Abstract
Background
Evidences have identified the correlation of 8-oxoguanine DNA glycosylase-1 (OGG1) and eph-receptor tyrosine kinase-type A2 (EPHA2) polymorphisms in age-related cataract (ARC) risk. However, the results were not consistent. The objective of this study was to examine the role of these two gene polymorphisms in ARC susceptibility.
Methods
Eligible case–control studies published between January 2000 and 2015 were searched and retrieved in the electronic databases. The odds ratio with 95 % confidence interval (CI) was employed to calculate the strength of the relationship.
Results
We totally screened out six articles, including 5971 cataract patients and 4189 matched controls. Three variants were contained (OGG1 rs1052133; EPHA2 rs7543472 and rs11260867). For OGG1 rs1052133, we detected a significant correlation between OGG1 polymorphism and ARC risk under the heterogenous model (CG vs. CC: OR = 1.34, 95 % CI = 1.06–1.70, P = 0.01) and dominant model (GG+CG vs. CC: OR = 1.45, 95 % CI = 1.16–1.81, P = 0.001), especially in patients with cortical cataract of subgroup analysis by phenotypes (P < 0.05). For EPHA2 rs7543472 and rs11260867, we did not find a positive association between these two mutations and ARC susceptibility in total cases. Subgroup analysis by phenotypes of cataract showed that only in cortical cataract, genotypes of rs7543472 under the allele model, homogenous model and recessive model; genotypes of rs11260867 under the heterogenous model and dominant model were associated with ARC risk.
Conclusions
OGG1 rs1052133 (CG and CG+GG genotypes) might be risk factor for ARC, particularly in cortical cataract risk. EPHA2 rs7543472 (T allele and TT genotype) and rs11260867 (CG and GG+CG genotypes) might be associated with cortical cataract.
Keywords: Age-related cataract, OGG1, EPHA2, Polymorphism, Meta-analysis
Background
Cataract is a leading cause of blindness and visual impairment throughout the world, increasing the public health and economic burden of this disease [1]. According to the Global estimates of visual impairment, approximate 51 % of blindness and 33 % of visual impairment were estimated due to cataract between 2000 and 2010 [2]. In addition, there are racial/ethnic disparities in the prevalence of cataract [3]: Cataract of Europeans accounts for 50 % (WHO criteria) to 65 % (US criteria) of unilateral visual impairment, and 45 % (US criteria) of 5-year incident bilateral visual impairment [4]; Asian populations had a higher prevalence and earlier age of onset of cataract than Europeans [5]; while the prevalence of cataract was lower in Africans compared with Europeans [6]. The major risk factors for cataract are age, as well as several demographic and lifestyle characteristics [7, 8]. Approximately 80 % of cataract is age-related cataract (ARC) [9]. Based on the location of the opacity in the lens, ARC is classified as cortical cataract, nuclear cataract, or posterior subcapsular cataract [10]. The prevalence of ARC is rising, with a prediction that 30.1 million Americans will be affected by 2020 [11]. However, its etiology is multi-factorial and not fully understood to date.
Etiological research have found that the set of genes were associated with cataract, especially for ARC [12]. In recent years, 8-oxoguanine DNA glycosylase-1 (OGG1) and eph-receptor tyrosine kinase-type A2 (EPHA2) have been identified as key regulators in lens clarity. OGG1 is located in human chromosome 3p26.2, and its protein is a key enzyme in base excision repair (BER) pathway [13]. It is involved in maintaining genome integrity and preventing cancer development [14]. OGG1 could be used as a therapeutic target for certain types of cancer in monotherapy or combination therapy [15]. OGG1 is also implicated in oxidative mechanisms which play an important role in the pathogenesis of ARC, and might increase the risk of developing ARC [16]. Single nucleotide polymorphism (SNP) of OGG1 was shown to be a risk factor for oxidative pathologies. The most studied variant was OGG1 gene rs1052133 (Ser326Cys), a C to G transversion at nucleotide 1245, leading to a serine to cysteine substitution at residue 326 located in the C-terminal domain of the protein. The Cys326 protein has been shown to have about 7 times weaker 8-hydroxyguanine-repair capacity than Ser326 protein [17]. Homozygous carriers of the S326C OGG1 polymorphism presented reduced repair activity, and C326 OGG1 homozygous carriers might be at increased risk of oxidative pathologies [18].
EPHA2, located in human chromosome 1p36, is a member of the Eph receptor tyrosine kinases family [19]. It is an important regulator of tumor initiation, neo-vascularization and metastasis in a wide range of epithelial and mesenchymal cancers [20, 21]. EPHA2 is highly expressed in aggressive human cancers, and also offers opportunities for Eph/ephrin-based targeted drug delivery and imaging [22, 23]. EPHA2 protein is expressed in human and mouse lens [24, 25]. Multiple mutations in the EPHA2 gene have been recently shown to cause cataracts in humans, contributing to the destabilization of the receptor and the loss of cell migration activity [26]. The TT genotype of rs7543472 was shown to be associated with ~2× increased risk for cataracts; rs3754334 might be a variant on the EPHA2 gene that is commonly associated with the risk for ARC in different ethnical and geographical populations [27].
Although epidemiologic studies have identified the correlation of these genes polymorphisms in ARC risk, however, the results remain inconclusive. Therefore, we conducted this meta-analysis to establish the true association between OGG1 and EPHA2 SNPs and the risk for ARC.
Methods
Identification of eligible articles
We performed a comprehensive literature search in the following electronic databases of Medline, PubMed, Springer and Elsevier to retrieve relevant articles published between January 2000 and 2015. The key terms were “cataract” or “age-related cataract”, “8-oxoguanine glycosylase-1 or OGG1 or DNA repair gene”, “Eph-receptor tyrosinekinase-type A2 or EPHA2”, and “variant or polymorphism” as well as their combinations. References of related studies were manually searched to obtain more sources. Only studies written in English were included in this meta-analysis.
