Abstract
Although non-small cell lung cancer (NSCLC) with malignant pleural effusion (M1a) is generally contraindicated for surgery, several reports have demonstrated favorable prognosis. This study aimed to describe the results of surgical intervention in this disease. In this retrospective study, we evaluated NSCLC patients with ipsilateral malignant pleural effusion selected from Surveillance Epidemiology and End-Results database (SEER). Primary tumor resection was compared to no tumor resection in the overall survival (OS) and lung cancer-specific survival (LCSS). Multivariate analyses and propensity score matching were applied to compare the two groups. The study included 2,217 eligible patients. Primary tumor resection group was significantly associated with better OS and LCSS compared to no tumor resection group (the median survival time (MST), 20 vs 7 months; OS, p <0.001; LCSS, p <0.001). Multivariable analyses indicated that no primary tumor resection was associated with decreased OS (Hazard Ratio (HR), 2.136; p<0.001) and LCSS (HR, 2.053; p<0.001). In propensity score-matched pairs, better OS and LCSS were further validated in patients with ipsilateral malignant pleural effusion who underwent primary tumor resection compared to no tumor resection (MST, 20 vs 6 months; OS, p <0.001; LCSS, p <0.001). Similarly, multivariable analyses also indicated that no primary tumor resection was associated with decreased OS (HR, 2.309; p <0.001) and LCSS (HR, 2.301; p <0.001) for patients with ipsilateral malignant pleural effusion. In conclusion, the prognosis after contraindicated surgery of NSCLC patients with malignant pleural effusion (M1a) may be better than expected. Thus, subsequent studies should aim to identify patients who could benefit from surgery.
Keywords: lung cancer, surgery, malignant pleural effusion, prognosis, surveillance epidemiology and end-results database
INTRODUCTION
Malignant pleural effusion, as one kind of non-small-cell lung cancer (NSCLC) with pleural dissemination, has been proved to have poor outcomes, and it is generally contraindicated for operations [1–3]. The International Association for the Study of Lung Cancer (IASLC) Staging Project had stated that the median survival time (MST) and the 5-year survival rate of patients with pleural dissemination were 8 months and 2%, respectively [3]. Therefore, NSCLC with malignant pleural effusion was staged as IV (M1a) in the new staging system of the Union for International Cancer Control [4].
Lim and colleagues reported that positive pleural lavage cytology during surgical resection is an independent predictor factor in predicting worse survival of NSCLC patients with a resectable-stage tumor [5]. Ryu and colleagues founded that prognostic impact effect of minimal pleural effusion was higher in early rather than advanced stages of NSCLC [6]. However, recently, many surgeons have reported that the postoperative prognosis of patients diagnosed with malignant pleural disease at thoracotomy is relatively favorable [7–12].
Hence, in the present study, we used the Surveillance, Epidemiology, and End Results (SEER) database to identify the large cohort of NSCLC patients with ipsilateral malignant pleural effusion reported up to date, and evaluated the prognostic correlates of overall survival (OS) and lung cancer-specific survival (LCSS) in this population.
RESULTS
This study included 2,217 patients with ipsilateral malignant pleural effusion from SEER registry patients diagnosed with NSCLC between 2004 and 2012 with no prior history of malignancy. Of these patients, 128 had primary tumor resection and 2,089 did not have primary tumor resection. The distribution of specific patient and tumor characteristics among patients with ipsilateral malignant pleural effusion is shown in Table 1. Surgeons seem more inclined to perform primary tumor resection in younger M1a patients with malignant pleural effusion and tumor size of less than 7cm.
