Figure 1. ERK2-directed phosphorylation of EBNA1 S383 in vitro.
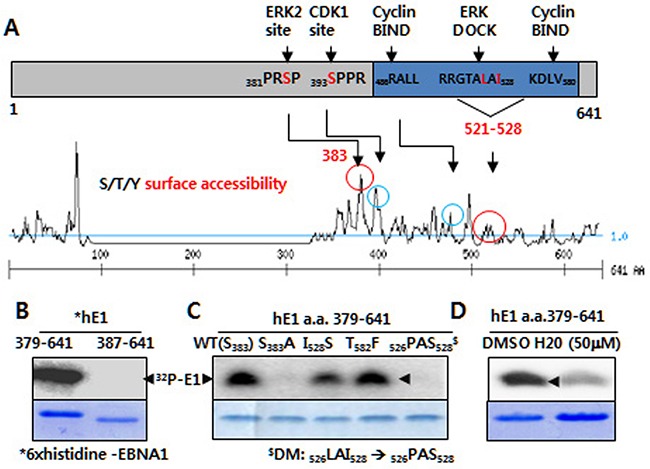
A. Putative ERK and CDK1 phosphorylation and bindings sites (circled) on EBNA1, as predicted by kinase surface accessibility plots. B. In vitro kinase assay reveals ERK2 catalytic activity in the presence of 6× histidine-tagged EBNA1 (hE1) a.a. 379-641, but not hE1 a. a. 387-641. Coomassie blue staining shows EBNA1 levels. C. Absence of ERK2-directed phosphorylation on hE1 S383A and 526PAS528 double mutant (DM), reduced phosphorylation on I528S mutant. D. H20 inhibits ERK-mediated EBNA1 phosphorylation.
