Summary
Aims
Hyponatraemia (HN) is the most common electrolyte balance disorder in clinical practice. Since the 1970s, demeclocycline has been used in some countries to treat chronic HN secondary to syndrome of inappropriate antidiuretic hormone secretion (SIADH). The precise mechanism of action of demeclocycline is unclear, but has been linked to the induction of nephrogenic diabetes insipidus. Furthermore, the safety profile of demeclocycline is variable with an inconsistent time to onset, and a potential for complications. There has been no systematic evaluation of the use of demeclocycline for the treatment of HN secondary to SIADH to date. A systematic literature review was performed to obtain an insight into the clinical safety and efficacy of demeclocycline for this condition.
Methods
Embase™, MEDLINE ®, MEDLINE ® In‐Process, and The Cochrane Library were searched on two occasions using MeSH terms combined with free‐text terms. References were screened by two independent reviewers. Relevant publications were then extracted by two independent reviewers, with a third reviewer collating and finalising extractions.
Results
The searches returned a total of 705 hits. 632 abstracts were screened after the removal of duplicates. Following screening, 35 full‐length publications were reviewed. Of these, 17 were excluded, resulting in 18 studies deemed relevant for data extraction. Two were randomised controlled trials (RCTs), 16 were non‐RCTs, and 10 were case reports.
Discussion
Although most reports suggest that demeclocycline can address serum sodium levels in specific patients with HN, efficacy is variable, and may depend upon the underlying aetiology. Demeclocycline dose adjustments can be complex, and as its use in clinical practice is not well defined, it can differ between healthcare professionals.
Conclusion
There is a lack of clinical and economic evidence supporting the use of demeclocycline for HN secondary to SIADH. Patients receiving demeclocycline for HN secondary to SIADH must be closely monitored.
Review criteria
Embase™, MEDLINE®, MEDLINE® In‐Process, and The Cochrane Library were searched on two occasions using relevant MeSH terms combined with free‐text terms. References were screened by two independent reviewers. Relevant publications were then extracted by two independent reviewers, with a third reviewer collating and finalising extractions.
Message for the clinic
There is a lack of high‐quality clinical evidence supporting the therapeutic use of demeclocycline for HN secondary to SIADH. The safety profile of demeclocycline is variable with an inconsistent time to onset, and a potential for complications. Economic evaluations or HRQoL studies of demeclocycline for the treatment of HN secondary to SIADH have not been performed. Patients receiving demeclocycline must be closely monitored.
Introduction
Hyponatraemia (HN), defined as a serum sodium concentration < 135 mmol/l, is the most common metabolic disorder of body water and total body sodium concentration. HN is usually associated with a disturbance in vasopressin [antidiuretic hormone (ADH)], which can be synthesised independently of serum osmolality or circulating fluid volume under specific pathological or pharmacological conditions 1. Clinical criteria for the syndrome of inappropriate antidiuretic hormone secretion (SIADH) were established by Bartter and Schwartz 2 and have remained largely unchanged ever since 1. SIADH accounts for one‐third of all cases of HN 3.
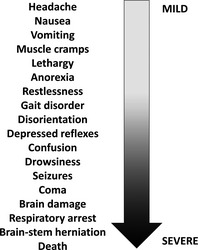
Hyponatraemia occurs in approximately 30% of hospitalised patients 1, 4 and is a clinical feature in 15–20% of emergency admissions 1. HN has a wide range of aetiologies across several patient populations. In the great majority of patients, its aetiology is multifactorial 5. HN secondary to SIADH is associated with significant morbidity 6, mortality 7, 8, 9, 10 and increased length of hospital stay 11. Symptoms are associated primarily with the severity of the HN, and range from confusion and vomiting in mild HN (< 135 mmol/l sodium) to seizures, coma and death in severe cases (< 125 mmol/l sodium; Figure 1) 4, 12, 13. In addition to symptoms, patients are subject to serious sequelae of HN, including gait abnormalities, falls 14, and an increased fracture risk 15. Such morbidities are clinically significant, as HN is particularly common in elderly people 12.
Figure 1.
There is a substantial economic healthcare burden associated with HN, which include significant increases in length of hospital stay, hospital costs and intensive care unit (ICU) costs, as well as an increase in the risk of ICU admission and 30‐day hospital readmission. Studies in the USA estimate that direct costs for treating HN range from $1.6 to $3.6 billion annually 16.
Current management strategies for acute symptomatic HN secondary to SIADH comprise hypertonic (3%) saline given either via bolus or continuous intravenous infusion, with or without diuretics. Patients with euvolaemic hypo‐osmolality because of SIADH do not respond to isotonic saline, and occasionally, this causes HN to worsen 16. In chronic HN (< 135 mmol/l for > 48 h) secondary to SIADH, fluid restriction is considered first‐line treatment in patients without severe symptoms or where hypovolaemia is not suspected 1, 16. Fluid restriction between 500 and 1000 ml/day is recommended, according to HN severity 17. Pharmacological therapy generally comprises a vaptan (specific vasopressin‐2 receptor antagonists) or demeclocycline in some countries (e.g. France and the UK); other options may include urea or loop diuretics or combinations of above treatments 16.
The aim of HN management was to normalise the serum sodium concentration whilst avoiding the risk of osmotic demyelination syndrome (ODS; previously known as central pontine myelinolysis). It is important to limit the correction of sodium to a maximum rate of 12 mmol/24 h, and to this end, it is important that patients are in an environment suitable for monitoring with regular estimations of sodium levels. If HN is known to be of short duration (< 48 h), correction may be carried out at a faster rate as the risk of cerebral oedema in a patient with severely symptomatic HN outweigh the risks of ODS. It should also be remembered that often, the aim was to restore the sodium to a ‘safe’ level rather than achieve sodium normalisation. Most physicians suggest regular review of sodium levels and less invasive action once sodium levels reach 120–125 mmol/l 16.
Demeclocycline is a tetracycline derivative antibiotic, with a marketing authorisation in the UK for the treatment of chronic HN associated with SIADH secondary to malignant disease, where water restriction is ineffective and the patient does not have concomitant cirrhosis 18. In France, demeclocycline is reimbursed for the treatment of SIADH, particularly with an origin of paraneoplastic syndrome with chronic hyponatraemia (< 125 mmol/l) associated with inappropriate natriuresis, and/or clinical signs associated with hyponatraemia resistant to fluid restriction 19. Although its mode of action has not yet been established, it is thought that demeclocycline induces nephrogenic diabetes insipidus in approximately 60% of patients with HN secondary to SIADH, resulting in decreased urine concentration and rebalancing of body sodium concentrations 12, 16. Onset of action is unpredictable, usually occurring after 2–5 days, but occasionally taking longer 3, 12, 16.
Treatment with demeclocycline has been associated with gastrointestinal intolerance, including nausea and vomiting, nephrotoxicity and renal failure 18; cases of reversible renal failure have also been reported 20, 21, 22. As with all antibiotics, overgrowth of resistant organisms may cause candidiasis 18.
To our knowledge, there has been no systematic evaluation of the use of demeclocycline for the treatment of HN secondary to SIADH to date, and its use appears to be supported by subjective familiarity rather than objective evidence. Therefore, a systematic literature review of all available evidence was performed to obtain an insight into the clinical safety and efficacy of demeclocycline for this condition. To further understand the financial impact of treating with demeclocycline, an economic component was included within the search criteria.
Methods
A comprehensive search strategy was employed following Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) standards 23. Embase™, MEDLINE®, MEDLINE® In‐Process, and The Cochrane Library were searched using Medical Subject Heading (MeSH) terms, combined with free‐text terms (see Appendix A for full search strings). Disease search terms comprised terminological variations of SIADH, HN and key clinical indicators (e.g. sodium serum and urine osmolality). References were then limited to those relating to demeclocycline, and considered relevant if primary data sources examined the use of demeclocycline in patients with HN because of SIADH.
Inclusion criteria for the search output were limited to any study examining the clinical efficacy or long‐term/real‐world effectiveness of demeclocycline, case reports with more than five patients where only clinical efficacy (rather than safety) was reported, or case reports with one or more patients reporting any safety outcomes. In addition, studies presenting cost‐effectiveness or health‐related quality of life (HRQoL) data for demeclocycline in the treatment of HN associated with SIADH were also included.
Potentially relevant references identified from the search output were screened electronically by two independent reviewers and any differences subsequently adjudicated by a third reviewer. Full publications of the resulting, potentially relevant studies were then reviewed before the included study list was finalised. Relevant publications were extracted into a predesigned data extraction table by two independent reviewers, with a third reviewer collating and finalising the extractions. Papers published in languages other than English or French were included only if sufficient data were available in English‐language abstracts. Quality assessment was conducted for randomised controlled trials (RCTs) and cohort studies using the 2011 Scottish Intercollegiate Guidelines Network checklists (www.sign.ac.uk).
The comprehensive literature search was performed on 28 May 2014. Subsequently, a second search was undertaken to identify relevant studies published between March and December 2014; searches occurred on the 9 December 2014 and were performed to an identical protocol.