Table 1. Baseline characteristics of M1a NSCLC patients only due to ipsilateral malignant pleural effusion.
| Characteristics | Primary tumor resection | None primary tumor resection | p Value |
|---|---|---|---|
| N=128 | N=2089 | ||
| Age group | <0.001 | ||
| ≤65 y | 65 (50.8) | 617 (29.5) | |
| >65 y | 63 (49.2) | 1472 (70.5) | |
| Gender | 0.375 | ||
| Male | 75 (58.6) | 1140 (54.6) | |
| Female | 53 (41.4) | 949 (45.4) | |
| Married | 75 (58.6) | 1022 (48.9) | 0.034 |
| Race/ethnicity | 0.569 | ||
| White | 100 (78.1) | 1624 (77.7) | |
| Black | 19 (14.8) | 268 (12.8) | |
| Other | 9 (7.0) | 197 (9.4) | |
| Tumor Size | <0.001 | ||
| ≤3cm | 56 (43.8) | 548 (26.2) | |
| ≤5cm | 35 (27.3) | 598 (28.6) | |
| ≤7cm | 17 (13.3) | 490 (23.5) | |
| >7cm | 20 (15.6) | 453 (21.7) | |
| Location | 0.292 | ||
| Main bronchus | 10 (7.8) | 150 (7.2) | |
| Upper | 55 (43.0) | 1104 (52.8) | |
| Middle | 9 (7.0) | 110 (5.3) | |
| Lower | 51 (39.8) | 687 (32.9) | |
| Overlap | 3 (2.3) | 38 (1.8) | |
| Lymph node status | <0.001 | ||
| N0 | 53 (41.4) | 641 (30.7) | |
| N1 | 15 (11.7) | 155 (7.4) | |
| N2 | 58 (45.3) | 978 (46.8) | |
| N3 | 1 (0.8) | 222 (10.6) | |
| NX | 1 (0.8) | 93 (4.5) | |
| Histology | 0.386 | ||
| Adenocarcinoma | 66 (51.6) | 1042 (49.9) | |
| Squamous cell carcinoma | 34 (26.6) | 482 (23.1) | |
| Other | 28 (21.9) | 565 (27.0) | |
| Radiotherapy | 0.795 | ||
| No | 94 (73.4) | 1512 (72.4) | |
| Yes | 34 (26.6) | 577 (27.6) | |
| Follow-up time, months | 20 (1-48) | 7 (1-60) | <0.001 |
Bold values corresponds to the comparisons with P < 0.001.
Kaplan-Meier analysis showed that for patients with ipsilateral malignant pleural effusion, primary tumor resection group showed significantly better OS and LCSS compared to no tumor resection group (MST, 20 vs 7 months; OS, p <0.001; LCSS, p <0.001) (Figure 1A, 1B).
Figure 1. Overall and lung cancer-specific survival in NSCLC patients with ipsilateral malignant pleural effusion before propensity score matching.
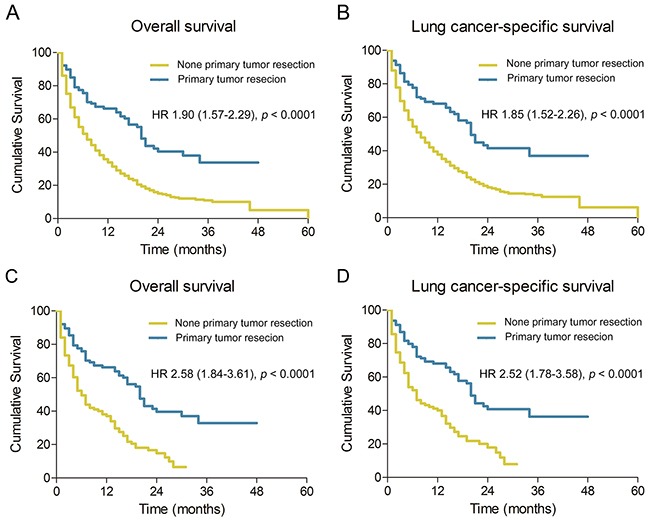
A. and B., and NSCLC patients with ipsilateral malignant pleural effusion after propensity score matching C. and D. HR, hazard ratio; NSCLC, non-small cell lung cancer
Multivariable analyses suggested that among patients with ipsilateral malignant pleural effusion, decreased OS and LCSS is associated with age > 65 years, unmarried, white or black race, and non-radiotherapy (Table 2). Notably, multivariable analyses results indicated that no primary tumor resection was associated with decreased OS (Hazard Ratio (HR), 2.136; 95% CI, 1.645-2.772; p <0.001) and LCSS (HR, 2.053; 95% CI, 1.568-2.690; p <0.001) for patients with ipsilateral malignant pleural effusion (Table 2).
