–1.11) | 0.08 | |
| Model 1 | – | 1.28 (1.06–1.54) | 1.39 (1.15–1.69) | 1.26 (1.03–1.55) | 0.007 | |
| Model 2 | – | 1.23 (1.02–1.50) | 1.22 (1.00–1.49) | 1.01 (0.80–1.26) | 0.05 | |
| Model 3 | – | 1.20 (0.98–1.47) | 1.21 (0.98–1.49) | 1.03 (0.82–1.30) | 0.1 | |
Bold indicates statistically significant at α ≤ 0.05 and α ≤ 0.10 for interaction.
There were 873 cancer deaths among 22,041 REGARDS participants.
Model 1 adjusted for sociodemographics (i.e., age, sex, race, education, income and region).
Model 2 is additionally adjusted for health behaviors (i.e., smoking status, alcohol use and physical activity).
Model 3 is additionally adjusted for chronic medical conditions (i.e., chronic lung disease, coronary artery disease, diabetes, dyslipidemia, hypertension, myocardial infarction, obesity, peripheral artery disease and stroke).
Estimated using Cox proportional hazards model.
(–) Q1 is reference group.
Dietary patterns and cancer mortality among Blacks
Among 7,317 Blacks included in this study, there were 291 cancer deaths over the 10-year study observation period (Table 3). In the unadjusted model, Black individuals within the third quartile of the sweets/fats dietary pattern were at increased risk of cancer mortality compared with those in the first quartile (HR: 1.51; 95% CI: 1.12–2.05). However, this association became attenuated in the adjusted model accounting for sociodemographics and baseline health behavior (HR: 1.40; 95% CI: 1.02–1.93). In addition, Black participants with greater adherence to the Southern dietary pattern were at a twofold increased risk of cancer mortality (HR: 2.07; 95% CI: 1.18–3.65), however after adjustment for socio-demographics and health behaviors, the association became attenuated and non-significant. There were no other significant associations between cancer mortality and dietary patterns (Figs. 2a, 2b and 2e) among Blacks.
Table 3. Hazard ratios1 (HRs) and associated 95% confidence intervals (CIs) for the association between diet patterns and cancer mortality in Blacks.
| Dietary pattern | Quartile of dietary pattern | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p Valuetrend1 | ||
| Convenience | Events | 106 | 75 | 64 | 46 | |
| Crude | – | 0.87 (0.65–1.17) | 1.00 (0.73–1.36) | 0.86 (0.61–1.21) | 0.7 | |
| Model 1 | – | 1.02 (0.76–1.38) | 1.20 (0.87–1.64) | 1.07 (0.75–1.52) | 0.7 | |
| Model 2 | – | 0.96 (0.71–1.31) | 1.17 (0.85–1.61) | 1.04 (0.72–1.50) | 0.7 | |
| Model 3 | – | 0.93 (0.67–1.28) | 1.10 (0.79–1.54) | 0.92 (0.62–1.36) | 0.8 | |
| Plant based | Events | 64 | 72 | 72 | 83 | |
| Crude | – | 0.89 (0.64–1.25) | 0.86 (0.61–1.20) | 0.91 (0.66–1.27) | 0.8 | |
| Model 1 | – | 0.80 (0.57–1.12) | 0.77 (0.55–1.08) | 0.84 (0.60–1.17) | 0.4 | |
| Model 2 | – | 0.83 (0.58–1.18) | 0.86 (0.60–1.22) | 1.03 (0.73–1.45) | 0.5 | |
| Model 3 | – | 0.87 (0.60–1.25) | 0.94 (0.65–1.36) | 1.00 (0.69–1.45) | 0.8 | |
| Sweets/fats | Events | 85 | 66 | 80 | 60 | |
| Crude | – | 1.05 (0.76–1.44) | 1.51 (1.12–2.05) | 1.17 (0.84–1.63) | 0.04 | |
| Model 1 | – | 0.98 (0.71–1.35) | 1.36 (1.00–1.85) | 1.06 (0.76–1.48) | 0.1 | |
| Model 2 | – | 1.01 (0.72–1.41) | 1.40 (1.02–1.93) | 1.04 (0.73–1.46) | 0.1 | |
| Model 3 | – | 1.04 (0.74–1.47) | 1.32 (0.95–1.84) | 0.92 (0.63–1.33) | 0.2 | |
| Southern | Events | 13 | 40 | 81 | 157 | |
| Crude | – | 1.28 (0.69–2.40) | 1.56 (0.87–2.80) | 2.07 (1.18–3.65) | 0.004 | |
| Model 1 | – | 1.22 (0.65–2.29) | 1.34 (0.74–2.41) | 1.67 (0.94–2.97) | 0.1 | |
| Model 2 | – | 1.37 (0.70–2.67) | 1.40 (0.74–2.65) | 1.74 (0.93–3.25) | 0.2 | |
| Model 3 | – | 1.61 (0.78–3.34) | 1.55 (0.77–3.12) | 1.92 (0.97–3.83) | 0.2 | |
| Alcohol/salads | Events | 101 | 83 | 68 | 39 | |
| Crude | – | 1.08 (0.81–1.44) | 1.24 (0.91–1.69) | 0.97 (0.67–1.41) | 0.5 | |
| Model 1 | – | 1.19 (0.89–1.59) | 1.36 (1.00–1.87) | 1.19 (0.81–1.73) | 0.3 | |
| Model 2 | – | 1.16 (0.86–1.57) | 1.24 (0.90–1.72) | 1.04 (0.69–1.55) | 0.5 | |
| Model 3 | – | 1.13 (0.82–1.54) | 1.16 (0.82–1.64) | 1.01 (0.66–1.54) | 0.8 | |
Bold indicates statistically significant at α ≤ 0.05.
