Abstract
Objective
To evaluate long‐term outcomes of adjunctive therapy with SPN‐804 (Oxtellar XR®, Supernus Pharmaceuticals), an extended‐release tablet formulation of oxcarbazepine (OXC), in adults with refractory partial‐onset seizures.
Methods
After completing a 16‐week double‐blind, placebo‐controlled trial of SPN‐804 at fixed dosages (1200 or 2400 mg QD), patients entering this open‐label extension study were converted in blinded fashion to 1200 mg QD SPN‐804 as a target starting dose for long‐term treatment. Patients were followed for 1 year, during which SPN‐804 dosages could be adjusted up to 2400 mg/day according to clinical response.
Results
Of 214 patients, 84% completed 1‐year open‐label treatment. Median maintenance SPN‐804 dosage was 1200 mg; <10% of patients required 2400 mg. Median 28‐day seizure frequency reduction from baseline was 59%; seizure frequency was reduced ≥50% in 58% of patients; 11% were seizure free ≥6 months; and 5% were seizure free ≥1 year. SPN‐804 was discontinued due to adverse events in 5% (n = 10). Incidences of each of the most common adverse events (dizziness, headache, diplopia, nausea, vomiting, balance disorder, blurred vision) were ≤15% during 1‐year follow‐up and occurred most frequently in patients previously naïve to SPN‐804. No new safety signals, no clinically significant changes in health status, and no deaths attributable to SPN‐804 were observed.
Conclusion
SPN‐804 administered once daily for 1 year was effective as adjunctive therapy in improving seizure control and maintaining therapeutic response in adults with refractory partial‐onset seizures. With dosage flexibility, SPN‐804 was well tolerated.
Keywords: Oxcarbazepine, extended‐release, partial‐onset seizures, adjunctive therapy
Oxcarbazepine (OXC) is an analogue of carbamazepine (CBZ) that is rapidly and almost completely converted presystemically after oral administration to 80% eslicarbazepine (S‐licarbazepine) and 20% R‐licarbazepine 1, 10‐monohydroxy derivative (MHD) enantiomers that have equal biologic activity 2. Oxcarbazepine retains the efficacy of CBZ 3 but has more favorable metabolic, pharmacokinetic, tolerability, and safety profiles 4. However, the usefulness of immediate‐release OXC (OXC‐IR) can often be compromised by poor tolerability, particularly at higher dosages (i.e., ≥1200 mg/day) 5.
SPN‐804 (Oxtellar XR®, Supernus Pharmaceuticals, Rockville, MD, USA) is a novel extended‐release tablet formulation of OXC. Its unique matrix delivery technology blunts peak MHD concentrations, produces more constant plasma concentration–time profiles, and allows once‐daily dosing. A multinational double‐blind placebo‐controlled study [Prospective Randomized Study of Oxcarbazepine XR in Subjects with Partial Epilepsy, Refractory (PROSPER); ClinicalTrials.gov identifier: NCT00772603] demonstrated the efficacy of once‐daily SPN‐804 administered as adjunctive therapy at fixed dosages (1200 and 2400 mg QD) in adults with refractory partial‐onset seizures 6. Consistent with the more favorable plasma concentration–time profile of SPN‐804 vs OXC‐IR, discontinuations due to adverse events (AEs) with SPN‐804 were threefold to fivefold lower in the PROSPER study than in a similarly designed study of OXC‐IR 600 and 1200 mg BID 5. Double‐blind, randomized, controlled, fixed‐dose trials such as PROSPER provide critical efficacy and safety information that informs regulatory approval. However, their designs do not closely mirror clinical practice when dosages can be individualized according to clinical response. Open‐label extensions of randomized controlled trials can prospectively and systematically evaluate outcomes of long‐term treatment in study conditions that more closely approximate usual clinical practices 7. We report results from the open‐label extension of the PROSPER study evaluating SPN‐804 QD.
