Abstract
Forkhead box (FOX) transcription factor family plays an important role in cancer growth and metastasis. This study aimed to determine the predictive ability of FOX genes in gastric carcinoma. A total of 360 patients with gastric from The Cancer Genome Atlas (TCGA) cohorts were collected in this study. The expression profile of FOX family were obtained from the TCGA RNAseq database. Clinicopathological characteristics, including age, gender, tumor node metastasis (TNM), tumor grade, and overall survival were collected. Univariate and multivariate Cox proportional hazards model were used to assess the risk factors for survival, and the results were further validated in in-house cohort. In the TCGA cohort, FOXO4 (HR = 0.613, 95%CI 0.452–0.832) and FOXD3 (HR = 1.704, 95%CI 1.212–2.397) were shown independently predictive of overall survival in gastric cancer after Cox proportional hazards analysis. The finding was validated in our in-house cohort, which demonstrated that both FOXO4 and FOXD3 were independent predictors for overall survival (FOXO4 high, HR: 0.445, 95%CI 0.277–0.715, P = 0.001, FOXD3 high, HR: 1.927, 95%CI 1.212–3.063, P = 0.006) and disease free survival (FOXO4 high, HR: 0.628, 95%CI 0.420–0.935, P = 0.022, FOXD3 high, HR: 1.698, 95%CI 1.136–2.540, P = 0.010). Collectively, FOX family paly critical roles in gastric cancer, and FOXO4 and FOXD3 were identified as independent prognostic factors for survival outcomes of gastric cancer. Further functional study is needed to understand more about FOX family in gastric cancer.
Keywords: gastric cancer, FOXD3, FOXO4, survival analysis
INTRODUCTION
Gastric cancer represents a major cause of cancer mortality because of its poor prognosis [1]. The only potentially curative treatment for gastric cancer is complete resection (R0). However, despite aggressive surgical intervention, more than 50% of patients undergoing radical resection will experience disease recurrence, usually in the form of metastatic disease. The development of metastatic disease is almost invariably lethal, and it is estimated in 2015 that over 10, 720 individuals in the United States will perish from metastatic gastric cancer in the United States [2]. Thus, a better understanding of the underlying mechanisms that promote the pathogenesis and progression of gastric cancer is urgently needed.
Forkhead box (FOX) transcription factors are a large evolutionarily conserved family of transcriptional regulators that share a highly conserved winged helix DNA binding domain. Outside of this domain, FOX family have diverged into sub-families and incorporated a variety of other domains conferring on them a plethora of functions [3]. FOX family has been recently reported to be involved in various cancer progression and metastasis [4–9], and they have been found operating as both oncogenes and tumor suppressors via a variety of mechanisms. For example, FoxM1c induces EMT by activating the uPA system/Slug pathway [5]. FoxC2 promote epithelial mesenchymal transition (EMT) and colorectal cancer metastasis through the Akt/GSK-3β/Snail Pathway [7]. Loss of FOXA1 is associated with high grade, late stage bladder cancer and increased tumor proliferation [8]. FOXO3a modulates WNT/β-catenin signaling and suppresses EMT in prostate cancer cells [9]. But the potential role of FOX family in gastric cancer and its biological functions on the initiation, progression, and outcome of the disease remains not fully understand.
To describe the characteristics of FOX genes in gastric cancer in depth, we analyzed all FOX family genes in 360 gastric cancer cases from The Cancer Genome Atlas (TCGA) cohort and an additional 226 cases in-house validated cohort. We also examined their associations with clinicopathologic characteristics and survival outcomes of gastric tumors.
RESULTS
Clinical factors in TCGA and validated cohorts
In the TCGA cohort, the median age of these 360 gastric patients was 65, ranging from 30 to 90 years old. Two hundred thirty-four (65.0%) were male patients and 126 (35.0%) were female patients. Gender, age of diagnosis, TNM, tumor grade, are shown in Table 1. The median length of follow-up was 16 months (range, 1 month-124 months) and 226 patients had died at the end of follow-up.