There were 291 cancer deaths among 7,317 Black REGARDS participants.
Model 1 adjusted for sociodemographics (i.e., age, sex, education, income and region).
Model 2 is additionally adjusted for health behaviors (i.e., smoking status, alcohol use and physical activity).
Model 3 is additionally adjusted for chronic medical conditions (i.e., chronic lung disease, coronary artery disease, diabetes, dyslipidemia, hypertension, myocardial infarction, obesity, peripheral artery disease and stroke).
Estimated using Cox proportional hazards model.
(–) Q1 is reference group.
Dietary patterns and cancer mortality among Whites
Among 14,724 White participants included in this study, there were 582 cancer deaths observed over the 10-year study observation period (Table 4). In the unadjusted model, Whites with greater adherence to the Convenience (4th quartile vs. 1st quartile) (HR: 0.58; 95% CI: 0.46–0.73; Fig. 3a: p valuesLog-rank <0.001) and Plant-based (HR: 0.61; 95% CI: 0.48–0.78; Fig. 3b: p valuesLog-rank = 0.001) dietary patterns were at reduced risk for cancer mortality. However, greater adherence to the Southern diet (HR: 2.27; 95% CI: 1.78–2.89) increased the risk of cancer mortality. After adjustment for socio-demographics and health behaviors, the associations became slightly attenuated, but remained statistically significant for the Convenience (HR: 0.74, 95% CI: 0.57–0.95), Plant-based (HR: 0.72; 95% CI: 0.56–0.94) and Southern (HR: 1.59, 95% CI: 1.22–2.09) dietary patterns.
Table 4. Hazard ratios1 (HRs) and associated 95% confidence intervals (CIs) for the association between diet patterns and cancer mortality in Whites.
| Dietary pattern | Quartile of dietary pattern | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p Valuetrend1 | ||
| Convenience | Events | 149 | 151 | 149 | 133 | |
| Crude | – | 0.85 (0.68–1.06) | 0.71 (0.56–0.89) | 0.58 (0.46–0.73) | <0.001 | |
| Model 1 | – | 0.95 (0.76–1.19) | 0.84 (0.67–1.06) | 0.79 (0.62–1.01) | 0.2 | |
| Model 2 | – | 0.93 (0.74–1.17) | 0.81 (0.64–1.02) | 0.74 (0.57–0.95) | 0.07 | |
| Model 3 | – | 0.85 (0.67–1.08) | 0.84 (0.66–1.07) | 0.73 (0.56–0.94) | 0.1 | |
| Plant based | Events | 192 | 148 | 139 | 103 | |
| Crude | – | 0.84 (0.68–1.04) | 0.80 (0.64–0.99) | 0.61 (0.48–0.78) | <0.001 | |
| Model 1 | – | 0.74 (0.59–0.91) | 0.71 (0.57–0.89) | 0.56 (0.43–0.71) | <0.001 | |
| Model 2 | – | 0.83 (0.67–1.04) | 0.88 (0.70–1.11) | 0.72 (0.56–0.94) | 0.09 | |
| Model 3 | – | 0.79 (0.63–1.01) | 0.88 (0.69–1.12) | 0.72 (0.55–0.93) | 0.07 | |
| Sweets/fats | Events | 121 | 149 | 147 | 165 | |
| Crude | – | 1.03 (0.81–1.31) | 0.93 (0.73–1.19) | 1.07 (0.84–1.35) | 0.7 | |
| Model 1 | – | 0.91 (0.71–1.16) | 0.80 (0.63–1.02) | 0.91 (0.72–1.15) | 0.3 | |
| Model 2 | – | 0.98 (0.76–1.26) | 0.81 (0.63–1.05) | 0.91 (0.71–1.17) | 0.3 | |
| Model 3 | – | 0.95 (0.73–1.23) | 0.85 (0.65–1.11) | 0.97 (0.75–1.27) | 0.6 | |
| Southern | Events | 133 | 160 | 161 | 128 | |
| Crude | – | 1.46 (1.16–1.83) | 1.89 (1.50–2.37) | 2.27 (1.78–2.89) | <0.001 | |
| Model 1 | – | 1.37 (1.08–1.72) | 1.55 (1.23–1.97) | 1.91 (1.47–2.47) | <0.001 | |
| Model 2 | – | 1.26 (0.99–1.60) | 1.48 (1.17–1.88) | 1.59 (1.22–2.08) | 0.002 | |
| Model 3 | – | 1.30 (1.01–1.67) | 1.53 (1.19–1.97) | 1.69 (1.28–2.23) | <0.001 | |
| Alcohol/salads | Events | 105 | 149 | 171 | 157 | |
