Abstract
Aims
To examine the efficacy and safety of once‐weekly dulaglutide monotherapy (0.75 mg) compared with placebo and once‐daily liraglutide (0.9 mg) in Japanese patients with type 2 diabetes.
Methods
This was a phase III, 52‐week (26‐week primary endpoint), randomized, double‐blind, placebo‐controlled, open‐label comparator (liraglutide) trial comparing 492 Japanese patients with type 2 diabetes (dulaglutide, n = 281; liraglutide, n = 141; and placebo, n = 70) who were aged ≥20 years. Patients and investigators were blinded to treatment assignment for dulaglutide and placebo but not for liraglutide. The primary objective evaluated the superiority of dulaglutide versus placebo on change from baseline in glycated haemoglobin (HbA1c) at 26 weeks. Analyses were performed on the full analysis set.
Results
At 26 weeks, once‐weekly dulaglutide was superior to placebo and non‐inferior to once‐daily liraglutide for HbA1c change from baseline [least squares mean difference: dulaglutide vs placebo −1.57% (95% confidence interval −1.79 to −1.35); dulaglutide vs liraglutide −0.10% (95% confidence interval −0.27 to 0.07)]. The most frequently reported adverse events were nasopharyngitis, constipation, diarrhoea, nausea, abdominal distension and decreased appetite; only decreased appetite was different between the dulaglutide and liraglutide groups [dulaglutide, n = 2 (0.7%); liraglutide, n = 8 (5.8%); p = 0.003]. Nine (1.8%) patients experienced hypoglycaemia [dulaglutide, n = 6 (2.1%); liraglutide, n = 2 (1.5%); placebo, n = 1 (1.4%)], with no event being severe.
Conclusions
In Japanese patients with type 2 diabetes, once‐weekly dulaglutide (0.75 mg) was superior to placebo and non‐inferior to once‐daily liraglutide (0.9 mg) for reduction in HbA1c at 26 weeks. Dulaglutide was safe and well tolerated.
Keywords: dulaglutide, GLP‐1 receptor agonist, liraglutide, placebo, type 2 diabetes
Introduction
Glucagon‐like peptide‐1 (GLP‐1) is a member of an endogenous class of incretin hormones synthesized in intestinal epithelial L‐cells as a response to gastrointestinal nutrients 1. GLP‐1 enhances glucose‐dependent secretion of insulin 2, 3, inhibits glucagon secretion 4, slows gastric emptying 5 and reduces food intake 6, 7.
Dulaglutide (Eli Lilly and Co., Indianapolis, IN, USA), a long‐acting GLP‐1 receptor agonist 8, mimics some endogenous GLP‐1 effects. Dulaglutide has been approved in the USA and European Union at once‐weekly doses of 0.75 and 1.5 mg as a subcutaneous injection to improve glycaemic control in patients with type 2 diabetes 9, 10, and has been approved in Japan at a once‐weekly dose of 0.75 mg to improve glycaemic control in patients with type 2 diabetes.
Dulaglutide has been modified to stabilize against dipeptidyl peptidase‐4 inactivation, increase the solubility of the peptide, reduce immunogenic potential and increase its activity duration. The pharmacokinetic half‐life of dulaglutide in Japanese patients is approximately 5 days, supporting once‐weekly dosing.
In global clinical trials completed to date, dulaglutide (1.5 mg) has been shown to be superior to metformin, sitagliptin and exenatide twice daily and non‐inferiority to liraglutide (1.8 mg) for glycated haemoglobin (HbA1c) changes, and has been associated with reductions in body weight in patients with type 2 diabetes 11, 12, 13, 14.
In the present study, we compared once‐weekly dulaglutide (0.75 mg) with placebo and once‐daily liraglutide (0.9 mg, the highest available dose in Japan), with regard to multiple efficacy and safety markers. The results from the present study were used to evaluate dulaglutide as a treatment for Type 2 diabetes in Japanese patients, and this study was the first comparison of a once‐weekly GLP‐1 receptor agonist with once‐daily liraglutide in Japanese patients.
Materials and Methods
Study Design and Participants
This study was a 52‐week, multicentre, randomized, placebo‐controlled, double‐blind (dulaglutide and placebo) and open‐label liraglutide comparator trial examining the efficacy and safety of once‐weekly dulaglutide monotherapy in Japanese patients with type 2 diabetes who were discontinued from their oral antidiabetic medication (OAM) monotherapy or were OAM‐naïve. These analyses present the data from this study through the 26‐week primary endpoint. These data were collected at 33 Japanese sites between April 2012 and October 2013. The study was registered with ClinicalTrials.gov (NCT01558271).
During the 2‐week screening period, patients were screened for eligibility and then entered a 2‐week lead‐in period for OAM‐naïve patients or an 8‐week wash‐out period for patients receiving OAM monotherapy.
Eligible Japanese subjects were male or female, aged ≥20 years, were OAM‐naïve (diet and exercise only) or had discontinued OAM monotherapy (excluding thiazolidinedione). Eligible patients had a body mass index (BMI) in the range of 18.5–35.0 kg/m2 and a confirmed HbA1c value at the randomization visit of 7.0–10.0%.The key exclusion criteria for patients screened were: type 1 diabetes, previous GLP‐1 receptor agonist treatment, treatment with more than half of the sulphonylurea maximum dose at screening, current insulin or thiazolidinedione use, chronic systemic glucocorticoid use, or gastric emptying abnormality. Eligible patients were randomized and treated during the 26‐week primary evaluation period. At 26 weeks, patients in the placebo group were switched to once‐weekly dulaglutide for the remainder of the 52‐week controlled study. At the completion of participation or early discontinuation, all patients were required to participate in a 30‐day safety follow‐up period.