Methods
Study design and patients
This was a multinational, multicenter, open‐label study (ClinicalTrials.gov identifier: NCT00908344) conducted between May 2009 and November 2011 at 68 sites in eight countries (United States, n = 21; Russia, n = 12; Mexico, n = 9; Poland, n = 9; Bulgaria, n = 7; Romania, n = 5; Croatia, n = 3; Canada, n = 2). The study was performed in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki, European Medicines Agency requirements, and the US Code of Federal Regulations. The protocol, amendments, and informed consent were reviewed and approved by the appropriate regulatory authorities in each country and Institutional Review Board or Independent Ethics Committee at each site. Patients provided written informed consent before participating in the extension.
Details of the core double‐blind PROSPER study are reported elsewhere 6. In brief, 366 adults (18–65 years) who averaged ≥3 partial‐onset seizures per 28 days despite stable therapy with 1–3 AEDs were randomized to double‐blind treatment with fixed dosages of SPN‐804 QD (1200 or 2400 mg) or to placebo. After an 8‐week prospective observation period to establish baseline seizure frequency, patients entered a double‐blind phase that comprised a 4‐week titration period and 12‐week maintenance period. Patients successfully completing the double‐blind study were eligible to enter the 1‐year extension.
Patients were screened for extension study eligibility at the end of the double‐blind maintenance period of the PROSPER study. For patients electing to continue into the extension study, the target starting open‐label SPN‐804 dosage was 1200 mg QD. In the 3‐week blinded conversion to open‐label treatment, patients initially assigned to placebo in the PROSPER study were up‐titrated to 1200 mg SPN‐804 (600 mg starting dose increased after 1 week to 1200 mg); those previously assigned to 2400 mg were down‐titrated to 1200 mg (600‐mg/week decrements). The SPN‐804 dosage was unchanged in patients entering the extension study from the 1200‐mg arm of the double‐blind trial.
During the 1‐year maintenance period, SPN‐804 dosages could be adjusted in 300‐ or 600‐mg increments/decrements. The maximum allowable dosage was 2400 mg SPN‐804 QD; patients unable to tolerate at least 600 mg were withdrawn. Concomitant AED therapy was to remain stable unless the investigator deemed it medically necessary to change dose or to withdraw and/or add AEDs. At study completion (Month 12), the SPN‐804 dosage was tapered 600 mg/week and withdrawn over ≤4 weeks, depending on dose. Changes in other AEDs during/after SPN‐804 tapering to accommodate withdrawal of study medication were based on investigator clinical judgment.
Office visits were scheduled for Day 1 and at Months 1, 3, 6, and 9 of open‐label treatment. Patients were contacted by telephone at Month 12 and at the end of SPN‐804 tapering; the end‐of‐study visit was conducted within 30 days of the last SPN‐804 dose. Patients maintained study diaries to record seizures, SPN‐804/AED dosages, and treatment‐emergent symptoms. Study procedures at each patient visit included physical and neurological examinations. Clinical laboratory (Day 1, Month 3, end of study) and 12‐lead ECG (Day 1, end of study) assessments were also conducted.
Assessments/statistical analyses
Efficacy endpoints of interest for the extension study included median percent change in monthly (28‐day) seizure frequency, proportion of treatment responders (patients with ≥50% seizure frequency reduction), and patients seizure free ≥6 months and ≥1 year. Seizure frequency change was based on last observation carried forward in the Intent‐to‐Treat (ITT) population (all patients who received ≥1 dose of open‐label SPN‐804 and had ≥1 day of seizure data recorded in the study diary). Changes in seizure frequency were analyzed relative to the double‐blind baseline period and to the double‐blind maintenance period. The 6‐month seizure‐free rate was any 6‐month interval without seizures. The population for the 1‐year seizure‐free rate was patients who completed all on‐treatment visits with seizure data at each visit.
