Abstract
Aims
Cardiopulmonary exercise test (CPET) provides parameters such as peak VO2 and ventilation/CO2 production (VE/VCO2) slope, which are strong prognostic predictors in patients with stable advanced chronic heart failure (ADHF). The study aim was to evaluate the effects of the inodilator levosimendan on CPET in patients with ADHF under stable clinical conditions.
Methods and results
We enrolled patients with ADHF (peak VO2 < 12 mL/min/kg) in a double‐blind, placebo‐controlled protocol. Patients were randomly assigned to i.v. infusion of placebo (500 mL 5% glucose; n = 19) or levosimendan (in 500 mL 5% glucose; n = 23). Before and 24 h after the end of the infusion, patients underwent determination of New York Heart Association class, B‐type natriuretic peptide (BNP), haemoglobin, serum creatinine, and blood urea nitrogen levels, as well as CPET, standard spirometry, and alveolar capillary gas diffusion. BNP showed no change with placebo (1042 ± 811 to 1043 ± 867 pg/mL), but it was decreased with levosimendan (1163 ± 897 to 509 ± 543 pg/mL, P < 0.001). No changes were observed for haemoglobin, creatinine, and blood urea nitrogen in either group. With levosimendan, a minor improvement was observed in spirometry measurements, but not in alveolar capillary gas diffusion. Peak VO2 showed a small, non‐significant increase with placebo (9.5 ± 1.7 to 10.0 ± 2.1 mL/kg/min, P = 0.12), and a greater increase with levosimendan (9.8 ± 1.7 to 11.0 ± 1.9 mL/kg/min, P < 0.005). The VE/VCO2 slope showed no change (44.0 ± 11 vs. 43.4 ± 10.3, P = 0.44), and a decrease (41.9 ± 10 vs. 36.6 ± 6.4, P < 0.001) in the placebo and in the levosimendan group, respectively.
Conclusion
Levosimendan treatment significantly improves peak VO2 and reduces VE/VCO2 slope and BNP in patients with ADHF.
Keywords: Advanced chronic heart failure, Cardiopulmonary exercise test, Levosimendan
Introduction
The efficacy of any treatment for heart failure (HF) is assessed according to both patient survival and quality of life, and through several analytical tools, which include laboratory, functional, and exercise‐derived parameters.1 As well as providing clinical efficacy, which is a qualitative evaluation, this has allowed a more quantitative and objective analysis. Among the different functional techniques, cardiopulmonary exercise test (CPET) provides multiple parameters, such as oxygen consumption (VO2) both at peak exercise and at anaerobic threshold, and the ventilatory equivalent ratio for carbon dioxide [i.e. ventilation vs. carbon dioxide production (VE/VCO2)]. VE/VCO2 is either determined as the ratio or as the slope of the relationship, and, along with VO2, it is used to estimate the prognosis of patients with HF,2, 3, 4 to evaluate the efficacy of any therapy,3 and to determine the way in which any intervention is effective.3 Spirometry and alveolar–capillary membrane diffusion, which is usually calculated as the carbon monoxide diffusing capacity (DLCO), are frequently combined with CPET to allow a more complete interpretation of CPET data. Of note, DLCO per se provides information on patient prognosis and on the mechanisms of drug action.5, 6, 7
In contrast, in acute HF, the efficacy of therapy is usually evaluated only in terms of survival, hospital stay, quality of life, haemodynamic measurements, and possible laboratory data, such as B‐type natriuretic peptide (BNP) levels.8, 9 However, these data are frequently inconsistent, and their interpretation can be difficult. For instance, repetitive and prolonged administration of clinical inotropes, such as dobutamine and milrinone, has been associated with favourable haemodynamic changes and symptom improvement,10 although serious safety concerns have emerged.11 In particular, two meta‐analyses of randomized trials of β‐adrenergic agonists and phosphodiesterase inhibitors have suggested that, despite acute clinical and haemodynamic improvements, there is increased mortality.12, 13
The inodilator levosimendan combines positive inotropic, vasodilatory, and cardioprotective effects, without evoking significant changes in myocardial oxygen requirements,14, 15, 16 and it has been shown to improve symptoms and haemodynamics in patients with acute HF.17 Phase III clinical studies with levosimendan have shown encouraging results in patients with acute HF.8, 18, 19 Some studies have suggested that levosimendan can reduce mortality in this setting,20, 21 although the evidence is not uniform.22, 23 An expert panel recently suggested that, based on the existing evidence, levosimendan might also be used in patients with advanced chronic HF (ADHF),24 although no evaluation of the effects of levosimendan on CPET parameters and DLCO has ever been carried out in this setting. Such an investigation would provide relevant information for the evaluation of levosimendan efficacy in patients with ADHF and possibly also provide some mechanistic information. We therefore analysed the effects of levosimendan infusion on CPET, standard spirometry, DLCO, and BNP values in a population of patients with ADHF who could tolerate an exercise test, with a peak VO2 < 12 mL/min/kg. The BNP values were used as an index of patients' haemodynamic status.1, 9
Methods
Patient selection
The present study was a single‐centre, double‐blinded, placebo‐controlled, randomized trial, and it has been registered at clinicaltrials.gov as NCT02261948.