A common protocol was approved at each site by the relevant institutional review board, and the study was performed in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice 15. Each patient provided written informed consent before participation.
Procedures
Eligible patients were randomized to treatment at a 4 : 2 : 1 ratio (dulaglutide: liraglutide: placebo). Randomization was stratified by pre‐study OAM status (yes/no), BMI group (<25 and ≥25 kg/m2), and HbA1c (≤8.5 or >8.5%). The randomization was carried out according to a computer‐generated random sequence with an interactive voice response system. Patients and investigators were masked to dulaglutide and placebo treatment assignments but were not masked to liraglutide treatment assignment.
Dulaglutide and placebo were provided as non‐identifiable solutions in prefilled syringes. Liraglutide was supplied as an open‐label pen. Subcutaneous blinded dulaglutide (or placebo) injections were initiated at the full dose. Subcutaneous liraglutide injections were uptitrated from 0.3 mg/day during week 1 to 0.6 mg/day during week 2 and 0.9 mg/day starting at week 3, according to the Japanese label. Patients not tolerating study treatment were discontinued from the study drug but remained in the study to collect safety data.
Hypoglycaemia was defined as a blood glucose concentration ≤3.9 mmol/l. Severe hypoglycaemia was defined as an episode that required the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions. Patients were allowed to initiate rescue therapy for severe, persistent hyperglycaemia according to predefined thresholds on fasting blood glucose for at least 2 weeks with no readily identifiable cause.
Deaths, cardiovascular adverse events and pancreatitis were adjudicated by separate independent, external committees, using prespecified criteria, study evidence and clinical knowledge and experience. All patients were tested for the development of dulaglutide antidrug antibodies.
Outcomes and Statistical Analyses
The primary objective was to show the superiority of dulaglutide vs placebo as measured by HbA1c change from baseline at 26 weeks. Key secondary objectives at 26 weeks were to show that dulaglutide was non‐inferior or superior to liraglutide on HbA1c change from baseline values using a serial gatekeeping strategy 16. Secondary objectives at 26 weeks evaluated the proportions of patients who achieved HbA1c targets (<7.0 or ≤6.5%), change in fasting serum glucose from baseline, change in seven‐point self‐monitored blood glucose (SMBG) profiles from baseline, and change in body weight from baseline. SMBG profiles were collected on two separate, non‐consecutive days within 2 weeks before baseline and weeks 14 and 26. Safety assessments included adverse events, vital signs (pulse rate and blood pressure), ECGs, laboratory variables and dulaglutide antidrug antibodies.
The sample size of at least 490 randomized patients was selected to provide >99% power to demonstrate superiority of dulaglutide to placebo. This assumed a true mean difference in HbA1c change from baseline between dulaglutide and placebo of 0.8%, a common standard deviation of 1.1%, a one‐sided significance level of 0.025, and a 9% drop‐out rate between randomization and week 26. Moreover, assuming no difference between dulaglutide and liraglutide, the given sample size provided at least 90% power to confirm non‐inferiority of dulaglutide to liraglutide with a margin of 0.4%.
Efficacy analyses used the full analysis set, defined as all randomized patients who took at least one dose of study drug. Safety analyses were conducted on the as‐treated population according to the patients' actual treatments (safety analysis set).
The primary efficacy analysis model was a mixed model for repeated measures for change from baseline to week 26 for HbA1c. The model included treatment, prestudy therapy (OAM: yes/no), baseline BMI (<25/≥25 kg/m2), visit, treatment by visit interaction as fixed effects, baseline HbA1c as a covariate, and patient as a random effect. An unstructured covariance structure was used to model the within‐patient errors. The 95% confidence interval (CI) for the treatment difference (dulaglutide − placebo) in the least‐squares (LS) mean at week 26 based on this model was used to assess the primary objective. If the primary objective was met, the key secondary hypotheses for non‐inferiority and superiority of dulaglutide compared with liraglutide were to be tested using a serial gatekeeping strategy to control the family‐wise type I error rate. Statistical analyses comparing liraglutide and placebo were not conducted.
For other continuous measurements, mixed model for repeated measures was performed with the same model as the primary efficacy analysis, with the relevant baseline value as a covariate. Seven‐point SMBG was analysed using an analysis of covariance model with terms for treatment, prestudy therapy and baseline BMI as fixed effects, and baseline value as a covariate.
For categorical measurements, the Cochran–Mantel–Haenszel test stratified by prestudy therapy and baseline BMI or Fisher's exact test was performed.
Results
Patients
Overall, 587 patients entered the study, 492 were randomized, 487 were treated with study drug, and 462 completed 26 weeks of treatment (Figure 1). Thirty patients discontinued the study [dulaglutide, n = 10 (3.6%); liraglutide, n = 13 (9.2%); and placebo, n = 7 (10.0%); p = 0.020], with ‘withdrawal by subject’ being the most common reason [dulaglutide, n = 6 (2.1%); liraglutide, n = 11 (7.8%); and placebo, n = 5 (7.1%); p = 0.011]. Patient demographics and baseline characteristics were similar between the groups (Table 1).
Figure 1.
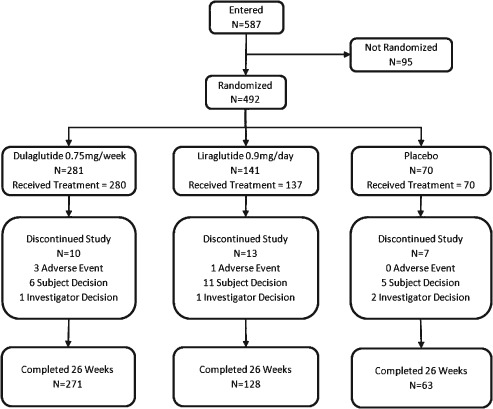
Trial profile. Patients were randomized to treatment at a 4 : 2 : 1 ratio (dulaglutide: liraglutide: placebo). N = number of patients.