Table 2. Multivariate analysis of overall survival and lung cancer-specific survival in M1a NSCLC patients only due to ipsilateral malignant pleural effusion.
| Variables | Overall survival | Lung cancer-specific survival | ||
|---|---|---|---|---|
| Hazard Ratios (95% CI) | p Value | Hazard Ratios (95% CI) | p Value | |
| Age | ||||
| ≤65 y | 1.00 (Reference) | 1.00 (Reference) | ||
| >65 y | 1.412 (1.259-1.585) | <0.001 | 1.368 (1.214-1.543) | <0.001 |
| Gender | ||||
| Female | 1.00 (Reference) | 1.00 (Reference) | ||
| Male | 1.118 (1.008-1.241) | 0.035 | 1.086 (0.974-1.212) | 0.138 |
| Married | ||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | ||
| No | 1.322 (1.192-1.466) | <0.001 | 1.279 (1.147-1.426) | <0.001 |
| Race | ||||
| White | 1.00 (Reference) | 1.00 (Reference) | ||
| Black | 0.968 (0.831-1.127) | 0.672 | 0.965 (0.822-1.134) | 0.668 |
| Other | 0.628 (0.517-0.762) | <0.001 | 0.612 (0.499-0.752) | <0.001 |
| Tumor Size | ||||
| ≤3cm | 1.00 (Reference) | 1.00 (Reference) | ||
| ≤5cm | 0.989 (0.862-1.135) | 0.880 | 0.999 (0.865-1.155) | 0.994 |
| ≤7cm | 1.144 (0.986-1.327) | 0.075 | 1.152 (0.985-1.347) | 0.077 |
| >7cm | 1.339 (1.150-1.560) | <0.001 | 1.348 (1.148-1.583) | <0.001 |
| Location | ||||
| Main bronchus | 1.00 (Reference) | 1.00 (Reference) | ||
| Upper | 1.021 (0.841-1.241) | 0.831 | 1.041 (0.848-1.279) | 0.700 |
| Middle | 0.949 (0.717-1.256) | 0.713 | 0.933 (0.693-1.257) | 0.649 |
| Lower | 0.911 (0.744-1.115) | 0.367 | 0.917 (0.741-1.135) | 0.428 |
| Overlap | 1.462 (1.012-2.115) | 0.043 | 1.416 (0.955-2.099) | 0.084 |
| Lymph node status | ||||
| N0 | 1.00 (Reference) | 1.00 (Reference) | ||
| N1 | 0.880 (0.715-1.082) | 0.225 | 0.871 (0.697-1.088) | 0.223 |
| N2 | 1.156 (1.027-1.301) | 0.016 | 1.216 (1.073-1.379) | 0.002 |
| N3 | 1.076 (0.898-1.291) | 0.427 | 1.130 (0.934-1.367) | 0.210 |
| NX | 1.134 (0.881-1.459) | 0.328 | 1.226 (0.944-1.591) | 0.126 |
| Histology | ||||
| Adenocarcinoma | 1.00 (Reference) | 1.00 (Reference) | ||
| Squamous cell carcinoma | 1.258 (1.094-1.447) | 0.001 | 1.255 (1.084-1.453) | 0.002 |
| Other | 1.360 (1.206-1.533) | <0.001 | 1.317 (1.160-1.495) | <0.001 |
| Primary tumor resection | ||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | ||
| No | 2.136 (1.645-2.772) | <0.001 | 2.053 (1.568-2.690) | <0.001 |
| Radiotherapy | ||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | ||
| No | 1.639 (1.447-1.856) | <0.001 | 1.612 (1.415-1.836) | <0.001 |
Bold values corresponds to the comparisons with P < 0.001.