Eligible patients had ADHF according to the European Society of Cardiology (ESC) definitions.1 Specifically, patients had severe HF symptoms [New York Heart Association (NYHA) classes III to IV], multiple episodes of fluid retention and/or peripheral hypoperfusion, and objective evidence of severe cardiac dysfunction. Moreover, patients had severe impairment of functional capacity, history of >1 HF hospitalizations in the past 6 months, and the presence of all the previous features despite optimal therapy.1 The infusions started approximately 3 h after the clinical, blood, and spirometry tests had been completed, which means after 48 to 72 h of clinical stabilization.
In the present study, patients were hospitalized for worsening HF, but, when recruited for the study, they had been returned to a stable clinical condition (NYHA III–IV), and they had been free from both inotrope support and other i.v. therapies, such as nitrate, nitroprusside, or dobutamine, for at least 48 h prior to study inclusion, except for diuretics where needed. The study inclusion criteria were a follows: left ventricular ejection fraction at echocardiography ≤35%, age ≥18 years, capability to perform CPET, peak VO2 < 12 mL/min/kg, peak respiratory quotient >1.05, and standard HF therapy.
The exclusion criteria were as follows: ongoing mechanical ventilation; recent or acute coronary and respiratory syndromes; recent sustained ventricular tachycardia or ventricular fibrillation; severe aortic or mitral valve disease, or known malfunctioning artificial heart valve; uncorrected obstructive valvular disease; hypertrophic cardiomyopathy; and uncorrected thyroid disease, or presence of any disease that might per se influence exercise performance. Patients with left ventricle assist devices, patients with a pacemaker‐guided heart rate at rest or during exercise, and patients in which levosimendan is contraindicated were also excluded. Finally, patients who had received levosimendan in the previous 6 months were excluded.
Patients were enrolled by specific study investigators (SM, PA), with randomization to placebo or levosimendan carried out by a designated employee of the hospital's pharmacy department who was not aware of the patients' data. The placebo and active drug infusions were indistinguishable, and both patients and investigators were blinded to treatment allocation. In parallel with the placebo infusion (500 mL 5% glucose solution), the active treatment (levosimendan 12.5 mg in 500 mL 5% glucose solution) was infused after 48 h of stable conditions, starting at 0.05 µg/kg/min (starting dose), and progressively increased up to 0.2 µg/kg/min, based on patient clinical status and blood pressure, until the entire infusion had been administered.
The protocol was approved by the Institutional Ethics Board (Cardiology Centre Ethical Committee, N° S199/312), and it was performed in compliance with institutional guidelines and with the Declaration of Helsinki. All patients provided written informed consent before entering the study.
Study procedures
A scheme of the study procedures is shown in Figure 1. All patients belonged to a cohort of HF patients regularly followed at our HF Unit. The patients had initially been hospitalized for worsening of their HF, and they were invited to participate in the study once they had been stabilized for at least 24 h, if they fulfilled the study inclusion/exclusion criteria. Their complete medical history, physical examination, and blood sample tests were recorded both before the treatment infusions and 24 h after the end of the treatment infusions, and the following were included: NYHA class, BNP, haemoglobin and creatinine levels, and blood urea nitrogen. Two‐dimensional standard echocardiography evaluation was also performed. Standard spirometry and DLCO measurements were performed before and 24 h after the treatment infusions, always before CPET. Forced expiratory volume in 1 s (FEV1) and vital capacity were measured in triplicate and calculated according to the American Thoracic Society criteria,25 using a mass flow sensor (SensorMedics 2200, Sensor Medics Co., Yorba Linda, CA, USA). DLCO was measured by the single‐breath constant‐expiratory‐flow technique (SensorMedics 229D, Sensor Medics Co.26). Dilution of CH4 was used to measure alveolar volume.
Figure 1.
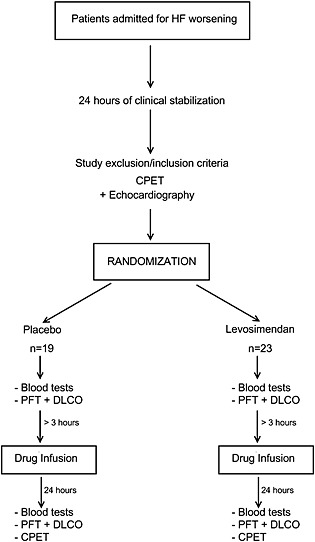
Scheme of the study protocol. CPET, cardiopulmonary exercise test; PFT, Pulmonary Function Test; HF, heart failure; DLCO, carbon monoxide diffusing capacity.
All patients had previously performed at least one CPET, which is routinely performed in our HF clinical follow‐up programme to optimize patient follow‐up. The prior CPET combined with the clinical conditions upon study entry was used to choose patients' personalized ramp exercise protocol, which was aimed at achieving peak exercise in 10 min.27 CPET was performed on a cycle ergometer (SensorMedics Ergo 800S, Sensor Medics Co.) before and 24 h after treatment infusions. If the exercise duration was <7 min, the CPET was repeated the following day; in these cases, the laboratory measurements were also repeated. Expiratory O2, CO2, and VE were measured breath by breath (Vmax 229D, Sensor Medics Co.). Peak VO2 was considered as the highest VO2 achieved during the exercise (mean, 20 s). The anaerobic threshold was measured as the V‐slope analysis of the plot for carbon dioxide production (VCO2) vs. VO2, on equal scales,28 and confirmed by changes in the ventilatory equivalent and end‐tidal oxygen pressure and carbon dioxide pressure (PetCO2). The VO2 vs. work‐rate relationship was calculated throughout the exercise. The VE vs. VCO2 relationship was calculated as the slope of the linear relationship between VE and VCO2 from the beginning of the loaded exercise to the end of the isocapnic buffering period. The ratios between VE and VCO2 and VO2 were calculated at the anaerobic threshold.29 Oxygen pulse was calculated as VO2/heart rate. Exercise‐induced periodic breathing was defined as a cyclic fluctuation of ventilation during exercise.30 Twelve‐lead electrocardiograms were also continuously recorded (Case 800, Marquette, Milwaukee, WI, USA). Blood pressure was measured during CPET every 2 min, by sphygmomanometer.