Criteria for inclusion and exclusion
The inclusion criteria were as follows: 1) case–control studies concerning the role of OGG1 or EPHA2 polymorphisms in ARC risk; 2) patients with ARC was defined as lens opacity along with disturbance of vision and were over 50 years old (cataract status was determined by lens examination using a slit-lamp biomicroscope; lens opacities were determined using the Lens Opacities Classification System III [28]); controls were age-, sex-, and ethnically matched individuals without history of cataract, hypertension, or other ocular diseases; 3) the genotype information were available to extract, and the results were expressed as odds ratio (OR) with 95 % confidence interval (CI); 4) when the same authors or laboratories reported the issue among the same populations in more articles, only the recent full-text articles were included; and 5) genotype distribution of control for a certain polymorphism must be in Hardy-Weinberg equilibrium (HWE).
The exclusion criteria were: 1) review reports or conference papers; 2) without control group; 3) studies with duplicate data; and 4) genotype information couldn’t be extracted.
Quality assessment and data extraction
Two investigators independently assessed the quality of related articles. Any disagreement was subsequently resolved by discussion with another expert to reach a consensus on all of the items. The following information was extracted from each article: first author, year of publication, country, ethnicity, mean age, sample size, genotyping method, and genotype distribution in cases and controls.
Statistical analysis
The strength of association between polymorphisms of OGG1, EPHA2 and ARC risk was measured by the pooled ORs with its 95 % CI. The Z test was employed to determine the significance of the pooled ORs, and a P value less than 0.05 was considered statistically significant. For all the genetic polymorphisms, the comparison models (allelic model; homogenous model; heterogenous model; dominant model; and recessive model) were examined. The I2 test and the Q-statistic test were used to estimate between-study heterogeneity. The random-effect model was employed when the P-value less than 0.10 for the Q-test and I2 more than 50 %; otherwise, the fixed-effects model was used. The evidence of publication bias was assessed by visual funnel plot inspection. Statistical analyses were conducted in Review Manager (RevMan version 5.3, the Cochrane Collaboration, Oxford, England). All the tests were two-sided.
Results
Literature search and meta-analysis databases
We finally screened out 6 relevant articles, including 5971 cataract patients and 4189 controls. Figure 1 presented the flow diagram of searching process. The six studies were conducted in five countries (USA [29], India [30], China [31, 32], Sweden [33], Egypt [34]) and contained three SNPs (one for OGG1: rs1052133; two for EPHA2: rs7543472 and rs11260867). All these articles were written in English, and genetic polymorphisms of OGG1 and EPHA2 were measured by polymerase chain reaction. The genotype distribution in controls were all in accord with HWE (P > 0.05). Tables 1 and 2 listed the essential information of included studies in this meta-analysis. Figure 2 presented the distribution of genotype information.
Fig. 1.
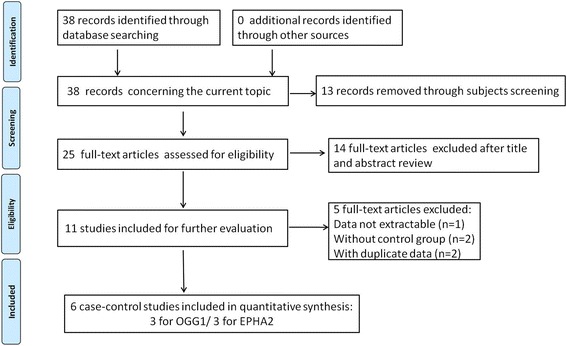
Flow chart of selection process
Table 1.
Main characteristics of included studies in this meta-analysis
| First author | Year | Country | Ethnicity | Mean age | Sample size | Genotype methods | ||
|---|---|---|---|---|---|---|---|---|
| ARCs | Controls | ARCs | Controls | |||||
| Shiels A [29] | 2008 | USA | European | 75.7 ± 7.9 | 74.5 ± 7.6 | 213 | 104 | PCR |
| Sundaresan P [30] | 2012 | India | Asian | – | – | 4198 | 3220 | PCR |
| Zhang Y [31] | 2012 | China | Asian | 67.17 ± 6.92 | 65.77 ± 6.49 | 415 | 386 | PCR-RFLP |
| Jiang SQ [32] | 2013 | China | Asian | 70.9 ± 8.2 | 60.2 ± 5.7 | 504 | 244 | PCR |
| Celojevic D [33] | 2014 | Sweden | European | 72 ± 8.7 | 66 ± 6.9 | 491 | 185 | PCR |
| Gharib AF [34] | 2014 | Egypt | African | 60.33 ± 6.22 | 67.83 ± 5.54 | 150 | 50 | PCR |
ARC age-related cataract, − not available, PCR-RFLP polymerase chain reaction-restriction fragment length polymorphism
Table 2.
Genotype distribution of OGG1 and EPHA2 polymorphisms in cataract cases and controls
| First author | ARCs | Controls | HWE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs1052133 | CC | CG | GG | C | G | CC | CG | GG | C | G | |
| Zhang Y [31] | 222 | 153 | 40 | 597 | 233 | 247 | 120 | 19 | 614 | 158 | 0.68 |
| Jiang SQ [32] | 72 | 222 | 210 | 366 | 642 | 40 | 103 | 101 | 183 | 305 | 0.29 |
| Gharib AF [34] | 77 | 51 | 22 | 205 | 95 | 32 | 16 | 2 | 80 | 20 | 1.00 |
| rs7543472 | TT | TC | CC | T | C | TT | TC | CC | T | C | |
| Shiels A [29] | 146 | 53 | 5 | 345 | 63 | 58 | 41 | 3 | 157 | 47 | 0.40 |
| Sundaresan P [30] | 202 | 1419 | 2569 | 1823 | 6557 | 128 | 1054 | 2028 | 1310 | 5110 | 0.83 |
| Celojevic D [33] | 298 | 163 | 30 | 759 | 223 | 115 | 58 | 12 | 288 | 82 | 0.46 |
| rs11260867 | CC | CG | GG | C | G | CC | CG | GG | C | G | |
| Shiels A [29] | 4 | 43 | 166 | 51 | 375 | 1 | 33 | 68 | 35 | 169 | 0.38 |
| Sundaresan P [30] | 25 | 623 | 3527 | 673 | 7677 | 24 | 448 | 2725 | 496 | 5898 | 0.50 |
| Celojevic D [33] | 317 | 158 | 16 | 792 | 190 | 121 | 58 | 6 | 300 | 70 | 0.96 |
ARC age-related cataract, HWE Hardy-Weinberg Equilibrium
Fig. 2.