Results
Search outputs
Searches outputs from the initial protocol (28 May 2014) returned 679 hits. After the electronic removal of 71 duplicate references, 608 abstracts were screened. Upon completion of dual electronic screening, the full‐length publications of 25 studies were ordered for full review; five further studies were available as conference abstracts only. Of these, 12 were excluded (Appendix B summarises the excluded papers), resulting in 18 studies deemed relevant for data extraction.
Results from the second, updated literature search (March to December 2014) produced 26 hits. After the removal of two duplicate references, 24 abstracts were screened; five studies were identified for full review following electronic screening. Of these, all abstracts were excluded (Appendix B), resulting in no additional studies deemed relevant for data extraction (Figure 2).
Figure 2.
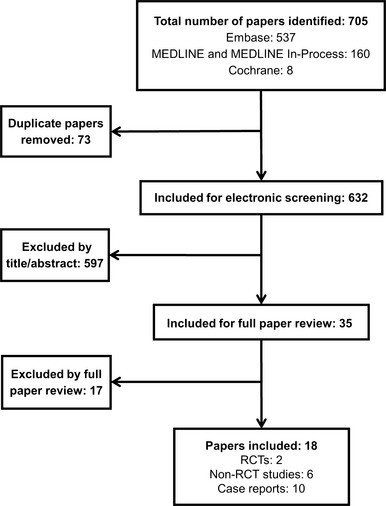
Combined PRISMA flow diagram for the original systematic review and update, showing the total combined publications detected in the original and updated literature searches. As there was a 3‐month overlap between search dates, some publications may be detected twice. An adjustment for this possibility was not made. RCT, randomised controlled trial
Systematic review
Of the 18 studies deemed relevant for data extraction, two RCTs were identified (Table 1) 24. Alexander et al. compared demeclocycline with placebo in nine patients with chronic psychiatric illness and episodic or chronic HN (< 135 mmol/l) associated with polydipsia 24; Horattas et al. compared demeclocycline with placebo in 30 patients undergoing elective coronary artery bypass grafting (CABG) 25.
Table 1.
Included randomised controlled trials – study design
| Author, year [study name] | Patient population | Study design | Eligibility criteria | Interventions | Study outcomes | Demeclocycline dose |
|---|---|---|---|---|---|---|
| Alexander et al. 1991 24 | Nine psychiatric patients (six male), aged 31–47 years (mean, 38.3 years), with polydipsia‐HN | Randomised, double‐blind, placebo‐controlled cross‐over trial | Chronic psychiatric illness and documented episodic or chronic HN (< 135 mmol/l) associated with polydipsia |
Demeclocycline or placebo Stable daily doses of neuroleptics |
Body weight; serum sodium levels; number of episodes of sodium levels <125 or < 135 mmol/l | 300 mg for 3 weeks (twice daily for 7 days; three times daily for 7 days; four time daily for 7 days) |
| Horattas et al. 1998 25 | Thirty patients (20 male), aged 40–70 years (mean, 61.4 years), undergoing elective coronary artery bypass grafting | Randomised, double‐blind, placebo‐controlled clinical study |
Normal electrolytes and renal function Exclusions: renal or hepatic dysfunction; endocrine abnormalities; pregnancy; recent antibiotic use; hypersensitivity to tetracycline |
600 mg DMC or placebo twice daily, beginning 5 days pre‐operatively and continuing through postoperative day 2 Pain medication and/or antihypertensives |
Serum electrolytes; complete blood counts; prothrombin time; partial thromboplastin time; arterial blood gases; urine electrolytes; osmolality; serum vasopressin | 600 mg (twice daily) from day 5 pre‐operation to day 2 post operation |
DMC, demeclocycline; HN, hyponatraemia.
In total, 16 non‐RCTs were considered relevant, comprising six cohort studies (four observational studies 20, 22, 26, 27, two retrospective chart analyses 21, 28, and 10 case reports 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 (Tables 2 and 3, respectively); no cost‐effectiveness or HRQoL studies were identified. Quality assessments for all studies other than case reports are presented (Appendix C).
Table 2.
Included cohort studies – study design
| Author, year | Patient population | Study design | Eligibility criteria | Interventions | Study outcomes | Demeclocycline dose |
|---|---|---|---|---|---|---|
| De Troyer et al. 1977 20 | Seven male patients with lung carcinoma, aged 48–76 years | Observational study | SIADH | DMC | Serum sodium; urine osmolality; blood urea, creatinine; water clearance | 300 mg DMC four times daily, reduced to 600 mg/day after 10 days. |
| Forrest et al. 1978 26 | Ten patients (eight male) with chronic SIADH, aged 6–68 years | Observational study | HN despite fluid restriction; SIADH |
DMC Three patients given lithium prior to DMC. |
Serum sodium; urine osmolality; urinary sodium excretion | 600–1200 mg DMC daily given to 10 patients. |
| Goldman and Luchins, 1985 27 | Eight psychiatric patients (seven male), aged 43–54 years, with polydipsia‐HN | Observational study | Compulsive water drinkers; hyponatraemic (115–130 mmol/l); not taking carbamazepine |
DMC Stable daily doses of neuroleptics |
Serum sodium; urine osmolality | 600 mg (twice daily) for 3 weeks |
| Perks et al. 1979 22 | Fourteen patients (seven male); mean age of 61 years. | Observational study | Diagnosis of SIADH based on De Troyer and Demanet (1976) criteria 37 | DMC | Serum electrolytes, urea, creatinine, osmolarity and packed cell volume | 1200 mg/day |
| Brewerton and Jackson, 1994 28 | Six psychiatric patients (one male), aged 37–80 years (mean 56.0 years) | Retrospective chart analysis | Patients taking carbamazepine who were forced to discontinue because of HN not associated with psychogenic polydipsia |
DMC Carbamazepine was administered concomitantly or initiated 3–7 days after DMC |
Mean serum sodium |
300 mg (twice daily), increased to 600 mg (twice daily) by day 3–5 Average duration of treatment was 11.3 days |
| Trump, 1981 21 | Patients with malignancies who received DMC for4 or more days forserious HN (n = 15) | Retrospective review of patient records | Serious HN (< 125 mmol/l); no oedema or dehydration; normal serum creatinine and urea nitrogen |
DMC Seven patients were additionally receiving corticosteroids |
Serum sodium, osmolality, urea nitrogen; urine sodium, osmolality; average daily intake/output; bilirubin, weight | 600–1200 mg/day |
DMC, demeclocycline; HN, hyponatraemia; SIADH, symptom of inappropriate antidiuretic hormone secretion.
Table 3.
Relevant case reports – study summary
| Author, year | Patient population | Interventions | Outcome | Author conclusion |
|---|---|---|---|---|
| Antonelli et al. 1993 29 |
A 59‐year‐old man Slight fever, dyspnoea, weight loss, asthenia and mental slowness |
300 mg DMC twice daily for about 3 weeks Water restriction Hypertonic saline iv Corticosteroid treatment for 10 months |
Patient developed phosphate diabetes after 4 days of DMC Progressive amelioration of the symptomatology and resolution of SIADH after DMC discontinuation and the start of corticosteroids |
Phosphate diabetes may be related to selective DMC‐induced tubulopathy |
| Curtis et al. 2002 30 |
A 94‐year‐old man hospitalised following collapse Diagnosis of SIADH |
300 mg DMC three times daily plus fluid restriction Nine days after admission, DMC increased to four times daily Metronizadole Benzydamine hydrochloride mouth washes |
Fatal acute renal failure |
Short‐term DMC can effectively control symptomatic HN but caution is required because of its potential nephrotoxicity Authors note a danger of dehydration in patients whose fluid intake has become compromised Authors suggest that had DMC been discontinued once the HN had resolved, the development of acute renal failure would have been avoided |
| Danovitch et al. 1978 31 |
Two patients: 1. A 51‐year‐old man with oat‐cell carcinoma of the lung. Diagnosis of SIADH 2. A 60‐year‐old‐man with 4‐week history of confusion and drowsiness. Diagnosis of SIADH |
1. 1200 mg DMC daily plus cyclophosphamide 2. 1200 mg DMC daily |
DMC treatment corrected HN and hypo‐osmolality, but was discontinued in both patients owing to deterioration of renal function |
Potentially dangerous side effects exclude routine use of DMC Overall, DMC is effective in the treatment of SIADH, but has a potential to lead to a deterioration of renal function |
| Decaux et al. 1981 33 |
A 76‐year‐old man admitted to hospital because of grand mal seizures SIADH diagnosed |
A single dose of DMC Water restriction Urea infusion iv Furosemide |
Patient had severe gastric intolerance to DMC and the drug was stopped after the first dose | HN was controlled with furosemide after intolerance to DMC |
| Decaux et al. 1985 32 |
A 62‐year‐old woman Heavy smoker reporting weakness and memory loss Diagnosis of SIADH Oat‐cell carcinoma |
300 mg DMC twice daily | DMC corrected HN but led to phosphate diabetes |
Phosphate diabetes appeared after therapy with DMC and persisted for 3 months The augmentation in phosphate clearance was unrelated to serum sodium levels Phosphate diabetes was related to selective DMC‐induced renal toxicity |
| Heim et al. 1977 34 |
75‐year‐old woman, decline in general condition and pleural effusion (Two further cases not receiving DMC are reported) |
DMC 1200 mg/day | HN was corrected by fluid restriction; addition of DMC resulted in increased fluid clearance, but the patient developed vomiting and diarrhoea on day 4 and DMC was stopped on day 7 |
DMC is a relatively non‐toxic antibiotic which was shown to be effective; however, treatment was interrupted on day 8 owing to vomiting and diarrhoea The dose of 1200 mg/day may have been too strong for this patient |