| Crude | – | 1.14 (0.89–1.46) | 1.10 (0.86–1.41) | 0.90 (0.70–1.15) | 0.2 | |
| Model 1 | – | 1.34 (1.04–1.72) | 1.44 (1.12–1.84) | 1.34 (1.04–1.74) | 0.03 | |
| Model 2 | – | 1.28 (0.99–1.66) | 1.22 (0.94–1.58) | 1.02 (0.77–1.36) | 0.1 | |
| Model 3 | – | 1.24 (0.95–1.62) | 1.25 (0.96–1.64) | 1.08 (0.81–1.45) | 0.2 | |
Bold indicates statistically significant at α ≤ 0.05.
There were 582 cancer deaths among 14,724 White REGARDS participants.
Model 1 adjusted for sociodemographics (i.e., age, sex, education, income and region).
Model 2 is additionally adjusted for health behaviors (i.e., smoking status, alcohol use and physical activity).
Model 3 is additionally adjusted for chronic medical conditions (i.e., chronic lung disease, coronary artery disease, diabetes, dyslipidemia, hypertension, myocardial infarction, obesity, peripheral artery disease and stroke).
Estimated using Cox proportional hazards model.
(–) Q1 is reference group.
Discussion
The objectives of this study were to determine the association between adherence to various dietary patterns and cancer mortality, and whether these associations were modified by race. In the large, racially diverse REGARDS cohort we observed that after adjusting for confounders, both the Convenience and Plant-based dietary patterns were associated with reduced risk of cancer mortality, while the Southern dietary pattern was associated with increased risk of cancer mortality. These associations were statistically significant and consistent among White participants. In contrast, no dietary pattern was significantly associated with cancer mortality in adjusted models among Black participants.
The Southern dietary pattern was characterized by high consumption of high-fat/fried foods, red/organ or processed meat and high-sugar/sweetened beverages, foods that are traditionally consumed by residents of the Southern US states.13 There is strong evidence supporting the link between Southern dietary pattern and increased chronic disease mortality, including cancer-specific mortality.21–26 In a meta-analysis of over 100 epidemiological studies, Huxley et al. reported that individuals with higher consumption of red and processed meats were at a 20% increased risk of colorectal cancer incidence.25 In addition, studies on both pre- and post-menopausal women have shown that women with diets high in total fat content were at more than a threefold increased risk of breast cancer mortality.23,26–28 Similar to our results, prior REGARDS studies have also found a link between the Southern dietary patterns to increased risk of stroke,29 acute coronary heart disease (CHD)8 and sepsis.15 Shikany et al. found that participants with greater adherence to the Southern dietary pattern were at 56% increased risk of incident CHD.8 What has been missing in the literature so far is consideration of racial differences in the association between dietary patterns and cancer outcomes. In this study, we address this limitation by examining dietary patterns separately among Blacks and Whites, and observed about a twofold increase in risk of cancer mortality associated with the Southern diet among Blacks and Whites, although the association among Blacks did not reach statistical significance in adjusted models, likely due to the small sample size in this group.