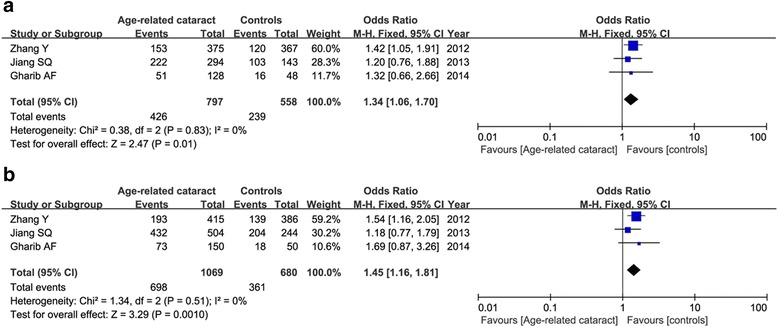
Meta-analysis of the relationship between the OGG1 rs1052133 and age-related cataract under the heterogenous model (a: CG vs. CC) and dominant model (b: GG+CG vs. CC)
Association between OGG1 rs1052133 (C/G) and ARC risk
Table 3 showed the results of test for relationship between OGG1 and EPHA2 polymorphisms and ARC risk based on different genetic models in total and subgroup analysis.
Table 3.
Meta-analysis of OGG1 and EPHA2 polymorphisms in ARC based on different genetic models
| Comparisons | Total | Cortical cataract | Nuclear cataract | PSC | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95 % CI) | P | OR (95 % CI) | P | OR (95 % CI) | P | OR (95 % CI) | P | |
| rs1052133 | ||||||||
| G vs. C | 1.36 (0.99, 1.87) | 0.05 | 1.50 (0.97, 2.32) | 0.07 | 1.23 (0.98, 1.55) | 0.07 | 1.26 (0.94, 1.68) | 0.12 |
| GG vs. CC | 1.88 (0.96, 3.71) | 0.07 | 2.18 (0.90, 5.28) | 0.08 | 1.45 (0.87, 2.44) | 0.16 | 1.61 (0.83, 3.09) | 0.16 |
| CG vs. CC | 1.34 (1.06, 1.70) | 0.01 | 1.43 (1.04, 1.96) | 0.03 | 1.32 (0.93, 1.86) | 0.12 | 1.22 (0.79, 1.88) | 0.37 |
| GG+CG vs. CC | 1.45 (1.16, 1.81) | 0.001 | 1.54 (1.15, 2.07) | 0.004 | 1.37 (0.99, 1.90) | 0.06 | 1.32 (0.88, 1.99) | 0.18 |
| GG vs. CG+CC | 1.65 (0.82, 3.32) | 0.16 | 1.83 (0.78, 4.29) | 0.17 | 1.18 (0.79, 1.75) | 0.43 | 1.30 (0.78, 2.17) | 0.31 |
| rs7543472 | ||||||||
| T vs. C | 1.13 (0.92, 1.38) | 0.25 | 1.16 (1.01, 1.33) | 0.03 | 1.04 (0.75, 1.43) | 0.83 | ||
| TT vs. CC | 1.23 (0.99, 1.52) | 0.06 | 1.52 (1.05, 2.18) | 0.03 | 1.08 (0.84, 1.39) | 0.55 | ||
| TC vs. CC | 1.06 (0.96, 1.17) | 0.22 | 0.99 (0.82, 1.20) | 0.94 | 1.03 (0.92, 1.15) | 0.61 | ||
| TT+TC vs. CC | 1.08 (0.99, 1.19) | 0.10 | 1.06 (0.88, 1.27) | 0.53 | 1.04 (0.93, 1.16) | 0.49 | ||
| TT vs. TC+CC | 1.24 (0.90, 1.72) | 0.18 | 1.54 (1.18, 2.01) | 0.001 | 1.11 (0.72, 1.70) | 0.63 | ||
| rs11260867 | ||||||||
| G vs. C | 0.99 (0.89, 1.11) | 0.88 | 0.89 (0.73, 1.07) | 0.20 | 1.09 (0.84, 1.40) | 0.52 | ||
| GG vs. CC | 1.14 (0.71, 1.83) | 0.59 | 0.62 (0.31, 1.24) | 0.18 | 1.38 (0.76, 2.49) | 0.29 | ||
| CG vs. CC | 1.08 (0.80, 1.47) | 0.60 | 0.66 (0.43, 1.00) | 0.05 | 1.46 (0.95, 2.23) | 0.08 | ||
| GG+CG vs. CC | 1.08 (0.80, 1.45) | 0.62 | 0.66 (0.44, 0.99) | 0.04 | 1.43 (0.94, 2.18) | 0.09 | ||
| GG vs. CG+CC | 1.16 (0.74, 1.81) | 0.52 | 1.19 (0.55, 2.59) | 0.66 | 0.95 (0.83, 1.10) | 0.50 | ||
PSC posterior subcapsular cataract, OR odds ratio, 95 % CI 95 % confidence interval
Three articles concerned the OGG1 variant, including 1069 patients and 680 controls. Although the frequency of G allele (minor) was shown to be higher in ARC cases than that in controls (45.4 versus 35.5 %), our result found that the G allele was not associated with ARC susceptibility in the random-effect model (OR = 1.36, 95 % CI = 0.99–1.87, P = 0.05). This insignificance was also found under the homogenous model and recessive model (P > 0.05). But we detected a significant correlation between OGG1 polymorphism and ARC risk in the heterogenous model (CG vs. CC: OR = 1.34, 95 % CI = 1.06–1.70, P = 0.01) and dominant model (GG+CG vs. CC: OR = 1.45, 95 % CI = 1.16–1.81, P = 0.001) in the fixed-effect model as shown in Fig. 2. Subgroup analysis by phenotypes of cataract showed that only in cortical, not nuclear or posterior subcapsular cataract, OGG1 variant was associated with increased the risk of ARC under the heterogenous model (CG vs. CC: OR = 1.43, 95 % CI = 1.04–1.96, P = 0.03) and dominant model (GG+CG vs. CC: OR = 1.54, 95 % CI = 1.15–2.07, P = 0.004). Figure 3 showed the relative strength of the association between OGG1 rs1052133 and different types of cataract under the heterogenous model.