Table 1.
Baseline demographics and characteristics
| Variable | Dulaglutide 0.75 mg (N = 280) | Liraglutide 0.9 mg (N = 137) | Placebo (N = 70) | Total (N = 487) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Men | 228 (81) | 113 (83) | 55 (79) | 396 (81) |
| Women | 52 (19) | 24 (18) | 15 (21) | 91 (19) |
| Mean (s.d.) age, years | 57.2 (9.6) | 57.9 (10.4) | 57.7 (8.3) | 57.4 (9.6) |
| Age ≥65 years, n (%) | 68 (24) | 39 (29) | 13 (19) | 120 (25) |
| Mean (s.d.) weight, kg | 71.3 (12.5) | 70.2 (12.5) | 69.3 (11.6) | 70.7 (12.4) |
| Mean (s.d.) BMI, kg/m2 | 25.6 (3.6) | 25.5 (3.5) | 25.2 (3.2) | 25.5 (3.5) |
| Mean (s.d.) diabetes duration, years | 6.8 (5.6) | 6.3 (6.0) | 6.3 (5.1) | 6.6 (5.6) |
| Mean (s.d.) HbA1c, % | 8.15 (0.77) | 8.08 (0.89) | 8.20 (0.83) | 8.14 (0.81) |
| HbA1c >8.5%, n (%) | 89 (32) | 42 (31) | 26 (37) | 157 (32) |
| Mean (s.d.) fasting serum glucose, mmol/l | 9.4 (1.9) | 9.0 (1.9) | 9.6 (2.2) | 9.3 (1.9) |
| Pre‐study OAM therapy, n (%) | 94 (34) | 48 (35) | 22 (31) | 164 (34) |
| OAM‐naïve, n (%) | 186 (66) | 89 (65) | 48 (69) | 323 (66) |
| Mean (s.d.) HOMA2‐%β (fasting insulin) | 34.5 (19.4) | 36.9 (20.3) | 33.0 (23.5) | 34.9 (20.3) |
| Mean (s.d.) HOMA2‐%S (fasting insulin) | 99.3 (53.8) | 100.7 (52.8) | 109.1 (57.8) | 101.1 (54.1) |
All patients were from Japan. BMI, body mass index; HbA1c, glycated haemoglobin; HOMA2‐%β, updated homeostasis model assessment of β‐cell function; HOMA2‐%S, updated homeostasis model assessment of insulin sensitivity; N, number of patients in full analysis set; OAM, oral antihyperglycaemic medication; s.d., standard deviation.
Efficacy
At 26 weeks, once‐weekly dulaglutide was superior to placebo for HbA1c change from baseline (p < 0.001; Figures 2A, 3A). Dulaglutide was also non‐inferior, but not superior, to once‐daily liraglutide (pnon‐inferiority < 0.001). The LS mean (standard error) changes in HbA1c from baseline to 26 weeks were −1.43% (0.05) for dulaglutide, −1.33% (0.07) for liraglutide, and 0.14% (0.10) for placebo. The LS mean difference between dulaglutide and placebo was −1.57% (95% CI −1.79, −1.35) and between dulaglutide and liraglutide was −0.10% (95% CI −0.27, 0.07). For each timepoint from baseline to primary endpoint, dulaglutide significantly reduced HbA1c compared with placebo (p < 0.001 all timepoints; Figure 2A).
Figure 2.

Glycated haemoglobin (HbA1c), fasting serum glucose and body weight baseline values up to 26 weeks. (A) HbA1c values from baseline to 26 weeks (%). (B) Fasting serum glucose values from baseline to 26 weeks (mmol/l). (C) Body weight change from baseline to 26 weeks. FSG, fasting serum glucose; LSM, least‐squares mean; s.e., standard error. *p < 0.001 dulaglutide vs placebo. §All dulaglutide comparisons vs placebo and liraglutide p > 0.05.
Figure 3.
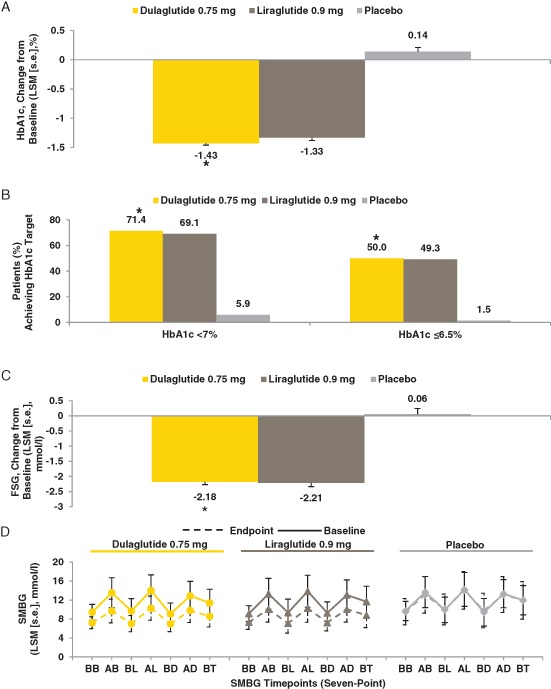
Glycated haemoglobin (HbA1c) change from baseline, HbA1c target, fasting serum glucose, and self‐monitored blood glucose. (A) Change in HbA1c from baseline at week 26 (%). (B) Proportions of patients achieving predefined HbA1c targets at week 26 (LOCF). (C) Change in fasting serum glucose from baseline at week 26 (mmol/l). (D) Seven‐point SMBG measurements at baseline and endpoint (week 26; LOCF) (mmol/l). AB, after breakfast; AD, after dinner; AL, after lunch; BB, before breakfast; BD, before dinner; BL, before lunch; BT, bedtime; FSG, fasting serum glucose; HbA1c, glycated haemoglobin; LOCF, last observation carried forward; LSM, least‐squares mean; s.e., standard error; SMBG, self‐monitored blood glucose. *p < 0.001 dulaglutide vs placebo.
