Abstract
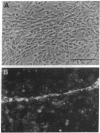
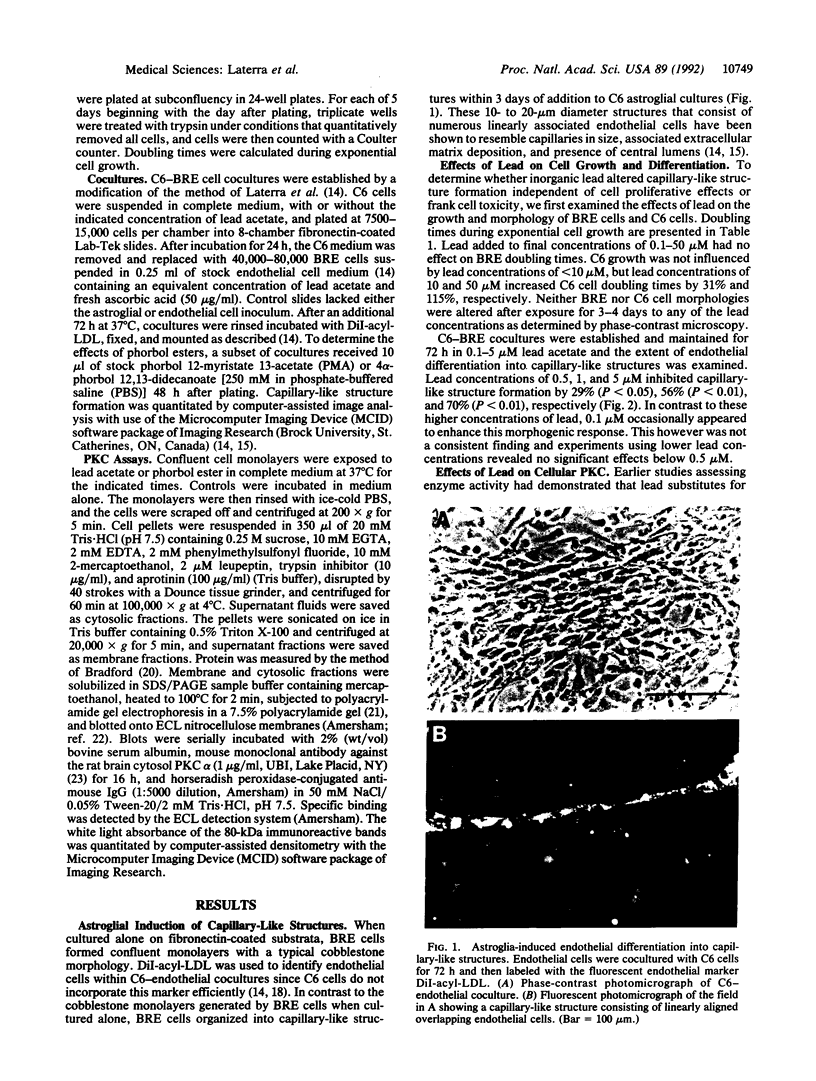
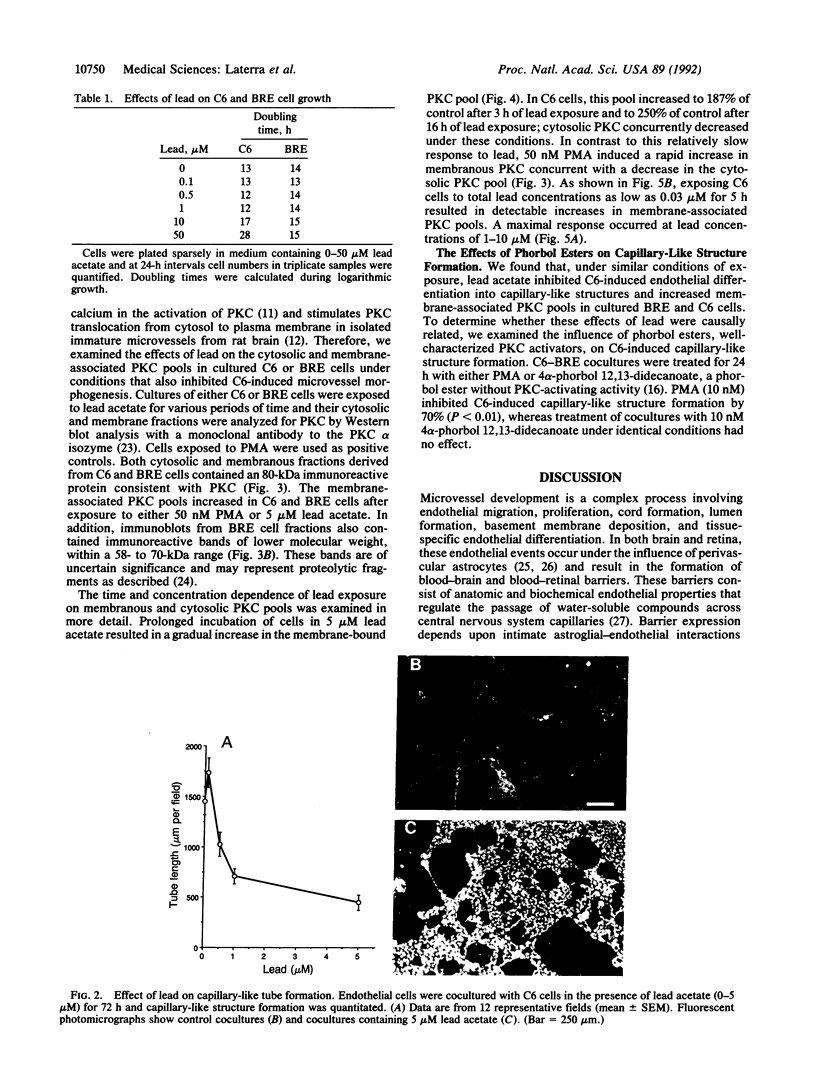
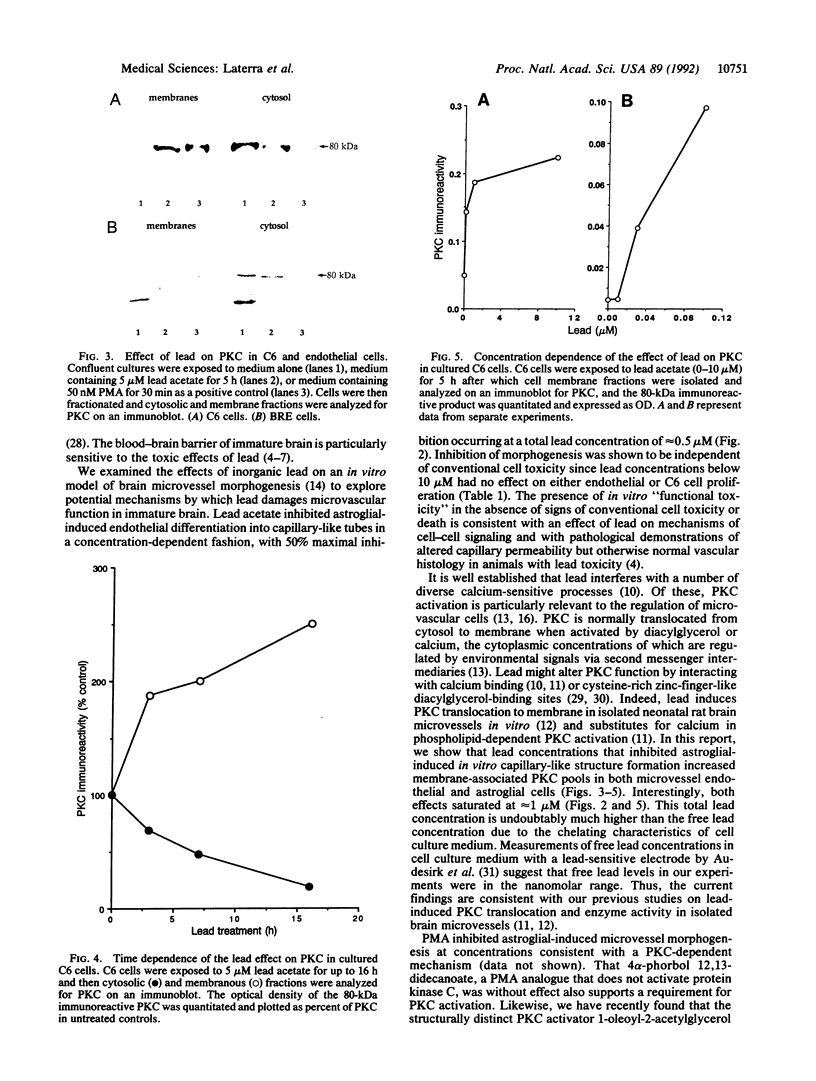
Microvascular endothelial function in developing brain is particularly sensitive to lead toxicity, and it has been hypothesized that this results from the modulation of protein kinase C (PKC) by lead. We examined the effects of inorganic lead on an in vitro model of central nervous system endothelial differentiation in which astroglial cells induce central nervous system endothelial cells to form capillary-like structures. Capillary-like structure formation within C6 astroglial-endothelial cocultures was inhibited by lead acetate with 50% maximal inhibition at 0.5 microM total lead. Inhibition was independent of effects on cell viability or growth. Under conditions that inhibited capillary-like structure formation, we found that lead increased membrane-associated PKC in both C6 astroglial and endothelial cells. Prolonged exposure of C6 cells to 5 microM lead for up to 16 h resulted in a time-dependent increase in membranous PKC as determined by immunoblot analysis. Membranous PKC increased after 5-h exposures to as little as 50 nM lead and was maximal at approximately 1 microM. Phorbol esters were used to determine whether PKC modulation was causally related to the inhibition of endothelial differentiation by lead. Phorbol 12-myristate 13-acetate (10 nM) inhibited capillary-like structure formation by 65 +/- 5%, whereas 4 alpha-phorbol 12,13-didecanoate was without effect. These findings suggest that inorganic lead induces cerebral microvessel dysfunction by interfering with PKC modulation in microvascular endothelial or perivascular astroglial cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audesirk G., Shugarts D., Nelson G., Przekwas J. Organic and inorganic lead inhibit neurite growth in vertebrate and invertebrate neurons in culture. In Vitro Cell Dev Biol. 1989 Dec;25(12):1121–1128. doi: 10.1007/BF02621263. [DOI] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Bowman P. D., Betz A. L., Goldstein G. W. Primary culture of microvascular endothelial cells from bovine retina: selective growth using fibronectin coated substrate and plasma derived serum. In Vitro. 