Maintenance SPN‐804 dosages were determined from an analysis of drug exposure data. Duration of exposure at individual dosages (600, 900, 1200, 1500, 1800, 2400 mg) was based on study medication records and patient reported compliance. Maintenance dosage was defined as ≥ 3‐month (84 days) exposure at a specified dosage.
Adverse events were classified according to the Medical Dictionary for Regulatory Activities (MedDRA). Safety assessments included physical and neurologic examinations, vital signs, laboratory tests, and ECGs. Patients who received ≥1 dose of open‐label SPN‐804 comprised the Safety Population.
Outcome parameters were summarized by descriptive statistics.
Results
Of 248 patients completing the double‐blind PROSPER study, 214 elected to continue into the open‐label extension and comprised the ITT and Safety Populations. Patient characteristics are summarized in Table 1. Patients averaged more than six seizures per month at entry into the PROSPER study; SPN‐804 was added to ≥2 AEDs in most (66%) patients. Of patients entering the extension study, 76 (36%) were from the placebo arm of the double‐blind study, 75 (35%) were from the 1200‐mg arm, and 63 (29%) had initially been assigned to the 2400‐mg group. A total of 179 (84%) patients completed 1 year of open‐label SPN‐804 treatment. The most common reasons for discontinuation were adverse events (n = 10; 5%) and withdrawal of consent (n = 9; 4%).
Table 1.
Patient characteristics: ITT/Safety Population (N = 214)
| Age (yrs), mean ± SD | 37 ± 12 |
| Female, n (%) | 111 (52) |
| Race, n (%) | |
| White | 178 (83) |
| Black | 4 (2) |
| Other | 36 (17) |
| Epilepsy duration (years), mean ± SDa | 19 ± 14 |
| Baseline seizure frequency (average seizures/28 days), mediana | 6.5 |
| Concomitant AEDs, n (%)a | |
| 1 AED | 72 (34) |
| 2 AEDs | 118 (55) |
| ≥3 AEDs | 24 (11) |
| Valproate | 111 (52) |
| Carbamazepine | 78 (36) |
| Lamotrigine | 53 (24) |
| Topiramate | 43 (20) |
| Levetiracetam | 35 (16) |
| Barbiturates | 16 (7) |
| Gabapentin/pregabalin | 15 (7) |
| Other | 24 (11) |
At baseline of core double‐blind (PROSPER) study.
During open‐label treatment, the median maintenance SPN‐804 dosage was 1200 mg (mean, 1394 ± 411 mg). Maintenance dosages were 1200–1800 mg QD for the majority (78%) of patients (Fig. 1).
Figure 1.
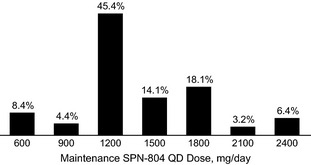
Distribution of Oxtellar XR (SPN‐804) QD maintenance dosages during open‐label treatment (N=214). Maintenance dosage defined as ≥ 3 month (84 days) exposure at specified dosage.
Efficacy
At the end of the 1‐year extension study, median percent seizure reduction in 28‐day seizure frequency was 59% relative to the baseline of the PROSPER study; 58% responded with ≥50% seizure reduction. In analyses of response by visit (Fig. 2), treatment effect at 3 months and beyond was relatively stable. A total of 24 patients (11%) were seizure free for ≥6 months; 10 patients (5%) were seizure free for the entire 1‐year extension study.
Figure 2.
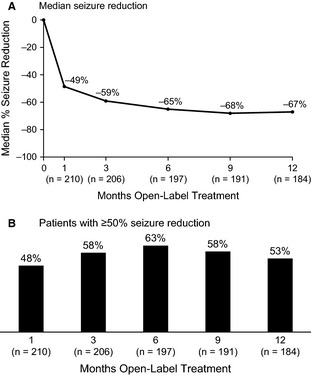
Median percent change from baseline 28‐day seizure frequency over time during open‐label Oxtellar XR (SPN‐804) treatment (A) and patients with ≥ 50% reduction from baseline seizure frequency (B). ITT population; last observation carried forward.