At 26 weeks (LOCF), a significantly greater proportion of patients on dulaglutide achieved HbA1c <7.0% compared with placebo [dulaglutide, 200/280 (71.4%); placebo, 4/68 (5.9%); p < 0.001; Figure 3B]. A significantly greater proportion of patients on dulaglutide [140/280 (50.0%)] achieved HbA1c ≤6.5% compared with placebo [1/68 (1.5%)] at 26 weeks (LOCF; p < 0.001; Figure 3B). Proportions of patients who achieved HbA1c <7.0 and ≤6.5% were similar between dulaglutide and liraglutide at 26 weeks [LOCF, 200/280 (71.4%) vs 94/136 (69.1%) and 140/280 (50.0%) vs 67/136 (49.3%), respectively].
The LS mean changes in fasting serum glucose from baseline at week 26 were −2.18, −2.21 and 0.06 mmol/l for dulaglutide, liraglutide and placebo, respectively (Figures 2B, 3C); dulaglutide significantly reduced fasting serum glucose compared with placebo at week 26 (p < 0.001). Dulaglutide significantly reduced SMBG values from baseline compared with placebo for all measures (seven‐point profile values, mean SMBG values, 2‐h excursion values for each meal, and circadian variation; all p < 0.05), except for the 2‐h excursion for the evening meal (Figure 3D and Table S1). Dulaglutide treatment resulted in similar LS mean decreases from baseline in all SMBG values compared with liraglutide.
Treatment did not result in significant changes in body weight from baseline at week 26 (LS mean changes: dulaglutide, −0.02 kg; liraglutide, −0.36 kg; and placebo, −0.63 kg), and there were no significant differences between groups (Figure 2C).
Dulaglutide significantly increased the updated homoeostasis model assessment of β‐cell function from baseline compared with placebo (p < 0.001) at week 26 (LOCF); the difference between dulaglutide and liraglutide was not significant. No significant differences were observed between dulaglutide and the other treatment groups for the updated homoeostasis model assessment of insulin sensitivity (Figure S1).
Safety
No deaths were reported during the treatment period (Table 2). The incidence rates of serious adverse events and treatment‐emergent adverse events were similar between the groups up to 26 weeks (Tables 2 and S2). The most frequently reported treatment‐emergent adverse events (≥5% of patients in any group) are shown in Table 2; of these, only decreased appetite was different between dulaglutide and liraglutide [dulaglutide, n = 2 (0.7%); liraglutide, n = 8 (5.8%); p = 0.003].
Table 2.
Safety assessments and vital signs up to 26 weeks of treatment
| Dulaglutide 0.75 mg (N = 280) | Liraglutide 0.9 mg (N = 137) | Placebo (N = 70) | p value | ||
|---|---|---|---|---|---|
| Dulaglutide 0.75 mg vs liraglutide 0.9 mg | Dulaglutide 0.75 mg vs placebo | ||||
| Deaths | 0 | 0 | 0 | N/A | N/A |
| Serious adverse events* | 3 (1.1) | 2 (1.5) | 2 (2.9) | 0.665 | 0.262 |
| Patients with at least one treatment‐emergent adverse event | 157 (56.1) | 76 (55.5) | 39 (55.7) | 0.917 | >0.999 |
| Treatment‐emergent adverse events (≥5% in any group) | |||||
| Nasopharyngitis | 37 (13.2) | 16 (11.7) | 4 (5.7) | 0.755 | 0.097 |
| Decreased appetite | 2 (0.7) | 8 (5.8) | 0 | 0.003 | >0.999 |
| Gastrointestinal disorders | 61 (21.8) | 42 (30.7) | 11 (15.7) | 0.054 | 0.322 |
| Constipation | 19 (6.8) | 8 (5.8) | 3 (4.3) | 0.834 | 0.587 |
| Diarrhoea | 16 (5.7) | 5 (3.6) | 1 (1.4) | 0.477 | 0.212 |
| Nausea | 15 (5.4) | 11 (8.0) | 1 (1.4) | 0.289 | 0.211 |
| Abdominal distension | 6 (2.1) | 7 (5.1) | 0 | 0.133 | 0.604 |
| Patients who discontinued study due to an adverse event | 3 (1.1) | 1 (0.7) | 0 | >0.999 | >0.999 |
| Vital signs, mean change from baseline (s.e.)† | |||||
| Seated systolic blood pressure, mmHg | 0.62 (0.62) | −2.10 (0.89) | 0.53 (1.25) | 0.013 | 0.944 |
| Seated diastolic blood pressure, mmHg | 1.09 (0.39) | 0.43 (0.56) | 0.29 (0.78) | 0.336 | 0.360 |
| Seated pulse rate, bpm | 3.35 (0.45) | 4.77 (0.64) | 1.49 (0.90) | 0.070 | 0.064 |
| ECG PR interval mean change from baseline (s.e.)†, ms | 2.20 (0.60) | 2.07 (0.88) | −0.45 (1.22) | 0.899 | 0.052 |
| Pancreatic enzymes, median change (IQR) | |||||
| Total amylase, U/l‡ | 7.0 (2.0–15.0) | 7.0 (1.0–15.0) | 0.0 (−6.0–6.0) | 0.605 | <0.001 |
| Lipase, U/l‡ | 7.0 (1.0–13.0) | 11.0 (5.0–21.0) | 1.0 (−6.0–5.0) | <0.001 | <0.001 |
| Patients with treatment‐emergent abnormal changes in pancreatic enzymes (>ULN) | |||||
| Total amylase§ | 11/261 (4.2) | 8/124 (6.5) | 4/62 (6.5) | 0.450 | 0.500 |
| Lipase§ | 47/254 (18.5) | 36/121 (29.8) | 3/61 (4.9) | 0.017 | 0.006 |
| Patients with pancreatic enzyme concentration >3 × ULN | |||||
| Total amylase | 0 | 0 | 1 (1.5) | N/A | 0.191 |
| Lipase | 4 (1.5) | 2 (1.5) | 0 | 1.000 | 1.000 |
| Treatment‐emergent dulaglutide antidrug antibodies¶ | |||||
| Dulaglutide antidrug antibodies | 3 (1.1) | 0 | 0 | N/A | N/A |