Table 1. Clinical characteristics of patients with gastric in TCGA and validated cohort.
| Variable | TCGA | Validated cohort | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Sex | |||||
| male | 234 | 65.0 | 122 | 54.0 | |
| female | 126 | 35.0 | 104 | 46.0 | |
| Age | 65 | 30–90 | 58 | 19–82 | |
| Primary site | |||||
| Antrum/Distal | 137 | 38.1 | 84 | 37.2 | |
| Cardia/Proximal | 48 | 13.3 | 77 | 34.1 | |
| Fundus/Body | 132 | 36.7 | 44 | 19.5 | |
| Gastroesophageal Junction | 37 | 10.3 | 21 | 9.3 | |
| Unspecific | 6 | 1.7 | 0 | 0 | |
| Grade | |||||
| G1/G2 | 133 | 36.9 | 96 | 42.5 | |
| G3 | 218 | 60.6 | 126 | 55.8 | |
| Gx | 9 | 2.5 | 4 | 1.8 | |
| T stage | |||||
| T1 | 17 | 4.7 | 3 | 1.3 | |
| T2 | 70 | 19.4 | 32 | 14.2 | |
| T3 | 167 | 46.4 | 100 | 44.2 | |
| T4 | 105 | 29.2 | 91 | 40.3 | |
| Tx | 1 | 0.3 | 0 | 0 | |
| N stage | |||||
| N0 | 113 | 31.4 | 62 | 27.4 | |
| N1 | 94 | 26.1 | 50 | 22.1 | |
| N2 | 72 | 20.0 | 50 | 22.1 | |
| N3 | 75 | 20.8 | 64 | 28.3 | |
| Nx | 6 | 1.7 | 0 | 0 | |
| M stage | |||||
| M0 | 328 | 91.1 | 226 | 100 | |
| M1 | 18 | 5.0 | 0 | 0 | |
| Mx | 14 | 3.9 | 0 | 0 | |
In the validated cohort, the median age of these 226 gastric cancer patients was 58, ranging from 19 to 82 years old. One hundred twenty-two (54.0%) were male and 104 (46.0%) were female patients. The expression levels of FOX family genes (FOXD3, FOXO4) in this cohorts were nearly normal distributed (data not shown); therefore, we divided the two cohorts into low or high expression groups according to median expression level. At last follow up, ninety-nine patients were relapsed and 76 were died. The median follow-up time of this cohort was 32 months. The characteristics of the samples are shown in Table 1.
FOXD3 and FOXO4 were independent prognostic factors for OS in the TCGA cohort
In univariate Cox proportion hazard ratio analysis, age, tumor(T) stage, Node(N) stage, metastasis(M) stage, FOXD3, FOXO4 and FOXS1 expression were significantly associated with prognosis in terms of OS of patients with gastric cancer in the TCGA cohorts (p < 0.05, Table 2). A reduced model was used in the multivariate Cox analysis, which means only variables that were significantly correlated with prognosis in univariate Cox proportion hazard ratio (HR) analysis were included in the next step. Multivariate analysis after adjustment for all the potential prognostic factors demonstrated that age (HR = 1.036, 95% CI 1.016–1.055, P < 0.001), T stage (HR = 1.326, 95% CI 1.028–1.709, P = 0.030), N stage (HR = 1.271, 95% CI 1.077–1.500, P = 0.005), FOXD3 (HR = 1.704, 95% CI 1.212–2.397, P = 0.002), and FOXO4 (HR = 0.613, 95% CI 0.452–0.832, P = 0.002) were independent predictors of OS (Table 2).