Fig. 3.
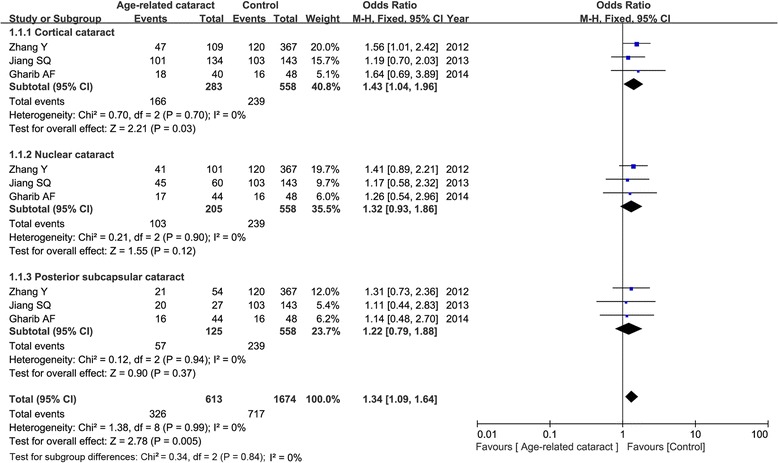
Forest plot of the relative strength of the association between OGG1 rs1052133 and different types of cataract under the heterogenous model (CG vs. CC)
Association between EPHA2 rs7543472 (C/T), rs11260867 (C/G) and ARC risk
Three articles contained 4902 cases and 3509 controls evaluating the association of EPHA2 genetic polymorphisms and ARC occurrence. For rs7543472, our result found that this variant was not associated with ARC risk under any genetic models (T vs. C: OR = 1.13, 95 % CI = 0.92–1.38, P = 0.25; TT vs. CC: OR = 1.23, 95 % CI = 0.99–1.52, P = 0.06; TC vs. CC: OR = 1.06, 95 % CI = 0.96–1.17, P = 0.22; TT+TC vs. CC: OR = 1.08, 95 % CI = 0.99–1.19, P = 0.10; TT vs. TC+CC: OR = 1.24, 95 % CI = 0.90–1.72, P = 0.18). Subgroup analysis by phenotypes of cataract showed a significant relationship between rs7543472 and cortical cataract under the allele model (OR = 1.16, 95 % CI = 1.01–1.33, P = 0.03), homogenous model (OR = 1.52, 95 % CI = 1.05–2.18, P = 0.03) and recessive model (OR = 1.54, 95 % CI = 1.18–2.01, P = 0.001) in the fixed-effect model as shown in Fig. 4. No association was detected between rs7543472 and patients with nuclear cataract under any genetic models.
Fig. 4.
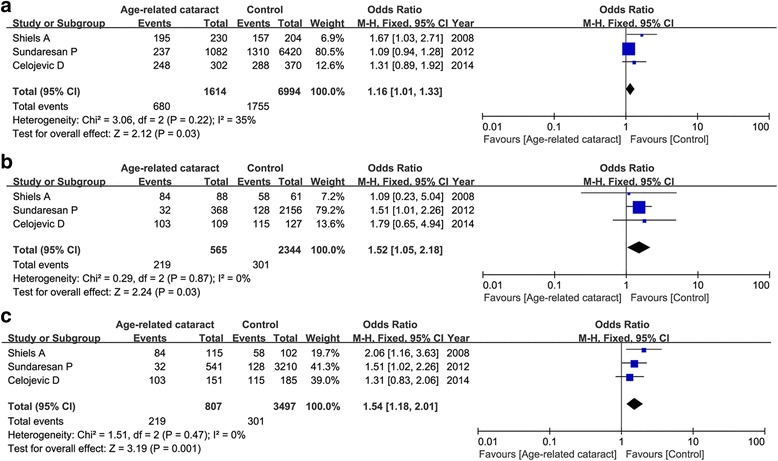
Meta-analysis of correlation of EPHA2 rs7543472 in cortical cataract under the allele model (a: T vs. C), homogenous model (b: TT vs. CC) and recessive model (c: TT vs. TC+CC)
For rs11260867, we did not observe a significant positive correlation between this variant and ARC risk under any genetic models as well (Table 3). Subgroup analysis by phenotypes of cataract showed that this variant was associated with increased the risk of cortical cataract, not nuclear cataract under the heterogenous model (CG vs. CC: OR = 0.66, 95 % CI = 0.43–1.00, P = 0.05) and dominant model (GG+CG vs. CC: OR = 0.66, 95 % CI = 0.44–0.99, P = 0.04) as shown in Fig. 5.