Following the propensity score matching, all variables, including patient characteristics, tumor features, and therapeutic managements, were calculated. Patients with ipsilateral malignant pleural effusion were classified into well matched groups, specifically, 125 patients were assigned to primary tumor resection group and 125 patients to no primary tumor resection group. Table 3 shows no significant difference in demographic, pathologic, and therapeutic variables between two groups. Consistent with the prior analyses, better OS and LCSS were observed among patients with ipsilateral malignant pleural effusion who underwent primary tumor resection when compared to those who did not (MST, 20 vs 6 months; OS, p <0.001; LCSS, p <0.001) (Figure 1C, 1D). Similarly, multivariable analyses also indicated that no primary tumor resection was associated with decreased OS (HR, 2.309; 95% CI, 1.636-3.259; p <0.001) and LCSS (HR, 2.301; 95% CI, 1.610-3.287; p <0.001) for patients with ipsilateral malignant pleural effusion (Table 4).
Table 3. Baseline characteristics of M1a NSCLC patients only due to ipsilateral malignant pleural effusion after propensity score matching.
| Characteristics | Primary tumor resection | None primary tumor resection | p Value |
|---|---|---|---|
| N=125 | N=125 | ||
| Age group | 0.800 | ||
| ≤65 y | 62 (49.6) | 59 (47.2) | |
| >65 y | 63 (50.4) | 66 (52.8) | |
| Gender | 0.373 | ||
| Male | 73 (58.4) | 60 (48.0) | |
| Female | 52 (41.6) | 65 (52.0) | |
| Married | 74 (59.2) | 63 (50.4) | 0.204 |
| Race/ethnicity | 0.939 | ||
| White | 98 (78.4 | 96 (76.8) | |
| Black | 18 (14.4) | 20 (16.0) | |
| Other | 9 (7.0) | 9 (9.2) | |
| Tumor Size | 0.908 | ||
| ≤3cm | 55 (44.0) | 57 (45.6) | |
| ≤5cm | 35 (28.0) | 32 (25.6) | |
| ≤7cm | 15 (12.0) | 18 (14.4) | |
| >7cm | 20 (16.0) | 18 (14.4) | |
| Location | 0.921 | ||
| Main bronchus | 9 (7.2) | 11 (8.8) | |
| Upper | 55 (44.0) | 50 (40.0) | |
| Middle | 9 (7.2) | 7 (5.6) | |
| Lower | 51 (40.8) | 56 (44.8) | |
| Overlap | 1 (0.8) | 1 (0.8) | |
| Lymph node status | 0.210 | ||
| N0 | 52 (41.6) | 53 (42.4) | |
| N1 | 14 (11.2) | 18 (14.4) | |
| N2 | 57 (45.6) | 48 (38.4) | |
| N3 | 1 (0.8) | 6 (4.8) | |
| NX | 1 (0.8) | 0 (0) | |
| Histology | 0.662 | ||
| Adenocarcinoma | 65 (52.0) | 68 (54.4) | |
| Squamous cell carcinoma | 33 (26.4) | 27 (21.6) | |
| Other | 27 (21.6) | 30 (24.0) | |
| Radiotherapy | 0.377 | ||
| No | 91 (72.8) | 98 (78.4) | |
| Yes | 34 (27.2) | 27 (21.6) | |
| Follow-up time, months | 20 (1-48) | 6 (1-31) | <0.001 |
Bold values corresponds to the comparisons with P < 0.001.
Table 4. Multivariate analysis of overall survival and lung cancer-specific survival in M1a NSCLC patients only due to ipsilateral malignant pleural effusion after propensity score matching.
