Summary
Aim
To develop a non‐invasive management strategy for men with lower urinary tract symptoms (LUTS) after treatment for pelvic cancer, that is suitable for use in a primary healthcare context.
Methods
PubMed literature searches of LUTS management in this patient group were carried out, together with obtaining a consensus of management strategies from a panel of authors for the management of LUTS from across the UK.
Results
Data from 41 articles were investigated and collated. Clinical experience was sought from authors where there was no clinical evidence. The findings discussed in this paper confirm that LUTS after the cancer treatment can significantly impair men's quality of life. While many men recover from LUTS spontaneously over time, a significant proportion require long‐term management. Despite the prevalence of LUTS, there is a lack of consensus on best management. This article offers a comprehensive treatment algorithm to manage patients with LUTS following pelvic cancer treatment.
Conclusion
Based on published research literature and clinical experience, recommendations are proposed for the standardisation of management strategies employed for men with LUTS after the pelvic cancer treatment. In addition to implementing the algorithm, understanding the rationale for the type and timing of LUTS management strategies is crucial for clinicians and patients.
What's known
Lower urinary Tract symptoms (LUTs) are a constellation of symptoms that are common in men who have been treated for pelvic malignancies, not only as a result of their disease but also as a consequence of cancer treatment. Symptoms such as urinary incontinence, frequency and urgency are often reported by men as the most bothersome. Pelvic therapies include treatment for prostate, bowel and bladder cancers. Many men continue to experience long term symptoms over many years and this can have a negative effect on recovery and subsequent quality of life. Conservative management strategies are defined for LUTS but these are mainly developed and evaluated in general populations with current guidelines based on benign disease. The evidence base for such conservative management of LUTS after pelvic cancer treatment is small and inconsistent and may not be appropriate for LUTS from different causality.
What's new
LUTS after cancer treatment is a significant problem for cancer survivors especially as more men are surviving cancer treatment. Symptoms can occur for many years after cancer therapy and incidence and timing of LUTs depends on treatment type and extent of predictive factors prior to treatment. LUTS after cancer treatment includes both urinary incontinence and lower urinary tract symptoms which can be concurrent and impacts on men's quality of life. Awareness of the treatments that men have received is important in defining LUTS management and the pathway of care. Assessment, appropriate pharmacotherapy, behavioural and lifestyle management can improve symptoms. While many men recover spontaneously over time a proportion of men require long term management of LUTs. Simple assessment, use of behavioural strategies, drug management and consistent follow up can help reduce the burden of this symptom for men after cancer treatment. Symptoms of LUTS that persist after 3 months of conservative treatment and impact on men's quality of life should be referred to specialist urology teams.
Introduction
There are currently more than 2 million people in England living with cancer and this number is increasing as cancer survival improves 1. Men with prostate cancer account for much of the male survival; 41,700 men were diagnosed in 2011 and 8 in 10 of these will survive for 5 or more years. Other pelvic cancers such as bladder and bowel cancer account for 30,500 men diagnosed per year, making pelvic cancer a substantive area of disease burden in the male population 1. Many of these men continue to experience symptoms that impact on quality of life such as urinary and bowel problems, haematuria, rectal bleeding, pain and sexual dysfunction 2. Common symptoms as a result of cancer therapy have been addressed in substantive reviews 3, 4, 5, 6. Urinary symptoms despite the high prevalence in men after cancer treatment and the links to negative effect on quality of life 7 have not yet been addressed.
Lower urinary tract symptoms (LUTS) may be divided into storage, voiding and postmicturition 8, 9. These are common problems and up to 3.4 million men in the United Kingdom live with LUTS and the prevalence rates for various types of LUTS after cancer therapy ranges from 3.7% to 52.2% 7, 10. Most of these men are managed within primary care, with either conservative lifestyle measures or medical treatment 10. LUTS is a complex group of symptoms, which are difficult to define. However, the NICE guidelines on LUTS published in 2010 define the symptoms as shown in Table 1 8. Generally, a symptom‐based approach is used in classifying LUTS. According to the International Continence Society (ICS), LUTS can be divided, similarly to NICE guidelines, into storage symptoms, voiding symptoms and symptoms experienced postmicturition, although ICS also includes urinary incontinence (UI) and postmicturition dribble in its definition (Table 1) 9. Symptoms such as UI, frequency, urgency and nocturia are often the most bothersome of LUTS 11. Most clinical trials in pelvic cancer patients tend to assess only UI as an outcome measure rather than LUTS as a cluster of symptoms. Overall, the management of LUTS remains an area requiring improvement for cancer survivors and impacts considerably on quality of life for men. In addition, the problem is under‐reported.
Table 1.
| LUTS | Storage | Voiding | Post‐micturition |
|---|---|---|---|
| NICE |
Urgency Increased daytime frequency Nocturia Urinary incontinence Altered bladder sensations |
Hesitancy Intermittency Slow stream Splitting or spraying Straining Terminal dribble |
Feeling of incomplete emptying |
| ICS |
Frequency Nocturia Urgency Urinary incontinence Stress incontinence Urge incontinence |
Slow stream Splitting or spraying Intermittent stream Hesitancy Straining |
Feeling of incomplete emptying Postmicturition dribble |
Lower urinary tract symptoms are closely associated with erectile dysfunction (ED) 12. A large multinational survey showed that the prevalence of ED increased with increasing severity of LUTS 13. Preclinical evidence suggests that there are common pathophysiological mechanisms underlying the development of both ED and LUTS 14. Indeed, in a recent review, Kirby et al. have recommended that physicians should be aware of the sexual adverse effects of many treatments, which are currently recommended for LUTS and that sexual function should be evaluated prior to commencement of treatment, and monitored throughout the treatment to ensure that the choice of drug is appropriate 14. The evidence‐base for conservative management of LUTS after treatment for pelvic cancers is small and characterised by variations in patient characteristics. Furthermore, although guidelines exist for treating men with LUTS, these are not specific to cancer patients and are based on benign disease causality 15. Here we review the conservative interventions, which can improve LUTS in men who have had treatment for pelvic cancers. We aim to provide recommendations based on clinical evidence and best clinical practice.
General predictive factors for LUTS
Predictive factors for postoperative and postradiotherapy LUTS are summarised in Table 2 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26. Known risk factors such as prior transurethral resection have been well evidenced, but probable risk factors such as central nervous system damage and/or cognitive impairment is less well defined in the cancer population but is essential in the assessment of LUTS. Many older cancer patients may already have high anticholinergic loading from other medications such as tricyclic antidepressants and ACE inhibitors which may impact on LUTS. These should be considered in the assessment intervention and management 27, 28.
Table 2.
Known and probable predictive factors for LUTS after prostate cancer treatment
| Known risk factors | Probable risk factors |
|---|---|
| Preoperative LUTS status | Clinical and pathologic stage of the tumour |
| Pelvic cancers and their treatments | Smoking |
| Age | Respiratory disease |
| Previous transurethral resection | Preoperative erectile dysfunction |
| Operative technique (prostatectomy patients)/preservation of the neurovascular bundles | Radiotherapy technique: dose‐volume (post radiotherapy) |
| Prostate volume | Number of needles in prostate brachytherapy |
| Obesity and low physical activity | Accuracy of radiotherapy |
LUTS after cancer treatment
Lower urinary tract symptoms can significantly reduce men's quality of life, and may point to serious pathology of the urogenital tract 8. Age is an important risk factor for LUTS and the prevalence of LUTS increases as men get older 8. LUTS can be indicative of prostate cancer and patients with other pelvic cancers should be assessed accordingly. LUTS can also be a complication following the treatment for pelvic cancers such as colorectal, bladder and prostate cancer 16. Indeed, cancer survivors are far more likely to suffer from UI than the general population, although very little data exist on the impact of UI on quality of life among cancer survivors, especially in elderly populations 29. The large Prostate Strategic Urologic Research Endeavor (CaPSURE) study in 3056 prostate cancer survivors demonstrated that decline in the urinary function was independently associated with satisfaction with prostate cancer care 30.