| Dulaglutide neutralising antidrug antibodies | 0 | 0 | 0 | N/A | N/A |
| nsGLP‐1 cross‐reactive antibodies | 2 (0.7) | 0 | 0 | N/A | N/A |
| nsGLP‐1 neutralizing antibodies | 0 | 0 | 0 | N/A | N/A |
| Both nsGLP‐1 neutralizing and cross‐reactive antibodies | 0 | 0 | 0 | N/A | N/A |
Data are n (%) unless otherwise specified. Treatment‐emergent adverse events coded using MedDRA Version 16.1. IQR, interquartile range; MedDRA, Medical Dictionary for Regulatory Activities; N, number of patients in safety analysis set; N/A, not applicable; nsGLP‐1, native‐sequence glucagon‐like peptide‐1; s.e., standard error; ULN, upper limit of normal.
Reported serious adverse events are listed in Table S2.
Data are least‐squares mean change (s.e.).
Data are LOCF, median change (IQR).
Data represent the number of patients with treatment‐emergent abnormal change in pancreatic enzymes at week 26 over the number of patients with normal results at baseline.
These values include all postbaseline observations including the safety follow‐up.
One patient, in the dulaglutide group, reported severe constipation; all other treatment‐emergent gastrointestinal adverse events up to week 26 were considered to be of mild or moderate intensity. The numbers of patients in each group who discontinued the study because of an adverse event were dulaglutide, n = 3 (1.1%), liraglutide, n = 1 (0.7%) and placebo, n = 0 (Table 2). Six patients discontinued the study drug within the first 26 weeks of treatment because of treatment‐emergent gastrointestinal adverse events: dulaglutide, n = 1 (gastroenteritis) and liraglutide, n = 5 (abdominal discomfort, n = 2; constipation, decreased appetite and nausea, n = 1 for each).
Up to 26 weeks, 9 patients (1.8%) experienced any hypoglycaemia [dulaglutide, n = 6 (2.1%); liraglutide, n = 2 (1.5%); placebo, n = 1 (1.4%)]; no episodes of severe hypoglycaemia were reported. Two patients (0.7%; both dulaglutide) experienced nocturnal hypoglycaemia up to 26 weeks.
In a pairwise comparison at 26 weeks, dulaglutide significantly increased total amylase and lipase levels compared with placebo (p < 0.001; Table 2). No difference was observed between dulaglutide and liraglutide in changes in total amylase level. Liraglutide significantly increased lipase levels compared with dulaglutide (median increases were 11.0 and 7.0 U/l, respectively; p < 0.001; Table 2). No patients in the dulaglutide or liraglutide groups had amylase levels >3 × upper limit of normal (ULN; Table 2). Four (1.5%) patients in the dulaglutide group and 2 (1.5%) in the liraglutide group had lipase levels >3 × ULN; the elevated values decreased below 3 × ULN while the patients continued on study medication. None of these patients discontinued study treatment because of pancreatic adverse events. There were no adjudicated events of confirmed pancreatitis.
One patient, a 67‐year‐old female who was treated with liraglutide for approximately 15 weeks, was diagnosed with pancreatic carcinoma.
Seated vital signs and ECG PR interval are summarized in Table 2. There were no differences in systolic or diastolic blood pressure change from baseline between dulaglutide and placebo; liraglutide resulted in a significantly greater decrease from baseline in seated systolic blood pressure compared with dulaglutide (p = 0.013). Seated pulse rates were increased in all groups, with no significant differences between the groups. ECG PR interval was prolonged from baseline in dulaglutide compared with placebo (p = 0.052); the ECG PR interval increase with liraglutide was similar to dulaglutide. No confirmed adjudicated cardiovascular events were observed.
From baseline up to 26 weeks, all patients had serum calcitonin values within normal limits. The patient with pancreatic carcinoma described previously also had a thyroid neoplasm treatment‐emergent adverse event, which was considered to be of mild severity and not related to study drug by the investigator.
Dulaglutide significantly reduced urine albumin : creatinine ratio (p < 0.001) from baseline to 26 weeks compared with placebo (Table S3). Dulaglutide treatment resulted in significant percent reductions in triglycerides (−8.0%; p = 0.020) and total cholesterol (−2.9%; p = 0.009) from baseline compared with placebo (Table S4).
Three patients in the dulaglutide group had treatment‐emergent dulaglutide antidrug antibodies (Table 2).
Discussion
The aim of the present study was to examine the efficacy and safety of once‐weekly dulaglutide (0.75 mg) in Japanese patients with type 2 diabetes. Overall, in the first head‐to‐head GLP‐1 receptor agonist study in Japanese patients with type 2 diabetes, once‐weekly dulaglutide (0.75 mg) was non‐inferior to once‐daily liraglutide (0.9 mg) and superior to placebo in change from baseline HbA1c.