Study endpoints
The primary endpoint was peak VO2 changes after the placebo and levosimendan infusions. The secondary endpoints were changes in BNP and VE/VCO2 slope after the treatment administrations.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 software (SPSS Inc, Chicago, IL, USA). Continuous variables were expressed as means ± standard deviation (SD), and discrete variables as absolute numbers and percentages. Comparisons between the two treatment groups at baseline were performed using unpaired t‐tests for normally distributed variables, and Mann–Whitney U‐test for non‐normally distributed variables. P < 0.05 was considered statistically significant. Differences between the effects of infusion in the two treatment groups were assessed by computing the interaction term (infusion × treatment) in a repeated measures analysis of covariance. Correlations between changes in BNP, peak VO2, and the other evaluated parameters were investigated using linear regression analysis. The required sample size was determined as 42, to be sufficient to detect a peak VO2 difference of 2 mL/min/kg with an SD of 2, with 90% power and α = 0.05.
Results
Characteristics of the study group
Forty‐two patients who fulfilled the study inclusion/exclusion criteria were enrolled in the study: 19 patients received placebo and 23 received levosimendan. At study run‐in, the mean NYHA classes were 3.2 ± 0.4 and 3.3 ± 0.4 for placebo and active treatment, respectively. The baseline characteristics were well balanced between the two groups (Table 1). The HF treatments were in accordance with international guidelines (Table 1), and they were maintained during the study.
Table 1.
Baseline characteristics of the study population, according to treatment group
| Characteristic | Treatment group | P‐values | |
|---|---|---|---|
| Placebo (n = 19) | Levosimendan (n = 23) | ||
| Male [n (%)] | 16 (84) | 19 (83) | ns |
| Age (years) | 68.2 ± 9 | 70.3 ± 9.4 | ns |
| Diabetes [n (%)] | 3 (16) | 4 (17) | ns |
| Hypertension [n (%)] | 11 (58) | 14 (61) | ns |
| Current smoker [n (%)] | 1 (5) | 2 (8) | ns |
| Ischaemic cardiomyopathy [n (%)] | 11 (58) | 12 (52) | ns |
| Left ventricle ejection fraction (%) | 25 ± 6 | 25 ± 7 | ns |
| Telediastolic volume (mL) | 213 ± 78 | 187 ± 63 | ns |
| Telesystolic volume (mL) | 161 ± 69 | 142 ± 59 | ns |
| Pulmonary artery systolic pressure (mmHg) | 48 ± 13 | 45 ± 14 | ns |
| NYHA class | 3.2 ± 0.4 | 3.3 ± 0.4 | ns |
| Angiotensin‐converting enzyme inhibitors [n (%)] | 11 (58) | 15 (65) | ns |
| Angiotensin receptor blockers [n (%)] | 8 (42) | 8 (35) | ns |
| Aldosterone‐blocking agents [n (%)] | 14 (74) | 18 (78) | ns |
| β‐blockers [n (%)] | 19 (100) | 23 (100) | ns |
| Diuretics [n (%)] | 19 (100) | 23 (100) | ns |
| Nitrates [n (%)] | 6 (31) | 7 (30) | ns |
| Cardioaspirin [n (%)] | 11 (58) | 12 (52) | ns |
Data are expressed as means ± standard deviation, or n and %.
NYHA, New York Heart Association; ns, not significant.
Clinical, laboratory, and spirometry parameters
The infusions started approximately 3 h after the clinical, blood, and spirometry tests had been completed, which means after 48 to 72 h of clinical stabilization. The mean infusion rates were 16 ± 4 mL/h for placebo and 14 ± 4 mL/h for levosimendan (equivalent to 0.82 µg/kg/min levosimendan) (P = 0.34). The mean infusion durations were 31.3 ± 3.5 and 37.7 ± 4.0 h for placebo and active treatments, respectively (P = 0.28). Clinical, blood, and spirometry parameters in the two groups of patients before and 24 h after the end of the treatment infusions are reported in Table 2. Before the infusions, there were no significant differences between the two study groups for any of the investigated parameters, although there was a tendency towards a worse kidney function in the placebo group.
Table 2.
