Abstract
Acute chest syndrome is a frequent cause of acute lung disease in children with sickle-cell disease. Asthma is common in children with sickle-cell disease and is associated with increased incidence of vaso-occlusive pain events, acute chest syndrome episodes, and earlier death. Risk factors for asthma exacerbation and an acute chest syndrome episode are similar, and both can present with shortness of breath, chest pain, cough, and wheezing. Despite overlapping risk factors and symptoms, an acute exacerbation of asthma or an episode of acute chest syndrome are two distinct entities that need disease-specific management strategies. Although understanding has increased about asthma as a comorbidity in sickle-cell disease and its effects on morbidity, substantial gaps remain in knowledge about best management.
Introduction
In high-income countries, children with sickle-cell disease are now expected to live through young adulthood.1–3 As mortality has decreased in these children, the primary focus has shifted to improvements in quality of life and decreases in the major causes of morbidity, particularly vaso-occlusive pain and acute chest syndrome, a potential life-threatening pulmonary disorder specific to sickle-cell disease. Asthma also causes increased morbidity in children with sickle-cell disease. Acute exacerbations of asthma can be difficult to distinguish from acute chest syndrome; although recognition of the importance of comorbid asthma in sickle-cell disease outcomes is increasing, asthma continues to be underdiagnosed, inadequately treated, and difficult to distinguish from lung complications distinct to sickle-cell disease. In this Series paper, we focus on asthma and acute chest syndrome in sickle-cell disease. Given that most of the published work focuses on children with sickle-cell anaemia (referred to as haemo globin SS or S beta-thalassaemia zero due to the challenge in distinguishing the two phenotypes without genetic testing), we will focus on this phenotype.
Acute chest syndrome in sickle-cell disease
Definitions
Many definitions of acute chest syndrome are used in clinical practice and research (table 1). Unfortunately, none of these definitions has included the rate of respiratory decline, a major component of the life-threatening nature of acute chest syndrome. The prototypical episode of acute chest syndrome is one in which the patient acutely develops fever, increased work of breathing, a new radiodensity on chest roentgenogram, and then a rapid decline in respiratory status, commonly within 24 h of symptom onset. A subacute course (which occurs in most patients) can mimic the clinical course of bacterial pneumonia, asthma exacerbation, or bronchiolitis. The poor specificity in the definition of acute chest syndrome means that a 2 year old child with sickle-cell disease who is admitted to a hospital for an acute wheezing illness due to respiratory syncytial virus (RSV) and sub-segmental atelectasis on chest roentgenogram is referred to as having acute chest syndrome; as is a 15 year old who presents to the hospital with mild difficulty breathing, fever, and a haemoglobin oxygen saturation of greater than 95% on room air, who is admitted to an intensive care unit 12 h later with acute respiratory distress syndrome and endotracheal intubation. A more precise diagnosis of acute chest syndrome is needed to improve the clinical homogeneity of the syndrome and, most importantly, enhance the opportunity for effective translational research. To improve the diagnostic criteria for acute chest syndrome specific to sickle-cell disease, we recommend that a more formal study be done to address the rate of decline of respiratory status. Despite the clear importance of research into therapy for acute chest syndrome, no international consensus statement for definition and validation of acute chest syndrome has been made.
Table 1.
Various definitions of acute chest syndrome from highly cited studies
| Acute chest syndrome definition | |
|---|---|
| Vichinsky et al4 | A new pulmonary infiltrate involving at least one complete lung segment that is consistent with the presence of alveolar consolidation, but excluding atelectasis Chest pain, tachypnoea, wheezing, or cough Temperature higher than 38·5°C |
| Vichinsky et al5; Castro et al6 | A new pulmonary infiltrate on chest radiograph |
| Charache et al7 | Chest pain, increased leucocytosis Fever Pulmonary infiltrate |
Individuals with sickle-cell disease, pneumonia, or bronchiolitis will typically meet the definition of acute chest syndrome.
Search strategy and selection criteria.
We searched PubMed for papers published in English between 1970 and 2015, and reviewed all of the abstracts. The search terms included (“anemia, sickle-cell”[MeSH Terms] OR (“anemia”[All Fields] AND “sickle”[All Fields] AND “cell”[All Fields]) OR “sickle-cell; “anemia, sickle-cell”[MeSH Terms] OR (“anemia”[All Fields] AND “sickle”[All Fields] AND “cell”[All Fields]) OR “sickle-cell anemia”[All Fields] OR (“sickle”[All Fields] AND “cell”[All Fields] AND “disease”[All Fields]) OR “sickle-cell disease”[All Fields]) AND (“asthma”[MeSH Terms] OR “asthma”[All Fields]). We subsequently selected articles that were focused on asthma and or asthma symptoms in children and adults with sickle-cell anaemia. We also reviewed the references of the selected papers. When possible, we chose data from large multicentre studies rather than studies from single centres to determine the prevalence of asthma in sickle-cell disease or to summarise the clinical findings. When a series of studies identified a similar pattern of results, we attempted to summarise the results.
The presentation, natural course, causal mechanisms, and mortality rate of acute chest syndrome differs considerably with age. Children are far more likely to present with a fever and cough with no distinct predilection for any of the lobes of the lungs on chest roentgenogram; whereas, adults are more likely to present with shortness of breath, chest pain, and chest roentgenogram findings in multiple lobes.5 In children, high clinical suspicion is needed for diagnosis of acute chest syndrome because up to 40% will have a normal physical examination.6 Clinicians who depend on history and physical signs to exclude acute chest syndrome and do not obtain a chest roentgenogram will miss 61% of episodes of acute chest syndrome in children.8 The causal mechanisms of acute chest syndrome in children and adults are mostly unknown; however, for patients in whom a cause is known, infections are commonly identified.4 Most importantly, acute chest syndrome in young children is rarely the proximal cause of death (<1% of episodes); whereas, in adults, about 9% of all episodes result in death.4
Risk factors
Several prospective and retrospective studies have shown that children with sickle-cell disease and asthma have a higher rate of acute chest syndrome events than do those with sickle-cell disease without asthma.9–13 Other clinical risk factors include baseline higher haemoglobin concentration and white blood cell count and lower haemoglobin F concentration.6 Genetic variation in multiple loci has been associated with the incidence of acute chest syndrome; they include the beta-globin gene cluster haplotype,14 a (GT)(n) dinucleotide repeat located in the promoter region of HMOX1 (protein HO-1),15 a single nucleotide roughly 8 kilobases upstream of COMMD7,16 and plasma concentrations of HO-1.17 However, neither laboratory findings nor genetic risk factors are sufficient to stratify children into groups of high or low risk for future acute chest syndrome episodes. Elucidation of an underlying genetic predisposition to acute chest syndrome could lead to identification of potential pathways that could be targeted for therapy.