In cancer patients, LUTS can have distinctive pathology and causality because of a combination of different factors than in patients with benign disease. Around 20% of patients develop or continue to have UI within 2 years of prostectomy for prostate cancer 16. The symptoms of LUTS can develop months to years after the treatment for pelvic cancers. Hence, regular assessment of LUTS in cancer survivors is necessary. Structural abnormalities of the bladder such as rigidity and changes in bladder size can also influence bladder capacity. Overactive bladder (OAB) is one of the main contributing factors of LUTS 32. This may be caused by the direct effects of cancer therapies or prior disease. This is because of injury to neural pathways to and from the bladder and from a partial denervation of the bladder muscle causing excitability and an involuntary rise in pressure within the bladder resulting in frequency and urgency of passing urine 32.
Postprostatectomy LUTS
Patients undergoing prostatectomy are more likely to have stress UI (SUI) than those undergoing radiotherapy (RT) at 2 and 5 years, although there are no significant between‐group differences at 15 years 30. Continence improves progressively until 2 years from Radical prostatectomy (RP) but some patients can become incontinent later 16, 33. In clinical practice, however, clinicians tend to observe that this improvement takes place over the first year; and therefore, surgical interventions should be considered after 1 year. Therefore, an important question to ask a patient during assessment is the duration of incontinence. The criterion of pad use discriminates well between men with a limited reduction in their QoL (no or one pad used) and those with a markedly affected QoL (> or =2 pads/day) 33. The 24‐h pad weight can also be employed, and is usually a better marker for severity of incontinence, allowing it to be divided into mild, moderate and severe 34. Furthermore, only one‐third of leak‐free and pad‐free continent patients prior to treatment return to the same state at 2 years after treatment 26. Postprostatectomy UI is most often caused by dysfunction of the urethral sphincter (either from injury of striated muscle fibres or the innervating nerve fibres) and/or detrusor dysfunction, leading to stress and/or urgency incontinence, respectively 35, 36, 37. The reported SUI rates one year after RP vary between 5% and 48.0% 38. In addition, especially during the first year after RP, OAB symptoms are common in up to 77% of patients, but generally resolve over time 39.
Finally, the relatively small number of men treated with RT before prostatectomy have higher incidence of incontinence compared with men treated with just external beam RT or RT after surgery. Fowler et al. found that the rate of incontinence was 5.5% when the surgery was performed before RT and 33% when performed after RT 40. In another study of 60 patients, given external RT after radical prostatectomy (RP), no difference was observed in terms of UI in the 24‐month follow‐up 41.
Post RT and chemotherapy LUTS
Radiotherapy with or without chemotherapy for pelvic malignancies may result in LUTS 21. Overall, severe late effects occur in ≤ 10% of patients with prostate or bladder cancer 21. Acute side effects that occur during RT usually resolve within a few months 21. Long‐term symptoms attributable to global injury include dysuria, frequency, urgency, contracture from fibrosis, spasm, reduced flow and incontinence 21. More focal injury includes haematuria, fistula, obstruction, ulceration and necrosis 21.
A prospective study of 614 patients with localised prostate cancer treated with RP, external conformal RT and brachytherapy (BT) was carried out to compare treatment impact on health‐related quality of life (HRQL) 42. In each treatment group, HRQL initially deteriorated after treatment with subsequent partial recovery. Compared with the BT group, RP patients had worse UI scores (p < 0.001). Prostatectomy patients had significantly better urinary irritation scores than BT patients (p < 0.001) 42.
The bladder is particularly sensitive to certain cytotoxic drugs, leading to cystitis, fibrosis and occasionally diminished bladder volume leading to symptoms of urinary frequency, dysuria, haematuria and sphincter dysfunction 4. LUTS occurs in an estimated 71% of patients receiving maintenance BCG for bladder cancer 4. Intravesical mitomycin C (MMC) has also been known to exacerbate LUTS in these patients 43, 44, 45. In general, patients on chemotherapy appear to be more prone to UTIs 46, 47, 48 and primary care physicians must be aware of this in order to assess and manage these patients appropriately.
Neo‐adjuvant and Adjuvant ADT in combination with RT LUTS
Adjuvant androgen suppression with hormonal therapy did not increase rectal or urinary dysfunction in the RADAR trial, designed to determine whether adjuvant androgen suppression, bisphosphonates and radiation dose escalation for localised prostate cancer may improve oncologic outcomes 49. Stone et al. showed that pretreatment ADT in patients receiving BT may decrease treatment‐related urinary symptoms in patients who have a large prostate and an International Prostate Symptom Score (IPSS) of 15 or greater 50. Grant et al. showed that patients on RT plus ADT achieved baseline urinary symptoms more rapidly than the patients on RT alone 51. However, Crook et al. demonstrated that despite 2–6 months of prior hormonal therapy before RT; late urinary morbidity was seen in 27% of men following prostate BT 52.
Treatment with degarelix or goserelin + bicalutamide has demonstrated relief of LUTS in patients with moderate and severe voiding problems at baseline 53, 54, 55. Another study of 104 patients, on 3.75 mg leuprolide acetate at 4 week intervals for a total of 12 weeks, demonstrated that leuprolide treatment significantly improved daytime urinary frequency, despite a deterioration in physical, role and sexual function 56.
LUTS after high‐intensity focused ultrasound and cryotherapy
Owing to technological advancements in high‐intensity focused ultrasound (HIFU) procedures, long‐term follow‐up of patients has demonstrated improved urinary symptoms after treatment 57. A recent study assessed the impact of HIFU on lower urinary tract by comparing pre‐ and postoperative symptoms and urodynamic changes. Following HIFU, detrusor overactivity, decreased bladder compliance and urge incontinence were observed. However, these symptoms were also observed in 20% of patients before surgery. There was a progressive improvement in all storage and voiding patterns at 6‐month follow‐up, although patients with high prostate volume and long procedure length suffered from urge incontinence during long‐term follow‐up 58. Limited urinary and rectal morbidity have been observed in other long‐term follow‐ups after HIFU (≥ 12 months) 59, 60, 61. According to clinical observations, LUTS tend to improve quite quickly after treatment.
Reported postsalvage cryosurgery UI rates range from 0% to 83% 62, 63, 64, 65, 66, 67. Generally, men treated with cryotherapy report higher prevalence of urinary symptoms compared with RP in the short term, but the symptoms improve or disappear after ≥ 3 months 68, 69. A 2‐year follow‐up observational study of 10,928 men comparing BT vs. cryotherapy demonstrated that cryotherapy was associated with more urinary complications than BT 70. In general, urinary complications after HIFU or cryotherapy are more common and more severe in patients previously treated for prostate cancer (usually by RT) vs. treatment naive patients 71.