This is the first study to demonstrate the efficacy and safety of dulaglutide monotherapy in Japanese patients with type 2 diabetes, and these findings may help inform treatment decisions for those patients. This is also the first study in Japanese patients to show non‐inferiority of once‐weekly dulaglutide (0.75 mg) to once‐daily liraglutide (0.9 mg), the maximum doses evaluated in Japanese phase III registration programmes for these compounds. In other once‐weekly GLP‐1 receptor agonist studies DURATION‐6 [26 weeks: exenatide once weekly (AstraZeneca, London, UK)] and HARMONY‐7 [32 weeks: albiglutide (GlaxoSmithKline, Wilmington, DE, USA)], neither exenatide once weekly nor albiglutide demonstrated non‐inferiority to liraglutide (1.8 mg) on HbA1c change from baseline values [LS mean differences 0.21% (95% CI 0.08, 0.33), non‐inferiority margin of 0.25%; and 0.21% (95% CI 0.08, 0.34), non‐inferiority margin of 0.3%, respectively] 17, 18. AWARD‐6 (26 weeks: dulaglutide 1.5 mg) has been the only phase III study to date to demonstrate non‐inferiority to liraglutide (1.8 mg) on HbA1c change from baseline measurements, in mainly Caucasian patients with type 2 diabetes [LS mean difference −0.06% (95% CI −0.19, 0.07), with a non‐inferiority margin of 0.4%] 12.
The HbA1c reduction observed at 26 weeks in the present study in Japanese patients with type 2 diabetes is consistent with the upper range of reductions seen in the global AWARD studies for dulaglutide (0.75 or 1.5 mg) 11, 12, 13, 14. It has been previously reported that GLP‐1 receptor agonists exert greater HbA1c‐lowering effects in Asian compared with non‐Asian people, and it has been suggested that differences in BMI between the ethnicities may be a contributing factor in the observed differential effects 19. In the present study, once‐weekly dulaglutide (0.75 mg) resulted in approximately 70 and 50% of patients achieving HbA1c targets of <7.0 or ≤6.5% at week 26 (LOCF), respectively. These results were also consistent with a phase II study of dulaglutide in Japanese patients 20.
It has been reported that Japanese patients with type 2 diabetes tend to have a pathophysiology of insulin secretion impairment rather than insulin resistance and are inclined to be less obese compared with Western populations 21. Because GLP‐1 receptor agonists have a potential for improving β‐cell function and increasing insulin secretion, this class may be particularly effective in lean East‐Asian patients with type 2 diabetes. In the present study, both dulaglutide and liraglutide resulted in improvement in β‐cell function, consistent with previous reports for liraglutide 0.9 mg in a study of Japanese patients with type 2 diabetes 22.
Notably, there was no clinically relevant reduction in weight in any group over the 26‐week period. This was not unexpected and is consistent with observations in studies of liraglutide (0.9 mg) in Japanese patients 23, 24. The reason for this is unclear. One could speculate that it might be related to concomitant background medications or to lower baseline body weights in the Japanese population, or that it might be an effect of improvement in β‐cell function, as described below. The mean baseline body weight was approximately 70 kg (mean BMI 25.6 kg/m2), which is typical of Japanese patients with type 2 diabetes and lower than in Caucasian patients. In such patients, dulaglutide improved HbA1c regardless of weight change during treatment, possibly through improvement in β‐cell function rather than insulin resistance.
Overall, once‐weekly dulaglutide (0.75 mg) was generally well tolerated, with a low number of overall discontinuations in each group. The safety profile of dulaglutide in this study (Table 2) was similar to that seen in previous GLP‐1 receptor agonist studies 11, 12, 13, 14, 17, 18. The most‐frequently reported adverse event was nasopharyngitis, reported by 12–13% of patients in the active treatment groups. The incidence of nasopharyngitis in this study was not considered clinically relevant, and the difference between the treatment groups was not statistically significant. The incidence of gastrointestinal symptoms was also similar between dulaglutide and liraglutide. Nausea was transient and occurred most often during the first 2 weeks of treatment. Notably, decreased appetite occurred significantly more often with liraglutide than with dulaglutide. The incidence of hypoglycaemia was similar between the groups and very low as a whole, with no events of severe hypoglycaemia observed. These results are similar to those seen in the class 11, 12, 13, 14, 17, 18, 19, 23, indicating that GLP‐1 receptor agonists, including dulaglutide, when used as monotherapy, show a low propensity to cause hypoglycaemia.
Vital signs at week 26 showed mean increases in seated pulse rate in the dulaglutide and liraglutide groups, and these changes appear to be a GLP‐1 receptor agonist class effect 25.
Compared with placebo, dulaglutide resulted in statistically significant increases in amylase and lipase levels at week 26. Liraglutide resulted in a statistically significant increase in lipase level compared with dulaglutide. These findings are also consistent with a recent meta‐analysis of GLP‐1 receptor agonist clinical trials 26. These types of elevations were not predictive of pancreatitis during the dulaglutide global development programme 11, 12, 13, 14; however, the long‐term clinical significance of these elevations remains unclear. Acute pancreatitis was not observed in the present study.