Prevention
Supportive measures are used to try to prevent acute chest syndrome in children presenting with vaso-occlusive pain and who are subsequently hospitalised. The most documented preventive strategy for acute chest syndrome is the use of incentive spirometry during hospital stays for vaso-occlusive pain events. In a study by Bellet and colleagues, 18 children and adolescents with sickle-cell disease that were hospitalised for vaso-occlusive pain were randomly allocated to receive either incentive spirometry with ten maximal inspirations every 2 h between 0800 h and 2200 h while awake (experimental group) or no spirometry (standard therapy). Children assigned to incentive spirometry had an 87% relative risk reduction of acute chest syndrome events. Another strategy to prevent acute chest syndrome includes standardising hospital medical care and educating physicians and nursing staff to ensure patients receive optimum doses of opioids to relieve pain (but not cause sedation leading to atelectasis) and adequate intravenous and oral fluids.19 When an inpatient multimodal strategy was implemented to manage vaso-occlusive pain, rates of acute chest syndrome decreased from 25% to 12% (p=0·003).19
Treatment
After acute chest syndrome has been diagnosed, management typically includes supportive measures. Broad-spectrum antibiotics effective against Streptococcus pneumoniae and a macrolide should be started for possible treatment of Chlamydia pneumoniae or Mycoplasma pneumoniae.20 Commonly, the haemoglobin oxygen saturation is kept higher than 92%, which might need supplemental oxygen. When a patient has a constellation of clinical findings that include at least one of the following: increasing respiratory rate with increasing work of breathing, increasing oxygen requirement to keep the oxygen saturation higher than 92%, and a decreasing haemoglobin concentration, a blood transfusion is given to raise the haemoglobin concentration up to approximately 100 g/L, if the patient has a baseline haemoglobin level that is lower than 90 g/L. In patients with a haemoglobin concentration of 90 g/L or lower with a deteriorating respiratory status, erythrocytapheresis is often done to raise the concentration to higher than 10·0 g/dL and bring the haemoglobin S concentration down to 30%. Individuals with acute chest syndrome initially managed at hospitals that do not have the capacity to quickly perform erythrocytapheresis should be transferred to a tertiary care medical centre because the patient’s respiratory status might decline rapidly. Erythrocytapheresis has associated risks in the setting of acute chest syndrome, including, but not limited to, posterior reversible encephalopathy syndrome, increased number of red blood cell unit exposures with associated red blood cell alloimmunisation, and iatrogenic complications associated with central line placement. Given the consensus that erythrocytapheresis is effective in attenuating the downward spiral of acute respiratory failure in individuals with severe acute chest syndrome, a randomised controlled trial is not likely to be done. Other respiratory support measures such as bi-level positive airway pressure devices might be started, but these do not replace the use of blood transfusion therapy.
Asthma and airway hyper-responsiveness in sickle-cell disease
Lung disorders in sickle-cell disease
Individuals with sickle-cell disease commonly have regular (even chronic) cough, shortness of breath, and wheeze in the absence of asthma. Airway hyper-responsiveness is another feature of sickle-cell lung disease.21 Various studies have defined hyper-responsiveness either as a decrease in lung function after exercise, cold air, or methacholine challenges or an increase after administration of a bronchodilator. Up to 70% of children with sickle-cell disease have airway hyper-responsiveness.22 In a cross-sectional study, Field and colleagues23 showed an association between the degree of airway responsiveness to methacholine and levels of lactic dehydrogenase concentrations in children with sickle-cell disease,23 suggesting an interaction between haemolysis in blood vessels of the lung and responsiveness of the airways. However, the basis for the association between hyper-responsiveness and the degree of haemolysis has not been extensively studied.
In adults, chronic lung disease is often listed as a cause of morbidity, but stages of sickle-cell chronic lung disease are based on a combination of clinical data (chest pain), laboratory evaluation (arterial blood gas), cardiac evaluation, and pulmonary function tests, mainly spirometry and lung volumes.24 Unfortunately, the originally proposed sickle-cell chronic lung disease classification has not been validated or broadly applied as an approach to determine the severity of lung disease in sickle-cell anaemia or as a predictor for premature death.
Asthma in sickle-cell disease
Given the substantial overlap in symptoms of acute asthma exacerbation and acute lung disease associated with sickle-cell disease, making a diagnosis of asthma in a child with sickle-cell disease can be difficult. The presence of sickle-cell disease alone could be sufficient to change the lung physiology and inflammatory status to resemble an asthma phenotype. The best evidence supporting this premise is from two separate studies in mice with sickle-cell disease, which show altered immune and lung physiology that most resembles experimental asthma. Andemariam and colleagues25 showed that lungs from sickle-cell disease mice have histological and cytochemical evidence of airway inflammation. Nandedkar and colleagues26 showed that transgenic sickle-cell disease mice have higher eosinophil counts, immunoglobulin E concentrations, and interleukin 13 concentrations compared with transgenic haemoglobin A mice. Both eosinophils and interleukin 13 concentrations are implicated in allergic inflammation; however, the biological basis for these findings is not known. In a large study of children with sickle-cell anaemia, approximately 50% (261 of 521) had total immunoglobulin E concentrations higher than the 90th percentile for age-matched healthy controls.27 Total immunoglobublin E level was associated with a diagnosis of asthma and with incidence of acute chest syndrome.27 These data suggest that children with sickle-cell anaemia could be primed for increased allergic inflammation.