Table 2. Univariate and multivariate Cox proportional hazards analysis of FOX gene expression and overall survival for patients with gastric cancer in the TCGA cohort.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Factor | HR (95% CI) | P | HR (95% CI) | P |
| Gender | 0.681 (0.457–1.016) | 0.060 | ||
| Age | 1.020 (0.585–1.792) | 0.014 | 1.036 (1.016–1.055) | < 0.001 |
| T category | 1.375 (1.094–1.727) | 0.007 | 1.326 (1.028–1.709) | 0.030 |
| N stage | 1.300 (1.113–1.518) | 0.001 | 1.271 (1.077–1.500) | 0.005 |
| M stage | 1.590 (1.132–2.234) | 0.007 | 1.384 (0.978–1.959) | 0.066 |
| Grade | 1.313 (0.933–1.848) | 0.119 | ||
| Tumor location | 0.971 (0.828–1.139) | 0.721 | ||
| FOXA1 | 0.985 (0.870–1.116) | 0.815 | ||
| FOXA2 | 0.979 (0.864–1.111) | 0.746 | ||
| FOXA3 | 0.884 (0.773–1.013) | 0.078 | ||
| FOXC1 | 0.986 (0.845–1.152) | 0.862 | ||
| FOXC2 | 1.139 (0.899–1.442) | 0.282 | ||
| FOXD1 | 0.955 (0.824–1.106) | 0.536 | ||
| FOXD2 | 0.818 (0.640–1.044) | 0.107 | ||
| FOXD3 | 1.419 (1.007–1.999) | 0.045 | 1.704 (1.212–2.397) | 0.002 |
| FOXD4 | 0.564 (0.284–1.117) | 0.101 | ||
| FOXF1 | 1.066 (0.923–1.239) | 0.370 | ||
| FOXF2 | 0.990 (0.848–1.157) | 0.903 | ||
| FOXH1 | 1.067 (0.861–1.323) | 0.552 | ||
| FOXI1 | 0.694 (0.413–1.164) | 0.167 | ||
| FOXJ1 | 0.971 (0.866–1.089) | 0.616 | ||
| FOXJ2 | 0.980 (0.668–1.438) | 0.919 | ||
| FOXJ3 | 0.897 (0.613–1.313) | 0.575 | ||
| FOXK1 | 1.001 (0.751–1.336) | 0.989 | ||
| FOXK2 | 0.972 (0.657–1.437) | 0.887 | ||
| FOXL1 | 1.138 (0.882–1.468) | 0.320 | ||
| FOXL2 | 0.998 (0.639–1.559) | 0.994 | ||
| FOXM1 | 1.000 (0.835–1.197) | 0.998 | ||
| FOXN2 | 0.948 (0.665–1.350) | 0.767 | ||
| FOXN3 | 1.179 (0.913–.522) | 0.208 | ||
| FOXO1 | 1.008 (0.753–1.349) | 0.958 | ||
| FOXO3 | 1.174 (0.891–1.547) | 0.255 | ||
| FOXO4 | 0.681 (0.513–0.904) | 0.008 | 0.613 (0.452–0.832) | 0.002 |
| FOXP1 | 1.018 (0.751–1.380) | 0.910 | ||
| FOXP2 | 1.144 (0.961–1.362) | 0.131 | ||
| FOXP3 | 0.807 (0.629–1.035) | 0.092 | ||
| FOXP4 | 0.938 (0.747–1.177) | 0.581 | ||
| FOXQ1 | 0.919 (0.817–1.035) | 0.165 | ||
| FOXS1 | 1.219 (1.008–1.474) | 0.041 | 1.180 (0.957–1.455) | 0.121 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Bold type indicates statistical significance.
FOXD3 and FOXO4 expressions were prognostic factors for OS and DFS in the validated cohort
These results should be treated with caution because they could be biased by confounding factors that were not specified in TCGA database, such as lymphovascular invasion, perineural invasion and quality of surgery (palliative resection or radical resection). To evaluate the reliability of TCGA results, we validated the results in 226 in-house eligible patients. Patient demographics and pathological features are summarized in Table 1. Likewise, we divided the cohort into low- and high expression groups according to median expression level.