When extension study data were analyzed according to patient assignment in the double‐blind trial, median percent seizure frequency change relative to the PROSPER study baseline was −53% (1200‐mg arm) and −59% (2400‐mg arm) in patients initially assigned to SPN‐804 and −64% in those initially assigned to placebo. In patients converted from placebo to SPN‐804 in blinded fashion, the median percent seizure frequency change was −30% in the first 3 weeks after SPN‐804 was added. The median percent reduction during just the open‐label extension period (i.e., reduction vs double‐blind maintenance) was 39% (placebo), 19% (1200 mg SPN‐804), and 12% (2400 mg SPN‐804), representing incremental improvement associated with investigators being able to adjust dosages according to clinical response.
Tolerability and safety
One or more treatment‐emergent AEs were recorded in 124 (58%) patients participating in the extension study. The incidence of any individual AE (Table 2) was ≤15%. In most (>90%) patients, AEs were mild‐to‐moderate in severity. The only severe AEs occurring in >1% of patients were asthenia and dizziness (n = 3 each). Adverse events considered possibly or definitely related to SPN‐804 were reported by 69 (32%) patients, the most common of which was dizziness (14%). Among the 15 patients (7%) with serious AEs, the only events occurring in >1 patient were seizures (n = 5) and non‐cardiac chest pain (n = 2). Other serious AEs reported (one patient each) were ataxia, dizziness, encephalopathy, transient ischemic attack, suicidal behavior, acute pyelonephritis, spinal fracture, nephrolithiasis, and increased prostate‐specific antigen. No deaths attributable to SPN‐804 were reported. One patient died due to bilateral pulmonary artery thrombosis that was unlikely related to study medication.
Table 2.
Most common adverse events (≥5% incidence): ITT/Safety Population (N = 214)
| Adverse event | n (%) |
|---|---|
| Dizziness | 33 (15) |
| Headache | 24 (11) |
| Diplopia | 20 (9) |
| Nausea | 16 (8) |
| Vomiting | 13 (6) |
| Somnolence | 12 (6) |
| Balance disorder | 10 (5) |
| Upper respiratory tract infection | 10 (5) |
Actions taken to manage AEs included modifying SPN‐804 dose in 16% of patients, temporarily interrupting SPN‐804 dosing in 4%, and discontinuing SPN‐804 in 5%. Among the 10 patients who discontinued due to AEs, the only events that led to SPN‐804 discontinuation in ≥2 patients were nausea and/or vomiting (n = 3), balance disorder (n = 2), and dizziness (n = 2). Actions such as SPN‐804 dose reduction or temporary interruption of therapy were most commonly taken to manage dizziness (6%, n = 13), diplopia (4%, n = 9), nausea and/or vomiting (3%, n = 6), and ataxia (2%, n = 4). Of the patients in whom an AE was the primary reason for discontinuing SPN‐804, 6 (60%) were patients who were previously naïve to SPN‐804 therapy (i.e., received placebo in the double‐blind study). These patients also accounted for 50–70% of patients experiencing diplopia, nausea, vomiting, and/or blurred vision.
Hyponatremia (Na+ <135 mmol/l) was reported in four (2%) patients, including one case of severe (125 mmol/l) hyponatremia; all patients were asymptomatic. There were no clinically significant changes in mean values for vital signs, ECGs, or laboratory investigations.
Discussion
Open‐label extension studies of AEDs answer important clinical questions that cannot be addressed by double‐blind, randomized, controlled trials with fixed‐dose designs and relatively short durations, for example, magnitude of the treatment effect when dosages can be adjusted according to clinical response, potential for long‐term seizure freedom, long‐term tolerability/safety, and overall effectiveness in terms of patients who continue therapy (retention rate). Results of the open‐label extension study reported here demonstrated that SPN‐804 as adjunctive therapy improved and maintained seizure control and was generally well tolerated when the starting open‐label dosage of 1200 mg QD was adjusted according to clinical response.