| Padfield et al. 1978 35 | A 64‐year‐old man with SIADH following head injury and meningitis |
300 mg DMC four times daily followed by 600 mg DMC four times daily Fixed fluid intake |
600 mg DMC four times daily rapidly corrected all biochemical features of SIADH Acute renal failure was possibly induced by DMC On discontinuation of DMC normal renal function returned. |
DMC corrected the biochemical abnormalities of SIADH Possible evidence of nephrotoxicity |
| Perks et al. 1976 36 |
A 61‐year‐old man with memory deficit Inoperable carcinoma diagnosed 7 months after original admission |
600 mg DMC daily Fluid restriction |
Treatment with DMC led to clinical and biochemical improvement |
DMC is a safe and effective treatment that in this case allowed discharge from hospital This regimen may simplify outpatient management of other patients with this syndrome |
| Shimoda et al. 1986 (note, published in Japanese) 38 |
A 63‐year‐old woman hospitalised after losing consciousness; prior surgery for ruptured anterior artery Diagnosis of SIADH |
900 mg/day DMC 16 mg/day dexamethasone |
Patient became comatose and developed quadriplegia after rapid correction of serum sodium levels Patient died of septic shock after 12 months of hospitalisation |
Computed tomography scans and brain stem auditory responses were indicative of ODS |
| Soudan and Qunibi, 2012 39 |
A 78‐year‐old woman with history of hypothyroidism, vitiligo, rheumatoid arthritis and early Alzheimer's dementia Hospitalised with severe HN diagnosed as secondary to SIADH. |
300 mg DMC three times daily. Fluid restriction Normal saline infusion Furosemide Existing medication: Methotrexate Memantine HCL Mirtazapine Sulfamethoxazole and trimethoprim |
Patient developed severe HN as a result of rigid fluid restriction and DMC therapy Serum sodium levels stabilised after discontinuation of DMC |
DMC should be reserved for patients unable tolerate or unwilling to follow strict fluid restriction |
DMC, demeclocycline; HN, hypernatraemia; iv, intravenous; ODS, osmotic demyelination syndrome; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
RCT evidence for the effectiveness and safety of demeclocycline
A comparison of demeclocycline with placebo in nine patients with chronic psychiatric illness suggested, there are no significant differences in mean serum sodium concentration after 3 weeks' treatment (131.4 mmol/l and 134.1 mmol/l, respectively). There were no significant differences between treatments in the frequency of episodes of serum sodium levels < 125 mmol/l (p = 0.78). All patients experienced episodes of HN during the study; no adverse events related to demeclocycline were reported 24.
Horattas et al. reported a prospective, double‐blind, placebo‐controlled study of 30 patients undergoing elective CABG. Patients were randomised to receive either placebo or demeclocycline 1200 mg/day at pre‐operative Day 5 and continuing through to postoperative Day 2. Following placebo, serum sodium concentration was significantly reduced from pre‐operative levels while serum sodium and osmolality was within normal range in the demeclocycline group (p < 0.01); urine osmolality increased significantly with placebo on postoperative Day 1 (p = 0.04). At postoperative Day 2, serum sodium concentrations in the placebo group continued at a reduced level compared with baseline, but not with demeclocycline 25. Two patients discontinued demeclocycline owing to skin hypersensitivity and gastrointestinal upset, and four reported mild gastrointestinal upset 25. RCT data for the effectiveness and safety of demeclocycline are presented in Table 4.
Table 4.
Efficacy and safety results – randomised controlled trial summary
| Author, year | Serum sodium (pretreatment) | Serum sodium (post treatment) | Urine sodium (pretreatment) | Urine sodium (post treatment) | AEs (total) | AEs (individual) | SAEs (total) | Author conclusion | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Alexander et al. 1991 24 |
On placebo: Mean of 131.4 mmol/l in third week Episodes of serum sodium <125 mmol/l, 13 of 110 measurements |
On DMC: Mean of 134.1 mmol/l in third week Episodes of serum sodium <125 mmol/l, 10 of 103 measurements |
NR | NR | 0 | 0 | 0 | No significant effect of DMC on serum sodium of in psychotic patients with polydipsia‐HN. | Patients not explicitly diagnosed with SIADH |
| Horattas et al. 1998 25 |
Pre‐operative: DMC mean: 138.9 mmol/l Placebo mean: 138.1 mmol/l |
Postoperative day 2: DMC mean: 138 mmol/l approx. Placebo mean: 135.5 mmol/l approx. |
NR | NR | Two patients discontinued |
Hypersensitivity (rash) leading to discontinuation: 1/30 GI upset leading to discontinuation: 1/30 Mild GI upset: 4/30 |
No specific SAEs reported, but 2 patients discontinued | Administration of DMC can inhibit vasopressin secretion following urgery | Postoperative length of stay was not affected by DMC |
AE, adverse event; DMC, demeclocycline; GI, gastrointestinal; HN, hyponatraemia; NR, not reported; SAE, serious adverse event; SIADH, symptom of inappropriate antidiuretic hormone secretion.
Non‐randomised cohort studies of demeclocycline
Four observational cohort studies were identified and reported a total of 39 patients with HN secondary to SIADH. All four studies reported an increase in mean serum sodium levels during treatment with demeclocycline at doses of up to 1200 mg/day 20, 22, 26, 27.
Two studies reported adverse events associated with demeclocycline, including azotemia (nine patients in total; two discontinued demeclocycline), renal function impairment (two patients) and nausea (one patient) 20, 22. Demeclocycline effectiveness and safety data reported in observational cohort studies are presented in Table 5. Two retrospective chart analyses reported the impact of demeclocycline in a further 21 patients 21, 28.
Table 5.
Efficacy and safety results – cohort studies summary
| Author, year | Serum sodium (pretreatment) | Serum sodium (post treatment) | Urine sodium (pretreatment) | Urine sodium (post treatment) | AEs (individual) | SAEs (total) | Author conclusion | Comments |
|---|---|---|---|---|---|---|---|---|
| De Troyer et al. 1977 20 | 120.4 ± 2.4 mmol/l |
135.7 ± 4.2 mmol/l (p < 0.0005 vs before) |
NR | NR |
One patient had a rise in blood urea level Two patients had renal function impairment because of treatment One patient experienced mild nausea |
NR | Although DMC moderately impairs renal function, it appears to be the treatment of choice in the chronic form of SIADH | Clinical benefits were reported in five of seven patients |
| Forrest et al. 1978 26 | 122 mmol/l | 139 mmol/l | 98 mmol/l | 40 mmol/l | NR | NR |
DMC is superior to lithium in the treatment of SIADH and may obviate the need for severe water restriction No side effects were observed in this study |
Seven patients had carcinoma; one had glioma of the hypothalamus, one had a basal skull fracture and one had SIADH of unknown cause |
| Goldman and Luchins, 1985 27 |
Mean: 128.1 mmol/l |
During treatment, mean: 131.9 mmol/l After treatment, mean: 128.2 mmol/l |
NR | NR | NR | NR | DMC reduces the frequency and severity of HN episodes in chronic psychotics | Only five of eight patients met criteria for SIADH |
| Perks et al. 1979 22 | 118 ± 8 mmol/l |
138 ± 7 mmol/l after a mean of 8.6 ± 5.3 days (In six patients who discontinued, serum sodium fell to 125 ± 7 mmol/l) |
54 ± 24 mmol/l (n = 7) |
29 ± 21 mmol/l (n = 7) |
Eight patients developed azotemia | Two patients discontinued DMC owing to azotemia |
The adverse effects of DMC may be more important than previously suggested The 1200 mg dose may be associated with an increased likelihood of impaired renal function |
|
| Brewerton and Jackson, 1994 28 |
Carbamazepine: 130.1 ± 5.9 mmol/l |
Carbamazepine plus DMC: 136.7 ± 6.1 mmol/l |
NR | NR | NR | NR | DMC appears to have prevented carbamazepine‐induced HN in five of six patients | |
| Trump, 1981 21 | 119 mmol/l | Mean peak serum sodium: 138.8 mmol/l by day 9 (peak achieved after 3–28 days) | 92 mmol/l | NR |
Azotemia: Serum urea nitrogen > 5 mg/dl: 12 of 15 patients Serum urea nitrogen > 25 mg/dl: 6 of 15 patients |
Three patients died within 10 days of receiving DMC 1200 mg/day; causes were deemed to be advanced infections or malignancy; all three had marked azotemia |
Authors suggest that renal dysfunction associated with DMC may have contributed to the deaths of three patients Most (five of six) patients with severe azotemia were also receiving other nephrotoxic or antianabolic agents. |
Patients not explicitly diagnosed with SIADH |
AE, adverse event; CI, confidence interval; CNS, central nervous system; DMC, demeclocycline; GI, gastrointestinal; HN, hyponatraemia; NR, not reported; SAE, serious adverse event; SIADH, symptom of inappropriate antidiuretic hormone secretion
Brewerton et al. reported on six psychiatric patients with a history of carbamazepine responsiveness who developed clinically significant carbamazepine‐induced HN. Treatment with demeclocycline was associated with significant increases in serum sodium levels in five patients compared with pretreatment concentrations (increases from 130.1 to 136.7 mmol/l). Safety data were not reported in this study 28.