χ2 tests demonstrated that FOXO4 mRNA expression level was inversely correlated with T stage (P < 0.01), N stage (P = 0.027), while FOXD3 expression was positively correlated with T stage (P = 0.043) (Supplementary Table S1). Five year OS and DFS were 61.5%, 37.3% and 56.5%, 34.6% for FOXO4 high and low expression, low FOXO4 expression was associated with poor prognosis for both OS (log-rank test, p = 0.001) and DFS (log-rank test, p = 0.022), 5-year OS and DFS were 43.1%, 55.9% and 37.2% and 46.7% for low and high expression of FOXD3, high level of FOXD3 expression was correlated with poor prognosis for OS (log-rank test, p = 0.006) and DFS (log-rank test, p = 0.010) (Table 4). The Kaplan–Meier curves are shown in Figure 1.
Table 4. Univariate and multivariate Cox proportional hazards analysis of FOX gene expression and disease free survival for patients with gastric cancer in the validated cohort.
| Factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | 0.832 (0.559–1.238) | 0.365 | ||
| Age | 1.268 (0.853–1.885) | 0.240 | ||
| T category | 1.827 (1.347–2.478) | < 0.001 | 1.374 (0.956–1.975) | 0.086 |
| N stage | 1.454 (1.223–1.728) | < 0.001 | 1.306 (1.059–1.610) | 0.012 |
| Grade | 1.829 (1.220–1.743) | 0.003 | 1.608 (1.059–2.441) | 0.026 |
| Lymphovascular invasion | 1.595 (1.049–2.424) | 0.029 | 1.719 (1.121–2.638) | 0.013 |
| Perineural invasion | 2.137 (1.390–3.285) | 0.001 | 1.602 (1.029–2.493) | 0.037 |
| Tumor location | 1.116 (0.938–1.328) | 0.216 | ||
| FOXO4 | 0.628 (0.420–0.935) | 0.022 | 0.451 (0.295–0. 691) | < 0.001 |
| FOXD3 | 1.698 (1.136–2.540) | 0.010 | 1.966 (1.282–3.014) | 0.002 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Bold type indicates statistical significance.
Figure 1. Influence of FOXO4 and FOXD3 expression patterns on overall survival and disease-free survival by Kaplan-Meier analyses in validated cohort.
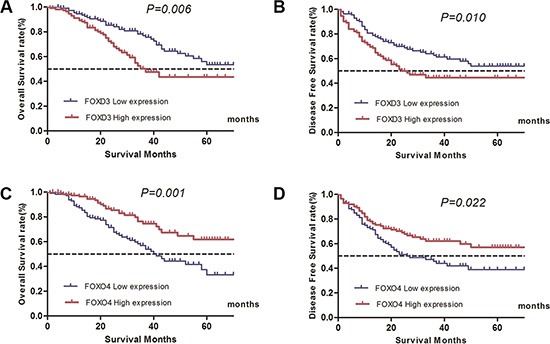
(A) FOXD3, OS: χ2 = 7.920, P = 0.006; (B) FOXD3, DFS: χ2 = 6.869, P = 0.010; (C) FOXO4, OS: χ2 = 11.786, P = 0.001; (D) FOXO4, DFS: χ2 = 5.378, P = 0.022.
Besides, in univariate Cox proportion hazard ratio analysis, tumor stage, N stage, tumor grade, present of lymphovascular invasion and perineural invasion were all significantly associated with poor prognosis in terms of OS and DFS (P < 0.05, Tables 3, 4). Multivariate analysis after adjustment for all the potential prognostic factors indicated that FOXO4 and FOXD3 expression level were the two strong predictors of OS (FOXO4 high, HR: 0.281, 95% CI 0.168–0.469, P < 0.001, FOXD3 high, HR: 2.576, 95% CI 1.553–4.274, P < 0.001) and DFS (FOXO4 high, HR: 0.451, 95% CI 0.295–0.691, P < 0.001, FOXD3 high, HR: 1.966, 95% CI 1.282–3.014, P = 0.002) (Tables 3, 4).