1982 Jul;18(7):626–632. doi: 10.1007/BF02796395. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bressler J. P., Goldstein G. W. Mechanisms of lead neurotoxicity. Biochem Pharmacol. 1991 Feb 15;41(4):479–484. doi: 10.1016/0006-2952(91)90617-e. [DOI] [PubMed] [Google Scholar]
- Bressler J. P., Weingarten D., Kornblith P. L. Glucocorticoid-mediated increases in glycerol phosphate dehydrogenase activity is inhibited by the phorbol ester tumor promoters. J Neurochem. 1985 Oct;45(4):1268–1272. doi: 10.1111/j.1471-4159.1985.tb05552.x. [DOI] [PubMed] [Google Scholar]
- Burns D. J., Bell R. M. Protein kinase C contains two phorbol ester binding domains. J Biol Chem. 1991 Sep 25;266(27):18330–18338. [PubMed] [Google Scholar]
- Bär T., Wolff J. R. The formation of capillary basement membranes during internal vascularization of the rat's cerebral cortex. Z Zellforsch Mikrosk Anat. 1972;133(2):231–248. doi: 10.1007/BF00307145. [DOI] [PubMed] [Google Scholar]
- Clasen R. A., Hartmann J. F., Starr A. J., Coogan P. S., Pandolfi S., Laing I., Becker R., Hass G. M. Electron microscopic and chemical studies of the vascular changes and edema of lead encephalopathy. A comparative study of the human and experimental disease. Am J Pathol. 1974 Feb;74(2):215–240. [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. W., Asbury A. K., Diamond I. Pathogenesis of lead encephalopathy. Uptake of lead and reaction of brain capillaries. Arch Neurol. 1974 Dec;31(6):382–389. doi: 10.1001/archneur.1974.00490420048005. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W., Betz A. L. The blood-brain barrier. Sci Am. 1986 Sep;255(3):74–83. doi: 10.1038/scientificamerican0986-74. [DOI] [PubMed] [Google Scholar]