Changes in the laboratory and spirometry parameters pre‐infusion and post‐infusion for the placebo and levosimendan treatments
| Parameter | Placebo | P‐value | Levosimendan | P‐value | P‐value between treatmentsa | ||
|---|---|---|---|---|---|---|---|
| Pre‐infusion | Post‐infusion | Pre‐infusion | Post‐infusion | ||||
| HR rest (bpm) | 72.2 ± 9.1 | 67 ± 9.1 | 0.04 | 72.9 ± 10.4 | 78.4 ± 13.8 | 0.015 | 0.002 |
| Hb (g/dL) | 12.4 ± 1.7 | 12.3 ± 1.8 | ns | 12.4 ± 1.5 | 12.2 ± 1.5 | ns | ns |
| BNP (pg/mL) | 1042 ± 811 | 1043 ± 867 | ns | 1163 ± 897 | 510 ± 543 | <0.001 | <0.001 |
| Creatinine (mg/dL) | 1.7 ± 0.6 | 1.8 ± 0.5 | ns | 1.5 ± 0.5 | 1.6 ± 0.5 | ns | ns |
| BUN (mg/dL) | 95 ± 46 | 90 ± 28 | ns | 77 ± 36 | 79 ± 40 | ns | ns |
| FEV1 (L) | 1.8 ± 0.4 | 1.89 ± 0.4 | ns | 2.0 ± 0.53 | 2.1 ± 0.6 | 0.02 | ns |
| FEV1 (%pred) | 70 ± 14 | 71 ± 13 | ns | 79 ± 17 | 85 ± 20 | 0.01 | ns |
| VC (L) | 2.65 ± 0.6 | 2.66 ± 0.6 | ns | 2.7 ± 0.71 | 2.8 ± 0.7 | ns | ns |
| VC (%pred) | 76 ± 16 | 75 ± 13 | ns | 82 ± 18 | 86 ± 19 | 0.02 | 0.03 |
| DLCO (mL/mmHg/min) | 16.3 ± 3.9 | 16.9 ± 3.9 | ns | 14.9 ± 4.2 | 14.3 ± 4.1 | ns | 0.003 |
| DLCO (%pred) | 66 ± 12 | 68 ± 12 | ns | 64 ± 16 | 61 ± 14 | ns | ns |
| Va (L) | 4.3 ± 0.8 | 4.4 ± 1.4 | ns | 4.3 ± 1.0 | 4.4 ± 1.0 | ns | ns |
Data are expressed as means ± standard deviation.
BNP, brain natriuretic peptide; BUN, blood urea nitrogen; DLCO, lung diffusion for carbon monoxide adjusted for haemoglobin; FEV1, forced expiratory volume in 1 s; HR, heart rate; Hb, haemoglobin; Va, alveolar volume; VC, vital capacity.
Assessed by the infusion × treatment interaction.
Following the treatments, there was no significant change in the placebo group for the mean NYHA class (3.2 ± 0.4 to 3.1 ± 0.5, P = 0.15), while it was significantly reduced in the levosimendan group (3.3 ± 0.4 to 2.1 ± 0.3, P < 0.001) (Figure 2).
Figure 2.
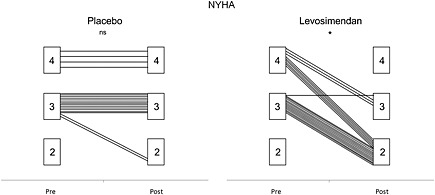
New York Heart Association (NYHA) class changes with placebo (left) and levosimendan (right). ns, non‐significant; asterisk, P < 0.001.
BNP also showed no changes with the placebo infusion (1042 ± 811 to 1043 ± 867 pg/mL, P = 0.9), but it was decreased significantly with the levosimendan infusion (1163 ± 897 to 509 ± 543 pg/mL, P <0.001) (Figure 3 A).
Figure 3.
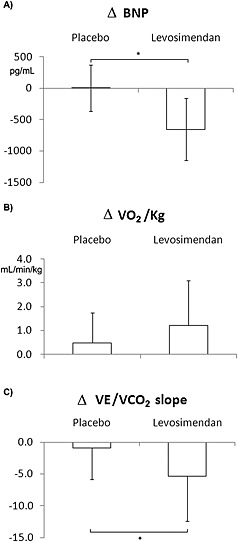
Changes in B‐type natriuretic peptide (BNP) (A), peak oxygen consumption (VO2) (B), and ventilation/CO2 production (VE/VCO2) slope (C) in patients treated with placebo and levosimendan. *, P < 0.02.
Improvements in FEV1 and VC, but not in DLCO, were also observed in the levosimendan‐treated patients (Table 2).
Cardiopulmonary exercise test parameters
The pre‐infusion and 24‐h post‐infusion CPET data are reported in Table 3. A minor peak VO2 increase was observed after the placebo treatment, although it did not reach statistical significance (P = 0.09), while the patients treated with levosimendan showed a significant peak VO2 improvement (Figure 3 B; Table 3; P = 0.005). Similarly, the peak workload showed no change after placebo treatment, but it significantly increased after levosimendan (Table 3; P = 0.002). The VE/VCO2 slope also showed no change after placebo treatment, but it significantly decreased after levosimendan (Figure 3 C; Table 3; P = 0.001).
Table 3.