Table 3. Univariate and multivariate Cox proportional hazards analysis of FOX gene expression and overall survival for patients with gastric cancer in the validated cohort.
| Factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | 0.750 (0.475–1.183) | 0.216 | ||
| Age | 1.238 (0.789–1.945) | 0.353 | ||
| T category | 1.735 (1.258–2.393) | 0.001 | 1.215 (0.801–1.843) | 0.359 |
| N stage | 1.559 (1.272–1.909) | < 0.001 | 1.373 (1.057–1.784) | 0.017 |
| Grade | 1.737 (1.096–2.751) | 0.019 | 1.705 (1.052–2.762) | 0.030 |
| Lymphovascular invasion | 1.808 (1.137–2.875) | 0.012 | 2.174 (1.348–3.506) | 0.001 |
| Perineural invasion | 1.930 (1.181–3.154) | 0.009 | 1.384 (0.837–2.290) | 0.206 |
| Tumor location | 0.170 (0.962–1.462) | 0.116 | ||
| FOXO4 | 0.445 (0.277–0.715) | 0.001 | 0.281 (0.168–0.469) | < 0.001 |
| FOXD3 | 1.927 (1.212–3.063) | 0.006 | 2.576 (1.553–4.274) | < 0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Bold type indicates statistical significance.
DISCUSSION
In this study, to our knowledge, for the first time, we comprehensive demonstrated that FOX family correlated with OS and DFS of gastric cancer patients. Members of this family, especially FOXO4 and FOXD3, are two independent prognostic factors for OS and DFS of gastric cancer patients.
FOX proteins are a family of transcription factors that play important roles in regulating the expression of genes involved in cell growth, proliferation, differentiation, and longevity. Many FOX proteins are important to embryonic development [10, 11]. FOX proteins have pioneering transcription activity by being able to bind condensed chromatin during cell differentiation processes [12]. Despite the highly conserved FOX DNA-binding domain, Fox protein regulation and function vary significantly between families [3]. For examples, ectopic expression of the FOXC2 accelerates the development, proliferation and growth of tumors in colorectal cancer [7]. By contrast, activation of the FoxO family of proteins is associated with cell cycle arrest and the induction of apoptosis [13, 14]. Given that FOX family genes control these essential developmental and homeostatic processes, it is not surprise that a loss or gain of Fox function can alter cell fate and lead to tumorigenesis. Despite the fact that our knowledge of FOX transcription factors is still in its infancy, several FOX subfamilies such as FOXA, FOXC, FOXM, FOXP, and FOXO have been linked to tumorigenesis and the progression of some cancers [3]. Here, we investigated the relevant of FOX genes and gastric cancer comprehensively.
The FOXO transcription factor family contains three members in mammalian cells, including FOXO1, FOXO3, and FOXO4. FOXO family members play important roles in cell cycle progression [14, 15], apoptosis [13], oxidative stress [14], DNA repair [16] and drug sensitivity [17]. Importantly. Loss of FOXO function has been observed in prostate cancer [18], non-small lung cancer [19], nasopharyngeal carcinoma [20], and breast cancer [21]. Recently study indicated that FOXO4 is down-regulated and inhibits tumor proliferation and metastasis in gastric cancer [22]. In light of these important studies, it is likely that FOXO4 has a tumor suppressive function that is inactivated in tumorigenesis. Our study give a new insight of FOXO4 gene functional role in gastric cancer and found it was an important prognostic biomarker in gastric.