- Holtzman D., De Vries C., Nguyen H., Jameson N., Olson J., Carrithers M., Bensch K. Development of resistance to lead encephalopathy during maturation in the rat pup. J Neuropathol Exp Neurol. 1982 Nov;41(6):652–663. doi: 10.1097/00005072-198211000-00008. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Arora P. K., Hanna E. E., Huang K. P. Proteolytic degradation of protein kinase C in the phorbol ester-induced interleukin-2 secreting thymoma cells. Arch Biochem Biophys. 1988 Dec;267(2):503–514. doi: 10.1016/0003-9861(88)90057-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laterra J., Goldstein G. W. Astroglial-induced in vitro angiogenesis: requirements for RNA and protein synthesis. J Neurochem. 1991 Oct;57(4):1231–1239. doi: 10.1111/j.1471-4159.1991.tb08284.x. [DOI] [PubMed] [Google Scholar]
- Laterra J., Guerin C., Goldstein G. W. Astrocytes induce neural microvascular endothelial cells to form capillary-like structures in vitro. J Cell Physiol. 1990 Aug;144(2):204–215. doi: 10.1002/jcp.1041440205. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Powers E. A., McGuire J. C., Dong L., Kiley S. C., Jaken S. Monoclonal antibodies specific for type 3 protein kinase C recognize distinct domains of protein kinase C and inhibit in vitro functional activity. J Biol Chem. 1988 Sep 15;263(26):13223–13230. [PubMed] [Google Scholar]
- Lee T. S., Saltsman K. A., Ohashi H., King G. L. Activation of protein kinase C by elevation of glucose concentration: proposal for a mechanism in the development of diabetic vascular complications. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5141–5145. doi: 10.1073/pnas.86.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Ling T. L., Mitrofanis J., Stone J. Origin of retinal astrocytes in the rat: evidence of migration from the optic nerve. J Comp Neurol. 1989 Aug 15;286(3):345–352. doi: 10.1002/cne.902860305. [DOI] [PubMed] [Google Scholar]
- Markovac J., Goldstein G. W. Lead activates protein kinase C in immature rat brain microvessels. Toxicol Appl Pharmacol. 1988 Oct;96(1):14–23. doi: 10.1016/0041-008x(88)90242-6. [DOI] [PubMed] [Google Scholar]