A retrospective review of 15 patients with malignancies treated with demeclocycline for serious HN, without a specific diagnosis of SIADH, reported an increase in mean serum sodium. From pretreatment values of 119 mmol/l, sodium levels were raised to a mean peak of 139 mmol/l after 3–28 days 21.
In total, 80% of patients (n = 12) developed azotemia and three died within 10 days of receiving demeclocycline. Although the deaths were because of advanced infections and malignancy, three presented with marked azotemia; the renal dysfunction associated with demeclocycline was considered as a possible contributor to the deaths. Of the six patients with severe azotemia (serum urea nitrogen > 25 mg/dl), five patients were receiving concomitant nephrotoxic or antianabolic agents: aminoglycoside antibiotics, amphotericin B and corticosteroid 21.
Case reports involving demeclocycline
Ten case reports, detailing 11 patients in total, presented safety data pertaining to demeclocycline 29, 30, 31, 32, 33, 34, 35, 36, 37, 38. Demeclocycline effectiveness and safety data reported in case studies are presented in Table 6.
Table 6.
Efficacy and safety results – case reports summary
| Author, year | Patient population | Interventions | Outcome | Author conclusion |
|---|---|---|---|---|
| Antonelli et al. 1993 29 | A 59‐year‐old man; slight fever, dyspnoea, weight loss, asthenia and mental slowness |
300 mg DMC twice daily for about 3 weeks Water restriction Hypertonic saline iv Corticosteroid treatment for 10 months. |
Patient developed phosphate diabetes after 4 days of DMC Progressive amelioration of the symptomatology and resolution of SIADH after DMC discontinuation and the start of corticosteroids | Phosphate diabetes may be related to selective DMC‐induced tubulopathy |
| Curtis et al. 2002 30 |
A 94‐year‐old man hospitalised following collapse Diagnosis of SIADH |
300 mg DMC three times daily plus fluid restriction Nine days after admission, DMC increased to four times daily Metronizadole Benzydamine hydrochloride mouth washes |
Fatal acute renal failure |
Short‐term DMC can effectively control symptomatic HN but caution is required because of its potential nephrotoxicity Authors note a danger of dehydration in patients whose fluid intake has become compromised Authors suggest that had DMC been discontinued once the HN had resolved, the development of acute renal failure would have been avoided |
| Danovitch et al. 1978 31 |
Two patients: 1. A 51‐year‐old man with oat‐cell carcinoma of the lung. Diagnosis of SIADH 2. A 60‐year‐old‐man with 4‐week history of confusion and drowsiness. Diagnosis of SIADH |
1. 1200 mg DMC daily plus cyclophosphamide 2. 1200 mg DMC daily |
DMC treatment corrected HN and hypo‐osmolality but was discontinued in both patients owing to deterioration of renal function |
Potentially dangerous side effects exclude routine use of DMC Overall, DMC is effective in the treatment of SIADH, but has a potential to lead to a deterioration of renal function |
| Decaux et al. 1981 33 |
A 76‐year‐old man admitted to hospital because of grand mal seizures SIADH diagnosed |
A single dose of DMC Water restriction Urea infusion iv Furosemide |
Patient had severe gastric intolerance to DMC and the drug was stopped after the first dose | HN was controlled with furosemide after intolerance to DMC |
| Decaux et al. 1985 32 |
A 62‐year‐old woman Heavy smoker reporting weakness and memory loss Diagnosis of SIADH Oat‐cell carcinoma |
300 mg DMC twice daily | DMC corrected HN but led to phosphate diabetes |
Phosphate diabetes appeared after therapy with DMC and persisted for 3 months The augmentation in phosphate clearance was unrelated to serum sodium levels Phosphate diabetes was related to selective DMC‐induced renal toxicity |
| Heim et al. 1977 34 |
75‐year‐old woman, decline in general condition and pleural effusion (Two further cases not receiving DMC are reported) |
DMC 1200 mg/day | HN was corrected by fluid restriction; addition of DMC resulted in increased fluid clearance, but the patient developed vomiting and diarrhoea on day 4 and DMC was stopped on day 7 |
DMC is a relatively non‐toxic antibiotic which was shown to be effective; however, treatment was interrupted on day 8 owing to vomiting and diarrhoea The dose of 1200 mg/day may have been too strong for this patient |
| Padfield et al. 1978 35 | A 64‐year‐old man with SIADH following head injury and meningitis |
300 mg DMC four times daily followed by 600 mg DMC four times daily Fixed fluid intake |
600 mg DMC four times daily rapidly corrected all biochemical features of SIADH Acute renal failure was possibly induced by DMC On discontinuation of DMC normal renal function returned. |
DMC corrected the biochemical abnormalities of SIADH Possible evidence of nephrotoxicity |
| Perks et al. 1976 36 |
A 61‐year‐old man with memory deficit Inoperable carcinoma diagnosed 7 months after original admission |
600 mg DMC daily Fluid restriction |
Treatment with DMC led to clinical and biochemical improvement |
DMC is a safe and effective treatment that in this case allowed discharge from hospital This regimen may simplify outpatient management of other patients with this syndrome |
| Shimoda et al. 1986 (published in Japanese) 38 |
A 63‐year‐old woman hospitalised after losing consciousness; prior surgery for ruptured anterior artery Diagnosis of SIADH |
900 mg/day DMC 16 mg/day dexamethasone |
Patient became comatose and developed quadriplegia after rapid correction of serum sodium levels Patient died of septic shock after 12 months of hospitalisation |
Computed tomography scans and brain stem auditory responses were indicative of ODS |
| Soudan and Qunibi, 2012 39 |
A 78‐year‐old woman with history of hypothyroidism, vitiligo, rheumatoid arthritis and early Alzheimer's dementia Hospitalised with severe HN diagnosed as secondary to SIADH. |
300 mg DMC three times daily. Fluid restriction Normal saline infusion Furosemide Existing medication: Methotrexate Memantine HCL Mirtazapine Sulfamethoxazole and trimethoprim |
Patient developed severe HN as a result of rigid fluid restriction and DMC therapy Serum sodium levels stabilised after discontinuation of DMC |
DMC should be reserved for patients unable tolerate or unwilling to follow strict fluid restriction |
DMC, demeclocycline; HN, hyponatraemia; ODS, osmotic demyelination syn drome; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
Three reports associated deterioration in renal function with demeclocycline in four patients with a diagnosis of SIADH 30, 31, 35. Distinct but reversible episodes of glomerular filtration rate (GFR) reduction were observed in two patients 31, with acute renal failure reported in a third receiving demeclocycline at up to 2400 mg/day 35. A fourth patient (94‐year‐old male hospitalised following collapse and subsequent diagnosis of SIADH) died of irreversible acute renal failure that authors attributed to dehydration associated with demeclocycline 1200 mg/day and a compromised fluid intake 30.
Two case reports described the development of hyperphosphaturia during demeclocycline treatment for HN secondary to SIADH. Upon follow‐up, both patients presented with malignancies considered related to elevated phosphate levels, which recovered following discontinuation of demeclocycline. Authors of both reports considered the phosphate diabetes related to selective demeclocycline‐induced renal toxicity 29, 32.
A further two reports describe severe gastric intolerance leading to discontinuation of demeclocycline 33, 34. In one case, a 76‐year‐old male admitted with grand mal seizures and a previous history of peptic ulcer, severe gastric intolerance occurred after a single dose of demeclocycline 33.
A single case study of a 61‐year‐old male with SIADH reported normalisation of serum sodium levels with demeclocycline (600 mg/day). After a month, demeclocycline was discontinued but followed by a rapid fall in both osmolality and level of consciousness. Reinstitution of treatment led to biochemical and clinical improvement; the patient was discharged on demeclocycline 3 months after admission 36.
A Japanese report of a 63‐year‐old female with SIADH treated by strict water restriction, and administration of sodium, dexamethasone and demeclocycline, highlights correction of serum sodium from 93 to 137 mmol/l within 3 days of treatment. Subsequently, the patient became comatose and developed quadriplegia. After 12 months of hospitalisation, the patient died of septic shock. Computed tomography scans and brain stem auditory responses were indicative of ODS 38. Data for this case study were obtained from the English language abstract and figures presented within the report.
A final study reported seizures and altered mental state in a 78‐year‐old female patient with a history of hypothyroidism, rheumatoid arthritis and early Alzheimer's disease admitted for severe HN, which was considered secondary to SIADH. The patient was treated with demeclocycline (900 mg/day) and discharged under fluid restriction. After 6 weeks, she was readmitted; laboratory investigation identified severe hypernatraemia (serum sodium, 185 mmol/l) resulting from over‐correction of HN. The authors recommended that demeclocycline should be reserved for patients for whom strict fluid restriction is unsuitable 39.