With individualized dosages, the treatment effect in the 1‐year extension was greater than in the 16‐week double‐blind PROSPER study (median 28‐day seizure frequency change: −59% vs −43% in 2400 mg SPN‐804 arm) 6. Discontinuations due to AEs were substantially lower during open‐label SPN‐804 treatment compared with forced‐dose double‐blind trial treatment (5% vs 22% in combined double‐blind SPN‐804 arms). In the subset of 76 patients previously naïve to SPN‐804, 8% (6/76) of patients discontinued due to AEs when dosages were individualized—a rate indistinguishable from that with placebo and half that in patients force‐titrated to 1200 mg in the PROSPER study. Taken together, the combination of beneficial treatment effects and tolerability allowed nearly 85% of patients to continue treatment for 1 year.
The selection of 1200 mg SPN‐804 QD as the starting dosage for open‐label treatment was based on the recommended total daily dosage for OXC‐IR 8, which was further validated by the PROSPER study once it was unblinded 6. The numerical difference favoring 1200 mg SPN‐804 over placebo was not statistically significant in the overall PROSPER population due to an inordinately high placebo response in study sites outside North America, but was significant in a post hoc analysis of study sites in North America. In addition, a concentration–effect analysis of data from the double‐blind trial demonstrated a significant treatment effect of 1200 mg QD SPN‐804. The results reported here for the subset of PROSPER study patients initially assigned to double‐blind placebo further support the evidence that 1200 mg QD is an appropriate target dosage when initiating SPN‐804 as adjunctive therapy. In this patient subset, median seizure frequency reduction was −30% during the 3‐week blinded transition from placebo to 1200 mg SPN‐804. Dosage flexibility in the open‐label maintenance phase was associated with incremental improvements in seizure control, regardless of initial assignment in the double‐blind phase. Based on maintenance dosages in the extension study, dosage needs for most adults may be ≤1800 mg SPN‐804 QD.
This open‐label extension study is the first such study to publish dosage needs and long‐term outcomes of adults with refractory partial‐onset seizures treated with OXC as adjunctive therapy. Although OXC‐IR has been evaluated in a trial very similar to the PROSPER study 5, data from its open‐label extension have not been published. However, a retrospective case record review provided potentially useful information about overall OXC‐IR effectiveness/success in refractory epilepsy 9. In 97 patients treated with OXC‐IR in clinical practice, ~60% were maintained on OXC‐IR ≥1 year.
Eslicarbazepine (ESL) acetate is metabolized to the same active enantiomers as OXC‐IR/SPN‐804, albeit in a different ratio (95% S‐licarbazepine; 5% R‐licarbazepine) 10. Double‐blind, placebo‐controlled trials of ESL 11, 12 were similar to the PROSPER study in design and study population and were followed by open‐label extension studies. In these extension studies, 69% 13 and 77% 14 of patients completed 1 year of open‐label treatment; discontinuations due to AEs were 4% and 11%, respectively. The seizure‐free rate among patients completing 1 year of open‐label therapy was 2.5% in the one study reporting this endpoint 14.
In a meta‐analysis of prospective, open‐label, long‐term studies of IR formulations of the ‘second‐generation’ AEDs gabapentin (GBP), levetiracetam (LEV), lamotrigine (LTG), and topiramate (TPM) 7, the proportion of patients seizure free ≥6 months ranged from 5% (GBP) to 16% (TPM); the rate of discontinuations ranged from 3% (GBP) to 24% (TPM). Levetiracetam was associated with the highest retention rates, with 60–75% of patients continuing treatment at 1 year. Most recently, results from open‐label extensions to Phase 3 double‐blind, placebo‐controlled trials have been reported for the ‘third‐generation’ AEDs lacosamide (LCM) 15 and perampanel (PER) 16. Outcomes were consistent with those reported in the meta‐analysis of second‐generation AEDs. With PER and LCM, 11% and 12% of patients, respectively, discontinued due to AEs. Although the 1‐year seizure‐free rate for PER was 7% vs 3% with LCM, only 40% of patients completed 1 year of PER treatment vs 75% with LCM.