Changes in the cardiopulmonary exercise test parameters pre‐infusion and post‐infusion for the placebo and levosimendan treatments
| Parameter | Placebo | P‐value | Levosimendan | P‐value | P‐value between treatmentsa | ||
|---|---|---|---|---|---|---|---|
| Pre‐infusion | Post‐infusion | Pre‐infusion | Post‐infusion | ||||
| VO2 anaerobic threshold (L/min) | 0.47 ± 0.14 | 0.56 ± 0.15 | 0.06 | 0.52 ± 0.13 | 0.54 ± 0.1 | ns | ns |
| Peak VO2 (L/min) | 0.71 ± 0.16 | 0.75 ± 0.18 | 0.07 | 0.68 ± 0.13 | 0.76 ± 0.13 | 0.006 | ns |
| Peak VO2 (mL/kg/min) | 9.5 ± 1.7 | 10.0 ± 2.1 | 0.09 | 9.8 ± 1.7 | 11.0 ± 1.9 | 0.005 | ns |
| Peak workload (W) | 41 ± 19 | 42 ± 20 | ns | 41 ± 14 | 48 ± 13 | 0.002 | 0.03 |
| Peak O2 pulse (mL/beat) | 8.4 ± 2.4 | 8.6 ± 2.2 | ns | 7.3 ± 2.2 | 7.4 ± 2.1 | ns | ns |
| Peak heart rate (beat/min) | 88 ± 18 | 90 ± 22 | ns | 98 ± 26 | 106 ± 27 | 0.004 | ns |
| Peak PetCO2 (mmHg) | 26 ± 4 | 26 ± 3 | ns | 27 ± 6 | 29 ± 4 | 0.04 | 0.06 |
| Peak PetO2 (mmHg) | 121 ± 3 | 121 ± 3 | ns | 120 ± 6 | 120 ± 6 | 0.06 | ns |
| Peak VE (L/min) | 39.2 ± 8.2 | 43.8 ± 7.1 | 0.08 | 39.2 ± 9.6 | 39.7 ± 9.6 | ns | ns |
| Peak respiratory quotient peak | 1.09 ± 0.15 | 1.10 ± 0.14 | ns | 1.13 ± 0.08 | 1.12 ± 0.11 | ns | ns |
| Peak VCO2 (L/min) | 0.79 ± 0.23 | 0.85 ± 0.26 | 0.06 | 0.77 ± 0.16 | 0.86 ± 0.19 | 0.015 | ns |
| VO2/workload slope (mL/min/W) | 9.0 ± 1.5 | 9.3 ± 1.1 | ns | 8.8 ± 1.8 | 9.2 ± 1.6 | ns | ns |
| VE/VCO2 slope | 44.0 ± 11 | 43.4 ± 10.3 | ns | 41.9 ± 10 | 36.6 ± 6.4 | 0.001 | 0.03 |
Data are expressed as means ± standard deviation.
VO2, oxygen consumption; Pet, end‐tidal pressure; VE, ventilation; VCO2, CO2 production.
Assessed by the infusion × treatment interaction.
There were some significant correlations between the pre‐infusion and post‐infusion BNP and peak VO2 changes for the patients in the levosimendan treatment group. In particular, the BNP reduction correlated with the reduction in the anaerobic threshold of VO2 (r = −0.398; P < 0.01), the VE/VCO2 slope reduction (r = −0.497; P < 0.001), and the VE/VO2 reduction (r = −0.488; P < 0.001). Similarly, the peak VO2 increase correlated with the VE/VCO2 slope reduction (r = −0.463; P < 0.002), the peak workload increase (r = 0.784; P < 0.001), and the peak PetCO2 increase (r = 0.444; P < 0.004).
Discussion
In the present study, we assessed the effects of a single levosimendan infusion vs. placebo in patients with ADHF, using CPET. With the levosimendan treatment, in parallel with a reduction in the resting BNP, there was an improvement in lung mechanics, but not in DLCO. We also observed a significant increase in peak VO2 and a significant decrease in VE/VCO2 slope. Of note, BNP, peak VO2, and VE/VCO2 parameters were all associated with HF severity and prognosis. Moreover, an improvement in peak VO2 is considered a pivotal marker to assess the efficacy of a therapy, and it is considered as a strong criterion to include, exclude, maintain, or withdraw a subject from a heart‐transplant list.31, 32
The populations that have been studied by most of the previous trials regarding inotropic agents were composed of patients with worsening HF, for whom it is difficult, if not impossible, to perform evaluations of functional capacity. Indeed, in this setting, any drug efficacy evaluation is limited to the clinical outcome, which is influenced by several uncontrolled variables, and to a few measured parameters and mainly haemodynamic variables and BNP values.
The patients included in the present study were all admitted to the HF Unit because of HF worsening, although when they were enrolled in this study, their ADHF was under stabilized conditions. We chose a peak VO2 < 12 mL/kg/min as the cut‐off for the inclusion in the present study because this is the cut‐off that has been used to assess patients who are candidates for heart transplantation if they are under treatment with β‐blockers, as all of the patients in the present study were.4, 31
At present, there remain some controversies about the efficacy of levosimendan in the setting of patients with ADHF. Indeed, in a recent study, Altenberger et al. 33 reported that intermittent ambulatory treatment with levosimendan in patients with ADHF improved functional capacity, as evaluated by 6‐min walk test, and quality of life, although this did not reach significance. However, the dose of levosimendan used by Altenberger et al. 33 was the lowest that has been used compared with other studies in the same patient population. Our patients and those of Altenberger et al. 33 shared some similarities; that is, in both cases, the patients with HF were able to exercise. However, there were also some differences with the Altenberger et al. 33 study, which included ambulatory patients who appeared likely to have had more prolonged stabilization compared with those in the present study. Moreover, we used a higher dose of levosimendan than Altenberger et al. 33 Finally, our study provides favourable results, but it was limited to a single levosimendan infusion, so that we do not know whether repeated infusions will provide similar results.