The urine albumin : creatinine ratio and triglyceride levels were both decreased with dulaglutide and liraglutide. These pleiotropic effects might be a class effect of GLP‐1 receptor agonists. A recent report showed that liraglutide protected against albuminuria in streptozotocin‐induced diabetic rats in a protein kinase A‐mediated manner, which was not related to lowered glucose levels 27. Meier et al. 28 and Xiao et al. 29 have reported that GLP‐1 improves postprandial lipidaemia because of delayed gastric emptying and insulin‐mediated inhibition of lipolysis and intestinal lipoprotein production. The combined effects of lowered urine albumin : creatinine ratio, triglycerides and cholesterol levels with dulaglutide treatment may provide protection against cardiovascular disease. GLP‐1 receptor agonists also exhibit numerous overlapping and distinct actions in the cardiovascular system 30, 31. Several studies of cardiovascular event outcomes after treatment with GLP‐1 receptor agonists are currently ongoing; the results will provide more information on the clinical relevance of GLP‐1 receptor agonist treatment with regard to the cardiovascular system.
Three (1.1%) patients in the dulaglutide group and no patients in the liraglutide or placebo groups had treatment‐emergent dulaglutide antidrug antibodies detected. The incidence of antibodies in the present study was lower than in other GLP‐1 compounds with an exendin‐4 backbone (exenatide twice daily, 44–60% 32; exenatide once weekly, 61–68% 33, 34; and lixisenatide, approximately 70% 35); the lower incidence in the present study is probably attributable to the design of the dulaglutide molecule 36.
The present study has several limitations. An open‐label format for liraglutide was used because placebo pens mimicking liraglutide were not commercially available. The length of the study was relatively short in clinical practice with type 2 diabetes; however, the 26‐week primary endpoint was long enough to reach steady‐state in order to evaluate the dulaglutide treatment effect for the primary and secondary objectives on HbA1c. This randomized, controlled study continued for another 26‐week period, during which all patients on placebo were switched to dulaglutide to gather further safety data; these results will be reported at a later date.
In conclusion, monotherapy with once‐weekly dulaglutide (0.75 mg) was safe and effective in Japanese patients with type 2 diabetes and resulted in similar safety and efficacy profiles compared with once‐daily liraglutide (0.9 mg).
Conflict of Interest
All authors participated in reviewing and interpreting the data and providing comments and revisions to the manuscript. All authors approved the final version of the manuscript and take full responsibility for the content. J. M. was a trial investigator, participated in data collection, and has received: grants from Boehringer Ingelheim and Eli Lilly; and research support from Astra Zeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Kowa Pharmaceutical, Novartis Pharma, Ono Pharmaceutical and Sanwa Kagaku Kenkyusho. M. O. was a trial investigator, participated in data collection, and has received: grants from Eli Lilly, Novo Nordisk and Sanofi; advisory panel fees from Eli Lilly, Novo Nordisk and Sanofi; research support from Eli Lilly, Novo Nordisk and Sanofi; and speaker's bureau fees from Eli Lilly, Novo Nordisk and Sanofi. T. T. was a trial investigator, participated in data collection, and has received a grant from Eli Lilly and speaker's bureau fees from Eli Lilly and Novo Nordisk. N. I. prepared the first draft of the manuscript, was responsible for trial design and medical oversight during the trial, and is an employee of Eli Lilly Japan K.K. Y. T. prepared the first draft of the manuscript, was responsible for the statistical consideration in the analysis and trial design, and is an employee of Eli Lilly Japan K.K. T. I. prepared the first draft of the manuscript, was responsible for trial design and medical oversight during the trial, crafted the discussion and research context, is an employee of Eli Lilly Japan K.K. and has the company stock option.
Supporting information
Figure S1. Updated homeostasis model assessment changes from baseline at 26 weeks (LOCF).
Table S1. Summary of least squares (± standard error) mean changes from baseline in self‐monitored blood glucose (mmol/l) values (LOCF at week 26).
Table S2. Summary of serious adverse events by preferred term and treatment for the treatment period from baseline to 26 weeks.
Table S3. Summary and analysis of renal analytes: median baseline values and median changes from baseline to 26 weeks (LOCF).
Table S4. Summary and analysis of lipid profiles: median baseline values and median absolute and percent changes from baseline to 26 weeks (LOCF).
Acknowledgements
The trial was sponsored by Eli Lilly and Company. We would like to thank the study investigators, staff, and participants for their needed contributions. We would also like to thank Miwa Sakaridani for clinical trial management of the study. The authors would like to thank David Meats (inVentiv Health Clinical) for his medical writing contributions for the submission of this manuscript.
References
- 1. Anini Y, Brubaker PL. Muscarinic receptors control glucagon‐like peptide 1 secretion by human endocrine L cells. Endocrinology 2003; 144: 3244–3250. [DOI] [PubMed] [Google Scholar]
- 2. Parkes DG, Pittner R, Jodka C, Smith P, Young A. Insulinotropic actions of exendin‐4 and glucagon‐like peptide‐1 in vivo and in vitro. Metabolism 2001; 50: 583–589. [DOI] [PubMed] [Google Scholar]
- 3. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 4. Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon‐like peptide I(7–36) amide in type I diabetic patients. Diabetes Care 1996; 19: 580–586. [DOI] [PubMed] [Google Scholar]
- 5. Tong J, D'Alessio D. Give the receptor a brake: slowing gastric emptying by GLP‐1. Diabetes 2014; 63: 407–409. [DOI] [PubMed] [Google Scholar]
- 6. Turton MD, O'Shea D, Gunn I et al. A role for glucagon‐like peptide‐1 in the central regulation of feeding. Nature 1996; 379: 69–72. [DOI] [PubMed] [Google Scholar]
- 7. Gutzwiller JP, Drewe J, Göke B et al. Glucagon‐like peptide‐1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol 1999; 276: R1541–1544. [DOI] [PubMed] [Google Scholar]
- 8. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 728–742. [DOI] [PubMed] [Google Scholar]
- 9. Eli Lilly and Company . Trulicity [Prescribing information]. 2014. Available from URL: http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed 11 March 2015.