Open‐label extensions of randomized, double‐blind, placebo‐controlled trials of AEDs as adjunctive therapy typically share very similar designs. Patients initially assigned to placebo are titrated to a starting dose of study medication during a blinded conversion lead‐in to open‐label treatment during which study medication dosages can be adjusted according to clinical response. The similar design of extension studies allows an indirect comparison of outcomes across studies. In the published meta‐analysis of second‐generation AEDs 7, GBP was the better tolerated therapy based on discontinuations due to AEs and TPM was associated with the highest seizure‐free rate, but LEV had the highest retention rate of the AEDs analyzed. Retention rate over the course of long‐term therapy is a global indicator of clinical effectiveness and the combination of treatment benefit (i.e., seizure control), tolerability, and adherence. Across second‐ and third‐generation AEDs, the highest reported retention rates were ~75% of patients completing 1 year of treatment. In the open‐label extension study reported here, nearly 85% of patients completed 1 year of SPN‐804 treatment, suggesting that SPN‐804 QD compares favorably with LEV, for example, in terms of patient retention during long‐term therapy.
As with virtually all of the open‐label extension studies of AEDs as adjunctive therapy, the limitations of the SPN‐804 extension to the PROSPER study include the lack of a comparator such as placebo or an active control. Efficacy outcomes may therefore reflect not only the AED's treatment effects but also spontaneous seizure remissions of variable duration. In addition, changes in AED co‐therapy (dosage adjustments, AED additions/withdrawals) may be underlying influences on seizure control. In terms of tolerability outcomes, a substantial proportion of patients entering open‐label extensions have already demonstrated an ability to tolerate study medication during double‐blind treatment and may therefore be less likely to withdraw due to AEs during an open‐label extension. Patient retention may also be influenced by having access to an investigational AED that is not otherwise available as well as the more intensive follow‐up that occurs in the setting of clinical studies. An open‐label extension study may therefore provide an imperfect profile of long‐term AED outcomes achieved when used in clinical practice in a broader patient population. However, results of open‐label extension studies combined with those of their companion double‐blind, randomized controlled trials provide clinicians with more complete information about an AED's potential usefulness in clinical practice.
The open‐label extension study reported here demonstrated that QD dosing with SPN‐804 improved and maintained seizure control during long‐term treatment as adjunctive therapy in adults with refractory partial‐onset seizures. Only 5% experienced treatment‐limiting AEs that resulted in discontinuation of study medication. The safety profile was favorable for long‐term therapy, with no new safety signals relative to short‐term exposure or to the known safety profile of OXC‐IR. With retention rate as a global measure of therapeutic effectiveness and usefulness, the finding that nearly 85% of patients continued SPN‐804 treatment for 1 year appears particularly noteworthy in light of similarly designed studies with second‐ and third‐generation AEDs as adjunctive therapy in adults with partial‐onset seizures.