Epidemiology and diagnosis of asthma in sickle-cell disease
Asthma is a common comorbidity in children with sickle-cell disease, occurring in 15–28% of participants in large multicentre cohort studies.9,27,28 In many of these studies, the asthma diagnosis is made based on a parental report of a physician diagnosis of asthma,29–31 a standard strategy for asthma epidemiology studies. Other definitions of asthma in children with sickle-cell disease include physician diagnosis of asthma and an episode of wheezing in the previous 12 months or receiving asthma medication, or both (table 2).32
Table 2.
Asthma definitions in individuals with sickle-cell disease and main findings of the article
| Asthma definition | Outcome associated with asthma | |
|---|---|---|
| Boyd et al9 | Older than 5 years; clinical diagnosis during any annual study visit or presentation with acute asthma during study or use of asthma medication reported on a clinic visit form | Asthma associated with more frequent and painful ACS episodes and earlier diagnosis of ACS |
| Knight-Madden et al10 | Parent report of ever or current asthma diagnosis, substantiated by medical record review; atopy defined by at least one positive skin-prick test | Asthma diagnosis more common in individuals with sickle-cell disease than in controls; atopy and asthma were more common in children with sickle-cell disease who had recurrent episodes of ACS compared with those who had one or no ACS episodes |
| Bernaudin et al11 | Doctor diagnosis of asthma in medical record | Significant increase in ACS episodes, but not pain |
| Sylvester et al21 | Parent report of a doctor diagnosis of asthma and taking an anti-asthma medication | Those taking anti-asthma medication more often had ACS episodes; asthma was diagnosed 3·5 years before first ACS episode |
| An et al27 | Parent report of a doctor diagnosis of asthma; when no asthma medication was reported, diagnosis of asthma was confirmed by chart audit finding of prescribed anti-asthma medication, hospitalisation, or urgent visit for asthma | Increased incidence of ACS and pain; elevations of serum immunoglobulin E and specific antibodies for Alternaria spp, cockroach, and dust mite |
| Strunk et al28 | Parent report of a doctor diagnosis and report of daily anti-asthma medication | Prospective ACS rate associated with having a parent with asthma, wheeze after exercise, wheeze causing shortness of breath |
| Knight-Madden et al32 | Current asthma diagnosed by a doctor, wheezed in previous 12 months or were receiving asthma medication; asthma in past if ever diagnosed but no wheeze or asthma medication; also analysed cigarette or marijuana smoking currently or in past | Increased risk of death with smoking or had current asthma |
| Boyd et al 33 | Medical record review indicated history of physician-diagnosed asthma during any admission to hospital prior to index admission for pain | Associated with increased risk of and longer hospitalisation for ACS |
| Paul et al34 | History of asthma | Patients with a history of acute pulmonary events more likely to have as independent variables a history of asthma, older age, severe episodes of pain in preceding 12 months, higher white blood cell count and tricuspid regurgitation velocity |
| Intzes et al35 | History of asthma | History of ACS significantly associated with either asthma or obstructive lung disease |
| Poulter et al36 | For children with sickle-cell disease: parent-reported asthma or routine use of asthma medications on admission history; or parent-reported or child complaint of wheezing at presentation | Asthma and wheeze significant risk factors for development of ACS |
| Cohen et al37 | Either a doctor diagnosis of asthma or reported use of anti-inflammatory asthma-controller medication in adults | Significantly associated with serum immunoglobulin E, family history of asthma, history of eczema, and wheeze symptoms; wheeze symptoms but not asthma associated with rates of pain and ACS events |
| Glassberg et al38 | Diagnosis of asthma documented in medical history or problem list with supporting evidence from medical record of prior health-care encounters; when asthma diagnosed but no asthma medication recorded, diagnosis confirmed in medical records for any hospital admission, emergency department visit, clinic visit, or medication for asthma | Wheezing and asthma independently associated with increased emergency department visits for pain; wheezing but not asthma associated with increased emergency department use for ACS |
| Boyd et al 39 | Clinical diagnosis during any annual study visit or presentation with acute asthma during study or use of asthma medication reported on a clinic visit form | After controlling for established risk factors, individuals with sickle-cell disease and asthma had increased risk of mortality |
ACS=acute chest syndrome.
In a rigorous, multicentre, prospective study of children with sickle-cell anaemia and asthma a multivariable logistic regression identified three factors associated with asthma: parent with asthma (p=0·006), wheezing causing shortness of breath (p=0·001), and wheezing after exercise (p<0·001).28 The model had a sensitivity of 100% when two or more features were present. When none of the features were present, model sensitivity was 0% at differentiation from children without asthma.28
Diagnosis of asthma in sickle-cell disease
On the basis of published work on acute chest syndrome, a reasonable definition of asthma in children with sickle-cell disease should include repetitive wheezing causing shortness of breath or wheezing with exercise, with confidence in the diagnosis of asthma strengthened by the presence of parental history of asthma, at least two positive aeroallergen skin tests, or both, in children 4 years of age or older.28 A diagnosis of repetitive acute chest syndrome episodes with a negative chest roentgenogram should also raise the suspicion of inadequately treated asthma. However, the presence of wheezing alone is not sufficient to make a diagnosis of asthma. Even in children with sickle-cell anaemia without an asthma diagnosis, 48% had a history of some wheezing symptoms and 13% had wheezing causing shortness of breath.28 Taken together, these data highlight the clinical challenge of making a diagnosis of asthma, and even an asthma exacerbation, in children with sickle-cell disease.