Discussion
Hyponatraemia can lead to a wide spectrum of clinical symptoms and presents in a range of conditions. Despite this, the diagnosis and management of patients remains problematic 1, 3, particularly because of the heterogeneous nature of HN 40. In addition, treatment guidance has been generally oversimplistic, without clear consensus, and not reflective of the range of clinical issues encountered in current daily practice 1.
The literature search and review demonstrates a paucity of available high‐quality data for the effectiveness and safety of demeclocycline in the treatment of HN secondary to SIADH; no relevant economic evaluations or HRQoL studies were identified. With the exception of case reports, no published studies have addressed the use of demeclocycline for SIADH‐associated HN for more than 15 years 20, 22, 24, 25, 26, 27. Despite its long history in the treatment of HN secondary to SIADH, the use of demeclocycline is largely based on clinical experience rather than objective evidence. Although a lack of robust evidence from RCTs is by no means uncommon in medicine, a systematic appraisal of all published studies and case reports was necessary to appraise the efficacy and safety of demeclocycline treatment.
Examining the studies that are available, only two compared demeclocycline with placebo 24, 25. Of these RCTs, one did not demonstrate any significant differences in mean serum sodium levels after 3‐weeks' treatment. However, this study enrolled patients with psychosis and episodic or chronic polydipsia‐associated HN (mean duration of 5.4 years) who did not have a specific diagnosis of SIADH 24. In addition, the dosing ranges of demeclocycline were initially relatively low (escalating from 600 mg/day to 1200 mg/day by week 3), compared with the recommended initial dose of 900–1200 mg/day 18. The second RCT identified also included patients without a diagnosis of SIADH, but concluded that demeclocycline prevented HN because of increased secretion of ADH, commonly seen in patients undergoing CABG procedures 25.
The mode of action of demeclocycline on ADH‐induced water flow is not well understood, but the mechanism of therapeutic effect involves induction of nephrogenic diabetes insipidus 12, 41. While no studies have systematically evaluated the side effects associated with demeclocycline, several studies have identified safety issues associated with the onset of nephrogenic diabetes insipidus, particularly renal dysfunction 20, 21, 22, 30, 31, 35. In some patients, polyuria can be profound and patients can become markedly symptomatic, occasionally developing hypernatraemia if access to water is severely restricted 12, 39. Patients with known liver disease should not receive more than 1 g/day demeclocycline 18. In addition, monitoring of both renal and hepatic function should be performed regularly 18, 42.
Onset of action for demeclocycline is unpredictable and reported to range from 2 to 5 days, complicating dose adjustments 12, 16. Two reports describe severe gastric intolerance leading to treatment discontinuation 33, 34; one incident occurred following a single dose of demeclocycline in a 76‐year‐old male with grand mal seizures and SIADH. Authors suggested a 1200 mg/day dose may have been too high for the second patient: a 75‐year‐old woman with decline in general condition and pleural effusion who developed vomiting and diarrhoea on Day 4 of treatment 33.
Overall, most reports suggest that demeclocycline is able to increase serum sodium levels in the majority (60%) of patients with HN secondary to SIADH 12; treatment effectiveness in patients without a specific SIADH diagnosis may be limited 24. The safety profile of demeclocycline appears variable with the potential for complications, such as severe hypernatraemia 39 and renal dysfunction 18. Recent European clinical practice guidelines for diagnosis and treatment of HN recommend against the use of demeclocycline 1. Thus, close monitoring may be warranted in patients receiving demeclocycline for HN secondary to SIADH.
Conclusions
The results from this systematic literature review highlight the paucity of clinical evidence and lack of economic evidence supporting the use of demeclocycline for HN secondary to SIADH. There is a clinical need to treat HN and manage patients with SIADH appropriately 3; in particular, the management of patients with cancer. For example, chemotherapy may need to be delayed until the normalisation of these patients' sodium levels 43, and delays to chemotherapy negatively influence outcomes 44. In addition, some chemotherapy regimens require aggressive hydration strategies 42, 45. Furthermore, patients may also require hospital admission for the symptomatic consequences of HN, adding to the burden of their cancer 42, 46, 47.
Although most reports suggest that demeclocycline can address serum sodium levels in HN 12, efficacy in patients without SIADH may be limited 24. Moreover, with a variable and unreliable time to onset of action, and a safety profile associated with induction of nephrogenic diabetes insipidus, demeclocycline dose adjustments can be complex 12, 16. Thus, it may be concluded that patients receiving demeclocycline for HN secondary to SIADH must be closely monitored.
Author contributions
All authors co‐wrote and critically reviewed each draft of the manuscript. Penny Dhanjal made recommendations on the original draft plan of inclusion of license and onset of action of demeclocycline as well as a critical review of efficacy and safety data to ensure all relevant adverse events were recorded within the manuscript. All authors contributed to and have approved the final manuscript.
Acknowledgements
The authors thank Oxford Pharmagenesis for assistance in conducting the literature searches, Nabh Rai for her assistance in the study design, and Allan Johnson PhD and Gil Bezzina PhD for medical writing and editorial support, which were funded by Otsuka Pharmaceutical Europe Ltd.
Appendix A. MESH search strings
| Embase | ||
| # | Searches | Results |
| #1 | exp inappropriate vasopressin secretion/ | 2829 |
| #2 | syndrome of inappropriate antidiuretic.mp. | 1001 |
| #3 | (inappropriate antidiuretic adj3 syndrome).mp. | 1057 |
| #4 | syndrome of immoderate antidiuresis.mp. | 0 |
| #5 | schwartz‐bartter syndrome.mp. | 754 |
| #6 | hyponatraemia.mp. or exp hyponatraemia/ | 20,570 |
| #7 | hyponatraemia.mp. | 2274 |
| #8 | sodium ion concentration.mp. | 261 |
| #9 | sodium serum.mp. | 165 |
| #10 | sodium blood level.mp. or exp sodium blood level/ | 9734 |
| #11 | hypovolemia.mp. or exp hypovolemia/ | 11,630 |
| #12 | hypovolaemia.mp. | 1263 |
| #13 | serum osmolality.mp. or exp serum osmolality/ | 2409 |
| #14 | urine osmolality.mp. or exp urine osmolality/ | 4223 |
| #15 | (osmolar or osmolarity).mp. | 23,152 |
| #16 | exp demeclocycline/ or demeclocycline.mp. | 2151 |
| #17 | declomycin.mp. | 98 |
| #18 | declostatin.mp. or exp demeclocycline plus nystatin/ | 0 |
| #19 | ledermycin.mp. | 159 |
| #20 | demeclotetracycline.mp. | 4 |
| #21 | (Demeclor or Elkamicina or Fidocin or Mexocine or Novotriclina or Perciclina or Rynabron).mp. | 17 |
| #22 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 66,834 |
| #23 | 16 or 17 or 18 or 19 or 20 or 21 | 2168 |
| #24 | #22 and #23 | 517 |
| Medline & Medline In‐Process | ||
| # | Searches | Results |
| #1 | exp Inappropriate ADH Syndrome/ or inappropriate vasopressin secretion.mp. | 2,275 |
| #2 | syndrome of inappropriate antidiuretic.mp. | 754 |
| #3 | (inappropriate antidiuretic adj3 syndrome).mp. | 811 |
| #4 | syndrome of immoderate antidiuresis.mp. | 0 |
| #5 | schwartz‐bartter syndrome.mp. | 97 |
| #6 | hyponatraemia.mp. or exp hyponatraemia/ | 9,892 |
| #7 | hyponatraemia.mp. | 1,707 |
| #8 | sodium ion concentration.mp. | 231 |
| #9 | sodium serum.mp. | 104 |
| #10 | sodium blood level.mp. | 2 |
| #11 | hypovolemia.mp. or exp Hypovolemia/ | 3,954 |
| #12 | exp Hypovolemia/ or hypovolaemia.mp. | 1,926 |
| #13 | hypovolaemia.mp. | 983 |
| #14 | exp Osmolar Concentration/ or serum osmolality.mp. | 60,404 |
| #15 | exp Osmolar Concentration/ or ((serum or urine) and osmolality).mp. | 62,530 |
| #16 | (osmolar or osmolarity).mp. | 53,453 |
| #17 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 | 82,327 |
| #18 | demeclocycline.mp. or exp Demeclocycline/ | 894 |
| #19 | declomycin.mp. | 13 |
| #20 | declostatin.mp. | 0 |
| #21 | ledermycin.mp. | 23 |
| #22 | demeclotetracycline.mp. | 1 |
| #23 | Demeclocyclinum.mp. | 0 |
| #24 | (Demeclor or Elkamicina or Fidocin or Mexocine or Novotriclina or Perciclina or Rynabron).mp. | 4 |
| #25 | 18 or 19 or 20 or 21 or 22 or 23 or 24 | 915 |
| #26 | #17 and #25 | 156 |
| Cochrane Library | ||
| # | Searches | Results |
| #1 | MeSH descriptor: [Inappropriate ADH Syndrome] explode all trees | 9 |
| #2 | inappropriate vasopressin secretion | 25 |
| #3 | syndrome of inappropriate antidiuretic | 19 |
| #4 | (inappropriate antidiuretic near/3 syndrome) | 16 |
| #5 | syndrome of immoderate antidiuresis | 0 |
| #6 | schwartz‐bartter syndrome | 0 |
| #7 | MeSH descriptor: [Hyponatraemia] explode all trees | 107 |
| #8 | hyponatraemia or hyponatraemia | 526 |
| #9 | sodium ion concentration | 634 |
| #10 | sodium serum | 2935 |
| #11 | sodium blood level | 3870 |
| #12 | MeSH descriptor: [Hypovolemia] explode all trees | 71 |
| #13 | hypovolemia or hypovolaemia | 349 |
| #14 | MeSH descriptor: [Osmolar Concentration] explode all trees | 1233 |
| #15 | serum osmolality | 298 |
| #16 | ((serum or urine) and osmolality) | 503 |
| #17 | osmolar or osmolarity | 1638 |
| #18 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 | 8019 |
| #19 | MeSH descriptor: [Demeclocycline] explode all trees | 45 |
| #20 | demeclocycline | 68 |
| #21 | declomycin or declostatin | 0 |
| #22 | ledermycin or demeclotetracycline or Demeclocyclinum | 12 |
| #23 | Demeclor or Elkamicina or Fidocin or Mexocine or Novotriclina or Perciclina or Rynabron | 0 |
| #24 | #19 or #20 or #21 or #22 or #23 | 78 |
| #25 | #18 and #24 | 6 |
Appendix B.