Participating Investigators
United States: Bassel Abou‐Khalil, Nashville, TN; Julio Cantero, Sarasota, FL; Warren Chumley, Lexington, KY; Steve Chung, Phoenix, AZ; John DeCerce, Jacksonville, FL; Stephen Evans, Springfield, IL; Mark Fisher, Oklahoma City, OK; Stephen Flitman, Phoenix, AZ; Richard Hull, Huntsville, AL; Waseem Ibrahim, Riverside, CA; Alan F. Jacobson, Miami, FL; Batool Kirmani, Temple, TX; Pavel Klein, Bethesda, MD; Ricardo Pardo, Baytown, TX; Bashir Shihabuddin, Little Rock, AR; Laura Strom, Aurora, CO; Thomas Swanson, Missoula, MT; David Teeple, Tucson, AZ; Alexandre Todorov, Northport, AL; Blanca Vazquez, New York, NY; Roi Ann Wallis, Los Angeles, CA. Canada: Jean‐Francois Clement, Greenfield Park; Neelan Pillay, Calgary. Mexico: Jose Aleman‐Pedroza, Zapopan; Freddy Castro‐Farfan, Mexico City; Eduardo Dıaz‐Juarez, Durango; Juan Perez‐Garcia, Puebla; Sarug Reyes‐Morales, Aguascalientes; Ildefonso Rodriguez‐Leyva, San Luis Postosi; Jose Ruiz‐Sandoval, Guadalajara; Sandra Silva‐Sanchez, Chihuahua; Felipe Vega‐Boada, Mexico City. Bulgaria: Nadezhda Deleva, Varna; Sasho Kastrev, Blogoevgrad; Dimitar Maslarov, Sofia; Neli Petrova, Ruse; Penko Shotekov, Sofia; Paraskeva Stamenova, Sofia; Plamen Tsvetanov, Pleven. Croatia: Silvio Basic, Zagreb; Josip Glavic, Dubrovnik; Zdravka Poljakovic, Zagreb. Poland: Piotr Czapinski, Krakow; Anna Czlonkowska, Warszawa; Wojciech Moskal, Wilkowice; Ewa Motta, Katowice; Marcin Nastaj, Lublin; Grzegorz Opala, Katowice; Artur Radman, Gizycko; Jacek Rozniecki, Lodz; Maria Zubiel, Lodz. Romania: Corneliu Angelo Bulboaca, Cluj‐Napoca; Mirela Chiru, Bucharest; Silviu Manescu, Campulung Muscel; Ioan Marginean, Cluj‐Napoca; Cornelia Zaharia, Craiova. Russia: Gagik Avakyan, Moscow; Anna Belova, Nizhny Novgorod; Boris Beyn, Kirov; Enver Bogdanov, Kazan; Alexander Gustov, Nizhny Novgorod; Vitaliy Laskov, Kursk; Natalia Maslova, Smolensk; Gennadiy Mishin, Pyatigorsk; Irina Poverenova, Samara; Alexander Skoromets, St. Petersburg; Elena Vostrikova, Novosibirsk; Eduard Yakupov, Kazan.
Conflicts of interest
Steve Chung is a consultant for Acorda Therapeutics, Eisai, Lundbeck, SK Life Science, Sunovion, Supernus Pharmaceuticals, UCB Pharma, and Upsher‐Smith Laboratories; is on the speaker's bureau of Cyberonics, Eisai, GlaxoSmithKline, Lundbeck, Sunovion, Supernus Pharmaceuticals, and UCB S.A.; and receives grant and research support from Acorda Therapeutics, Eisai, GlaxoSmithKline, Medtronics, Lundbeck, SK Life Science, UCB Pharma, and Upsher‐Smith Laboratories. Janet K. Johnson and Scott T. Brittain are employees of Supernus Pharmaceuticals, Inc. Paolo Baroldi was an employee of Supernus Pharmaceuticals, Inc., at the time of this study.
Acknowledgements
This study was sponsored by Supernus Pharmaceuticals, Inc. Alan Blackburn and Verna Ilacqua, ID&A, provided editorial services, which were funded by Supernus Pharmaceuticals, Inc.
Chung SS, Johnson JK, Brittain ST, Baroldi P. Long‐term efficacy and safety of adjunctive extended‐release oxcarbazepine (Oxtellar XR®) in adults with partial‐onset seizures. Acta Neurol Scand 2016: 133: 124–130. © 2015 The Authors. Acta Neurologica Scandinavica Published by John Wiley & Sons Ltd.