Asthma and acute chest syndrome in sickle-cell disease
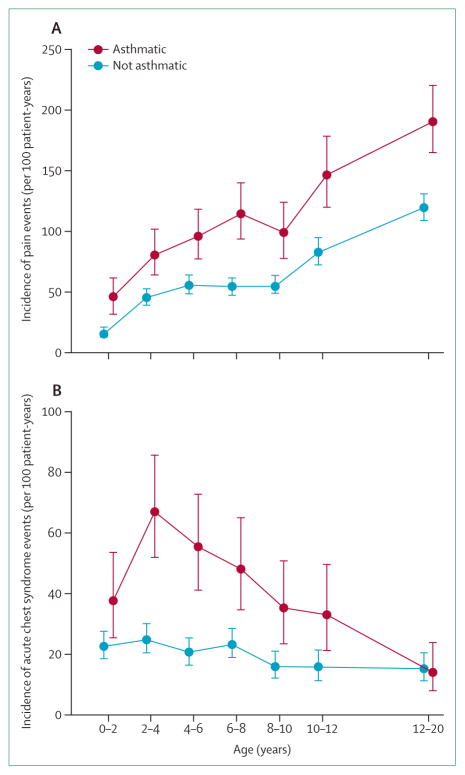
A confirmed asthma diagnosis is associated with increased incidence of acute chest syndrome and pain. The most rigorous study to show the relationship between asthma and acute chest syndrome was the Cooperative Study of Sickle-Cell Disease (CSSCD), a prospective cohort of 291 infants with sickle-cell anaemia observed for a mean of 14 years.9 Children with asthma had increased incidence of acute chest syndrome, pain, and an earlier onset of the first episode of acute chest syndrome when compared with children in the cohort who did not have asthma (figure 1).9 Other single institution and multi-institutional studies have consistently confirmed the association between asthma and increased incidence of acute chest syndrome and pain events in children with either sickle-cell anaemia10–12,27,32–35 or haemoglobin SC disease.36 Also three separate prospective studies in adults with sickle-cell disease have shown asthma or recurrent wheezing to be associated with an increased rate of pain or acute chest syndrome.32,37,38
Figure 1. Relationship between asthma and increased rate of pain events (A) and acute chest syndrome (B) in children with sickle-cell anaemia.
Overall incidence rate of painful events (A) is higher in children with asthma (1·39 events per patient-year) than in those without asthma (0·47 events per patient-year; p<0·001). Overall incidence rate of acute chest syndrome events (B) is higher in children with asthma (0·39 events per patient-year) than in children without asthma (0·20 events per patient-year, p<0·001).
*Line segments are point-wise exact 95% CI. Reproduced with permission of the American Haematology Society.
As would be expected with the higher incidences of pain and acute chest syndrome events, which are both independent risk factors for mortality,40,41 a diagnosis of asthma in individuals with sickle-cell anaemia is associated with earlier death when compared with individuals without asthma. One prospective study in children and adults with sickle-cell anaemia and two prospective studies in adults with sickle-cell anaemia and sickle-celll disease have shown the link between asthma39 or recurrent wheezing and earlier death.32,37 Two case series identified asthma exacerbations to be a proximal cause of death in young adults with sickle-cell disease,23,42 further bolstering the strength of the relationship between asthma and death. Collectively, these data show that a diagnosis of asthma or recurrent wheezing should be screened for, detected, and treated, not only to prevent morbidity, but also perhaps mortality.
One of the biggest risk factors for future acute chest syndrome is history of a previous episode, particularly for children younger than 4 years. This risk factor was first shown by Quinn and colleagues43 in the Dallas sickle-cell anaemia cohort, a large retrospective cohort of children with sickle-cell anaemia (n=264, with a mean follow-up of 12 years).43 Early acute chest syndrome explained 12% of the variability in late acute chest syndrome and was associated with late acute chest syndrome with an odds ratio of 2·51 (95% CI 1·67–3·86; p <0·001) when compared to the reference category. Early pain, early dactylitis, sex, and sickle-cell disease genotype did not predict incidence of late acute chest syndrome. However, this study did not define the timing of when the repeat event was likely to occur. More recently, in a multicentre cohort of children with sickle-cell anaemia observed in clinical centres since birth (n=125 children; median follow-up 15 years), Vance and colleagues44 reported that the interval with the highest incidence rate of recurrence of serious vaso-occlusive events (either pain or acute chest syndrome alone or both, requiring hospitalisation) was within 6 months of a child’s last event.44
Children younger than 4 years with sickle-cell anaemia who have been hospitalised for acute chest syndromes have a distinctly different natural history of acute lung disease when compared with older children at the time of their first episode. Three observations from multicentre studies strongly suggest that early onset of acute chest syndrome is a phenotype associated with an increased risk for future lung disease (acute chest syndrome events and perhaps asthma diagnosis). First, as mentioned, presence of an initial acute chest syndrome episode in children younger than 4 years is a very strong risk factor for future acute chest syndrome episodes (incidence rate ratio 2·84, 95% CI 1·84–4·37; p<0·001).13 Second, 80% of the children with a diagnosis of asthma that was made after age 5 years had at least one episode of acute chest syndrome when younger than 4 years.9 Third, children who had their first episode when younger than 4 years had a higher rate of severe vaso-occlusive disease requiring hospitalisation (pain or acute chest syndrome) 1 year after the event (62%) than did children older than 4 years at their first episode (39%; p=0·009).44 Together these findings provide compelling evidence that at the very least clinicians should identify children with sickle-cell anaemia who were younger than 4 years at their first episode because this group will most likely have asthma,9 have a higher risk of hospitalisation for vaso-occlusive events within a year,44 and have a higher incidence rate of future acute chest syndrome episodes.13
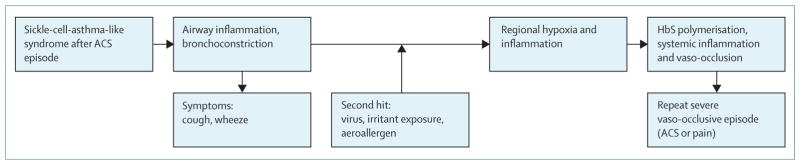
We postulate that young children with sickle-cell disease have the same frequency of respiratory viral illnesses in early life as do similarly aged children in the general population, approximately six respiratory infections per year.45 In the general population, young children admitted to hospital with viral-associated wheezing are likely to have repeat hospitalisation for wheezing within a year46 and have a predisposition to asthma.46,47 We posit that children with sickle-cell anaemia are susceptible to the same effects of early respiratory tract viral infections requiring hospitalisation, including a predisposition to asthma and future (pulmonary) acute chest syndrome events (figure 2). No results from prospective cohort studies have been reported to validate our hypothesis that early onset respiratory viral disease requiring hospitalisation predisposes to future asthma in children with sickle-cell disease.