Rationale for guidance development
The management of male LUTS after pelvic cancer therapy consists of three different approaches: conservative management, pharmacotherapy and surgical treatment. Existing guidance for the management of LUTS in primary care is based on benign prostatic disease 28 and therefore does not take into account the causality and differences in treatment‐related effects from cancer treatment. There is a need for specific guidance to manage LUTS symptoms in the population affected by the spectrum of male pelvic cancers. This review critically explores the evidence‐base for the assessment and management of LUTS in male cancer patients and provides guidance regarding the non‐invasive interventions (conservative management and pharmacotherapy) which can be used in primary care specifically and advice on when referral to specialist urological services is warranted. Our aim was to extract only the most recent studies and not to overlap with the evidence published in NICE LUTS Clinical Guideline 8. This guide is aimed at non‐specialist clinicians working with men after pelvic cancer therapy in primary care follow‐up. It discusses LUTS in men following the most commonly used treatments for cancer.
Methods
Literature analysis
A systematic review of the literature was conducted to investigate the evidence‐base for the non‐invasive management of LUTS in men following pelvic cancer treatment. The interventions covered by this guidance include lifestyle changes, exercise and oral medications.
Web of science, Medline, Cinahl, Psycinfo, Cochrane database and Embase were searched using various combinations of the following terms: lower urinary tract symptoms and/or treatment and/or bladder cancer and/or rectal cancer and/or prostate cancer and/or (names of specific drugs) and/or brachytherapy/radiotherapy/cryotherapy/HIFU/androgen deprivation therapy and urinary incontinence.
Only original publications and systematic reviews were sourced; however, literature reviews were also retrieved and hand searched for individual studies and all relevant papers extracted. Publications were included if they described an intervention for any area of LUTS. Interventions included were professional guided management such as pharmacological treatment, as well as self‐management interventions including all behavioural management approaches. Publications, which did not include at least one intervention for treating LUTS after pelvic cancer treatment, were excluded. The search included papers published from 2000 to 2014. The studies identified and used in this literature analysis were graded using the Oxford Centre for Evidence‐based Medicine Levels of Evidence. The findings of the literature analysis were integrated with authors' clinical experience to provide recommendations outlined in this review.
Results
Literature search overview
The literature search identified 41 articles for the final analysis. Twenty‐four papers were included that concerned the behavioural interventions using Pelvic Floor Muscle Exercises (PFME), 10 described pharmacological interventions, six articles described other interventions and one article described containment devices. The selection criteria for the 17 studies included: articles in English, studies which utilised an intervention for LUTS, studies with adult male patients treated for pelvic cancers. Non‐invasive treatments were included (conservative management, e.g. lifestyle, behavioural interventions such as exercise or diet and pharmacological interventions). Both randomised and non‐randomised studies were used (Table 3). A meta‐analyses of the data was not performed due to; Inconsistent definitions, measurement tools & diverse timings used in identified studies; as well as low number of articles identified for each intervention.
Table 3.
Study and patient characteristics selected from the literature analysis
| First author | LUTS | No. of patients | Study design | Level of evidence | Intervention | Start of treatment (after RP/RT) | Active treatment mean follow‐up |
|---|---|---|---|---|---|---|---|
| Conservative management | |||||||
| Pelvic floor muscle training (PFMT) | |||||||
| Campbell et al. 72 | UI | 1937 | Systematic review | 2A | Conservative management: PFMT | Varied (post‐surgery) | Varied |
| Centemero et al. 73 | UI | 118 | RCT | 1B | Pre and post‐op PFMT vs. Post‐op PFMT | 30 d before surgery vs. at catheter removal | 3 mo |
| Centemero et al. 74 | UI | 143 | RCT | 1B | Structured PFMT pre‐op vs. post‐op PFMT | 30 and 15 d pre‐op | 3 mo |
| Dieperink et al. 75 | Urinary, bowel, sexual, and hormonal symptoms | 161 | RCT | 1B | TG guided sessions vs. standard care | Pre and 4 wk post RT/ADT | 20 wk |
| Dubbelman et al. 76 | UI | 70 | RCT | 1B | Physiotherapist‐guided PFMT vs. information only folder | Immediately after surgery | 26 wk = 6 mo |
| Faithfull et al. 77 | LUTS | 71 | Case series | 4 | Self‐management:
|
≥ 3 mo post RT | 4 mo |
| Filocamo et al. 78 | UI | 300 | RCT | 1B | Structured PFMT program vs. no formal instructions re PFMT | After catheter removal post‐RP | 6 mo |
| Geraerts et al. 79 | UI | 180 | RCT | 1B | PFMT pre‐op and continued after surgery vs. PFMT post‐op | 3 wk before RP vs. at catheter removal | 6 mo |
| Glazener et al. 80 | UI | 787 (2 trials; 411 after RP and 442 after TURP) | RCT | 1B | PFMT with physiotherapist vs. lifestyle advise only (no PFMT info) | 6 wk after surgery | 12 mo |
| Goode et al. 81 | UI | 208 | RCT | 1B | Behavioural therapy vs. behavioural therapy + in‐office, dual‐channel electromyograph biofeedback and daily pelvic floor electrical stimulation vs. delayed treatment* | ≥ 1 yr after RP | 12 mo |
| Khoder et al. 17 | UI | 911 | Retrospective cohort analysis | 4 |
|
Varied (post‐RP) | Varied |
| Lin et al. 76 | UI | 67 | RCT | 1B | PFMT vs. no exercise | At catheter removal after surgery | 6 mo |
| Marchiori 18 | UI | 332 | RCT | 1B | Tutored and guided pelvic training program vs. no program (patients asked to perform same PFMT at home) | 1 mo after RP | 12 mo |
| Mariotti et al. 19 | UI | 60 | RCT | 1B | PFES + biofeedback vs. control (no treatment) | 7 d after surgery | 6 mo |
| Nilssen et al. 83 (based on Overgard subjects) | Urinary, sexual and bowel function | 85 | RCT | 1B | Physiotherapist‐guided PFMT vs. self‐training | Within 12 mo of RP | 12 mo |
| Overgard et al. 16 | UI | 85 | RCT | 1B | Physiotherapist‐guided PFMT vs. self‐training | Within 12 mo of RP | 12 mo |
| Park et al. 31 | UI | 49 | RCT | 1B | Combined exercise intervention vs. only Kegel exercises | Week 3 after RP | 12 wk |
| Patel et al. 84 | UI | 284 | Retrospective cohort analysis | 4 |
Preop Physiotherapist‐guided PFMT vs. information only Post‐op – both groups had Physiotherapist‐guided PFMT |
4 wk pre‐op vs. after surgery | 3 mo |
| Ribeiro et al. 85 | UI | 73 | RCT | 1B | PFMT‐biofeedback vs. brief advise on PFMT | 15 d after surgery | 12 mo |
| Serdà 86 | UI | 66 | RCT | 1B | PFMT | Time n/a (after RP) | 24 wk |
| Tienforti et al. 87 | UI | 32 | RCT | 1B | Training session + BFB, supervised PFMT and structured post‐op programme (+post op control visit) vs. post op info only | 1 d before surgery vs. At catheter removal | 6 mo |
| Van Kampen et al. 88 | UI | 102 | RCT | 1B | PFMT vs. placebo | At catheter removal | 1 yr |
| Wille et al. 89 | UI | 139 | RCT | 1B | Instructions about PFMT vs. instructions and ES† for 15 min twice daily vs. biofeedback for 15 min twice daily | At catheter removal post RP | 3 mo |
| Zahariou et al. 90 | UI | 58 | RCT | 1B | Nurse‐supported structured program vs. information only | At catheter removal after RP | 6 mo |
| Oral medication | |||||||
| Serotonin‐norepinephrine reuptake inhibitor (Duloxetine) | |||||||
| Cornu et al. 91 | Stress UI (SUI) | 31 | RCT | 1B | 80 mg duloxetine daily vs. placebo | >1 yr after RP | 3 mo |
| Filocamo et al. 92 | SUI | 112 | RCT | 1B | Duloxetine + rehabilitation vs. rehabilitation | After catheter removal (post RP) for 16 wk | 24 wk |