In the present study, NYHA class and BNP significantly decreased after the levosimendan infusion. Both are relevant clinical findings showing the efficacy of levosimendan infusion in this setting of ADHF patients. The NYHA class and >50% BNP reduction were observed over a very short time (2.5–3.0 days), and both are relevant and unexpected in their extent for a population of clinically stable patients. Previously, few reports have shown any reduction in BNP after levosimendan infusion, although the previous observations were on unstable patients.19, 20, 21, 22 Of note, the BNP reduction we report here showed strong correlations with VO2, VE/VO2, and VE/VCO2 at the anaerobic threshold. This confirms the strong dependence of the anaerobic threshold on the haemodynamic pattern.34, 35 Resting heart rate increased after levosimendan infusion and decreased with placebo. The former is an expected finding for a vasodilating drug as is levosimendan, while the limited heart rate reduction with placebo is a likely effect of concomitant therapy.
FEV1, VC, and DLCO are reduced in chronic HF patients, who usually show a restrictive pattern and alveolar capillary membrane dysfunction.35 Indeed, DLCO has been reported to correlate with HF prognosis and severity.6, 35 In terms of the spirometry data in the present study, we observed a small but significant improvement in the lung mechanics in the levosimendan‐treated patients, although there were no changes in DLCO. In the HF lung, mechanical improvements are frequently observed as a consequence of lung fluid reduction.36, 37 However, we did not observe DLCO changes. This finding is not unexpected, and indeed, the same result has been observed shortly after ultrafiltration, where lung mechanics improved and DLCO was unchanged.35 Similar findings have been reported during high‐altitude exposure, where DLCO decreases shortly after high‐altitude exposure because of lung fluid accumulation, whereas restoration of gas exchange needs prolonged exposure.38, 39
To the best of our knowledge, CPET has never been performed before to assess levosimendan efficacy. We observed a relevant and parallel amelioration of all of the major CPET‐derived parameters after levosimendan infusion. Of note, VO2 at peak exercise and at anaerobic threshold, VE/VCO2 slope, and PetCO2 are strong tools to assess HF severity.3 In contrast, in the placebo group, there was only a trend toward VO2 increase both at anaerobic threshold and under peak exercise, which is likely to be an effect of concomitant therapy for post‐acute decompensation.
A few study limitations should be acknowledged here. Firstly, we limited our observation to a single drug infusion, so that we do not know if it applies to subjects needing repeated levosimendan infusions. Secondly, we have performed a short‐term evaluation of levosimendan infusion. Therefore, we cannot say whether its benefit persists and how long it lasts in our population. Thirdly, we studied patients who were able to perform CPET. Accordingly, we excluded patients with more severe HF. Finally, our patients were free from HF comorbidities, which might have per se influenced their outcomes.
In conclusion, to the best of our knowledge, the present study is the first to describe the effects of levosimendan in terms of CPET. Our results show that levosimendan promoted peak VO2 and VE/VCO2 slope amelioration in parallel with a reduction in BNP. Our observations provide the basis for the use of levosimendan in the post‐acute phase of HF. Although the present study was limited to a single levosimendan infusion, it now provides a strong rationale to test the effects of multiple infusions of levosimendan at regular intervals in a population of patients with ADHF.
Conflict of interest
Orion Pharma supplied the placebo and levosimendan but had no role in the study design, data collection, data analysis, interpretation, or writing of this manuscript. Daniele Andreini and Gianluca Pontone are members of the speaker bureau of GE Healthcare. Gianluca Pontone is a member of the speaker bureau of Bayer, HeartFlow, and Medtronic. Piergiuseppe Agostoni is a consultant for Bayer and Menarini.
Mushtaq, S. , Andreini, D. , Farina, S. , Salvioni, E. , Pontone, G. , Sciomer, S. , Volpato, V. , and Agostoni, P. (2015) Levosimendan improves exercise performance in patients with advanced chronic heart failure. ESC Heart Failure, 2: 133–140. doi: 10.1002/ehf2.12047.