Figure 2. Proposed pathophysiology in children with sickle-cell anaemia with early-onset ACS.
Postulated mechanism for why early-onset ACS in children with sickle-cell anaemia younger than 4 years is associated with both short-term and long-term acute lung morbidity (ACS, asthma, or both). The initial ACS event results in airway inflammation and hyper-responsiveness, which predispose the airways to narrowing from a second hit, either a repeat viral infection (young children have about six viral infections a year), exposure to an environmental irritant (eg, cigarette smoke), or a history of atopy with exposure to an aeroallergen to which there is sensitivity. A second event then leads to a sequence of events that result in a repeat severe vaso-occlusive episode. ACS=acute chest syndrome. HbS=haemoglobin S.
Two cases (panels 1 and 2) identified in our joint paediatric sickle-cell disease and asthma clinic highlight the challenge of determination of whether presentation of a child in respiratory distress is due to sickle-cell disease-associated acute chest syndrome or an asthma exacerbation. Three abnormal lung disease phenotypes in children with sickle-cell anaemia (early onset acute chest syndromes, asthma, and sickle-cell-related lung disease) all overlap with the common asthma feature of wheezing.
Panel 1. Case one—asthma exacerbations misinterpreted as episodes of acute chest syndrome.
Multiple episodes of respiratory distress in a young child that were labelled acute chest syndrome events, but were asthma exacerbations
A 1-year-old boy with sickle-cell anaemia was seen during a hospitalisation for acute respiratory disease initially diagnosed as an acute chest syndrome event. He had severe eczema and persistent rhinorrhoea. Both parents had eczema and a sibling had asthma. On examination he was wheezing and had a prolonged expiratory phase. His blood pressure was 100/82 mm Hg, temperature 40·6°C, pulse 140 beats per min, respiratory rate 38 breaths per min, and haemoglobin oxygen saturation was 100% in ambient room air. On the basis of his wheezing without an upper respiratory illness and the strong family history of atopic disease, he was diagnosed as having asthma. His treatment in this hospitalisation at age 1 year was augmented with oral corticosteroids and regular use of albuterol. At discharge he was started on regular nebulised inhaled corticosteroid. Additional testing supported the diagnosis of asthma, with infant pulmonary function tests demonstrating airway obstruction and bronchodilator response (forced expiratory volume in 0·5 s was 57% predicted, with an increase by 34% after albuterol). With use of daily inhaled corticosteroids and oral corticosteroids initiated as the first signs of an exacerbation (increased wheeze and cough in the context of an upper respiratory infection), he had no hospital admissions for respiratory disease over the next 3 years.
Summary
This boy had asthma given his typical symptoms of wheeze without a cold and his strong personal and family history of atopy.28 His clinical course changed with asthma therapy. He represents a situation where asthma exacerbations can be misinterpreted as episodes of acute chest syndrome. Recognition of his clinical course and his family history allowed re-interpretation of his disease as asthma and treatment altered his clinical course.
Panel 2. Case two—sickle-cell-related lung disease that mimics asthma.
Recurrent respiratory exacerbations in the context of upper respiratory infections each associated with wheeze
A 9-year-old girl with sickle-cell anaemia had frequent hospitalisations (average three to four times per year), for acute respiratory illnesses with wheezing. Evaluation at baseline showed no evidence of atopy, with negative skin testing to aeroallergens, normal total serum immunoglobulin E levels, and no family and personal history negative for asthma, eczema, or allergic rhinitis. Pulmonary function testing did not show obstructive lung disease. Due to her history of wheezing with colds, she was prescribed inhaled corticosteroid just at times when she had increased upper respiratory symptoms, for the possibility that she had infection-induced asthma. On a subsequent hospitalisation with onset of wheeze after increased upper respiratory symptoms, her blood pressure was 108/68 mm Hg, temperature 38·1°C, pulse 190 beats per min, respiratory rate 60 breaths per min, and oxygen haemoglobin saturation 94% on 0·5 L supplemental oxygen. She had new radiodensities in the right middle and right lower lobes of her chest roentgenogram. Her wheeze was responsive to albuterol therapy. She was treated with intravenous antibiotics and prednisolone 2 mg/kg per day with a planned course of 5 days. She had resolution of symptoms within 24 h, but 2 days after discharge she had recurrence of respiratory symptoms now associated with severe pain. Overall her clinical course was not aided by use of inhaled corticosteroid upper respiratory symptoms developed and this therapy was stopped.