| Variables | Overall survival | Lung cancer-specific survival | ||
|---|---|---|---|---|
| Hazard Ratios (95% CI) | p Value | Hazard Ratios (95% CI) | p Value | |
| Age | ||||
| ≤65 y | 1.00 (Reference) | 1.00 (Reference) | ||
| >65 y | 1.063 (0.746-1.515) | 0.735 | 1.013 (0.700-1.465) | 0.947 |
| Gender | ||||
| Female | 1.00 (Reference) | 1.00 (Reference) | ||
| Male | 1.233 (0.871-1.745) | 0.237 | 1.226 (0.855-1.758) | 0.268 |
| Married | ||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | ||
| No | 1.353 (0.946-1.936) | 0.098 | 1.261 (0.870-1.827) | 0.220 |
| Race | ||||
| White | 1.00 (Reference) | 1.00 (Reference) | ||
| Black | 1.056 (0.643-1.733) | 0.831 | 1.087 (0.652-1.812) | 0.748 |
| Other | 1.254 (0.632-2.488) | 0.517 | 1.187 (0.583-2.419) | 0.636 |
| Tumor Size | ||||
| ≤3cm | 1.00 (Reference) | 1.00 (Reference) | ||
| ≤5cm | 0.670 (0.438-1.024) | 0.064 | 0.622 (0.400-0.968) | 0.035 |
| ≤7cm | 1.157 (0.667-2.007) | 0.603 | 1.048 (0.591-1.856) | 0.873 |
| >7cm | 0.891 (0.508-1.562) | 0.687 | 0.798 (0.439-1.449) | 0.458 |
| Location | ||||
| Main bronchus | 1.00 (Reference) | 1.00 (Reference) | ||
| Upper | 0.706 (0.373-1.339) | 0.286 | 0.724 (0.372-1.407) | 0.340 |
| Middle | 1.012 (0.440-2.328) | 0.977 | 1.012 (0.425-2.419) | 0.976 |
| Lower | 0.935 (0.485-1.801) | 0.840 | 0.915 (0.461-1.817) | 0.801 |
| Overlap | 6.215 (1.159-33.325) | 0.033 | 7.041 (1.279-38.772) | 0.025 |
| Lymph node status | ||||
| N0 | 1.00 (Reference) | 1.00 (Reference) | ||
| N1 | 1.238 (0.726-2.111) | 0.434 | 1.211 (0.686-2.137) | 0.510 |
| N2 | 1.697 (1.144-2.517) | 0.009 | 1.844 (1.225-2.774) | 0.003 |
| N3 | 2.235 (0.871-5.734) | 0.094 | 2.577 (0.991-6.698) | 0.052 |
| NX | <0.001 | 0.966 | <0.001 | 0.968 |
| Histology | ||||
| Adenocarcinoma | 1.00 (Reference) | 1.00 (Reference) | ||
| Squamous cell carcinoma | 0.924 (0.566-1.509) | 0.752 | 1.014 (0.609-1.688) | 0.959 |
| Other | 1.121 (0.738-1.704) | 0.592 | 1.128 (0.728-1.748) | 0.589 |
| Primary tumor resection | ||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | ||
| No | 2.309 (1.636-3.259) | <0.001 | 2.301 (1.610-3.287) | <0.001 |
| Radiotherapy | ||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | ||
| No | 1.275 (0.816-1.992) | 0.286 | 1.391 (0.868-2.230) | 0.170 |
Bold values corresponds to the comparisons with P < 0.001.
DISCUSSION
In the seventh version of TNM staging for NSCLC, malignant pleural effusion is defined as M1a [13]; therefore, it is generally contraindicated for surgery. However, malignant pleural effusion can be unexpectedly identified during operations when primary tumor removal seems feasible and does not add overloaded pressure to the patients. Dr. Ohta and colleagues reported that for 42 surgically resected cases with confirmed malignant pleural dissemination, 5-year survivals and MST were 13.1% and 17 months, respectively [10]. Dr. Iid and colleagues found that 5-year survival rate and MST of 313 pleural dissemination patients were 29.3% and 34.0 months, respectively. Primary tumor resection was performed in 256 (81.8%) patients, and 152 (48.6%) patients underwent macroscopic complete resection with 5-year survival rates of 33.1% and 37.1%, respectively [12]. In addition, prior results of surgical interventions treating this special disease category provided additional evidence for tumor resection [11, 14]. Therefore, it remains controversial whether attempts should be made to resect the primary tumor when confronting an unexpected malignant pleural effusion case.
Using the SEER database, we analyzed the largest cohort of NSCLC patients with malignant pleural effusion (n = 2,217) reported to date, although the incidence was slightly lower compared to that in previous studies [12, 15]. Also, the analyses of treatment modalities and survival were performed with patients without other M1a and/or M1b disease and with only one primary tumor.