The link between the Southern diet and cancer-specific mortality is likely driven by obesity, diabetes, hypertension, dyslipidemia and physical inactivity, risk factors that are highly prevalent in the Southern US states.30,31 Strong evidence indicates that individuals with obesity and diabetes are also likely to have other chronic health conditions including insulin resistance, pro-inflammatory cytokines and chronic vascular inflammation–all essential factors in the causal pathway between diet and cancer development.22 However, despite adjustment for these factors in our fully adjusted model, there was still an independent influence of Southern dietary patterns on increased cancer mortality. This suggests that efforts to identify and modify specific food items or food preparation techniques that render southern-style foods unhealthy may go along way in reducing adverse health outcomes. In addition, these findings highlight the need for studies focused on identifying race- or region-specific risk factors for cancer mortality in the US, including unique dietary patterns, and a “one size fits all” approach to cancer prevention may be inadequate particularly for reducing racial disparities in cancer mortality.
In contrast to the Southern dietary pattern, Plant-based and Convenience dietary patterns were each associated with reduced risk of cancer mortality in the overall sample population. The Plant-based diet includes multiple sources of protein from fish, meat and beans in addition to large amounts of fresh grains, fruits and vegetables, and was associated with significantly reduced risk of cancer mortality in the overall sample after adjustment for sociodemographic characteristics. Other studies have found similar results, showing that diets high in fiber, vegetables and fruit were associated with nearly a 23% reduced risk of cancer mortality.32 The food items that loaded highly on the convenience dietary pattern in factor analysis provide some clues into the protective association with cancer mortality that was observed in this study. Specifically, food items such as beans, mixed vegetable dishes, Chinese food, fish, Mexican dishes, mixed dishes with meat, pasta dishes, poultry, salty snacks, shellfish and soups loaded highly on the convenience dietary pattern, suggesting that convenience foods may not be inherently unhealthy. Some pre-packaged or fast-casual food items include healthy options including vegetables and lean meats that may be incorporated as part of a healthy diets, although future studies will be needed to assess the quantity and quality of micro-nutrient components, e.g., sodium content, before such recommendations can be made in clinical practice.
We further assessed whether the protective effect of Plant-based diets existed for both Blacks and Whites, after accounting for important confounders such as baseline health behavior and medical conditions. Despite the fact that a high proportion of participants at the highest quartile of adherence to Plant-based diets were Black (37.5%), we did not observe a protective effect for the Plant-based diet among Blacks in this study. In addition, the Convenience dietary pattern was not associated with reduced risk of cancer mortality among Black participants, but was strongly associated among Whites. This may be due to racial and socio-economic differences in the availability of healthy convenience food items. For instance, convenient food options for Blacks may be restricted to cheap, less healthy options that are high in fat and calorie-dense but nutritionally poor compared with the convenient food options available for Whites. This likely stems from the geographic distribution of food supplies in the US, with Blacks and residents of low-income communities more likely to reside in food deserts characterized by lack of grocery stores and poor access to fresh fruits and vegetables.33,34 Efforts to reduce the prevalence of diet-associated adverse health outcomes such as obesity, diabetes and cancer mortality will require a renewed focus on improving access to affordable, healthy food options through grocery stores or farmers markets, as well as improving the quality of convenient or fast-food options within neighborhoods.35 Together with improved access to safe, walking environment or green space, these highly modifiable factors may go a long way in improving diets and reducing the prevalence of obesity, and especially accelerate progress towards eliminating racial disparities in these chronic conditions, including cancer mortality.
There are several strengths and limitations that must be considered in evaluating these findings. First, as with most large epidemiologic studies, there is potential for recall or information bias in some of the self-reported covariates. Second, the total number of cancer deaths may be underestimated in this population as the REGARDS study was primarily intended to identify incident stroke events, and was not specifically focused on cancer outcomes. We did not have information regarding cancer type, stage or treatment and therefore were unable to make statistical adjustments for these differences in our models. However, the REGARDS cohort provided data on a large population of community-dwelling adults and allowed the examination of individual characteristics with subsequent cancer events. Our results are in alignment with current cancer prevention recommendations from the American Cancer Society (ACS) affirming that the risk of overall cancer mortality may be reduced by improving dietary quality and maintaining a healthy weight.36 Larger prospective studies are likely needed to better assess specific food items, micronutrients or food preparation techniques that may vary by race and confer either protective or adverse effects on health outcomes.