| Neff et al. 93 | Stress UI | 94 | Case series | 4 | Duloxetine 30 mg once a week, then 60 mg thereafter | Not specified (Post‐RP) | 1 mo |
| Alpha blockers | |||||||
| Jang et al. 94 | Voiding function | 94 | RCT | 1B | Tamsulosin (0.2 mg/day for 7 days) vs. placebo | 3 days after rectal surgery | 7 days |
| Oyama et al. 95 | LUTS | 116 | Case series | 4 | Alpha 1‐adrenoceptor antagonists (tamsulosin, silodosin and naftopidil) | ≥ 1 yr after BT | |
| Shimizu et al. 96 | LUTS | 105 | Randomised comparative study | 2A | Silodosin 8 mg/day daily for 6 mo | Immediately after BT | 12 mo |
| Tsumura et al. 97 | Urinary symptoms | 212 | RCT | 1B | Naftopidil vs. tamsulosin vs. vs. silodosin | 1 d after BT | 12 mo |
| Antimuscarinics | |||||||
| Zhang et al. 98 | Urinary symptoms | 116 | RCT | 1B | Solifenacin 5 mg for 2 wk vs. placebo | 6 h pre‐surgery and daily post‐surgery | 2 wk |
| Phosphodiesterase type 5 inhibitor (PDE5‐I) | |||||||
| Gacci et al. 99 | UI | 39 | RCT | 1B | Vardenafil on demand vs. Vardenafil nightly vs. Placebo | Immediately after RP | 12 mo |
| Gandaglia et al. 20 | Recovery of sphincter and pelvic floor function | 705 | Review | 4 | No PDE5‐I use vs. On‐demand PDE5‐I use vs. Daily PDE5‐I use | 30 d after surgery | Mean follow up 29 mo |
| Other treatments | |||||||
| Bonetta and Di Pierro 100 | UTIs | 370 | Randomised comparative study | IIA | Cranberry extract vs. no extract | During RT | 6–7 wk |
| Campbell et al. 101 | Urinary symptoms | 112 | Comparative study | IIA | Cranberry juice vs. apple juice 354 ml/day | While receiving RT | 9 wk (incl 7 wk on RT) |
| Cowan et al. 102 | Urinary symptoms | 128 | RCT | 1B | Cranberry juice vs. placebo beverage | During and post‐RT | 6 wk |
| Matsushita et al. 103 | Urinary symptoms | 54 | Comparative study | IIA | Mecobalamin (Vit B12) vs. no treatment | Pre‐ and post‐RP | 12 mo |
| Sommariva et al. 104 | Cystitis | 69 | Case series | 4 | Weekly sodium hyaluronate, 40 mg/50 ml + dexamethasone 32 mg (initial 4 wk) | Post RP and post chemo for bladder cancer | 24 wk |
| Tanaka et al. 105 | LUTS | 37 | Comparative study | IIA | Eviprostat vs. no treatment | 3 mo pre and 3 mo post BT | 6 mo |
| Containment devices | |||||||
| Fader et al. 106 | UI | 80 | Comparative study | IIA | Penile compression devices (clamp) vs. Sheath drainage systems vs. Body‐worn urinals | ≥ 1 yr post‐surgery | 3 mo |
PFMT, Pelvic floor muscle training; BT, Brachytherapy; RT, Radiotherapy; UTI, Urinary tract infection; TG, Therapist guided; mo, month; wk, week; d, day. *Offered treatment after 8 wk but not during study period. †Electrical stimulation.
Studies and patient characteristics from the literature analysis
In total, 8951 patients were included in the 41 selected studies (Table 3). Most of the studies were randomised controlled studies. Follow‐up ranged from 1 week up to 12 months while management duration ranged from 30 days before treatment to ≥ 1 year after treatment (Table 3).
Of the 30 studies assessed for management of UI after surgery, one study assessed recovery of sphincter/pelvic function after surgery and one study assessed management of cystitis after surgery. Four studies evaluated management of UI after RT/BT; one study evaluated management of urinary tract infections after RT and four studies specifically looked at management of LUTS after RT/BT. A wide range of assessments were used across the studies identified, and all the studies were conducted within differing ranges of time points; hence, the outcomes of the studies are difficult to compare directly. Instead, narratives of their key findings are presented in the discussion with author recommendations. Trial participants were adult males, who had received treatment for pelvic cancers, and who had experienced some type of LUTS subsequent to the cancer therapy. Much of the literature was prostate cancer specific and fewer studies explored LUTS in men with bladder and bowel cancer. Table 4 summarises efficacy analysis of current management strategies for LUTS in men after pelvic cancer treatment, as identified by the literature analysis.
Table 4.
Current management strategies for LUTS post pelvic cancer treatment from literature analysis
| First author | LUTS | Primary outcome | Time to symptom improvement | Adverse events | Summary |
|---|---|---|---|---|---|
| Conservative treatment | |||||
| Pelvic floor muscle training (PFMT) | |||||
| Campbell et al. 72 | UI |
UI symptoms
Treated vs. control: No significant benefit of therapists teaching PFMT for either prevention or treatment |
N/a | None | No significant benefit from pelvic floor exercises for UI |
| Centemero et al. 73 | UI |
1 mo: Significantly more patients in pre‐op PFMT were continent 3 mo: Significantly more patients in pre‐op PFMT were continent Pre‐op PFMT also decreased risk of becoming incontinent at 1 month post op |
1 mo | None | Preoperative PFMT may improve early continence and QoL outcomes after RP |
| Centemero et al. 74 | UI | 1 and 3 mo: UI symptoms significantly improved in pre‐op PFMT group | 1 mo | None | Pre‐op PFMT hastens the return to continence more than post‐op alone and decreases the severity of UI following RRP |
| Dieperink et al. 75 | Urinary, bowel, sexual, and hormonal symptoms |
TG guided intervention vs. standard care improved urinary symptoms significantly Patients with more severe impairment gained most |
4 wk | None | Multidisciplinary rehabilitation in irradiated PCa patients improved urinary and hormonal symptoms, and QoL |
| Dubbelman et al. 76 | UI | No significant difference in recovery of continence between physiotherapist assisted PFMT and self‐training with information folder | 6 mo | None | Physiotherapist assisted PFMT seems to have no beneficial effect on the recovery of continence over an information only approach |
| Faithfull et al. 77 | LUTS |
IPSS: Significant improvement in LUTS symptoms and voiding volume*
Improvement in QoL |
4 mo | None | Self‐management provided benefits for men |
| Filocamo et al. 78 | UI | At 1 mo significantly more patients in structured PFMT group achieved continence | 1 mo | None | After RRP an early supportive rehabilitation PFMT programme significantly reduces continence recovery time |
| Geraerts et al. 79 | UI |
No significant improvement re duration of UI between pre‐op and post‐op PFMT QoL better with pre‐op patients |
30 d | None | Three preop sessions of PFMT did not improve duration of incontinence but may impact QoL positively |
| Glazener et al. 80 | UI | Trials 1 and 2: Rates of UI not significantly different between PFMT vs. advise only | 12 mo | None | One‐to‐one PFMT is unlikely to be effective or cost effective |
| Goode et al. 81 | UI | Mean UI episodes decreased significantly in both behaviour and behaviour + stimulation groups vs. controls (p = 0.001) | 8 wk | None |
Behavioural therapy, compared with a delayed‐treatment control, resulted in fewer incontinence episodes Addition of BF and PFES † didn't result in greater effectiveness |
| Khoder et al. 17 | UI |
Grade of incontinence after RP
Significantly improved after PFMT, AES, or combinations* |
3 wk | None | |
| Lin et al. 82 | UI |
Urinary control in the exercise group was better than in the non‐exercise group Urine leakage decreased over time regardless of the group |
1 mo | None | Patient education regarding PFMT by a nurse prior to and after surgery has a significant impact on the early recovery of UI |
| Marchori 18 | UI |
Median time of continence recovery
Significantly quicker time to recovery with pelvic floor re‐educational dedicated program vs. no education* |
44 (treatment) vs. 76 (control) days (p < 0.01) | None | PFMT supported significantly improves time to recovery of continence |
| Mariotti et al. 19 | UI |
The mean leakage weight became significantly lower (p < 0.05) in group 1 than Median time of continence recovery:
Reductions in UI in treatment vs. control* |