FOXD3, one member of the FOXD transcription factor family, is originally identified in embryonic stem cells [23] and plays crucial roles in the neural crest development and stem cell biology through specifying the cell lineage [24, 25]. FOXD3 knockout results in early embryonic death in mice [24]. These indicate that FOXD3 plays a primary role in embryonic development, and it is interesting to investigate the potential roles of FOXD3 in the tumors. To date, the results seems controversies, previous study indicate that FOXD3 exhibits tumor suppressive activity that affects the growth, aggressiveness and angiogenesis of neuroblastoma [26]. Promoter hypermethylation could slicing FOXD3 expression and significantly promotor gastric cancer cell proliferation and invasion [27]. However, in mutant B-RAF melanoma cells, adaptive upregulation of FOXD3 can cause resistance to PLX4032/4720 (a target therapy regent)-induced cell death. In our study, the FOXD3 expression level was determined at transcriptional level, as the RNAseq in TCGA database and qRT-PCR analysis in validated database, we confirmed low FOXD3 expression was favorable prognostic factor in gastric. Our results seems controversies with previous functional study in vitro [27], and it will deserved further study.
A major strength of this study is that the information was obtained from two independent populations with a relative long-time follow up, but there are certain limitations. The prognosis of gastric is affected by many factors such as patients' immune status, surgical techniques, and response to adjuvant therapy, so biomarkers from a single gene family is not enough. Second, the data from TCGA was public available and patients' number was large, which make our results reliable, however, for the complicated interactive network and signaling pathways in vivo, the FOX family genes which were not validated as biomarkers in this study may also play critical role in gastric cancer, and it need further study. In addition, information regarding disease recurrence and metastasis is unavailable in the TCGA cohort, and therefore only OS could be evaluated.
In conclusion, FOX family paly critical roles in gastric cancer, and FOXO4 and FOXD3 were identified as independent prognostic factors for survival outcomes of gastric cancer. Further functional study is needed to understand more about FOX family in gastric cancer.
MATERIALS AND METHODS
Patients and samples
This study received Institutional Review Board approval from Yangzhou No.1 people's hospital. Written informed consent was obtained from all subjects. The methods were carried out in accordance with the approved guidelines.
For the TCGA cohort, FOX genes expression and clinical data of TCGA database are available from the website of Cancer Genomics Browser of University of California Santa Cruz (UCSC) (https://genome-cancer.ucsc.edu/). Eleven members of the FOX family were excluded from the study for extremely low mRNA copy number (FOXB1, FOXE1, FOXE3, FOXI2, FOXN1, FOXN4), or the copy number was 0 in more than 2/3 patients (FOXB2, FOXG1, FOXI3, FOXR1, FOXR2). As results, thirty-two members of the FOX family are included in the database as is shown in Table 1. Other inclusion criteria were: patients with no pretreatment, with fully characterized tumors and intact overall survival (OS) information. Follow-up was completed on Dec 21, 2014.
The validated cohort consists of 226 patients with histologically confirmed invasive gastric cancer who had undergone radical surgical resection between January 1, 2003 and December 31, 2009. All patients received no pretreatment, and only patients without any evidence of metastasis at the time of diagnosis were enrolled. Demographic and clinical characteristics, such as age, sex, age at initial diagnosis, and stage at diagnosis (tumor, node, metastasis [TNM] classification) were obtained from electronic records and summarized in Table 1.
RNA extraction, reverse transcription, and qRT-PCR analysis
Total RNA was isolated from 226 gastric cancer samples using TRIzol® reagent (15596026, Invitrogen). A PrimeScript™ RT Master Mix (Perfect Real Time) kit (RR036A, Takara) was used to synthesize first-strand cDNA from total RNA. After that, SYBR Green real-time PCR assays were performed using an ABI 7900HT (Applied Biosystems, USA). The expression level of RNA was normalized to the level of GAPDH. The primers for RT-PCR analysis were synthesized by Huagene (Shanghai, People's Republic of China), the sequences of which are shown in Supplementary Table S2.