In summary, while the Southern diet was associated with higher risk of cancer mortality, the Plant-based and Convenience dietary patterns were associated with reduced risk of cancer mortality, but only among Whites. Further studies are needed to identify specific aspects of the Southern diet that may drive the increased mortality risk, with prevention strategies focused on eliminating those. Further studies are also urgently needed to characterize protective risk factors for cancer mortality among Blacks, since identifying those factors and developing strategies to promote them in Black communities may go a long way in reducing the burden of cancer mortality in this racial group.
What's new?
In the United States, obesity and cancer mortality differ between races, raising questions about whether interactions between dietary patterns and race influence cancer outcomes. In this investigation of Black and White adults in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort, greater adherence to “Southern” dietary patterns, characterized by consumption of added fats, fried foods and sugar-sweetened beverages, was associated with a twofold increase in cancer mortality in both racial groups. In whites only, “plant-based” and “convenience” dietary patterns were associated with reduced cancer mortality. Avoiding aspects of the Southern diet could improve cancer outcomes for Blacks and Whites.
Acknowledgments
Dr Akinyemiju was supported by grant U54 CA118948 from the NIH. Mr Moore received grant support from grant R25 CA47888 from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Grant sponsor: National Institute for Nursing Research; Grant number: R01-NR012726; Grant sponsor: National Center for Research Resources; Grant number: UL1-RR025777; Grant sponsor: National Heart, Lung and Blood Institute; Grant number: K08HL096841; Grant sponsors: Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham; Grant sponsor: National Institute of Neurological Disorders and Stroke; Grant number: U01-NS041588; Grant sponsors: National Institutes of Health, Department of Health and Human Service (REGARDS study); Grant sponsor: NIH (Dr Akinyemiju); Grant number: U54 CA118948; Grant sponsor: NIH (Mr. Moore); Grant number: R25 CA47888
References
- 1.O'Keefe EB, Meltzer JP, Bethea TN. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front Pub Health. 2015;3:1–15. doi: 10.3389/fpubh.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang M, Kenfield SA, Van Blarigan EL, et al. Dietary patterns after prostate cancer diagnosis in relation to disease-specific and total mortality. Cancer Prev Res (Phila) 2015;8:545–51. doi: 10.1158/1940-6207.CAPR-14-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlich MJ, Singh PN, Sabate J, et al. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern Med. 2015;175:767–76. doi: 10.1001/jamainternmed.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang B, Jacobs EJ, Gapstur SM, et al. Active smoking and mortality among colorectal cancer survivors: the Cancer Prevention Study II nutrition cohort. J Clin Oncol. 2015;33:885–93. doi: 10.1200/JCO.2014.58.3831. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari P, Licaj I, Muller DC, et al. Lifetime alcohol use and overall and cause-specific mortality in the European Prospective Investigation into Cancer and nutrition (EPIC) study. BMJ Open. 2014;4:e005245. doi: 10.1136/bmjopen-2014-005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emaus MJ, Peeters PH, Bakker MF, et al. Vegetable and fruit consumption and the risk of hormone receptor-defined breast cancer in the EPIC cohort. Am J Clin Nutr. 2016;103:168–77. doi: 10.3945/ajcn.114.101436. [DOI] [PubMed] [Google Scholar]
- 7.American Institute for Cancer Research, Recommendations for Cancer Prevention. American Institute for Cancer Research; [Accessed Feb 2016]. 2015. Online report at: http://www.aicr.org/reduce-your-cancer-risk/recommendations-for-cancer-prevention/ [Google Scholar]
- 8.Shikany JM, Safford MM, Newby PK, et al. Southern dietary pattern is associated with hazard of acute coronary heart disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Circulation. 2015;132:804–14. doi: 10.1161/CIRCULATIONAHA.114.014421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zucchetto A, Gini A, Shivappa N, et al. Dietary inflammatory index and prostate cancer survival. Int J Cancer. 2016 doi: 10.1002/ijc.30208. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeke CE, Eliassen AH, Chen WY, et al. Dietary fat intake in relation to lethal breast cancer in two large prospective cohort studies. Breast Cancer Res Treat. 