4 wk | None | Early, pelvic floor electrical stimulation plus biofeedback have a significant positive impact on the early recovery of UI |
| Nilssen et al. (based on Overgard subjects) 83 | Urinary, sexual and bowel function | No statistically significant difference in HRQoL was found between treatment groups | 12 mo | None | No significant difference between physiotherapist‐guided training vs. standard self‐training |
| Overgard et al. 16 | UI |
3 mo: no statistically significant difference in continence status 6 mo: guided PFMT significantly better continence than self‐training 12 mo: clinically and statistically significant improvements with guided training |
6 mo | None | Physiotherapist‐guided PFMT training for up to 6 mo significantly improves continence status vs. self or standard training |
| Park et al. 31 | UI | 12 wk: Except for grip strength, all physical functions were better in the exercise group than in the control group. Better continence recovery and improved QoL in exercise group | 12 wk | None | 12‐wk combined exercise intervention after RP results in improvement of physical function, continence rate, and QoL |
| Patel et al. 84 | UI |
6 wk: UI symptoms significantly lower in physiotherapist‐guided preop group 3 mo: No significant difference Physiotherapist‐guided PFMT reduced time to continence significantly |
6 wk | None | Physiotherapist‐guided PFMT 4 wk preoperatively, significantly reduces the time to continence and it significantly reduces the duration and severity of early UI after RP |
| Ribeiro et al. 85 | UI |
Number of pads used daily 96.15% (PFMT) vs. 75.0% (control) continent at 12 mo* + improvements in other LUTS symptoms* |
12 mo | None | Early biofeedback‐PFMT is beneficial for reducing duration and severity of UI |
| Serdà 86 | UI |
UI symptom, intensity, frequency, difficulty and limitation of activity were significantly improved QoL correlated with UI improvement |
24 wk | None | Improvement in QoL is mediated by improvement in UI symptoms |
| Tienforti et al. 87 | UI | 3 and 6 mo: UI symptoms significantly improved in pre‐op PFMT group and better NS QoL scores | 1 mo | None | Pre‐op PFMT, even if started a day before surgery, can confer significant benefits in terms of UI symproms |
| Van Kampen et al. 88 | UI | Continence achieved in both groups but duration and degree of incontinence significantly better with PFMT vs. placebo | 3 mo | None | PFMT improved UI if started at catheter removal |
| Wille et al. 89 | UI | UI symptoms: No significant difference among the three groups | N/A | None | PFMT, electrical stimulation (ES) and biofeedback did not affect continence |
| Zahariou et al. 90 | UI |
1 mo: No difference between groups 3 and 6 mo: Significantly higher number of continent patients in treatment vs. control group |
3 mo | None | Nurse‐trained patients achieve higher continence rates vs. patients who were just informed re PFMT |
| Oral medication | |||||
| Serotonin‐norepinephrine reuptake inhibitor | |||||
| Cornu et al. 91 | SUI | Significant reduction in urinary symptoms as well as QoL improvements with duloxetine vs. placebo | 3 mo | Both treatments well tolerated (fatigue was the only AE associated with duloxetine) | Duloxetine is effective in the treatment of SUI & improves QoL |
| Filocamo et al. 92 | SUI |
Duloxetine + rehab: Significant decrease in pad use and significantly more dry patients at 16 wk At 24 wk no significant difference between groups in dry rates |
16 wk | 15.2% had adverse effects | Duloxetine improves continence temporarily after RP |
| Neff et al. 93 | Stress UI | Significant decrease in daily pad use and Incontinence Impact Questionnaire (IIQ‐7) | 1 mo |
Intolerable side effects in 14/94 (15%) Fatigue, light‐headedness, insomnia, nausea and dry mouth |
Duloxetine improved post‐prostatectomy SUI though drop out rate was high |
| Alpha blockers | |||||
| Jang et al. 94 | Voiding function | Postop voiding parameters were not better with tamsulosin vs. control | 7 d | Well tolerated | Tamsulosin 0.2 mg/day does not prevent acute voiding difficulty |
| Oyama et al. 95 | LUTS | Better IPSS scores and recovery with silodosin compared with tamsulosin or naftopidil | 3 mo | Not specified | Silodosin may provide a favourable improvement of LUTS after BT |
| Shimizu et al. 96 | LUTS |
6 mo; Significant improvements in the IPSS with silodosin vs patients not on it 3 and 12 mo:Silodosin significantly enlarged the bladder capacity No improvement of bladder outlet obstruction index (BOOI) |
3 mo | Well tolerated | Silodosin temporarily improves LUTS |
| Tsumura et al. 97 | Urinary symptoms |
1 mo: Significantly greater decreases in urinary symptoms with silodosin than naftopidil 6 mo: Silodosin showed a significant improvement in the PVR vs. tamsulosin |
1 mo | Well tolerated | Silodosin has a greater impact on improving PI‐induced LUTS vs. naftopidil and tamsulosin |
| Antimuscarinics | |||||
| Zhang et al. 98 | Urinary symptoms |
Significant reductions in overactive bladder symptom scores with solifenacin Episodes of daytime, frequency, nocturia, urgency, and urge urinary incontinence were significantly lower than with solifenacin (p < 0.05) |
2 wk | Well tolerated | Solifenacin can be beneficial for the management of urinary symptoms after surgery for bladder tumours |
| Phosphodiesterase type 5 inhibitor (PDE5‐I) | |||||
| Gacci et al. 99 | UI |
Urinary function (UF) improved significantly in all arms Nightly resulted in greater UF at 3, 6, and 9 mo vs. placebo |
1 mo | Well tolerated | Daily use of vardenafil provides better continence rate |
| Gandaglia et al. 20 | Recovery of sphincter and pelvic floor function |
Significantly lower rates of continence recovery with no PDE5‐I Daily PDE5‐I associated with higher continence recovery at vs. on demand |
1 yr | Well tolerated |
PDE5‐I use significantly improved continence recovery Effect is significantly better with daily vs. on demand use |
| Other treatments | |||||
| Bonetta and Di Pierro 100 | UTIs | Significantly more LUTS without cranberry extract observed | Preventative study – lasted 7 wk | Gastric pain | Cranberry extracts reduced the incidence of LUTIs when given during RT |
| Campbell et al. 101 | Urinary symptoms | No significant difference in urinary symptoms | 2 wk | None | No significant difference in urinary symptoms during EBRT with cranberry juice vs. apple juice |
| Cowan et al. 102 | Urinary symptoms | Non‐significant increase in urinary symptoms with placebo vs. cranberry | 6 wk | None | Cranberry juice did not affect urinary symptoms though the study was of limited size and duration |
| Matsushita et al. 103 | Urinary symptoms | No difference between the groups in terms of urinary function | 3 mo | None | Vitamin B12 doesn't improve urinary function significantly after RP |
| Sommariva et al. 104 | Cystitis |
4 wk – bladder capacity and urinary symptoms improved in all patients 8 wk – significant improvements in urinary symptoms and pain |
4 wk | None | Intravescical sodium hyaluronate seems a valid and quick therapeutic solution for cystitis from chemo or RT |
| Tanaka et al. 105 | LUTS | Eviprostat‐treated patients showed significantly better recovery compared to Eviprostat‐untreated control at 6 mo | 3 mo | None | Eviprostat demonstrated benefits in post‐op LUTS after BT |
| Containment devices | |||||
| Fader et al. 106 | UI |
Pads most highly rated vs. Sheaths, clamps and BWUs BWU rated worse than the sheath Sheath rated highest for extended period use ~50% used combination of these over 3 mo |
N/A | Clamp rated as significantly more painful than other devices |
Male devices can help men with UI Most men prefer to use a combination of devices and pads in order to meet their lifestyle needs |
PFMT, Pelvic floor muscle training; AES, anal electrical stimulation; IPSS, International Prostate Symptom Scores; d, day; wk, week; mo, month; yr, year. *p ≤ 0.05. †Pelvic muscle electrical stimulation.