- 10. Eli Lilly and Company . Trulicity [Summary of product characteristics]. 2014. Available from URL: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002825/human_med_001821.jsp&mid=WC0b01ac058001d124. Accessed 11 March 2015.
- 11. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care 2014; 37: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dungan KM, Povedano ST, Forst T et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 13. Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care 2014; 37: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 14. Wysham C, Blevins T, Arakaki R et al. Efficacy and safety of dulaglutide added on to pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care 2014; 37: 2159–2167. [DOI] [PubMed] [Google Scholar]
- 15. World Medical Association . World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997; 277: 925–926. [PubMed] [Google Scholar]
- 16. Dmitrienko A, Tamhane A, Wiens B. General multistage gatekeeping procedures. Biom J 2008; 50: 667–677. [DOI] [PubMed] [Google Scholar]
- 17. Buse JB, Nauck M, Forst T et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION‐6): a randomised, open‐label study. Lancet 2013; 381: 117–124. [DOI] [PubMed] [Google Scholar]
- 18. Pratley RE, Nauck MA, Barnett AH et al. Once‐weekly albiglutide versus once‐daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open‐label, multicentre, non‐inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 2: 289–297. [DOI] [PubMed] [Google Scholar]
- 19. Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c‐lowering efficacy of glucagon‐like peptide‐1 analogues between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetes Obes Metab 2014; 16: 900–909. [DOI] [PubMed] [Google Scholar]
- 20. Terauchi Y, Satoi Y, Takeuchi M, Imaoka T. Monotherapy with the once‐weekly GLP‐1 receptor agonist dulaglutide for 12 weeks in Japanese patients with type 2 diabetes: dose‐dependent effects on glycaemic control in a randomised, double‐blind, placebo‐controlled study. Endocr J 2014; 61: 949–959. [DOI] [PubMed] [Google Scholar]
- 21. Hirose T, Kawamori R. Diabetes in Japan. Curr Diab Rep 2005; 5: 226–229. [DOI] [PubMed] [Google Scholar]
- 22. Seino Y, Rasmussen MF, Clauson P, Kaku K. The once‐daily human glucagon‐like peptide‐1 analog, liraglutide, improves B‐cell function in Japanese patients with type 2 diabetes. J Diabetes Investig 2012; 3: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose‐dependent improvement in glycemia with once‐daily liraglutide without hypoglycemia or weight gain: a double‐blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 81: 161–168. [DOI] [PubMed] [Google Scholar]
- 24. Kaku K, Rasmussen MF, Clauson P, Seino Y. Improved glycaemic control with minimal hypoglycaemia and no weight change with the once‐daily human glucagon‐like peptide‐1 analogue liraglutide as add‐on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 341–347. [DOI] [PubMed] [Google Scholar]
- 25. Robinson LE, Holt TA, Rees K, Randeva HS, O'Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta‐analysis. BMJ Open 2013; 3: e001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Glucagon‐like peptide‐1 receptor agonists and pancreatitis: a meta‐analysis of randomized clinical trials. Diabetes Res Clin Pract 2014; 103: 269–275. [DOI] [PubMed] [Google Scholar]
- 27. Hendarto H, Inoguchi T, Maeda Y et al. GLP‐1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin‐induced diabetic rats via protein kinase A‐mediated inhibition of renal NAD(P)H oxidases. Metabolism 2012; 61: 1422–1434. [DOI] [PubMed] [Google Scholar]
- 28. Meier JJ, Gethman A, Gotze O et al. Glucagon‐like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non‐esterified fatty acids in humans. Diabetologia 2006; 49: 452–458. [DOI] [PubMed] [Google Scholar]
- 29. Xiao C, Bandsma RHJ, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon‐like peptide receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol 2012; 32: 1513–1519. [DOI] [PubMed] [Google Scholar]
- 30. Seino Y, Yabe D. Glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1: incretin actions beyond the pancreas. J Diabetes Investig 2013; 4: 108–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ussher JR, Drucker DJ. Cardiovascular actions of incretin‐based therapies. Circ Res 2014; 114: 1788–1803. [DOI] [PubMed] [Google Scholar]
- 32. Kadowaki T, Namba M, Imaoka T et al. Improved glycemic control and reduced body weight with exenatide: a double‐blind, randomized, phase 3 study in Japanese patients with suboptimally controlled type 2 diabetes over 24 weeks. J Diabetes Investig 2011; 2: 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inagaki N, Atsumi Y, Oura T, Saito H, Imaoka T. Efficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral antidiabetes drug(s): results from a 26‐week, randomized, open‐label, parallel‐group, multicenter, noninferiority study. Clin Ther 2012; 34: 1892–1908.e1. [DOI] [PubMed] [Google Scholar]
- 34. Ji L, Onishi Y, Ahn CW et al. Efficacy and safety of exenatide once‐weekly vs exenatide twice‐daily in Asian patients with type 2 diabetes mellitus. J Diabetes Investig 2013; 4: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riddle MC, Aronson R, Home P et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care 2013; 36: 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glaesner W, Vick AM, Millican R et al. Engineering and characterization of the long‐acting glucagon‐like peptide‐1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev 2010; 26: 287–296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Updated homeostasis model assessment changes from baseline at 26 weeks (LOCF).
Table S1. Summary of least squares (± standard error) mean changes from baseline in self‐monitored blood glucose (mmol/l) values (LOCF at week 26).
Table S2. Summary of serious adverse events by preferred term and treatment for the treatment period from baseline to 26 weeks.
Table S3. Summary and analysis of renal analytes: median baseline values and median changes from baseline to 26 weeks (LOCF).
Table S4. Summary and analysis of lipid profiles: median baseline values and median absolute and percent changes from baseline to 26 weeks (LOCF).