We observed that patients who underwent primary tumor resection, especially NSCLC patients with ipsilateral malignant pleural effusion, collectively exhibited greater OS and LCCS compared to those with no tumor resection (Figure 1). The multivariable analyses showed that primary tumor resection group had a significantly more favorable prognosis compared to no primary tumor resection group (Tables 2, 4). The underlying reason included possible reduction of the tumor burden. Clinical support might be found in a study by Dr. Iid and his colleagues. Considering pleural dissemination patients, they found that 5-year survival rate of macroscopic complete resection group (37.1%) was significantly better compared to macroscopic incomplete resection group (22.7%, p = 0.009) and exploratory thoracotomy group (12.2%, p < 0.001), respectively [12]. Dr. Ren and colleagues found that for patients with intra-operatively proven malignant nodules and minimal pleural effusion (<300ml), primary tumor resection had better survival rate compared to biopsy (MST, 27 vs 7 months, p = 0.003). Theoretical support might be found in Dr. Rashid and his colleagues' study on metastatic breast cancer resection. The authors utilized biotechnology to monitor overall breast cancer load under direct vision mouse model [16] from which they found that only primary tumor resection significantly reduced tumor burden.
Moreover, a relevant published study from Shanghai Pulmonary Hospital also suggested that M1a NSCLC patients with pleural dissemination might not be entirely excluded from the surgery [17]. All surgical NSCLC cases (9,576) of Shanghai Pulmonary Hospital between January 2005 and December 2013 were reviewed. Among them, 83 cases (0.9%) met the definition of “unexpected” macroscopic malignant pleural dissemination, despite routine preoperative evaluations for tumor metastasis. Patients with primary tumor resection had significantly better outcome compared to biopsy (MST: respectively, 35 vs. 17 months, p=0.001). Also, multivariate analysis showed that primary tumor resection (HR: 3.678, p=0.014) were favorable prognostic factors in patients with malignant pleural dissemination.
This study has some limitations. First, the SEER database was generated retrospectively, and inevitably, our analyses of this data are subject to the influence of patient and treatment selection bias. We have attempted to control for this by using some advanced statistical methods to balance the variables between arms. Second, the SEER database set was not integrated for malignant pleural effusion, important factors, such as the pre-operation TNM stage, the amount of pleural effusion, lung function, symptoms, and perhaps the tumor burden. The prognosis for surgery for NSCLC patients with malignant pleural effusion may depend on the factors mentioned above. Because of these limitations, a more complete prospective cohort study is warranted to confirm the role of primary tumor resection in NSCLCs patients, especially in those with intra-operatively proven malignant pleural effusion. Notably, SEER does not provide information about chemotherapy treatment, which is a very fundamental issue in the survival of NSCLC patients with malignant pleural effusion.
Briefly, the results of this study revealed that the prognosis after contraindicated surgery of NSCLC patients with malignant pleural effusion (M1a) may be better than expected. Thus, subsequent studies should aim to identify patients who could benefit from surgery.
METHODS
Study population
SEER-18 registry data were used to identify patients that met the inclusion criteria (site = lung and bronchus, behavior = malignant, age = 25-84, and year of diagnosis = 2004-2012) [18]. In addition, we included the patients who had (1) pathologically confirmed NSCLC, (2) M1a disease with ipsilateral malignant pleural effusion (SEER code: CS mets at dx “15”), and (3) only one malignant primary tumor.
We collected the demographic characteristics of patients (age, gender, marriage, and race), pathological features of tumors (size, location, histological type, and lymph node status, location of malignant pleural effusion), and types of therapeutic management (surgical type, and radiotherapy) from SEER database. In this study, pathological types were classified as adenocarcinoma (SEER codes 8140, 8230, 8255, 8260, 8310, 8333, 8470, 8480, 8481, 8490 and 8550), squamous cell carcinoma (SEER codes 8052, 8070, 8071, 8072, 8073, 8083 and 8084), and other histological types with low incidence rate (large cell carcinoma and etc.). Since OS and LCSS were also included in SEER database, both of them were regarded as the outcomes of interest. Patient outcomes were obtained until December 31, 2012. OS was defined as the survival time from diagnosis until death for any reason or until the last follow-up, and LCSS from diagnosis until cause-specific death with lung cancer or until the last follow-up.