Discussion
The literature analysis identified a range of intervention studies, but it must be noted that most of the research have focused on UI rather than the wider extent of LUTS. Therefore, few studies explore the full range of LUTS symptoms as defined.
Assessment of LUTS
Assessment is fundamental in the management of LUTS as well as recognising the impact and bother urinary symptoms may have for the individual 71. The majority of the studies identified in this review assess UI rather than LUTS and the wider perspective of the patient. Pad tests/daily pad usage, IPSS and self‐reported continence were generally used to assess LUTS/UI in most of the clinical studies identified. NICE guidelines recommend the use of bladder diaries as a cost‐effective tool for assessing incontinence (though not specific to cancer patients), and this gives information on voiding and frequency 8. These tools are developed for generic LUTS and are research focused and so, may be difficult to use routinely in practice. The need for objective measurement and to provide a baseline for assessing change and patient outcomes is essential.
In clinical practice, the IPSS is routinely used to assess LUTS, and contains three questions regarding storage symptoms and four on obstructive voiding symptoms and one on LUTS impact on quality of life. However, IPSS does not assess incontinence, which is why more detailed questionnaires are often needed. The International Consultation on Incontinence Questionnaire (ICIQ) and ICIQ‐LUTS is a validated questionnaire for evaluating quality of life and urinary symptoms. It explores in detail the impact on patients' lives of LUTS and can be used as an outcome measure to assess impact of different treatment modalities. Hence, the ICIQ‐LUTS is useful for assessing QoL; ICIQ UI for incontinence and ICIQ OAB for storage and LUTS symptoms. Asking patient about their symptoms is also important as questioning which often identifies the impact and adherence with interventions that may not be measured in LUTS scores 107. Common questions which GPs can use include:
Do you experience any loss of urine when coughing or sneezing?
Do you experience any loss of urine after voiding completion?
Do you have to arrange your day around finding toilets because of urinary frequency?
Do you experience disturbed sleep at night because of needing to pass urine frequently?
The aims of assessment are to: identify reversible factors that may be contributing to or causing symptoms contributing to LUTS, understand the level of distress or bother and impact for the individual, identify those men who may need more specialist assessment or intervention such as urology or clinical nurse specialist, or continence adviser referral and to develop a baseline prior to referral and as an evidence‐based plan of treatment for the individual.
Assessment recommendation
General assessment including self‐reported incontinence.
Self‐reported continence can be complemented with one of the validated questionnaires, e.g. IPSS, ICIQ‐LUTS QoL for QoL; ICIQ UI for incontinence and ICIQ OAB for storage LUTS symptoms.
3–7 day bladder diary.
Consider pad usage.
Dipstick urinalysis for leucocytes and nitrites to rule out infection.
Dipstick analysis for haematuria.
Additional assessment
Bladder ultrasound for identifying residual and structural issues.
Flow rate and measurement of urodynamics (usually available through community Continence nurse services).
Conservative management
Conservative management of LUTS (specifically UI – the most studied LUTS symptom) includes lifestyle interventions, pelvic floor muscle training (PFMT) with or without biofeedback, and bladder training. Lifestyle interventions include moderating fluid intake, avoidance of known bladder irritants such as caffeine and alcohol, weight loss, and smoking cessation; however, these interventions are less researched 108.
Current guidelines recommend behavioural therapies and lifestyle changes as first line treatments for urinary problems although there is no specific guidance for cancer patients 8, 28. Behavioural techniques include bladder retraining techniques for example progressive voiding schedule together with relaxation and distraction for urinary urgency. Patient education on promoting healthy bladder habits, reducing bladder irritants from the diet, fluid intake management, weight control, smoking cessation and management of bowel regularity 109. These common techniques were not considered in the review. Behavioural interventions which have multicomponent elements of training such as PFME were considered in this review as part of treatment approaches.
Pelvic floor muscle training
Most of the publications found in this analysis primarily focus on UI rather than LUTS and the interventions have been studied mainly in prostate cancer patients rather than other pelvic cancers. Therefore, this may under report the impact of PFMT on the wider profile of urinary symptoms. In addition, the recommendations proposed for management of LUTS after pelvic cancer treatment are based on clinical practice as well as clinical evidence‐base.
PFMT after radiotherapy
Two small studies have assessed the effects of PFMT and multidisciplinary rehabilitation after RT/ADT, and both demonstrated significant improvements with PFMT in this patient group 75, 77. Faithfull et al. demonstrated that PFMT, in conjunction with bladder retraining, patient education and problem solving and coping strategies, resulted in significant improvement in IPSS (p < 0.005) as well as a positive impact on HRQL after RT treatment 77. Serda et al. 86 demonstrated improvements in variables related to the UI symptom, intensity, frequency, difficulty and limitation of activity after 24 weeks of PFMT (p ≤ 0.0001). These results were further confirmed by Dieperink in an RCT of Danish population (n = 161) stratified to multidisciplinary rehabilitation after RT/ADT, where nursing counselling sessions and therapist‐guided instructive sessions resulted in significant improvements of LUTS symptoms vs. standard care 75.
PFMT before/after surgery
Most of the randomised controlled trials identified contain information on PFMT after prostatectomy. Strengthening PFMs plays a significant role in recovery after surgery.
A Cochrane review, published in 2012, which assessed the effects of ‘conservative’ management for UI after prostatectomy, concluded that there remains no clear support that conservative management of any type for postprostatectomy UI is either helpful or harmful, whether delivered as treatment to men who are incontinent or as prevention to all men undergoing RP 72. It must be noted that the Cochrane review did not stratify studies in early vs. late initiation PFMT or preoperative vs. postoperative PFMT or physiotherapist‐guided (with/without biofeedback) vs. standard care PFMT. These factors have been addressed briefly below.