- Markovac J., Goldstein G. W. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 1988 Jul 7;334(6177):71–73. doi: 10.1038/334071a0. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985 Sep;42(2):469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Press M. F. Lead encephalopathy in neonatal Long-Evans rats: morphologic studies. J Neuropathol Exp Neurol. 1977 Jan;36(1):169–193. doi: 10.1097/00005072-197701000-00014. [DOI] [PubMed] [Google Scholar]
- Robertson P. L., Du Bois M., Bowman P. D., Goldstein G. W. Angiogenesis in developing rat brain: an in vivo and in vitro study. Brain Res. 1985 Dec;355(2):219–223. doi: 10.1016/0165-3806(85)90044-6. [DOI] [PubMed] [Google Scholar]
- Rubin L. L., Hall D. E., Porter S., Barbu K., Cannon C., Horner H. C., Janatpour M., Liaw C. W., Manning K., Morales J. A cell culture model of the blood-brain barrier. J Cell Biol. 1991 Dec;115(6):1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström R., Müntzing K., Kalimo H., Sourander P. Changes in the integrity of the blood-brain barrier in suckling rats with low dose lead encephalopathy. Acta Neuropathol. 1985;68(1):1–9. doi: 10.1007/BF00688948. [DOI] [PubMed] [Google Scholar]
- Toews A. D., Kolber A., Hayward J., Krigman M. R., Morell P. Experimental lead encephalopathy in the suckling rat: concentration of lead in cellular fractions enriched in brain capillaries. Brain Res. 1978 May 19;147(1):131–138. doi: 10.1016/0006-8993(78)90777-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. E., Laterra J., Goldstein G. W. Steroid inhibition of neural microvessel morphogenesis in vitro: receptor mediation and astroglial dependence. J Neurochem. 1992 Mar;58(3):1023–1032. doi: 10.1111/j.1471-4159.1992.tb09357.x. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Furumichi T., Furui H., Yokoi T., Ito T., Yamauchi K., Yokota M., Hayashi H., Saito H. Roles of calcium, cyclic nucleotides, and protein kinase C in regulation of endothelial permeability. Arteriosclerosis. 1990 May-Jun;10(3):410–420. doi: 10.1161/01.atv.10.3.410. [DOI] [PubMed] [Google Scholar]