Summary
This case represents sickle-cell-related lung disease that mimics asthma. Even though her wheeze responded to bronchodilator therapy, there was no evidence of atopy, no family history of asthma, and no pulmonary obstruction on spirometry. Use of oral corticosteroids did not seem to be useful and in fact might have precipitated the pain episode that occurred after her hospitalisation for primarily respiratory symptoms. Other investigators have reported either vaso-occlusive pain, readmission to hospital, or both temporally associated with short use corticosteroids in children with sickle-cell anaemia.48–50
Role of spirometry in evaluation of sickle-cell disease
In 2014, the National Institutes of Health, Heart, Lung and Blood Institute (NHLBI) convened an expert panel to review the published work and make evidence-based recommendations for primary care providers on the management of sickle-cell disease.51 The expert panel did not recommend routine use of spirometry in children or adults with sickle-cell disease. However, on the basis of evidence that asthma and recurrent wheezing are associated with increased morbidity and mortality in sickle-cell disease, this non-endorsement is without strong merit. The case in panel 3 from our clinic shows how spirometry evaluation can alter acute care in sickle-cell disease. As in children with asthma and cystic fibrosis, obtaining spirometry evaluations improves the ability to assess the degree of airway obstruction, monitor treatment response, and assess acute obstructive airway disease during an acute illness. Furthermore, the added value of spirometry in a clinic with children with sickle-cell disease is expected when as many as 28% have asthma28 and at least 60% of the children without asthma have airway hyper-reactivity.22
Panel 3. Case three—overlapping asthma and sickle-cell pain.
Pain and acute respiratory disease without the presence of wheezing, but with reversible lower airway obstruction
An 11-year-old boy with sickle-cell anaemia presented to the clinic with complaint of an acute body pain for 3 weeks with no relief after oxycodone at home. He had a diagnosis of asthma and was prescribed albuterol to use as needed. On presentation to clinic during his episode of pain, he was afebrile with haemoglobin oxygen saturation 94% in ambient room air, lower than his usual 97%. He was sent to have pulmonary function testing based on his previous history of asthma, even though examination demonstrated only tenderness to palpation of his back, chest, and extremities, with no wheeze. On spirometry, his forced expiratory volume in 1 s (FEV1) was 66% predicted and improved by 23% to 82% predicted after administration of albuterol via metered-dose-inhaler. His pain resolved after the administration of the albuterol. Based on the initial lower airway obstruction, reversibility of the obstruction with a short-acting bronchodilator, and the resolution of his pain during testing, he was instructed to take albuterol every 4 h for 48 h and also started on inhaled corticosteroids twice a day for chronic use. He was seen 1 week later and had normal lung function results and continued to be without vaso-occlusive pain.
Summary
These results indicate the patient was having an asthma exacerbation with clinically significant reversible airway disease52 and a pain event. The observation of an acute increase in FEV1 after administering β2 agonist has been repeated in other children with sickle-cell disease presenting with pain or mild upper respiratory disease symptoms that did not have wheezing.
The evidence to support routine use of spirometry in sickle-cell disease includes, but is not limited to, the high pre-test probability of asthma coupled with the challenge of making the diagnosis of asthma in sickle-cell disease; the associated morbidity and mortality associated with asthma in sickle-cell disease; the high risk of deaths associated with asthma,39 recurrent wheezing,32,37 or cardiopulmonary disease;53–55 and the fact that in adults with sickle-cell anaemia a forced expiratory volume in 1 s (FEV1) lower than 70% predicted predicts earlier mortality.56 These observations indicate that routine spirometry evaluation is an important adjunct to the clinical care of this population with a high prevalence of pulmonary disease. Although adding spirometry to the routine care of children with sickle-cell disease and using evidenced-based asthma guidelines should improve asthma management, this might not be sufficient to decrease sickle-cell disease-related morbidity. McClain and colleagues57 showed that asthma care could be improved to qualities suggested by the NHLBI with a combined asthma and sickle-cell disease clinic. However, implementing the NHLBI guidelines did not decrease the rate of asthma exacerbation, pain, or acute chest syndromes.57 Research is needed to specifically address the best therapy for children and adults with sickle-cell disease who have asthma, recurrent wheezing, or both. Furthermore, more studies are needed from sub-Saharan Africa and India where most children with sickle-cell disease are born; so far only one cross-sectional study has been published describing respiratory symptoms in children with sickle-cell disease.58
Conclusion
Asthma is prevalent in children with sickle-cell disease and results in increased rates of severe vaso-occlusive pain and episodes of acute chest syndrome. Given the high rate of sickle-cell-associated lung disease, with high IgE concentrations, common clinical features mimicking asthma (eg, wheezing, airway hyper-responsiveness), and recent evidence that low FEV1 % predicted is associated with earlier death in adults with sickle-cell anaemia, sufficient evidence now exists to include in each clinic visit a routine history to ascertain asthma risk factors and symptoms. With the high prevalence of asthma and airway hyper-responsive disease without asthma in children with sickle-cell disease, spirometry should be used to complement the respiratory history, identify and monitor lower airway obstruction, and monitor response to therapy.
More research is needed to define effective treatment for asthma in the context of sickle-cell disease. Federal funding agencies and international professional societies in haematology and pulmonology have sufficient data to initiate consensus statements to define and manage acute lung disease (asthma and acute chest syndrome), identify key gaps in knowledge, and pledge research to improve evidence-based care for acute lung disease and their sequelae in children with sickle-cell disease.
Acknowledgments
We received funding from the National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) grant number 5R01-HL079937-08 (MRD and RCS) and the Burroughs Wellcome Foundation (MRD). We thank the members of the DeBaun laboratory that have provided helpful comments regarding this manuscript and Robyn Cohen. Figure 2 was adapted from an earlier version designed by Leah Vance while working in DeBaun’s Laboratory (the figure has never been published). This manuscript and work was inspired by a 15 year collaboration between Robert Strunk, a paediatric asthma specialist, and Michael DeBaun, a sickle-cell disease specialist. Strunk died unexpectedly in the spring of 2016.
Footnotes
Contributors
MRD and RCS contributed equally to the design and concept; the searches; and the writing, revision, and approval of the final manuscript.
Declaration of interests
We declare no competing interests.
Contributor Information
Michael R DeBaun, Department of Pediatrics and Medicine, Division of Hematology/Oncology, and Vanderbilt-Meharry Sickle Cell Center for Excellence, Vanderbilt University School of Medicine, Nashville, TN, USA.
Prof. Robert C Strunk, Division of Allergy, Immunology, and Pulmonary Medicine, Department of Pediatrics, Washington University School of Medicine, St Louis, MO, USA.