Fig. 5.
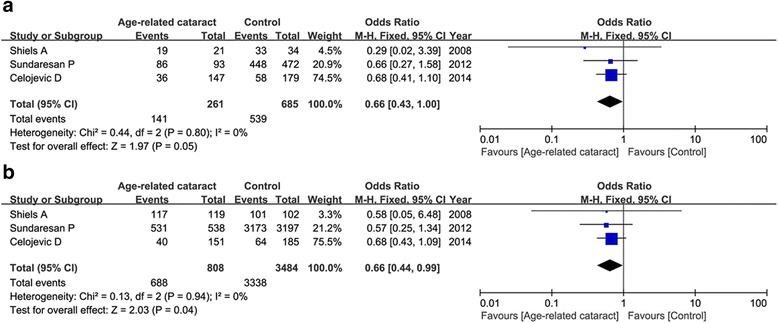
Forest plot of the association between EPHA2 rs11260867 and cortical cataract risk under the heterogenous model (a: CG vs. CC) and dominant model (b: GG+CG vs. CC)
Sensitivity analysis and publication bias evaluation
To confirm whether each included study influences the overall results or not, we successively omitted each single study, respectively. Our result found that the pooled ORs were not significantly changed. The funnel plots were used to evaluate the publication bias. All the plots were found to be roughly symmetrical, indicating no publication bias presented as shown in Fig. 6.
Fig. 6.
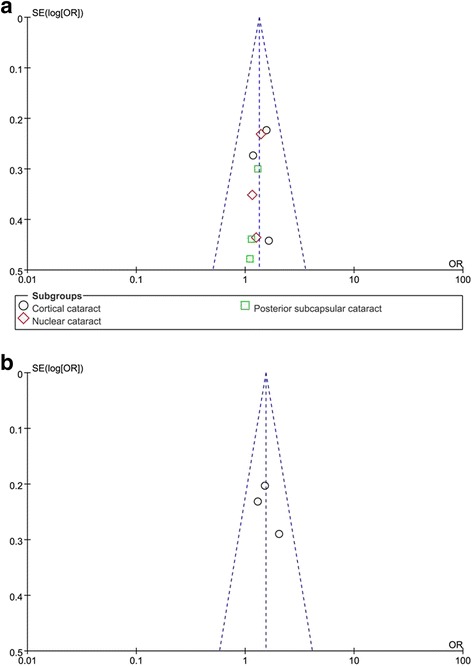
Funnel plot of a: OGG1 rs1052133 under the heterogenous model (CG vs. CC); b: EPHA2 rs7543472 under the recessive model (TT vs. TC+CC) in cataract risk
Discussion
In this meta-analysis, we totally identified six articles concerning three genetic polymorphisms. Our results showed that CG genotype and GG+CG genotype of OGG1 rs1052133 were associated with increased the risk of ARC, in particular with cortical cataract. This significant relationship was not found in EPHA2 polymorphisms (rs7543472 and rs11260867), however, subgroup analysis by phenotypes of cataract showed that only in cortical cataract, the genotypes of rs7543472 under the allele model, homogenous model and recessive model; genotypes of rs11260867 under the heterogenous model and dominant model were associated with ARC risk. This was the first meta-analysis concerning these three SNPs in ARC risk.
Cataract is the single largest contributor to blindness in the world. Age is the major risk factor for cataract. Even though several measures have been identified as a solution for cataract treatment [35, 36], there is no obvious therapeutic benefits, and established medical treatment for better prevention and treatment of cataract was not built [37]. Moreover, this disease has a strong genetic component. Therefore, understanding of genetic polymorphisms within the lens may provide an insight into the process of cataract onset.
OGG1 is involved in multiple vital processes. Recent studies showed that OGG1 was highly expressed in the embryonic brain, and lack of OGG1 might cause severe brain defects in brain integrity, balance and mobility [38]. The acetylation of OGG1 was shown to play an important etiologic role in regulating its function in response to DNA damage [39], and could be one of the mechanisms for ARC development [40]. Xu et al. proved that OGG1 might increase in lens epithelium cells with ARC, and the alteration of OGG1 level was associated with the location and opaque degrees of lens [41]. Wang et al. proved that the reduced OGG1 expression was correlated with hypermethylation of a CpG island of OGG1 in lens cortex of ARC [42]. In addition, OGG1 mutations might delay the repair of oxidative DNA damage, and be associated with increased disease risk [43]. Ali et al. showed that OGG1 mutation may prove to be a good candidate of better diagnosis, treatment, and prevention of breast cancer [44]. Zhang et al. suggested that OGG1 Ser326Cys polymorphism might be associated with increased risk of ARC [31]. While Su et al. did not find a correlation between OGG1 variants and ARC risk in Han Chinese from the Jiangsu Eye Study [45].
EPHA2 is an epithelial cell tyrosine kinase, and was shown to be enriched in adult tissues [46]. It is highly expressed in aggressive human cancers. During tumor progression, EPHA2 receptor can gain ligand-independent pro-oncogenic functions due to Akt activation and reduced ephrin-A ligand engagement [47]. Moreover, EPHA2 can function as a therapeutic target for antibody therapy of cancers and diseases [48, 49]. Dunne et al. found that EPHA2 was a key driver of invasion and migration and a synthetically lethal target in KRASMT colorectal cancer, indicating that EPHA2 was a poor prognostic marker [50]. Kato et al. showed a promising role for EPHA2 as a target for antibody treatment in melanoma and enhanced the therapeutic effect as an agonistic antibody to EPHA2 [51]. Genetic and pharmacological inhibition of EPHA2 induces apoptosis and abrogates tumorigenic growth of tumor cells [52]. Recent studies have identified EPHA2 as a surprisingly abundant plasma membrane component in cells of the ocular lens [53]. Mutations in EPHA2 have been shown to underlie inherited forms of cataract in humans [54, 55]. Common variants in EPHA2 have been associated with the much more prevalent age-related form of cataract. Dave et al. showed that mutations in EPHA2 accounted for 4.7 % of inherited cataract cases in South-Eastern Australia, and a rare variant rs139787163 was potentially associated with increased susceptibility to cataract, providing a link between congenital and age-related cataract [56]. Furthermore, the cytoprotective and antiapoptotic function of EPHA2 in lens epithelial cells was abolished by the functional polymorphisms [52]. These results indicated the potential role of EPHA2 in maintaining lens clarity during aging by promoting cell viability.