Statistical analysis
The data were presented as frequencies (percent) or median (range) deviation. The comparison of demographic, pathologic, and therapeutic features between patients who underwent primary tumor resection or those who did not was performed using unpaired t test for continuous variables and Pearson χ2 test for categorical variables. The OS and LCSS were estimated using the Kaplan-Meier method and the log-rank test comparing survival in two or more groups. Multivariate Cox proportional hazard analyses were applied to adjust the potential confounders related to patients, tumors, and therapies in the survival analysis. For both Kaplan-Meier and Cox analyses, patients were censored using the SEER.
We used propensity score matching method to balance the differences in the basic clinical characteristics of patients with ipsilateral malignant pleural effusion who underwent primary tumor resection group and those who did not. As an alternative method to compare survival outcomes among surgical procedures, propensity score matching was performed with one-to-three nearest-neighbor matching without replacement to identify matched cohorts representing the two treatment modalities. Specifically, to control the potential difference in the basic clinical characteristics of patients and tumors (variables in the propensity score matching including age, gender, race/ethnical group, lesion site and pathological classification), we made a comparative examination of survival between primary tumor resection group and those who did not to verify better prognosis for patients who underwent primary tumor resection. Covariate balance was evaluated by using standardized differences in means. Finally, OS and LCSS were compared in matched patients with ipsilateral malignant pleural effusion who underwent primary tumor resection group and those who did not by log-rank test.
In this study, a two-sided p value < 0.05 was regarded statistically significant. All analyses were conducted using SPSS 23.0 (SPSS Inc. Chicago, IL), and survival curve was drawn using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA).
Acknowledgments
We would like to thank to all the staff members of the National Cancer Institute, who have been involved with the Surveillance, Epidemiology and End Results (SEER) Program.
Footnotes
CONFLICTS OF INTEREST
No conflict of interest exits in the submission of this manuscript, and all authors for publication approve manuscript. I would like to declare on behalf of my co-authors that the work described presents an original research that has not been published previously, and it is not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
GRANT SUPPORT
The Shanghai Municipal Commission of health and family planning, special emphasis, 2013ZYJB0003, and by the Science and Technology Commission of Shanghai Municipality, 15411968400, supported the work. This publication's contents are the sole responsibility of the authors and do not necessarily represent the official views of the Shanghai Municipal Commission of health and family planning or the Science and Technology Commission of Shanghai Municipality. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Sugiura S, Ando Y, Minami H, Ando M, Sakai S, Shimokata K. Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clinical cancer research. 1997;3:47–50. [PubMed] [Google Scholar]
- 2.Jett JR, Scott WJ, Rivera MP, Sause WT. Guidelines on treatment of stage IIIB non-small cell lung cancer. Chest. 2003;123:221s–225s. doi: 10.1378/chest.123.1_suppl.221s. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. Journal of thoracic oncology. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Webber C, Gospodarowicz M, Sobin LH, Wittekind C, Greene FL, Mason MD, Compton C, Brierley J, Groome PA. Improving the TNM classification: findings from a 10-year continuous literature review. International journal of cancer. 2014;135:371–378. doi: 10.1002/ijc.28683. [DOI] [PubMed] [Google Scholar]