Statistical analysis
All statistical analysis was performed using SPSS software (version 21.0, IBM Corp., Armonk, NY, USA). Independent t-tests (for continuous variables) and Pearson's χ2 tests (for categorical variables) were used. The cut-point of FOX genes mRNA expression was defined as the median. The overall survival (OS) was defined as the time from surgery to death due to any cause. The disease-free survival (DFS) was defined as the time of surgery to tumor recurrence, progression or metastasis in localized gastric cancer. The difference in survival between the groups was compared by the log rank test. Variables that seemed to be significantly associated with survival on univariate analysis were entered into multivariate analysis, which was performed with Cox proportional hazard model. Patients without events or death were recorded as censored at the time of last follow-up. A two-sided P-value < 0.05 was considered to indicate statistical significance.
SUPPLEMENTARY MATERIALS TABLES
ACKNOWLEDGMENTS AND FUNDING
We would like to thank the TCGA database for its open access.
We thanked doctor Rongliang Shi, at Department of General Surgery, Minhang hospital, Minhang district, Shanghai, China, for technical help and data preparing for the study.
Footnotes
CONFLICTS OF INTEREST
None of the authors have any Conflicts of Interest to declare.
Authors' contributions
JL, ZHJ, XY and JDT conceived of and designed the study. JL, ZHJ and FH performed the analyses. FH, ZHJ, SXL and XY prepared all figures and tables. JL and ZHJ wrote the main manuscript. All authors reviewed the manuscript.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nature reviews Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 4.Ni D, Ma X, Li HZ, Gao Y, Li XT, Zhang Y, Ai Q, Zhang P, Song EL, Huang QB, Fan Y, Zhang X. Downregulation of FOXO3a promotes tumor metastasis and is associated with metastasis-free survival of patients with clear cell renal cell carcinoma. Clin Cancer Res. 2014;20:1779–1790. doi: 10.1158/1078-0432.CCR-13-1687. [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Xie D, Cui J, Li Q, Gao Y, Xie K. FOXM1c promotes pancreatic cancer epithelial-to-mesenchymal transition and metastasis via upregulation of expression of the urokinase plasminogen activator system. Clin Cancer Res. 2014;20:1477–1488. doi: 10.1158/1078-0432.CCR-13-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollier BG, Tinnirello AA, Werden SJ, Evans KW, Taube JH, Sarkar TR, Sphyris N, Shariati M, Kumar SV, Battula VL, Herschkowitz JI, Guerra R, Chang JT, et al. FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73:1981–1992. doi: 10.1158/0008-5472.CAN-12-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Wu J, Wei P, Xu Y, Zhuo C, Wang Y, Li D, Cai S. Overexpression of forkhead Box C2 promotes tumor metastasis and indicates poor prognosis in colon cancer via regulating epithelial-mesenchymal transition. American journal of cancer research. 2015;5:2022–2034. [PMC free article] [PubMed] [Google Scholar]
- 8.DeGraff DJ, Clark PE, Cates JM, Yamashita H, Robinson VL, Yu X, Smolkin ME, Chang SS, Cookson MS, Herrick MK, Shariat SF, Steinberg GD, Frierson HF, et al. Loss of the urothelial differentiation marker FOXA1 is associated with high grade, late stage bladder cancer and increased tumor proliferation. PLoS One. 2012;7:e36669. doi: 10.1371/journal.pone.0036669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Yin J, Wang H, Jiang G, Deng M, Zhang G, Bu X, Cai S, Du J, He Z. FOXO3a modulates WNT/beta-catenin signaling and suppresses epithelial-to-mesenchymal transition in prostate cancer cells. Cellular signalling. 2015;27:510–518. doi: 10.1016/j.cellsig.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Tuteja G, Kaestner KH. SnapShot: forkhead transcription factors I. Cell. 2007;130:1160. doi: 10.1016/j.cell.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Tuteja G, Kaestner KH. Forkhead transcription factors II. Cell. 2007;131:192. doi: 10.1016/j.cell.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Giraud P, Morvan E, Claude L, Mornex F, Le Pechoux C, Bachaud JM, Boisselier P, Beckendorf V, Morelle M, Carrere MO, Centers SS. Respiratory gating techniques for optimization of lung cancer radiotherapy. Journal of thoracic oncology. 2011;6:2058–2068. doi: 10.1097/JTO.0b013e3182307ec2. [DOI] [PubMed] [Google Scholar]