References
- 1.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–52. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–76. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 3.Hirst C, Owusu-Ofori S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. Cochrane Database Syst Rev. 2014;11:CD003427. doi: 10.1002/14651858.CD003427.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Vichinsky EP, Neumayr LD, Earles AN, et al. for the National Acute Chest Syndrome Study Group. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342:1855–65. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 5.Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B the Cooperative Study of Sickle Cell Disease. Acute chest syndrome in sickle cell disease: clinical presentation and course. Blood. 1997;89:1787–92. [PubMed] [Google Scholar]
- 6.Castro O, Brambilla DJ, Thorington B, et al. the The Cooperative Study of Sickle Cell Disease. The acute chest syndrome in sickle cell disease: incidence and risk factors. Blood. 1994;84:643–49. [PubMed] [Google Scholar]
- 7.Charache S, Scott JC, Charache P. “Acute chest syndrome” in adults with sickle cell anemia. Microbiology, treatment, and prevention. Arch Intern Med. 1979;139:67–69. [PubMed] [Google Scholar]
- 8.Morris C, Vichinsky E, Styles L. Clinician assessment for acute chest syndrome in febrile patients with sickle cell disease: is it accurate enough? Ann Emerg Med. 1999;34:64–69. doi: 10.1016/s0196-0644(99)70273-8. [DOI] [PubMed] [Google Scholar]
- 9.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108:2923–27. doi: 10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60:206–10. doi: 10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernaudin F, Strunk RC, Kamdem A, et al. Asthma is associated with acute chest syndrome, but not with an increased rate of hospitalization for pain among children in France with sickle cell anemia: a retrospective cohort study. Haematologica. 2008;93:1917–18. doi: 10.3324/haematol.13090. [DOI] [PubMed] [Google Scholar]
- 12.Sylvester KP, Patey RA, Broughton S, et al. Temporal relationship of asthma to acute chest syndrome in sickle cell disease. Pediatr Pulmonol. 2007;42:103–06. doi: 10.1002/ppul.20430. [DOI] [PubMed] [Google Scholar]
- 13.DeBaun MR, Rodeghier M, Cohen R, et al. Factors predicting future ACS episodes in children with sickle cell anemia. Am J Hematol. 2014;89:E212–17. doi: 10.1002/ajh.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bean CJ, Boulet SL, Yang G, et al. Acute chest syndrome is associated with single nucleotide polymorphism-defined beta globin cluster haplotype in children with sickle cell anaemia. Br J Haematol. 2013;163:268–76. doi: 10.1111/bjh.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bean CJ, Boulet SL, Ellingsen D, et al. Heme oxygenase-1 gene promoter polymorphism is associated with reduced incidence of acute chest syndrome among children with sickle cell disease. Blood. 2012;120:3822–28. doi: 10.1182/blood-2011-06-361642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galarneau G, Coady S, Garrett ME, et al. Gene-centric association study of acute chest syndrome and painful crisis in sickle cell disease patients. Blood. 2013;122:434–42. doi: 10.1182/blood-2013-01-478776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adisa OA, Hu Y, Ghosh S, Aryee D, Osunkwo I, Ofori-Acquah SF. Association between plasma free haem and incidence of vaso-occlusive episodes and acute chest syndrome in children with sickle cell disease. Br J Haematol. 2013;162:702–05. doi: 10.1111/bjh.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellet PS, Kalinyak KA, Shukla R, Gelfand MJ, Rucknagel DL. Incentive spirometry to prevent acute pulmonary complications in sickle cell diseases. N Engl J Med. 1995;333:699–703. doi: 10.1056/NEJM199509143331104. [DOI] [PubMed] [Google Scholar]
- 19.Reagan MM, DeBaun MR, Frei-Jones MJ. Multi-modal intervention for the inpatient management of sickle cell pain significantly decreases the rate of acute chest syndrome. Pediatr Blood Cancer. 2011;56:262–66. doi: 10.1002/pbc.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean D, Neumayr L, Kelly DM, et al. the Acute Chest Syndrome Study Group. Chlamydia pneumoniae and acute chest syndrome in patients with sickle cell disease. J Pediatr Hematol Oncol. 2003;25:46–55. doi: 10.1097/00043426-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Ozbek OY, Malbora B, Sen N, Yazici AC, Ozyurek E, Ozbek N. Airway hyperreactivity detected by methacholine challenge in children with sickle cell disease. Pediatr Pulmonol. 2007;42:1187–92. doi: 10.1002/ppul.20716. [DOI] [PubMed] [Google Scholar]
- 22.Leong MA, Dampier C, Varlotta L, Allen JL. Airway hyperreactivity in children with sickle cell disease. J Pediatr. 1997;131:278–83. doi: 10.1016/s0022-3476(97)70166-5. [DOI] [PubMed] [Google Scholar]
- 23.Field JJ, Horst J, Strunk RC, White FV, DeBaun MR. Death due to asthma in two adolescents with sickle cell disease. Pediatr Blood Cancer. 2011;56:454–57. doi: 10.1002/pbc.22891. [DOI] [PubMed] [Google Scholar]
- 24.Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine (Baltimore) 1988;67:66–76. [PubMed] [Google Scholar]
- 25.Andemariam B, Adami AJ, Singh A, et al. The sickle cell mouse lung: proinflammatory and primed for allergic inflammation. Transl Res. 2015;166:254–68. doi: 10.1016/j.trsl.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nandedkar SD, Feroah TR, Hutchins W, et al. Histopathology of experimentally induced asthma in a murine model of sickle cell disease. Blood. 2008;112:2529–38. doi: 10.1182/blood-2008-01-132506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An P, Barron-Casella EA, Strunk RC, Hamilton RG, Casella JF, DeBaun MR. Elevation of IgE in children with sickle cell disease is associated with doctor diagnosis of asthma and increased morbidity. J Allergy Clin Immunol. 2011;127:1440–46. doi: 10.1016/j.jaci.2010.12.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strunk RC, Cohen RT, Cooper BP, et al. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia. J Pediatr. 2014;164:821–26. e1. doi: 10.1016/j.jpeds.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persky VW, Slezak J, Contreras A, et al. Relationships of race and socioeconomic status with prevalence, severity, and symptoms of asthma in Chicago school children. Ann Allergy Asthma Immunol. 1998;81:266–71. doi: 10.1016/S1081-1206(10)62824-4. [DOI] [PubMed] [Google Scholar]