Several limitations were presented in this meta-analysis. First of all, the number of included studies for each SNP was small, future large-scale researches with more ethnicities are needed to further evaluate the relationship. Secondly, between-study heterogeneity was presented in several comparisons. Thirdly, we did not conduct the subgroup analysis by ethnicities due to the less data, which should be concerned in the future. Lastly, gene-gene and gene-environment interactions were not addressed in our meta-analysis.
Conclusions
Our results indicated that CG genotype and GG+CG genotype of OGG1 rs1052133 might be risk factor for ARC, especially in cortical cataract risk. The T allele and TT genotype of EPHA2 rs7543472; CG genotype and GG+CG genotype of EPHA2 rs11260867 were associated with cortical cataract risk. Future studies are still required to re-evaluate the results of OGG1 and EPHA2 polymorphisms in ARC risk.
Acknowledgment
None.
Funding
This study was supported by National Natural Science Foundation of China (Grant No.: 81472511).
Availability of data and materials
All data has been shared in the Figures and Tables.
Authors’ contributions
YH made substantial contributions to conception and design of this study; HZ, JZ and ZB made substantial contributions to acquisition of data, or analysis and interpretation of data; HZ, XF and YP drafted the manuscript; YH revised it critically for important intellectual content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have given the final approval of the version to be published.
Competing interests
No conflict of interest exists.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- ARC
Age-related cataract
- BER
Base excision repair
- CI
Confidence interval
- EPHA2
Eph-receptor tyrosine kinase-type A2
- HWE
Hardy-Weinberg equilibrium
- OGG1
8-oxoguanine DNA glycosylase-1
- OR
Odds ratio
- SNP
Single nucleotide polymorphism
References
- 1.Javitt JC, Wang F, West SK. Blindness due to cataract: epidemiology and prevention. Annu Rev Public Health. 1996;17(1):159–177. doi: 10.1146/annurev.pu.17.050196.001111. [DOI] [PubMed] [Google Scholar]
- 2.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2011:bjophthalmol-2011-300539 [DOI] [PubMed]
- 3.Storey P, Munoz B, Friedman D, West S. Racial differences in lens opacity incidence and progression: the Salisbury Eye Evaluation (SEE) study. Invest Ophthalmol Vis Sci. 2013;54:3010–3018. doi: 10.1167/iovs.12-11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunnlaugsdottir E, Arnarsson A, Jonasson F. Five‐year incidence of visual impairment and blindness in older Icelanders: the Reykjavik Eye Study. Acta Ophthalmol. 2010;88(3):358–366. doi: 10.1111/j.1755-3768.2008.01445.x. [DOI] [PubMed] [Google Scholar]
- 5.Chua J, Koh JY, Tan AG, Zhao W, Lamoureux E, Mitchell P, Wang JJ, Wong TY, Cheng C-Y. Ancestry, socioeconomic status, and age-related cataract in Asians: the Singapore Epidemiology of Eye Diseases study. Ophthalmology. 2015;122(11):2169–2178. doi: 10.1016/j.ophtha.2015.06.052. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF5 [DOI] [PMC free article] [PubMed]
- 7.Virgolici B, Popescu L. [Risk factors in cataract] Oftalmologia (Bucharest, Romania: 1990) 2005;50(2):3–9. [PubMed] [Google Scholar]
- 8.Daien V, Le Pape A, Heve D, Carriere I, Villain M. Incidence, risk factors, and impact of age on retinal detachment after cataract surgery in France: a national population study. Ophthalmology. 2015;122(11):2179–2185. doi: 10.1016/j.ophtha.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 9.West S. Epidemiology of cataract: accomplishments over 25 years and future directions. Ophthalmic Epidemiol. 2007;14(4):173–178. doi: 10.1080/09286580701423151. [DOI] [PubMed] [Google Scholar]
- 10.Domalpally A, Danis RP, Chew EY, Clemons TE, Reed S, SanGiovanni JP, Ferris FL., III Evaluation of optimized digital fundus reflex photographs for lens opacities in the age-related eye disease study 2: AREDS2 report 7. Invest Ophthalmol Vis Sci. 2013;54(9):5989. doi: 10.1167/iovs.13-12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman HB, Kiffel S, Weinstock FJ. Cataract surgery and the primary care practitioner. Geriatrics. 2009;64(5):19–22. [PubMed] [Google Scholar]
- 12.Shiels A, Hejtmancik J. Genetics of human cataract. Clin Genet. 2013;84(2):120–127. doi: 10.1111/cge.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith TB, Dun MD, Smith ND, Curry BJ, Connaughton HS, Aitken RJ. The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J Cell Sci. 2013;126(6):1488–1497. doi: 10.1242/jcs.121657. [DOI] [PubMed] [Google Scholar]
- 14.Yuzefovych LV, Kahn AG, Schuler MA, Eide L, Arora R, Wilson GL, Tan M, Rachek LI. Mitochondrial DNA repair through OGG1 activity attenuates breast cancer progression and metastasis. Cancer Res. 2015:canres. 0692.2015 [DOI] [PMC free article] [PubMed]