| Summaries of excluded full‐length papers | |
| Reference | Exclusion reason |
| Boissonnas A, Casassus P, Caquet R, Laroche C. Hyponatraemia in a cirrhotic suffering from late cutaneous porphyria: A drug to avoid, demeclocycline. [French]. Nouvelle Presse Medicale 1979; 8(3): 210. | Not SIADH |
| Burst V, Verbalis J, Greenberg A et al. Hyponatraemia in the hospital setting: Interim results from a prospective, observational, multi‐center, global registry. Pneumologie Conference 2013; 54. | Conference abstract – no full text available |
| Cawley MJ. Hyponatraemia: Current treatment strategies and the role of vasopressin antagonists. Annals of Pharmacotherapy 2007; 41(5): 840–50. | Review with no relevant data |
| Jellett L, O'Hare J, McAleese J. Syndrome of inappropriate antidiuretic hormone (SIADH) in patients with small cell lung cancer & incidence and response to treatment. Lung Cancer 2011;Conference: 9th Annual BTOG Conference 2011 Dublin Ireland. Conference Publication: 71: S41. | Conference abstract – no full text available |
| Kamoi K, Ebe T, Kobayashi O et al. Atrial natriuretic peptide in patients with the syndrome of inappropriate antidiuretic hormone secretion and with diabetes insipidus. Journal of Clinical Endocrinology and Metabolism 1990; 70(5):1385–90. | Small case study – three patients treated and no safety issues reported |
| Kamoi K, Toyama M, Takagi M et al. Osmoregulation of vasopressin secretion in patients with the syndrome of inappropriate antidiuresis associated with central nervous system disorders. Endocrine Journal 1999; 46(2): 269–77. | No specific demeclocycline results |
| Laszlo FA, Varga C, Doczi T. Cerebral oedema after subarachnoid haemorrhage. Pathogenetic significance of vasopressin. Acta Neurochirurgica 1995; 133 (3–4): 122–33. | No treatment data |
| Miller PD, Linas SL, Schrier RW. Plasma demeclocycline levels and nephrotoxicity. Correlation in hyponatremic cirrhotic patients. JAMA 1980; 243(24): 2513–5. | Not SIADH |
| Nagler EV, Haller MC, Van Biesen W et al. Treatments for chronic hyponatraemia: A systematic review of randomised controlled trials. Nephrology Dialysis Transplantation 2013;Conference: 50th ERA‐EDTA Congress Istanbul Turkey. Conference Publication: 28: i387. | Conference abstract – no full text available |
| Philip T, Souillet G, Gharib C et al. Inappropriate secretion of antiduiuretic hormone during acute leukaemia treated with vincristine. Two cases (author's transl). [French]. La Nouvelle presse medicale 1979; 8(26): 2181–5. | Small case study – one patients treated and no safety issues reported |
| Shakher J, Thompson J. Tolvaptan cost effective treatment of SIADH in malignancies. Lung Cancer 2013;Conference: 11th Annual British Thoracic Oncology Group Conference, BTOG 2013 Dublin Ireland. Conference Publication: 79: S66. | Conference abstract – no full text available |
| Walter HS, Ahmed SI. The incidence of hyponatraemia in small cell lung cancer: Prognostic and predictive potential in a retrospective single centre analysis. Lung Cancer 2013;Conference: 11th Annual British Thoracic Oncology Group Conference, BTOG 2013 Dublin Ireland. Conference Publication: 79: S65–S6. | Conference abstract – no full text available |
Appendix C. Quality assessments
| Quality assessment of included randomised controlled trials | ||
| Quality assessment question | Yes/No/Unclear/NA | Notes |
| Alexander RC et al. 1991 24 | ||
| Internal validity | ||
| Does the study address an appropriate and clearly focused question? | Yes | Quote: ‘we undertook a double‐blind, placebo‐controlled study of demeclocycline in psychiatric patients with polydipsia‐hyponatraemia’ |
| Is the assignment of subjects to treatment groups randomised? | Yes | Order of treatment was randomly assigned; study used a cross‐over method |
| Is an adequate concealment method used? | Unclear | Study was double‐blind and placebo‐controlled, but details on method of concealment were unclear |
| Are subjects and investigators kept ‘blind’ about treatment allocation? | Unclear | Study was double‐blind and placebo‐controlled, but details on method of blinding were unclear |
| Are the treatment and control groups similar at the start of the trial? | NA | Crossover design, not applicable |
| Is the only difference between groups the treatment under investigation? | Yes | Crossover design; groups are identical aside from order of treatment |
| Are all relevant outcomes measured in a standard, valid and reliable way? | Yes | Quote: ‘serum sodium levels were obtained in the morning twice a week (routine sodium levels) and also following episodes of acute weight gain (sporadic sodium levels)’Further details on serum sampling and sodium measurement not provided |
| What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed? | No patients dropped out | |
| Are all the subjects analysed in the groups to which they were randomly allocated (often referred to as intention‐to‐treat analysis)? | NA | Crossover design, not applicable |
| Where the study is carried out at more than one site, are results comparable for all sites? | NA | Single site study |
| Overall assessment | ||
| How well was the study done to minimise bias? (high quality/acceptable/reject) | ||
| Taking into account clinical considerations, your evaluation of the methodology used, and the statistical power of the study, are you certain that the overall effect is due to the study intervention? | ||
| Are the results of this study directly applicable to the patient group targeted by this guideline? | ||
| Horratas MC et al. 1998 25 | ||
| Internal validity | ||
| Does the study address an appropriate and clearly focused question? | ||
| Is the assignment of subjects to treatment groups randomised? | Yes | Patients were randomly assigned to treatment or placebo; details of exact method used to randomise not reported |
| Is an adequate concealment method used? | Unclear | Study was double‐blind and placebo‐controlled, but details on method of concealment were unclear |
| Are subjects and investigators kept ‘blind’ about treatment allocation? | Yes | Quote: ‘Neither the patients, caregivers, nor the investigators were aware of the assigned patient group. The study was blinded until its completion.’ |
| Are the treatment and control groups similar at the start of the trial? | Unclear | No summary of baseline characteristics providedQuote: ‘the two groups remained matched for age, sex, and weight’ |
| Is the only difference between groups the treatment under investigation? | Unclear | Exclusion criteria were defined and many patients not included if they had certain comorbidities, but insufficient information provided on baseline characteristics of included patients |
| Are all relevant outcomes measured in a standard, valid and reliable way? | Unclear | Limited information on outcome collection; authors state there is no commercial assay for demeclocycline |
| What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed? | Two patients in the demeclocycline group discontinued (2/15; 13.3%); no patients in the placebo group discontinued | |
| Are all the subjects analysed in the groups to which they were randomly allocated (often referred to as intention‐to‐treat analysis)? | No | No mention of intention‐to‐treat analysis analysis; it appears the two patients who dropped out were not included in the analysis |
| Where the study is carried out at more than one site, are results comparable for all sites? | NA | Single site study |
| Overall assessment | ||
| How well was the study done to minimise bias? (high quality/acceptable/reject) | ||
| Taking into account clinical considerations, your evaluation of the methodology used, and the statistical power of the study, are you certain that the overall effect is due to the study intervention? | ||
| Are the results of this study directly applicable to the patient group targeted by this guideline? | ||
| Quality assessment of included cohort studies | ||
| Quality assessment question | Yes/No/Unclear | Notes |
| Brewerton T et al. 1994 28 | ||
| Internal validity | ||
| Does the study address an appropriate and clearly focused question? | Yes | Quote: ‘We would like to extend these observations of demeclocycline's reversal of carbamazepine‐induced hyponatraemia in a group of six psychiatric inpatients’ |
| Are the two groups being studied selected from source populations that are comparable in all respects other than the factor under investigation? | NA | Single cohort/case series; no controls |
| Does the study indicate how many of the people asked to take part did so, in each of the groups being studied? | NA | Retrospective analysis of single cohort/case series; no controls |
| Is the likelihood that some eligible subjects might have the outcome at the time of enrolment assessed and taken into account in the analysis? | NA | All patients had a diagnosis of hyponatraemia (< 135 mmol/l) while taking carbamazepine and were subsequently treated with demeclocycline |
| What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed? | Retrospective analysis; all patients completed | |
| Is the comparison made between full participants and those lost to follow up, by exposure status? | NA | No patients lost to follow‐up |
| Are the outcomes clearly defined? | Yes | |
| Is the assessment of outcome made blind to exposure status? (if the study is retrospective this may not be applicable) | NA | Retrospective analysis |
| Where blinding was not possible, is there some recognition that knowledge of exposure status could have influenced the assessment of outcome? | No | |
| Is the method of assessment of exposure is reliable? | Yes | Chart review |
| Is evidence from other sources used to demonstrate that the method of outcome assessment is valid and reliable? | No | |
| Is the exposure level or prognostic factor assessed more than once? | No | |
| Are the main potential confounders identified and taken into account in the design and analysis? | No | |
| Have confidence intervals been provided? | No | Confidence intervals not provided but standard deviations reported |
| De Troyer A, 1977 20 | ||
| Internal validity | ||
| Does the study address an appropriate and clearly focused question? | Yes | Aim of study was to test ‘the efficacy of demeclocycline hydrochloride in suppressing the tubular action of tumoral antidiuretic products’ |