References
- 1. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 2. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167: 211–277. [DOI] [PubMed] [Google Scholar]
- 3. Piepoli MF, Corra U, Agostoni PG, Belardinelli R, Cohen‐Solal A, Hambrecht R, Vanhees L. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction. Recommendations for performance and interpretation. Eur J Cardiovasc Prev Rehabil 2006; 13: 10–12. [DOI] [PubMed] [Google Scholar]
- 4. Cattadori G, Agostoni P, Corra U, Di Lenarda A, Sinagra G, Veglia F, Salvioni E, La Gioia R, Scardovi AB, Emdin M, Metra M, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Magri D, Fiorentini C, Mezzani A, Scrutinio D, Pacileo G, Apostolo A, Iorio A, Paolillo S, Palermo P, Contini M, Giannuzzi P, Battaia E, Cicoira M, Passino C, Piepoli MF. Severe heart failure prognosis evaluation for transplant selection in the era of beta‐blockers: role of peak oxygen consumption. Int J Cardiol 2013; 168: 5078–5081. [DOI] [PubMed] [Google Scholar]
- 5. Agostoni P, Guazzi M, Bussotti M, De Vita S, Palermo P. Carvedilol reduces the inappropriate increase of ventilation during exercise in heart failure patients. Chest 2002; 122: 2062–2067. [DOI] [PubMed] [Google Scholar]
- 6. Agostoni P, Cattadori G, Bianchi M, Wasserman K. Exercise‐induced pulmonary edema in heart failure. Circulation 2003; 108: 2666–2671. [DOI] [PubMed] [Google Scholar]
- 7. Contini M, Apostolo A, Cattadori G, Paolillo S, Iorio A, Bertella E, Salvioni E, Alimento M, Farina S, Palermo P, Loguercio M, Mantegazza V, Karsten M, Sciomer S, Magri D, Fiorentini C, Agostoni P. Multiparametric comparison of CARvedilol, vs. NEbivolol, vs. BIsoprolol in moderate heart failure: the CARNEBI trial. Int J Cardiol 2013; 168: 2134–2140. [DOI] [PubMed] [Google Scholar]
- 8. Parissis JT, Panou F, Farmakis D, Adamopoulos S, Filippatos G, Paraskevaidis I, Venetsanou K, Lekakis J, Kremastinos DT. Effects of levosimendan on markers of left ventricular diastolic function and neurohormonal activation in patients with advanced heart failure. Am J Cardiol 2005; 96: 423–426. [DOI] [PubMed] [Google Scholar]
- 9. Avgeropoulou C, Andreadou I, Markantonis‐Kyroudis S, Demopoulou M, Missovoulos P, Androulakis A, Kallikazaros I. The Ca2+‐sensitizer levosimendan improves oxidative damage, BNP and pro‐inflammatory cytokine levels in patients with advanced decompensated heart failure in comparison to dobutamine. Eur J Heart Fail 2005; 7: 882–887. [DOI] [PubMed] [Google Scholar]
- 10. Young JB, Moen EK. Outpatient parenteral inotropic therapy for advanced heart failure. J Heart Lung Transplant 2000; 19: S49–57. [DOI] [PubMed] [Google Scholar]
- 11. Rapezzi C, Bracchetti G, Branzi A, Magnani B. The case against outpatient parenteral inotropic therapy for advanced heart failure. J Heart Lung Transplant 2000; 19: S58–63. [PubMed] [Google Scholar]
- 12. Thackray S, Easthaugh J, Freemantle N, Cleland JG. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure—a meta‐regression analysis. Eur J Heart Fail 2002; 4: 515–529. [DOI] [PubMed] [Google Scholar]
- 13. Tacon CL, McCaffrey J, Delaney A. Dobutamine for patients with severe heart failure: a systematic review and meta‐analysis of randomised controlled trials. Intensive Care Med 2012; 38: 359–367. [DOI] [PubMed] [Google Scholar]
- 14. Papp Z, Edes I, Fruhwald S, De Hert SG, Salmenpera M, Leppikangas H, Mebazaa A, Landoni G, Grossini E, Caimmi P, Morelli A, Guarracino F, Schwinger RH, Meyer S, Algotsson L, Wikstrom BG, Jorgensen K, Filippatos G, Parissis JT, Gonzalez MJ, Parkhomenko A, Yilmaz MB, Kivikko M, Pollesello P, Follath F. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol 2012; 159: 82–87. [DOI] [PubMed] [Google Scholar]
- 15. Lepran I, Pollesello P, Vajda S, Varro A, Papp JG. Preconditioning effects of levosimendan in a rabbit cardiac ischemia‐reperfusion model. J Cardiovasc Pharmacol 2006; 48: 148–152. [DOI] [PubMed] [Google Scholar]
- 16. Levijoki J, Pollesello P, Kaheinen P, Haikala H. Improved survival with simendan after experimental myocardial infarction in rats. Eur J Pharmacol 2001; 419: 243–248. [DOI] [PubMed] [Google Scholar]
- 17. Nieminen MS, Fruhwald S, Heunks LM, Suominen PK, Gordon AC, Kivikko M, Pollesello P. Levosimendan: current data, clinical use and future development. Heart, Lung and Vessels 2013; 5: 227–245. [PMC free article] [PubMed] [Google Scholar]
- 18. Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, Nyquist O, Remme WJ. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol 2000; 36: 1903–1912. [DOI] [PubMed] [Google Scholar]
- 19. Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, Hutchins S, Leier CV, LeJemtel TH, Loh E, Nicklas J, Ogilby D, Singh BN, Smith W. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Study investigators. Circulation 2000; 102: 2222–2227. [DOI] [PubMed] [Google Scholar]
- 20. Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low‐output heart failure (the LIDO study): a randomised double‐blind trial. Lancet 2002; 360: 196–202. [DOI] [PubMed] [Google Scholar]