- 15.Donley N, Jaruga P, Coskun E, Dizdaroglu M, McCullough AK, Lloyd RS. Small molecule inhibitors of 8-oxoguanine DNA glycosylase-1 (OGG1) ACS Chem Biol. 2015;10(10):2334–2343. doi: 10.1021/acschembio.5b00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selin JZ, Lindblad BE, Rautiainen S, Michaëlsson K, Morgenstern R, Bottai M, Basu S, Wolk A. Are increased levels of systemic oxidative stress and inflammation associated with age-related cataract? Antioxid Redox Signal. 2014;21(5):700–704. doi: 10.1089/ars.2014.5853. [DOI] [PubMed] [Google Scholar]
- 17.Kohno T, Shinmura K, Tosaka M, Tani M, Kim S-R, Sugimura H, Nohmi T, Kasai H, Yokota J. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene. 1998;16(25):3219–3225. doi: 10.1038/sj.onc.1201872. [DOI] [PubMed] [Google Scholar]
- 18.Simonelli V, Camerini S, Mazzei F, Van Loon B, Allione A, D’Errico M, Barone F, Minoprio A, Ricceri F, Guarrera S. Genotype–phenotype analysis of S326C OGG1 polymorphism: a risk factor for oxidative pathologies. Free Radic Biol Med. 2013;63:401–409. doi: 10.1016/j.freeradbiomed.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Son AI, Park JE, Zhou R. The role of Eph receptors in lens function and disease. Science China Life Sciences. 2012;55(5):434–444. doi: 10.1007/s11427-012-4318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koshikawa N, Hoshino D, Taniguchi H, Minegishi T, Tomari T, Nam S-O, Aoki M, Sueta T, Nakagawa T, Miyamoto S. Proteolysis of EphA2 converts it from a tumor suppressor to an oncoprotein. Cancer Res. 2015;75(16):3327–3339. doi: 10.1158/0008-5472.CAN-14-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borthakur S, Lee H, Kim S, Wang B-C, Buck M. Binding and function of phosphotyrosines of the Ephrin A2 (EphA2) receptor using synthetic Sterile α Motif (SAM) domains. J Biol Chem. 2014;289(28):19694–19703. doi: 10.1074/jbc.M114.567602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barquilla A, Pasquale EB. Eph receptors and ephrins: therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2015;55:465–487. doi: 10.1146/annurev-pharmtox-011112-140226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tandon M, Vemula SV, Mittal SK. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin Ther Targets. 2011;15(1):31–51. doi: 10.1517/14728222.2011.538682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jun G, Guo H, Klein B, Klein R, Wang JJ, Mitchell P, Miao H, Lee KE, Joshi T, Buck M. EPHA2 is associated with age-related cortical cataract in mice and humans. PLoS Genet. 2009;5(7):e1000584. doi: 10.1371/journal.pgen.1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan W, Hou S, Jiang Z, Hu Z, Yang P, Ye J. Association of EPHA2 polymorphisms and age-related cortical cataract in a Han Chinese population. Mol Vis. 2011;17:1553. [PMC free article] [PubMed] [Google Scholar]
- 26.Park JE, Son AI, Hua R, Wang L, Zhang X, Zhou R. Human cataract mutations in EPHA2 SAM domain alter receptor stability and function. PLoS One. 2012;7(5):e36564. doi: 10.1371/journal.pone.0036564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Luo J, Zhou P, Fan Q, Luo Y, Lu Y. Association of the ephreceptor tyrosinekinase-type A2 (EPHA2) gene polymorphism rs3754334 with age-related cataract risk: a meta-analysis. PLoS One. 2013;8(8):e71003. [DOI] [PMC free article] [PubMed]
- 28.Chylack LT, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu S-Y. The lens opacities classification system III. Arch Ophthalmol. 1993;111(6):831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 29.Shiels A, Bennett TM, Knopf HL, Maraini G, Li A, Jiao X, Hejtmancik JF. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol Vis. 2008;14:2042. [PMC free article] [PubMed] [Google Scholar]
- 30.Sundaresan P, Ravindran RD, Vashist P, Shanker A, Nitsch D, Talwar B, Maraini G, Camparini M, Nonyane BAS, Smeeth L. EPHA2 polymorphisms and age-related cataract in India. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Zhang L, Song Z, Sun DL, Liu HR, Fu SB, Liu DR, Liu P. Genetic polymorphisms in DNA repair genes OGG1, APE1, XRCC1, and XPD and the risk of age-related cataract. Ophthalmology. 2012;119(5):900–906. doi: 10.1016/j.ophtha.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Jiang S, Hu N, Zhou J, Zhang J, Gao R, Hu J, Guan H. Polymorphisms of the WRN gene and DNA damage of peripheral lymphocytes in age-elated cataract in a Han Chinese population. Age. 2013;35(6):2435–44. [DOI] [PMC free article] [PubMed]
- 33.Celojevic D, Abramsson A, Seibt Palmér M, Tasa G, Juronen E, Zetterberg H, Zetterberg M. EPHA2 polymorphisms in Estonian patients with age-related cataract. Ophthalmic Genet. 2014(0):1–5 [DOI] [PubMed]
- 34.Gharib AF, Dabour SA, Etewa RL, Fouad RA. Polymorphisms of DNA repair genes OGG1 and XPD and the risk of age-related cataract in Egyptians. Mol Vis. 2014;20:661. [PMC free article] [PubMed] [Google Scholar]
- 35.Laroche L. Actuality in cataract treatment. La Revue du praticien. 2013;63(1):43–47. [PubMed] [Google Scholar]