| Are the two groups being studied selected from source populations that are comparable in all respects other than the factor under investigation? | NA | Single cohort/case series; no controls |
| Does the study indicate how many of the people asked to take part did so, in each of the groups being studied? | No | Patients were consecutive, but unclear if any patients were asked to take part and did not provide consent |
| Is the likelihood that some eligible subjects might have the outcome at the time of enrolment assessed and taken into account in the analysis? | NA | All patients had SIADH at the start of the trial; no separate control group |
| What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed? | No patients lost to follow‐up | |
| Is the comparison made between full participants and those lost to follow up, by exposure status? | NA | No patients lost to follow‐up |
| Are the outcomes clearly defined? | Yes | |
| Is the assessment of outcome made blind to exposure status? (if the study is retrospective this may not be applicable) | No | |
| Where blinding was not possible, is there some recognition that knowledge of exposure status could have influenced the assessment of outcome? | No | |
| Is the method of assessment of exposure is reliable? | Yes | No other therapy given throughout the study; fluid and sodium intake ad libitum |
| Is evidence from other sources used to demonstrate that the method of outcome assessment is valid and reliable? | No | |
| Is the exposure level or prognostic factor assessed more than once? | No | |
| Are the main potential confounders identified and taken into account in the design and analysis? | No | |
| Have confidence intervals been provided? | No | Confidence intervals not provided but standard deviations reported |
| Forrest JN et al. 1978 26 | ||
| Internal validity | ||
| Does the study address an appropriate and clearly focused question? | Yes | Quote: ‘We compared the responses to demeclocycline and lithium in a series of 10 patients with [SIADH]’ |
| Are the two groups being studied selected from source populations that are comparable in all respects other than the factor under investigation? | NA | Single cohort/case series; no controls |
| Does the study indicate how many of the people asked to take part did so, in each of the groups being studied? | NA | Retrospective analysis of single cohort/case series; no controls |
| Is the likelihood that some eligible subjects might have the outcome at the time of enrolment assessed and taken into account in the analysis? | NA | All patients had chronic SIADH as identified by medical records |
| What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed? | Retrospective analysis; all patients completed | |
| Is the comparison made between full participants and those lost to follow up, by exposure status? | NA | No patients lost to follow‐up |
| Are the outcomes clearly defined? | Yes | |
| Is the assessment of outcome made blind to exposure status? (if the study is retrospective this may not be applicable) | No | Retrospective analysis |
| Where blinding was not possible, is there some recognition that knowledge of exposure status could have influenced the assessment of outcome? | No | |
| Is the method of assessment of exposure is reliable? | Yes | Chart review |
| Is evidence from other sources used to demonstrate that the method of outcome assessment is valid and reliable? | No | |
| Is the exposure level or prognostic factor assessed more than once? | No | |
| Are the main potential confounders identified and taken into account in the design and analysis? | No | Some patients received lithium prior to demeclocycline and others did not; unclear if proper washout period undertaken |
| Have confidence intervals been provided? | No | Confidence intervals not provided but standard errors of the mean reported (graph only) |
| Goldman MB et al. 1985 27 | ||
| Internal validity | ||
| Does the study address an appropriate and clearly focused question? | Yes | The study attempts to replicate previous findings from a case report using a larger sample size |
| Are the two groups being studied selected from source populations that are comparable in all respects other than the factor under investigation? | NA | Single cohort/case series; no controls |
| Does the study indicate how many of the people asked to take part did so, in each of the groups being studied? | Unclear | Unclear if any patients refused to participate |
| Is the likelihood that some eligible subjects might have the outcome at the time of enrolment assessed and taken into account in the analysis? | Unclear | All patients had hyponatraemia at time of enrolment but only 5/8 met criteria for SIADH |
| What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed? | No patients lost to follow‐up | |
| Is the comparison made between full participants and those lost to follow up, by exposure status? | NA | No patients lost to follow‐up |
| Are the outcomes clearly defined? | Yes | |
| Is the assessment of outcome made blind to exposure status? (if the study is retrospective this may not be applicable) | No | |
| Where blinding was not possible, is there some recognition that knowledge of exposure status could have influenced the assessment of outcome? | No | |
| Is the method of assessment of exposure is reliable? | Yes | |
| Is evidence from other sources used to demonstrate that the method of outcome assessment is valid and reliable? | No | |
| Is the exposure level or prognostic factor assessed more than once? | No | |
| Are the main potential confounders identified and taken into account in the design and analysis? | No | |
| Have confidence intervals been provided? | No | Confidence intervals not provided but standard deviations reported |
| Perks WH et al. 1979 22 | ||
| Internal validity | ||
| Does the study address an appropriate and clearly focused question? | Yes | The authors determined the effect of demeclocycline on patients with SIADH, especially renal function |
| Are the two groups being studied selected from source populations that are comparable in all respects other than the factor under investigation? | NA | Single cohort/case series; no controls |
| Does the study indicate how many of the people asked to take part did so, in each of the groups being studied? | Unclear | Unclear if any patients refused to participate |
| Is the likelihood that some eligible subjects might have the outcome at the time of enrolment assessed and taken into account in the analysis? | NA | All patients had SIADH based on specific criteria |
| What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed? | 7/14 patients (50%) discontinued, including one death, however it appears that all patients were taking demeclocycline at the analysis timepoint of 10 days | |
| Is the comparison made between full participants and those lost to follow up, by exposure status? | Unclear | Only comparison between serum sodium levels in all patients (pre‐discontinuation) are compared with levels in patients who discontinued |
| Are the outcomes clearly defined? | Yes | |
| Is the assessment of outcome made blind to exposure status? (if the study is retrospective this may not be applicable) | No | |
| Where blinding was not possible, is there some recognition that knowledge of exposure status could have influenced the assessment of outcome? | No | |
| Is the method of assessment of exposure is reliable? | Yes | |
| Is evidence from other sources used to demonstrate that the method of outcome assessment is valid and reliable? | No | |
| Is the exposure level or prognostic factor assessed more than once? | No | |
| Are the main potential confounders identified and taken into account in the design and analysis? | Unclear | Discontinuation is taken into account during analysis; no indication of other confounding factors accounted for |
| Have confidence intervals been provided? | No | Confidence intervals not provided but standard deviations reported |
| Trump DL et al. 1981 21 | ||
| Internal validity | ||
| Does the study address an appropriate and clearly focused question? | Yes | To assess the efficacy of demeclocycline in the treatment of patients with water intoxication and cancer |
| Are the two groups being studied selected from source populations that are comparable in all respects other than the factor under investigation? | NA | Single cohort/case series; no controls |
| Does the study indicate how many of the people who asked to take part did so, in each of the groups being studied? | NA | Retrospective analysis of single cohort/case series; no controls |
| Is the likelihood that some eligible subjects might have the outcome at the time of enrolment assessed and taken into account in the analysis? | NA | All patients had cancer and hyponatraemia as identified by medical records |
| What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed? | Retrospective analysis; all patients completed | |
| Is the comparison made between full participants and those lost to follow up, by exposure status? | NA | No patients lost to follow up |
| Are the outcomes clearly defined? | Yes | |
| Is the assessment of outcome made blind to exposure status? (if the study is retrospective this may not be applicable) | No | Retrospective analysis |
| Where blinding was not possible, is there some recognition that knowledge of exposure status could have influenced the assessment of outcome? | No | |
| Is the method of assessment of exposure is reliable? | Yes | Chart review |
| Is evidence from other sources used to demonstrate that the method of outcome assessment is valid and reliable? | No | |
| Is the exposure level or prognostic factor assessed more than once? | No | |
| Are the main potential confounders identified and taken into account in the design and analysis? | No | All patients had different total doses of demeclocycline and different treatment periods; these are listed but not accounted for in the analysis |
| Have confidence intervals been provided? | No | Confidence intervals not provided but ranges reported |
Disclosures
Dr John Miell has received lecture fees and an honorarium for his contribution to the writing of this manuscript from Otsuka Pharmaceuticals.