- 21. Mebazaa A, Nieminen MS, Packer M, Cohen‐Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Poder P, Kivikko M. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA 2007; 297: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 22. Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, Padley RJ, Thakkar R, Delgado‐Herrera L, Salon J, Garratt C, Huang B, Sarapohja T. Effect of levosimendan on the short‐term clinical course of patients with acutely decompensated heart failure. JACC Heart Failure 2013; 1: 103–111. [DOI] [PubMed] [Google Scholar]
- 23. Landoni G, Biondi‐Zoccai G, Greco M, Greco T, Bignami E, Morelli A, Guarracino F, Zangrillo A. Effects of levosimendan on mortality and hospitalization. A meta‐analysis of randomized controlled studies. Crit Care Med 2012; 40: 634–646. [DOI] [PubMed] [Google Scholar]
- 24. Nieminen MS, Altenberger J, Ben‐Gal T, Bohmer A, Comin‐Colet J, Dickstein K, Edes I, Fedele F, Fonseca C, Garcia‐Gonzalez MJ, Giannakoulas G, Iakobishvili Z, Jaaskelainen P, Karavidas A, Kettner J, Kivikko M, Lund LH, Matskeplishvili ST, Metra M, Morandi F, Oliva F, Parkhomenko A, Parissis J, Pollesello P, Polzl G, Schwinger RH, Segovia J, Seidel M, Vrtovec B, Wikstrom G. Repetitive use of levosimendan for treatment of chronic advanced heart failure: clinical evidence, practical considerations, and perspectives: an expert panel consensus. Int J Cardiol 2014; 174: 360–367. [DOI] [PubMed] [Google Scholar]
- 25. Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 1957; 11: 290–302. [DOI] [PubMed] [Google Scholar]
- 26. Huang YC, Helms MJ, MacIntyre NR. Normal values for single exhalation diffusing capacity and pulmonary capillary blood flow in sitting, supine positions, and during mild exercise. Chest 1994; 105: 501–508. [DOI] [PubMed] [Google Scholar]
- 27. Agostoni P, Bianchi M, Moraschi A, Palermo P, Cattadori G, La Gioia R, Bussotti M, Wasserman K. Work‐rate affects cardiopulmonary exercise test results in heart failure. Eur J Heart Fail 2005; 7: 498–504. [DOI] [PubMed] [Google Scholar]
- 28. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986; 60: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 29. Sun XG, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med 2002; 166: 1443–1448. [DOI] [PubMed] [Google Scholar]
- 30. Corra U, Pistono M, Mezzani A, Braghiroli A, Giordano A, Lanfranchi P, Bosimini E, Gnemmi M, Giannuzzi P. Sleep and exertional periodic breathing in chronic heart failure: prognostic importance and interdependence. Circulation 2006; 113: 44–50. [DOI] [PubMed] [Google Scholar]
- 31. Costanzo MR, Augustine S, Bourge R, Bristow M, O'Connell JB, Driscoll D, Rose E. Selection and treatment of candidates for heart transplantation. A statement for health professionals from the Committee on Heart Failure and Cardiac Transplantation of the Council on Clinical Cardiology, American Heart Association. Circulation 1995; 92: 3593–3612. [DOI] [PubMed] [Google Scholar]
- 32. Florea VG, Henein MY, Anker SD, Francis DP, Chambers JS, Ponikowski P, Coats AJ. Prognostic value of changes over time in exercise capacity and echocardiographic measurements in patients with chronic heart failure. Eur Heart J 2000; 21: 146–153. [DOI] [PubMed] [Google Scholar]
- 33. Altenberger J, Parissis JT, Costard‐Jaeckle A, Winter A, Ebner C, Karavidas A, Sihorsch K, Avgeropoulou E, Weber T, Dimopoulos L, Ulmer H, Poelzl G. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail 2014; 16: 898–906. [DOI] [PubMed] [Google Scholar]
- 34. Koike A, Weiler‐Ravell D, McKenzie DK, Zanconato S, Wasserman K. Evidence that the metabolic acidosis threshold is the anaerobic threshold. J Appl Physiol (1985) 1990; 68: 2521–2526. [DOI] [PubMed] [Google Scholar]
- 35. Agostoni PG, Wasserman K, Perego GB, Guazzi M, Cattadori G, Palermo P, Lauri G, Marenzi G. Non‐invasive measurement of stroke volume during exercise in heart failure patients. Clin Sci (Lond) 2000; 98: 545–551. [PubMed] [Google Scholar]
- 36. Marenzi G, Muratori M, Cosentino ER, Rinaldi ER, Donghi V, Milazzo V, Ferramosca E, Borghi C, Santoro A, Agostoni P. Continuous ultrafiltration for congestive heart failure: the CUORE trial. J Card Fail 2014; 20: 378.e371–379. [PubMed] [Google Scholar]
- 37. Agostoni PG, Marenzi GC, Pepi M, Doria E, Salvioni A, Perego G, Lauri G, Giraldi F, Grazi S, Guazzi MD. Isolated ultrafiltration in moderate congestive heart failure. J Am Coll Cardiol 1993; 21: 424–431. [DOI] [PubMed] [Google Scholar]
- 38. Agostoni P, Swenson ER, Fumagalli R, Salvioni E, Cattadori G, Bussotti M, Tamplenizza M, Lombardi C, Bonacina D, Brioschi M, Caravita S, Modesti P, Revera M, Giuliano A, Meriggi P, Faini A, Bilo G, Banfi C, Parati G. Acute high‐altitude exposure reduces lung diffusion: data from the HIGHCARE Alps project. Respir Physiol Neurobiol 2013; 188: 223–228. [DOI] [PubMed] [Google Scholar]
- 39. Agostoni P, Swenson ER, Bussotti M, Revera M, Meriggi P, Faini A, Lombardi C, Bilo G, Giuliano A, Bonacina D, Modesti PA, Mancia G, Parati G. High‐altitude exposure of three weeks duration increases lung diffusing capacity in humans. J Appl Physiol (1985) 2011; 110: 1564–1571. [DOI] [PubMed] [Google Scholar]