Early vs. late PFMT
In a quasi‐experimental study of 47 postsurgery patients randomised to PFMT vs. no PFMT, Lin et al. showed that that urinary control in the exercise group was better than in the non‐exercise group although UI decreased significantly in both groups 79. The difference observed between the two groups was attributed to patient education regarding pelvic floor exercises by a nurse prior to and after surgery. Patients were stratified in the two groups after catheter removal, suggesting early management may improve outcomes. Similarly, Van Kampen, et al. demonstrated significant improvements in the duration and degree of continence with PFMT vs. placebo therapy if PFMT was initiated at catheter removal 88. Other studies also demonstrate beneficial effects of PFMT if initiated early after RP 19, 85, 92.
Five studies identified in our review investigated the effectiveness on UI if PFMT is initiated up to 30 days preoperatively 73, 74, 84. One study even demonstrated benefits of PFMT if initiated a day before RP, although the sample size was quite small and a larger study would be needed to investigate this further 87. In addition, the investigators compared physiotherapist‐assisted vs. non‐physiotherapist‐assisted PFMT, hence the role of starting PFMT one day before surgery is not clear. A trial of 180 men, however, demonstrated no significant benefits in terms of UI symptom improvement between PFMT initiated 3 weeks preoperatively (3 sessions) or at catheter removal 79. However, the QoL trend was in favour of preoperative PFMT (non‐significant) 79.
In the Men After Prostate Surgery randomised control trial over 700 men underwent PFMT (four sessions with a therapist over 3 months vs. standard care and lifestyle advice only) 6 weeks after surgery. In this trial, PFMT was not shown to be therapeutic or cost‐effective in improving urinary continence 80. Of the patients in the intervention group, 148 of the 196 patients reported some form of incontinence at the 12‐month mark. In the control group, 151 of the 195 patients reported some UI (difference not significant) 80. However, it must be noted that patients often buy containment devices themselves and costs of those were not included in this study and the study authors recommend that their cost‐effectiveness data should be interpreted with caution 80.
In another study (n = 208), PFMT intervention for persistent long‐term UI after RP (initiated ≥ 1 year after surgery) showed that 8 weeks of behavioural intervention (with or without biofeedback and pelvic floor muscle stimulation), resulted in significantly fewer incontinence episodes compared with a delayed‐treatment control 81. The effect was durable up to 12 months after treatment. Wille et al. found that PFMT, electrical stimulation and biofeedback did not affect continence even when initiated at catheter removal 89. Taken together, these studies suggest some evidence for PFMT if initiated just before or soon after RP, e.g. at catheter removal, but further studies are needed to verify this. Factors to take into account include, among others, number of PFMT sessions, assessment of UI and biofeedback.
In a post‐RP evaluation of UI, Song et al. demonstrated that patients with better developed pelvic floor muscles, especially in relation to the size of the prostate, can be expected to achieve earlier recovery of continence after RP 110.
Physiotherapist‐guided sessions vs. patients training independently (standard care)
Marchiori et al. showed that PFMT in post‐RP patients, supported by physician and nurse experts in continence disorders, can help improve continence 18. Zahariou et al. also demonstrated significant improvements with healthcare professional‐assisted structured PFMT programme vs. standard training after RP 90. Dieperink et al. demonstrated that in post‐RT/ADT patients, therapist‐guided instructive sessions resulted in significant improvements of LUTS symptoms vs. standard care 75.
Two studies have shown no significant difference in improvement of urinary symptoms between physiotherapist‐guided training of the pelvic floor muscles after RP compared to standard care/training or self‐training approach 76, 82. However, in one of these studies, PFMT was imitated within 12 months of surgery rather than soon after the surgery 82.
Biofeedback, in conjunction with PFMT, may also play a role in improving LUTS. Biofeedback is a technique in which physiological activity is monitored, amplified and conveyed to the patient as visual or acoustic signals, thereby providing the patient with information about unconscious physiological processes 111. According to a recent review, the biofeedback for PFMT may improve the patients' ability to isolate the PFM and differentiate between muscle contraction and relaxation 108. In one trial, a single session of biofeedback‐assisted behavioural training reduced the duration of UI as well as the severity of symptoms in the 6 months post‐RP 22. In post‐RP patients, intense preoperative biofeedback‐assisted PFMT session which given one day before RP, – session immediately following catheter removal – and then monthly, combined with an assisted, low‐intensity postoperative programme has demonstrated reductions in the duration and severity of UI as well as improvements in QoL 87.
In OAB, the 5th International Consultation on Incontinence (ICI) guidelines recommend the inclusion of biofeedback in the treatment of urgency syndrome, but the decision is a therapist/patient decision based on economics and preference 111.
Summary
Preoperative or immediate postoperative PFMT is useful. In general, for both RP and RT, earlier return to continence was observed if PFMT was started early in the post‐treatment period.
Therapist‐guided PFMT can significantly improve time to return of continence, especially after prostate surgery. Example of a protocol is shown in Box 1. PFMT key objective is to build tone in the muscles by repeated exercise so that muscles can respond in time to the increase in intra‐abdominal pressure. Note that the actual numbers of exercises are not as important as inclusion of some fast and some slow repetitions (on account of the presence of both slow and fast twitch activity in the pelvic floor muscle). The exercises must be conducted on several occasions throughout the day in order to condition the brain to recognise this as tonic and not as phasic activity.
Box 1. PFMT protocol.
PFMT suggested programme (see Pelvic, Obstetric and Gynaecological Physiotherapy (POGP) guide at http://www.csp.org.uk/sites/files/csp/secure/acpwh-pelvicmen_1.pdf for details of PFMT protocol):
PFMT consists of repeated high‐intensity contractions, encouraged to tighten and lift the pelvic floor muscles as if as in the control of flatus. These can be practiced in front of a mirror to observe a visible withdrawal of the penis.
PFMT structured home programme: 10 min each day of 5‐s muscle contractions with 5‐s muscle relaxation performed in three different positions 87: The protocol consists of 5 slow exercises i.e. to hold and count to 10 and then 5 fast exercises, which are useful for urge incontinence.
The exercises should be conducted sitting, standing‐up and lying down up to three times a day i.e. in total 60 PFM contractions per day. In the lying down position men should have their knees bent or apart. In the standing position PFMs should be conducted with feet apart; and in the sitting position, PFMs should be conducted with the knees apart. Evidence suggests it is the intensity rather than the frequency of the PFMs that is important 87, 111.
PFMT recommendation [Table 3 evidence grade of 1B (partly based on consensus clinical opinion)]
Start PFMT pretreatment (ideally 1 month before surgery in the case of RP) or within one month of RT/ADT treatment/catheter removal after surgery.
Physiotherapist assisted programme has the greatest benefit. Consider using a physiotherapist or at least a DVD with a physiotherapist demonstrating the exercises.
Continue on PFMT for at least 6 weeks.
Can be provided in combination with biofeedback, if possible.
Oral medication
Alpha blockers
Alpha blockers can be used to treat LUTS such as urge UI or OAB as they relax smooth muscles 112. Tsumura et al. compared the efficacy of tamsulosin, silodosin and naftopidil in treating LUTS after BT 97. In this study, 212 patients received one of three alpha 1‐adrenoceptor antagonists for 1 year after BT. The results demonstrated significantly greater decreases with silodosin vs. naftopidil at 1 month in the total IPSS. Silodosin showed a significant improvement in the postvoid residual at 6 months vs. tamsulosin. The authors concluded that silodosin has a greater impact on improving LUTS after BT than tamsulosin or naftopidil 97. Oyama et al. more recently also demonstrated better improvements in IPSS score with silodosin vs. tamsulosin or naftopidil up to 9 months after BT 95. Shimizu et al., however, demonstrated that the effects of silodosin are temporary in a 12‐month follow‐up study of 105 patients given sildonosin daily for 6 months immediately after BT 96. In clinical experience, incontinence – either stress or urge – is uncommon after BT and occurs in less than 2% of patients in the first 2 years after implantation. LUTS following BT are generally driven by the temporary swelling/obstruction that the implant causes, hence the need for an alpha blocker.