References
- 1. Spasovski G, Vanholder R, Allolio B et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 2014; 170: G1–47. [DOI] [PubMed] [Google Scholar]
- 2. Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med 1967; 42: 790–806. [DOI] [PubMed] [Google Scholar]
- 3. Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab 2012; 3: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 2007; 356: 2064–72. [DOI] [PubMed] [Google Scholar]
- 5. Clayton JA, Le Jeune IR, Hall IP. Severe hyponatraemia in medical in‐patients: aetiology, assessment and outcome. QJM 2006; 99: 505–11. [DOI] [PubMed] [Google Scholar]
- 6. Verbalis J. Hyponatraemia In: Baylis PH, ed. Water and Salt Homeostasis in Health and Disease, 1st edn. London: Baillière Tindall, 1989: 499–530. [Google Scholar]
- 7. Gill G, Huda B, Boyd A et al. Characteristics and mortality of severe hyponatraemia – a hospital‐based study. Clin Endocrinol 2006; 65: 246–9. [DOI] [PubMed] [Google Scholar]
- 8. Sajadieh A, Binici Z, Mouridsen MR et al. Mild hyponatremia carries a poor prognosis in community subjects. Am J Med 2009; 122: 679–86. [DOI] [PubMed] [Google Scholar]
- 9. Stelfox HT, Ahmed SB, Khandwala F et al. The epidemiology of intensive care unit‐acquired hyponatraemia and hypernatraemia in medical‐surgical intensive care units. Crit Care 2008; 12: R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 2009; 122: 857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sherlock M, O'Sullivan E, Agha A et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol 2006; 64: 250–4. [DOI] [PubMed] [Google Scholar]
- 12. Sherlock M, Thompson CJ. The syndrome of inappropriate antidiuretic hormone: current and future management options. Eur J Endocrinol 2010; 162(Suppl. 1): S13–8. [DOI] [PubMed] [Google Scholar]
- 13. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med 2000; 342: 1581–9. [DOI] [PubMed] [Google Scholar]
- 14. Renneboog B, Musch W, Vandemergel X et al. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 2006; 119: 71–8. [DOI] [PubMed] [Google Scholar]
- 15. Gankam KF, Andres C, Sattar L et al. Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM 2008; 101: 583–8. [DOI] [PubMed] [Google Scholar]
- 16. Verbalis JG, Goldsmith SR, Greenberg A et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 2013; 126(10 Suppl. 1): S1–42. [DOI] [PubMed] [Google Scholar]
- 17. Furst H, Hallows KR, Post J et al. The urine/plasma electrolyte ratio: a predictive guide to water restriction. Am J Med Sci 2000; 319: 240–4. [DOI] [PubMed] [Google Scholar]
- 18. Mercury Pharmaceuticals Ltd . Ledermycin (demeclocycline hydrochloride). Summary of Product Characteristics (UK), April 2013.
- 19. Primius Lab Ltd . Alkonatrem (demeclocycline hydrochloride). Summary of Product Characteristics (France), December 2013.
- 20. De Troyer A. Demeclocycline. Treatment for syndrome of inappropriate antidiuretic hormone secretion. JAMA 1977; 237: 2723–6. [DOI] [PubMed] [Google Scholar]
- 21. Trump DL. Serious hyponatraemia in patients with cancer: management with demeclocycline. Cancer 1981; 47: 2908–12. [DOI] [PubMed] [Google Scholar]
- 22. Perks WH, Walters EH, Tams IP et al. Demeclocycline in the treatment of the syndrome of inappropriate secretion of antidiuretic hormone. Thorax 1979; 34: 324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alexander RC, Karp BI, Thompson S et al. A double blind, placebo‐controlled trial of demeclocycline treatment of polydipsia‐hyponatraemia in chronically psychotic patients. Biol Psychiatry 1991; 30: 417–20. [DOI] [PubMed] [Google Scholar]
- 25. Horattas MC, Evasovich MR, Muakkassa FF et al. Perioperative vasopressin secretion treated by demeclocycline. Am Surg 1998; 64: 281–6. [PubMed] [Google Scholar]
- 26. Forrest JN Jr, Cox M, Hong C. Superiority of demeclocycline over lithium in the treatment of chronic syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med 1978; 298: 173–7. [DOI] [PubMed] [Google Scholar]
- 27. Goldman MB, Luchins DJ. Demeclocycline improves hyponatraemia in chronic schizophrenics. Biol Psychiatry 1985; 20: 1149–55. [DOI] [PubMed] [Google Scholar]
- 28. Brewerton TD, Jackson CW. Prophylaxis of carbamazepine‐induced hyponatraemia by demeclocycline in six patients. J Clin Psychiatry 1994; 55: 249–51. [PubMed] [Google Scholar]
- 29. Antonelli A, Carmassi F, Alberti B et al. Demeclocycline‐induced phosphate diabetes in a patient with inappropriate ADH secretion and systemic sarcoidosis. Nephron 1993; 63: 226–9. [DOI] [PubMed] [Google Scholar]
- 30. Curtis NJ, van Heyningen C, Turner JJ. Irreversible nephrotoxicity from demeclocycline in the treatment of hyponatraemia. Age Ageing 2002; 31: 151–2. [DOI] [PubMed] [Google Scholar]
- 31. Danovitch GM, Le Roith D, Glick S. Renal function during treatment of inappropriate secretion of antidiuretic hormone with demeclocycline. Isr J Med Sci 1978; 14: 852–7. [PubMed] [Google Scholar]
- 32. Decaux G, Soupart A, Unger J et al. Demeclocycline‐induced phosphate diabetes in patients with inappropriate secretion of antidiuretic hormone. N Engl J Med 1985; 313: 1480–1. [DOI] [PubMed] [Google Scholar]
- 33. Decaux G, Waterlot Y, Genette F et al. Treatment of the syndrome of inappropriate secretion of antidiuretic hormone with furosemide. N Engl J Med 1981; 304: 329–30. [DOI] [PubMed] [Google Scholar]
- 34. Heim M, Conte‐Devolx B, Pin G et al. Syndrome of inappropriate secretion of vasopressin. Apropos of 3 cases. Sem Hop Paris 1977; 53: 1155–9. [PubMed] [Google Scholar]
- 35. Padfield PL, Morton JJ, Hodsman GP. Demeclocycline in the treatment of the syndrome of inappropriate antidiuretic hormone release: with measurement of plasma ADH. Postgrad Med J 1978; 54: 623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perks WH, Mohr P, Liversedge LA. Demeclocycline in inappropriate A.D.H. syndrome. Lancet 1976; 2: 1414. [DOI] [PubMed] [Google Scholar]
- 37. De Troyer A, Demanet JC. Clinical, biological and pathogenic features of the syndrome of inappropriate secretion of antidiuretic hormone. A review of 26 cases with marked hyponatraemia. Q J Med 1976; 45: 521–31. [PubMed] [Google Scholar]
- 38. Shimoda M, Matsuoka T, Kato T et al. A case of central pontine myelinolysis. [English abstract]. No Shinkei Geka 1986; 14(8): 1031–7. [PubMed] [Google Scholar]
- 39. Soudan K, Qunibi W. Severe hypernatremia following treatment of the syndrome of inappropriate antidiuretic hormone secretion. Am J Med Sci 2012; 343: 507–9. [DOI] [PubMed] [Google Scholar]
- 40. Laville M, Burst V, Peri A, Verbalis JG. Hyponatremia secondary to the syndrome of inappropriate secretion of antidiuretic hormone (SIADH): therapeutic decision‐making in real‐life cases. Clin Kidney J 2013; 6(Suppl. 1): i1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singer I, Rotenberg D. Demeclocycline‐induced nephrogenic diabetes insipidus. In‐vivo and in‐vitro studies. Ann Intern Med 1973; 79: 679–83. [DOI] [PubMed] [Google Scholar]
- 42. Castillo JJ, Vincent M, Justice E. Diagnosis and management of hyponatremia in cancer patients. Oncologist 2012; 17: 756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petereit C, Zaba O, Teber I et al. A rapid and efficient way to manage hyponatremia in patients with SIADH and small cell lung cancer: treatment with tolvaptan. BMC Pulm Med 2013; 13: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu KD, Huang S, Zhang J‐X et al. Association between delayed initiation of adjuvant CMF or anthracycline‐based chemotherapy and survival in breast cancer: a systematic review and meta‐analysis. BMC Cancer 2013; 13: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rosner MH, Dalkin AC. Electrolyte disorders associated with cancer. Adv Chronic Kidney Dis 2014; 21: 7–17. [DOI] [PubMed] [Google Scholar]
- 46. Onitilo AA, Kio E, Doi SA. Tumor‐Related Hyponatremia. Clin Med Res 2007; 5: 228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lewis MA, Wahner Hendrickson A, Moynihan TJ. Oncologic emergencies: pathophysiology, presentation, diagnosis, and treatment. CA Cancer J Clin 2011; 61: 287–314. [DOI] [PubMed] [Google Scholar]