- 13.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 14.Kops GJ, Medema RH, Glassford J, Essers MA, Dijkers PF, Coffer PJ, Lam EW, Burgering BM. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Molecular and cellular biology. 2002;22:2025–2036. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 16.Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, Jr, DiStefano PS, Chiang LW, Greenberg ME. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 17.Lupertz R, Chovolou Y, Unfried K, Kampkotter A, Watjen W, Kahl R. The forkhead transcription factor FOXO4 sensitizes cancer cells to doxorubicin-mediated cytotoxicity. Carcinogenesis. 2008;29:2045–2052. doi: 10.1093/carcin/bgn184. [DOI] [PubMed] [Google Scholar]
- 18.Su B, Gao L, Baranowski C, Gillard B, Wang J, Ransom R, Ko HK, Gelman IH. A genome-wide RNAi screen identifies FOXO4 as a metastasis-suppressor through counteracting PI3K/AKT signal pathway in prostate cancer. PLoS One. 2014;9:e101411. doi: 10.1371/journal.pone.0101411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu MM, Mao GX, Liu J, Li JC, Huang H, Liu YF, Liu JH. Low expression of the FoxO4 gene may contribute to the phenomenon of EMT in non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:4013–4018. doi: 10.7314/apjcp.2014.15.9.4013. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Tang Y, Wang J, Yan Z, Xu R. miR-421 induces cell proliferation and apoptosis resistance in human nasopharyngeal carcinoma via downregulation of FOXO4. Biochem Biophys Res Commun. 2013;435:745–750. doi: 10.1016/j.bbrc.2013.05.056. [DOI] [PubMed] [Google Scholar]
- 21.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 22.Su L, Liu X, Chai N, Lv L, Wang R, Li X, Nie Y, Shi Y, Fan D. The transcription factor FOXO4 is down-regulated and inhibits tumor proliferation and metastasis in gastric cancer. BMC Cancer. 2014;14:378. doi: 10.1186/1471-2407-14-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada DA, Fletcher CF, Jenkins NA, Copeland NG, Klemsz M, Hromas R. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J Biol Chem. 1996;271:23126–23133. doi: 10.1074/jbc.271.38.23126. [DOI] [PubMed] [Google Scholar]
- 24.Tompers DM, Foreman RK, Wang Q, Kumanova M, Labosky PA. Foxd3 is required in the trophoblast progenitor cell lineage of the mouse embryo. Developmental biology. 2005;285:126–137. doi: 10.1016/j.ydbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Teng L, Mundell NA, Frist AY, Wang Q, Labosky PA. Requirement for Foxd3 in the maintenance of neural crest progenitors. Development. 2008;135:1615–1624. doi: 10.1242/dev.012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D, Mei H, Qi M, Yang D, Zhao X, Xiang X, Pu J, Huang K, Zheng L, Tong Q. FOXD3 is a novel tumor suppressor that affects growth, invasion, metastasis and angiogenesis of neuroblastoma. Oncotarget. 2013;4:2021–2044. doi: 10.18632/oncotarget.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng AS, Li MS, Kang W, Cheng VY, Chou JL, Lau SS, Go MY, Lee CC, Ling TK, Ng EK, Yu J, Huang TH, To KF, et al. Helicobacter pylori causes epigenetic dysregulation of FOXD3 to promote gastric carcinogenesis. Gastroenterology. 2013;144:122–133. doi: 10.1053/j.gastro.2012.10.002. e129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