- 36.Lin H, Chen W, Luo L, Congdon N, Zhang X, Zhong X, Liu Z, Chen W, Wu C, Zheng D. Effectiveness of a short message reminder in increasing compliance with pediatric cataract treatment: a randomized trial. Ophthalmology. 2012;119(12):2463–2470. doi: 10.1016/j.ophtha.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 37.Boyd S. Preoperative selection for cataract surgery. Recent Trends in Cataract Management. 2013;1:1. [Google Scholar]
- 38.Gu A, Ji G, Yan L, Zhou Y. The 8-oxoguanine DNA glycosylase 1 (ogg1) decreases the vulnerability of the developing brain to DNA damage. DNA Repair. 2013;12(12):1094–1104. doi: 10.1016/j.dnarep.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Scott TL, Rangaswamy S, Wicker CA, Izumi T. Repair of oxidative DNA damage and cancer: recent progress in DNA base excision repair. Antioxid Redox Signal. 2014;20(4):708–726. doi: 10.1089/ars.2013.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang L, Zhao W, Zhang G, Wu J, Guan H. Acetylated 8-oxoguanine DNA glycosylase 1 and its relationship with p300 and SIRT1 in lens epithelium cells from age-related cataract. Exp Eye Res. 2015;135:102–108. doi: 10.1016/j.exer.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Xu B, Kang L, Zhang G, Wu J, Zhu R, Yang M, Guan H. The changes of 8-OHdG, hOGG1, APE1 and Pol β in lenses of patients with age-related cataract. Curr Eye Res. 2014;40(4):378–385. doi: 10.3109/02713683.2014.924148. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Li F, Zhang G, Kang L, Qin B, Guan H. Altered DNA methylation and expression profiles of 8-oxoguanine DNA glycosylase 1 in lens tissue from age-related cataract patients. Curr Eye Res. 2014(0):1–7 [DOI] [PubMed]
- 43.Kershaw RM, Hodges NJ. Repair of oxidative DNA damage is delayed in the Ser326Cys polymorphic variant of the base excision repair protein OGG1. Mutagenesis. 2012:ges012 [DOI] [PubMed]
- 44.Ali K, Mahjabeen I, Sabir M, Mehmood H, Kayani MA. OGG1 mutations and risk of female breast cancer: meta-analysis and experimental data. Dis Markers. 2015;2015. [DOI] [PMC free article] [PubMed]
- 45.Su S, Yao Y, Zhu R, Liang C, Jiang S, Hu N, Zhou J, Yang M, Xing Q, Guan H. The associations between single nucleotide polymorphisms of DNA repair genes, DNA damage, and age-related cataract: Jiangsu Eye Study. Invest Ophthalmol Vis Sci. 2013;54(2):1201–1207. doi: 10.1167/iovs.12-10940. [DOI] [PubMed] [Google Scholar]
- 46.Mori T, Wanaka A, Taguchi A, Matsumoto K, Tohyama M. Differential expressions of the eph family of receptor tyrosine kinase genes (sek, elk, eck) in the developing nervous system of the mouse. Mol Brain Res. 1995;29(2):325–335. doi: 10.1016/0169-328X(94)00263-E. [DOI] [PubMed] [Google Scholar]
- 47.Petty A, Myshkin E, Qin H, Guo H, Miao H, Tochtrop GP, Hsieh J-T, Page P, Liu L, Lindner DJ. A small molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor cell migration in vitro and prostate cancer metastasis in vivo. PLoS One. 2012;7(8):e42120. doi: 10.1371/journal.pone.0042120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinch MS, Zantek ND, Hein PW. EPHA2 as a therapeutic target for cancer. In: Google Patents; 2013
- 49.Xiao Z, Jackson D, Tice DA. EphA2 Immunoconjugate. In: Antibody-Drug Conjugates and Immunotoxins. Springer; 2013. p 241–253
- 50.Dunne PD, McArt DG, Blayney JK, Dasgupta S, Salto-Tellez M, Johnston PG, Van Schaeybroeck S. EpHA2 is an essential driver of invasion and a novel target in KRAS mutant colorectal cancer. Cancer Res. 2014;74(19 Supplement):2079. doi: 10.1158/1538-7445.AM2014-2079. [DOI] [Google Scholar]
- 51.Kato K, Sakamoto A, Kojima T, Hasegawa T, Ikeda S. An agonistic antibody to EphA2 exhibits anti-tumor effect to human melanoma. Cancer Res. 2015;75(15 Supplement):1676. doi: 10.1158/1538-7445.AM2015-1676. [DOI] [Google Scholar]
- 52.Yang J, Li D, Fan Q, Cai L, Qiu X, Zhou P, Lu Y. The polymorphisms with cataract susceptibility impair the EPHA2 receptor stability and its cytoprotective function. J Ophthalmol. 2015;2015. [DOI] [PMC free article] [PubMed]
- 53.Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol Vis. 2009;15:2448. [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang T, Hua R, Xiao W, Burdon KP, Bhattacharya SS, Craig JE, Shang D, Zhao X, Mackey DA, Moore AT. Mutations of the EPHA2 receptor tyrosine kinase gene cause autosomal dominant congenital cataract. Hum Mutat. 2009;30(5):E603–E611. doi: 10.1002/humu.20995. [DOI] [PubMed] [Google Scholar]
- 55.Kaul H, Riazuddin SA, Shahid M, Kousar S, Butt NH, Zafar AU, Khan SN, Husnain T, Akram J, Hejtmancik JF. Autosomal recessive congenital cataract linked to EPHA2 in a consanguineous Pakistani family. Mol Vis. 2010;16:511. [PMC free article] [PubMed] [Google Scholar]
- 56.Dave A, Laurie K, Staffieri SE, Taranath D, Mackey DA, Mitchell P, Wang JJ, Craig JE, Burdon KP, Sharma S. Mutations in the EPHA2 gene are a major contributor to inherited cataracts in South-Eastern Australia. PLoS One. 2013;8(8):e72518. doi: 10.1371/journal.pone.0072518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data has been shared in the Figures and Tables.