References
- 1. Flesch G, Czendlik C, Renard D et al. Pharmacokinetics of the monohydroxy derivative of oxcarbazepine and its enantiomers after a single intravenous dose given as racemate compared with a single oral dose of oxcarbazepine. Drug Metab Dispos 2011;39:1103–10. [DOI] [PubMed] [Google Scholar]
- 2. Schmutz M, Brugger F, Gentsch C et al. Oxcarbazepine: preclinical anticonvulsant profile and putative mechanisms of action. Epilepsia 1994;35(Suppl 5):S47–50. [DOI] [PubMed] [Google Scholar]
- 3. Koch MW, Polman SK. Oxcarbazepine versus carbamazepine monotherapy for partial onset seizures. Cochrane Database Syst Rev 2009;CD006453. [DOI] [PubMed] [Google Scholar]
- 4. Schmidt D, Elger CE. What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? Epilepsy Behav 2005;5:627–35. [DOI] [PubMed] [Google Scholar]
- 5. Barcs G, Walker E, Elger C et al. Oxcarbazepine placebo‐controlled, dose‐ranging trial in refractory partial epilepsy. Epilepsia 2000;41:1597–607. [DOI] [PubMed] [Google Scholar]
- 6. French JA, Baroldi P, Brittain ST et al. Efficacy and safety of extended‐release oxcarbazepine (Oxtellar XR) as adjunctive therapy in patients with refractory partial‐onset seizures: a randomized controlled trial. Acta Neurol Scand 2014;129:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaccara G, Messori A, Cincotta M et al. Comparison of the efficacy and tolerability of new antiepileptic drugs: what can we learn from long‐term studies? Acta Neurol Scand 2006;114:157–68. [DOI] [PubMed] [Google Scholar]
- 8. Novartis Pharmaceuticals Corporation . Trileptal® (Oxcarbazepine) [package insert]. East Hanover, New Jersey: Novartis Pharmaceuticals Corporation. Rev. March 2011. [Google Scholar]
- 9. Chung S, Wang N, Hank N. Comparative retention rates and long‐term tolerability of new antiepileptic drugs. Seizure 2007;16:296–304. [DOI] [PubMed] [Google Scholar]
- 10. Bialer M, Soares‐da‐Silva P. Pharmacokinetics and drug interactions of eslicarbazepine acetate. Epilepsia 2012;53:935–46. [DOI] [PubMed] [Google Scholar]
- 11. Ben‐Menachem E, Gabbai A, Hufnagel A et al. Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res 2010;89:278–85. [DOI] [PubMed] [Google Scholar]
- 12. Elger C, Halász P, Maia J et al. Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial‐onset seizures: a randomized, double‐blind, placebo‐controlled, parallel‐group phase III study. Epilepsia 2009;50:454–63. [DOI] [PubMed] [Google Scholar]
- 13. Halasz P, Cramer JA, Hodoba D et al. Long‐term efficacy and safety of eslicarbazepine acetate: results of a 1‐year open‐label extension study in partial‐onset seizures in adults with epilepsy. Epilepsia 2010;51:1963–9. [DOI] [PubMed] [Google Scholar]
- 14. Hufnagel A, Ben‐Menachem E, Gabbai AA et al. Long‐term safety and efficacy of eslicarbazepine acetate as adjunctive therapy in the treatment of partial‐onset seizures in adults with epilepsy: results of a 1‐year open‐label extension study. Epilepsy Res 2013;103:262–9. [DOI] [PubMed] [Google Scholar]
- 15. Husain A, Chung S, Faught E et al. Long‐term safety and efficacy in patients with uncontrolled partial‐onset seizures treated with adjunctive lacosamide: results from a phase III open‐label extension trial. Epilepsia 2012;53:521–8. [DOI] [PubMed] [Google Scholar]
- 16. Krauss GL, Perucca E, Ben‐Menachem E et al. Perampanel, a selective, noncompetitive alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor antagonist, as adjunctive therapy for refractory partial‐onset seizures: interim results from phase III, extension study 307. Epilepsia 2013;54:126–34. [DOI] [PubMed] [Google Scholar]