2014;146:383–92. doi: 10.1007/s10549-014-3005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reedy J, Krebs-Smith SM, Miller PE, et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144:881–9. doi: 10.3945/jn.113.189407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 13.Judd SE, Letter AJ, Shikany JM, et al. Dietary patterns derived using exploratory and confirmatory factor analysis are stable and generalizable across race, region, and gender subgroups in the REGARDS study. Front Nutr. 2014;1:1–10. doi: 10.3389/fnut.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newby PK, Noel SE, Grant R, et al. Race and region are associated with nutrient intakes among Black and White men in the United States. J Nutr. 2011;141:296–303. doi: 10.3945/jn.110.130583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez OM, Judd SE, Voeks JH, et al. Diet patterns and risk of sepsis in community-dwelling adults: a cohort study. BMC Infect Dis. 2015;15:1–9. doi: 10.1186/s12879-015-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62:177–203. doi: 10.1301/nr.2004.may.177-203. [DOI] [PubMed] [Google Scholar]
- 17.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–74. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care physicians. Am Fam Phys. 2009;80:44–50. [PubMed] [Google Scholar]
- 19.Moore JX, Donnelly JP, Griffin R, et al. Black–White racial disparities in sepsis: a prospective analysis of the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. Crit Care. 2015;19:1–10. doi: 10.1186/s13054-015-0992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navaranjan D, Rosella LC, Kwong JC, et al. Ethnic disparities in acquiring 2009 pandemic H1N1 influenza: a case–control study. BMC Pub Health. 2014;14:1–10. doi: 10.1186/1471-2458-14-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson RE, Cadmus LA, Emond JA, et al. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010;66:5–15. doi: 10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz RB, Hernandez PS. Diet and cancer: risk factors and epidemiological evidence. Maturitas. 2014;77:202–8. doi: 10.1016/j.maturitas.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 23.McEligot AJ, Largent J, Ziogas A, et al. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutr Cancer. 2006;55:132–40. doi: 10.1207/s15327914nc5502_3. [DOI] [PubMed] [Google Scholar]
- 24.Perera PS, Thompson RL, Wiseman MJ. Recent evidence for colorectal cancer prevention through healthy food, nutrition, and physical activity: implications for recommendations. Curr Nutr Rep. 2014;1:44–54. [Google Scholar]
- 25.Huxley RR, Ansary-Moghaddam A, Clifton P, et al. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–80. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 26.Borugian MJ, Sheps SB, Kim-Sing C, et al. Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev. 2004;13:1163–72. [PubMed] [Google Scholar]
- 27.Zhang S, Folsom AR, Sellers TA, et al. Better breast cancer survival for postmenopausal women who are less overweight and eat less fat. The Iowa Women's Health Study. Cancer. 1995;76:275–83. doi: 10.1002/1097-0142(19950715)76:2<275::aid-cncr2820760218>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke CH, Fung TT, Hu FB, et al. Dietary patterns and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:9295–303. doi: 10.1200/JCO.2005.02.0198. [DOI] [PubMed] [Google Scholar]
- 29.Judd SE, Gutierrez OM, Newby PK, et al. Dietary patterns are associated with incident stroke and contribute to excess risk of stroke in Black Americans. Stroke. 2013;44:3305–11. doi: 10.1161/STROKEAHA.113.002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gebreab SY, Davis SK, Symanzik J, et al. Geographic variations in cardiovascular health in the United States: contributions of state- and individual-level factors. J Am Heart Assoc. 2015;4:e001673. doi: 10.1161/JAHA.114.001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferdinand KC, Rodriguez F, Nasser SA, et al. Cardiorenal metabolic syndrome and cardiometabolic risks in minority populations. Cardiorenal Med. 2014;4:1–11. doi: 10.1159/000357236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YJY. Dietary fibre intake and mortality from cardiovascular disease and all cancers: a meta-analysis of prospective cohort studies. Arch Cardiovasc Dis. 2016;109:39–54. doi: 10.1016/j.acvd.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Thiebaut AC, Kipnis V, Chang SC, et al. Dietary fat and postmenopausal invasive breast cancer in the National Institutes of Health-AARP Diet and Health Study cohort. J Natl Cancer Inst. 2007;99:451–62. doi: 10.1093/jnci/djk094. [DOI] [PubMed] [Google Scholar]
- 34.Powell LM, Han E, Chaloupka FJ. Economic contextual factors, food consumption, and obesity among U.S. adolescents. J Nutr. 2010;140:1175–80. doi: 10.3945/jn.109.111526. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh-Dastidar B, Cohen D, Hunter G, et al. Distance to store, food prices, and obesity in urban food deserts. Am J Prev Med. 2014;47:587–95. doi: 10.1016/j.amepre.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]