- 30.Grant EN, Daugherty SR, Moy JN, Nelson SG, Piorkowski JM, Weiss KB. Prevalence and burden of illness for asthma and related symptoms among kindergartners in Chicago public schools. Ann Allergy Asthma Immunol. 1999;83:113–20. doi: 10.1016/S1081-1206(10)62621-X. [DOI] [PubMed] [Google Scholar]
- 31.Miller JE. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. Am J Public Health. 2000;90:428–30. doi: 10.2105/ajph.90.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight-Madden JM, Barton-Gooden A, Weaver SR, Reid M, Greenough A. Mortality, asthma, smoking and acute chest syndrome in young adults with sickle cell disease. Lung. 2013;191:95–100. doi: 10.1007/s00408-012-9435-3. [DOI] [PubMed] [Google Scholar]
- 33.Boyd JH, Moinuddin A, Strunk RC, DeBaun MR. Asthma and acute chest in sickle-cell disease. Pediatr Pulmonol. 2004;38:229–32. doi: 10.1002/ppul.20066. [DOI] [PubMed] [Google Scholar]
- 34.Paul R, Minniti CP, Nouraie M, et al. Clinical correlates of acute pulmonary events in children and adolescents with sickle cell disease. Eur J Haematol. 2013;91:62–68. doi: 10.1111/ejh.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Intzes S, Kalpatthi RV, Short R, Imran H. Pulmonary function abnormalities and asthma are prevalent in children with sickle cell disease and are associated with acute chest syndrome. Pediatr Hematol Oncol. 2013;30:726–32. doi: 10.3109/08880018.2012.756961. [DOI] [PubMed] [Google Scholar]
- 36.Poulter EY, Truszkowski P, Thompson AA, Liem RI. Acute chest syndrome is associated with history of asthma in hemoglobin SC disease. Pediatr Blood Cancer. 2011;57:289–93. doi: 10.1002/pbc.22900. [DOI] [PubMed] [Google Scholar]
- 37.Cohen RT, Madadi A, Blinder MA, DeBaun MR, Strunk RC, Field JJ. Recurrent, severe wheezing is associated with morbidity and mortality in adults with sickle cell disease. Am J Hematol. 2011;86:756–61. doi: 10.1002/ajh.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glassberg JA, Chow A, Wisnivesky J, Hoffman R, Debaun MR, Richardson LD. Wheezing and asthma are independent risk factors for increased sickle cell disease morbidity. Br J Haematol. 2012;159:472–79. doi: 10.1111/bjh.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92:1115–18. doi: 10.3324/haematol.11213. [DOI] [PubMed] [Google Scholar]
- 40.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 41.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 42.Dhakal B, Giese K, Santo-Thomas L, Field JJ. Death during an asthma exacerbation in an adult with sickle cell disease: an autopsy case study. Am J Hematol. 2013;88:824. doi: 10.1002/ajh.23492. [DOI] [PubMed] [Google Scholar]
- 43.Quinn CT, Shull EP, Ahmad N, Lee NJ, Rogers ZR, Buchanan GR. Prognostic significance of early vaso-occlusive complications in children with sickle cell anemia. Blood. 2007;109:40–45. doi: 10.1182/blood-2006-02-005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vance LD, Rodeghier M, Cohen RT, et al. Increased risk of severe vaso-occlusive episodes after initial acute chest syndrome in children with sickle cell anemia less than 4 years old: sleep and asthma cohort. Am J Hematol. 2015;90:371–75. doi: 10.1002/ajh.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112(suppl 6A):4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 46.Stensballe LG, Simonsen JB, Thomsen SF, et al. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. J Allergy Clin Immunol. 2009;123:131–37. e1. doi: 10.1016/j.jaci.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 47.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strouse JJ, Takemoto CM, Keefer JR, Kato GJ, Casella JF. Corticosteroids and increased risk of readmission after acute chest syndrome in children with sickle cell disease. Pediatr Blood Cancer. 2008;50:1006–12. doi: 10.1002/pbc.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sobota A, Graham DA, Neufeld EJ, Heeney MM. Thirty-day readmission rates following hospitalization for pediatric sickle cell crisis at freestanding children’s hospitals: risk factors and hospital variation. Pediatr Blood Cancer. 2012;58:61–65. doi: 10.1002/pbc.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernini JC, Rogers ZR, Sandler ES, Reisch JS, Quinn CT, Buchanan GR. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 1998;92:3082–89. [PubMed] [Google Scholar]
- 51.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033–48. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 52.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 53.Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 2010;85:36–40. doi: 10.1002/ajh.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–63. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 55.Ogun GO, Ebili H, Kotila TR. Autopsy findings and pattern of mortality in Nigerian sickle cell disease patients. Pan Afr Med J. 2014;18:30. doi: 10.11604/pamj.2014.18.30.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kassim AA, Payne AB, Rodeghier M, Macklin EA, Strunk RC, DeBaun MR. Forced expiratory volume in 1 second is associated with earlier death in sickle cell anemia. Blood. 2015;126:1544–50. doi: 10.1182/blood-2015-05-644435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClain BL, Ivy AK, Bryant V, Rodeghier M, DeBaun MR. Improved guideline adherence with integrated sickle cell disease and asthma care. J Prevent Med. doi: 10.1016/j.amepre.2016.03.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galadanci NA, Liang WH, Galadanci AA, et al. Wheezing is common in children with sickle cell disease when compared with controls. J Pediatr Hematol Oncol. 2015;37:16–19. doi: 10.1097/MPH.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]