- 5.Lim E, Clough R, Goldstraw P, Edmonds L, Aokage K, Yoshida J, Nagai K, Shintani Y, Ohta M, Okumura M, Iwasaki T, Yasumitsu T, Okada M, Mimura T, Tsubota N, Nakagawa T, et al. Impact of positive pleural lavage cytology on survival in patients having lung resection for non-small-cell lung cancer: An international individual patient data meta-analysis. The Journal of thoracic and cardiovascular surgery. 2010;139:1441–1446. doi: 10.1016/j.jtcvs.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 6.Ryu JS, Ryu HJ, Lee SN, Memon A, Lee SK, Nam HS, Kim HJ, Lee KH, Cho JH, Hwang SS. Prognostic impact of minimal pleural effusion in non-small-cell lung cancer. Journal of clinical oncology. 2014;32:960–967. doi: 10.1200/JCO.2013.50.5453. [DOI] [PubMed] [Google Scholar]
- 7.Ichinose Y, Tsuchiya R, Koike T, Kuwahara O, Nakagawa K, Yamato Y, Kobayashi K, Watanabe Y, Kase M, Yokoi K. The prognosis of patients with non-small cell lung cancer found to have carcinomatous pleuritis at thoracotomy. Surgery today. 2000;30:1062–1066. doi: 10.1007/s005950070002. [DOI] [PubMed] [Google Scholar]
- 8.Fukuse T, Hirata T, Tanaka F, Wada H. The prognostic significance of malignant pleural effusion at the time of thoracotomy in patients with non-small cell lung cancer. Lung cancer. 2001;34:75–81. doi: 10.1016/s0169-5002(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 9.Shiba M, Kakizawa K, Kohno H, Shibuya K, Yamakawa H, Hiroshima K, Fujisawa T. Prognostic implication of Ki-67 immunostaining in treating subclinical pleural cancer found at thoracotomy in lung cancer patients. The Annals of thoracic surgery. 2001;71:1765–1771. doi: 10.1016/s0003-4975(01)02589-9. [DOI] [PubMed] [Google Scholar]
- 10.Ohta Y, Shimizu Y, Matsumoto I, Tamura M, Oda M, Watanabe G. Retrospective review of lung cancer patients with pleural dissemination after limited operations combined with parietal pleurectomy. Journal of surgical oncology. 2005;91:237–242. doi: 10.1002/jso.20333. [DOI] [PubMed] [Google Scholar]
- 11.Sawabata N, Matsumura A, Motohiro A, Osaka Y, Gennga K, Fukai S, Mori T. Malignant minor pleural effusion detected on thoracotomy for patients with non-small cell lung cancer: is tumor resection beneficial for prognosis? The Annals of thoracic surgery. 2002;73:412–415. doi: 10.1016/s0003-4975(01)03426-9. [DOI] [PubMed] [Google Scholar]
- 12.Iida T, Shiba M, Yoshino I, Miyaoka E, Asamura H, Date H, Okumura M, Tada H, Nakanishi Y, Dosaka-Akita H. Surgical Intervention for Non–Small-Cell Lung Cancer Patients with Pleural Carcinomatosis: Results From the Japanese Lung Cancer Registry in 2004. Journal of Thoracic Oncology. 2015;10:1076–1082. doi: 10.1097/JTO.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 13.Detterbeck FC, Boffa DJ, Tanoue LT, Wilson LD. Details and difficulties regarding the new lung cancer staging system. Chest. 2010;137:1172–1180. doi: 10.1378/chest.09-2626. [DOI] [PubMed] [Google Scholar]
- 14.Bernard A, de Dompsure RB, Hagry O, Favre JP. Early and late mortality after pleurodesis for malignant pleural effusion. The Annals of thoracic surgery. 2002;74:213–217. doi: 10.1016/s0003-4975(02)03599-3. [DOI] [PubMed] [Google Scholar]
- 15.Tönnies M, Kollmeier J, Bauer T, Griff S, Kaiser D. Curative surgical treatment options for patients with non-small cell lung cancer (NSCLC) and solitary pulmonary metastasis [Article in German] Pneumologie. 2012;66:218–223. doi: 10.1055/s-0032-1308917. [DOI] [PubMed] [Google Scholar]
- 16.Rashid OM, Nagahashi M, Ramachandran S, Graham L, Yamada A, Spiegel S, Bear HD, Takabe K. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surgery. 2013;153:771–778. doi: 10.1016/j.surg.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren YJ, She YL, Dai CY, Jiang GN, Fei K, Chen C. Primary tumour resection showed survival benefits for non-small-cell lung cancers with unexpected malignant pleural dissemination. Interactive cardiovascular and thoracic surgery. 2016;22:321–326. doi: 10.1093/icvts/ivv353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamo M, Johnson C, Ruhl J, Dickie L. SEER program coding and staging manual. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]