Jang et al. investigated the efficacy of 0.2 mg/day tamsulosin (for 7 days) in preventing acute voiding difficulty after rectal cancer surgery in 94 rectal cancer patients 94. The results demonstrated similar reinsertion rate of the urinary catheter in the tamsulosin and control groups (p = 0.804) and similar effects on voiding parameters and IPSS. The authors concluded that tamsulosin did not prevent acute voiding difficulty after rectal cancer surgery.
However, alpha blockers can exacerbate stress incontinence 113, 114 and hence, cannot be recommended after surgery in this review.
Summary alpha blockers
Evidence grade ranging from 1B to 2A (Table 3 )
- Post‐BT: The most effective appears to be silodosin after BT though the effects are temporary (1–6 months)
-
oSilodosin is the only alpha blocker which has demonstrated improvements in LUTS after BT, but its effects only last up to 6 months. However, silodosin is not licensed for use in the UK and hence other alpha blockers can be used.
-
oTamsulosin is commonly used after BT for 3–6 months before symptoms return to base line.
-
o
Postsurgery: Cannot be recommended as they may exacerbate stress incontinence.
Antimuscarinics
Data on antimuscarinics for LUTS in male cancer patients are scarce. In a study of 116 patients, the antimuscarinic agent solifenacin was shown to provide symptomatic comfort after transurethral resection of the bladder tumour and chemotherapy 98. Patients who received solifenacin 6 h before surgery and every day for 2 weeks after the procedure reported significantly lower OAB symptom scores (5.67 vs. 7.86; p < 0.001) compared with patients who received placebo.
In a review, published in 2011, to evaluate contemporary non‐invasive and invasive treatment options for postprostatectomy incontinence, the authors recommended use of antimuscarinic therapy for urgency or urge incontinence alongside or after PFMT 39. For patients suffering from OAB symptoms +/− urgency incontinence after prostate surgery, antimuscarinic medications have been recommended in the European Association of Urology (EAU) guidelines 38. However, antimuscarinics may cause cognitive impairment and should be avoided in patients at risk 27. Other side effects of antimuscarinics include constipation, transient bradycardia (followed by tachycardia, palpitation and arrhythmias), reduced bronchial secretions, urinary urgency and retention, dilatation of the pupils with loss of accommodation, photophobia, dry mouth, flushing and dryness of the skin 115. It is important to note here that these adverse effects are less common with the newer antimuscarinic agents.
Antimuscarinics are also most commonly associated with dry mouth, which many patients find uncomfortable, and discontinue the therapy 116. In a 12‐month UK study looking at persistence with antimuscarinic treatment, solifenacin was associated with higher levels of persistence compared with other prescribed antimuscarinic agents 116. Mirabegron has been recommended by NICE as an option for treating the symptoms of OAB only for people in whom antimuscarinic drugs are contraindicated or clinically ineffective, or have unacceptable side effects, because of its better adverse event profile and similar efficacy to the antimuscarinics 117.
Antimuscarinics recommendations
Evidence grade of 1B (see Table 3 ) (Recommendation here are based on consensus opinion as well as evidence‐base)
The EAU guidelines recommend antimuscarinic drugs as initial drug therapy for adults with urgency UI. The guidance also states that there is no consistent evidence that one antimuscarinic drug is superior to an alternative antimuscarinic drug for cure or improvement of UI or QoL 118.
We recommend initiating antimuscarinics (tolterodine or solifenacin (Vesicare) most commonly used) if the main bothersome symptom of LUTS is urgency UI, followed by mirabegron if antimuscarinic drugs are contraindicated or clinically ineffective.
PDE5‐Is
Evidence from epidemiological studies suggests that LUTS are closely associated with ED 12, 13, 119. Oelke et al. demonstrated in a non‐cancer clinical study that that PDE5‐Is can improve LUTS as well as erectile function 119. Based on evidence in non‐cancer patients, the 2013 EAU guidelines treatment recommend use of PDE5‐Is in men with LUTS 15.
However, there are few postcancer studies in men which demonstrate improvements with PDE5‐Is. Gacci et al. demonstrated a potential therapeutic role for daily administration of PDE5‐Is in continence recovery after bilateral nerve‐sparing prostatectomy in 39 patients 99. A review of 705 patients further corroborated the efficacy of daily PDE5‐I use on urinary continence 1 year after RP vs. on demand use 20. Increased blood flow and oxygen supply by PDE5‐Is may be beneficial for recovery of sphincter and pelvic floor muscles 20.
The EAU treatment guidelines for LUTS include the use of the PDE‐5I tadalafil for LUTS 15. The NICE guidance states that there is there is no statistically significant difference between PDE5‐I and alpha blockers in improving symptom scores or nocturia at 3‐month follow‐up, though alpha blockers are more effective than PDE5‐I in decreasing urinary frequency at 3‐month follow‐up 8. However, it must be noted that the NICE guidance is based on older data compared with EAU guidelines (published in 2013). The NICE guidance also states that there is no statistically significant difference between combination treatment of alpha blockers plus PDE5‐I and alpha blockers in improving symptom scores, quality of life (IPSS question), Qmax (ml/s), nocturia or frequency at up to 3‐month follow‐up. Furthermore, the guidance states that there is no statistically significant difference between combination treatment of alpha blockers plus PDE5‐I and PDE5‐I in improving symptom scores, quality of life (IPSS question), nocturia or frequency at up to 3‐month follow‐up. However, large comparative studies are probably needed to investigate this more thoroughly. Taking into account the data to date, PDE5‐Is should be considered first in patients with LUTS who also suffer from ED.
PDE5‐I recommendation
Evidence grading of 1B to 4 (Table 3 ) (Recommendation here are based on consensus opinion as well as evidence‐base)
Recommend first line daily use of PDE5‐I in patients suffering from ED as well as LUTS.
PDE5‐Is should be used for as long as needed by the patients.
Serotonin‐norepinephrine reuptake inhibitor – duloxetine
Three studies examined SUI in patients treated with duloxetine, and both demonstrated that duloxetine improved postprostatectomy SUI up to 3 months postsurgery, but the benefits were not sustained in one of these studies up to 24 weeks (~5 months) 78, 91, 93. In addition, the drug intolerance and dropout rates are ~15–35% with duloxetine after ≥ 1 month of use 78, 93.
However, duloxetine is rarely used in clinical practice as patients often feel nauseous with this medication and it may put the patients at increased suicide risk 120.
SNRI (duloxetine) recommendation
Evidence grading of 1B–4 (Table 3 ) (Recommendation here are based on consensus opinion as well as evidence‐base)
Not routinely used in clinical practice. There is insufficient evidence for its use and hence cannot be recommended for LUTS.
Summary oral treatment recommendations
Oral treatment recommendation
- The sequencing of medication is generally bound by local prescribing guidance. Generally alpha blockers are given first, followed by antimuscarinics. However, our recommendation is to tailor the treatment based on the patient's needs, i.e. first line treatment should depend on what is the most bothersome symptom of LUTS identified on assessment.
- An alpha blocker (commonly tamsulosin) + antimuscarinic to be used first after RT if urge with/without leak incontinence. Stricture should be excluded prior to starting alpha blockers (Flow rate is often not available to primary care, but if the patient is at higher risk of a stricture or it is a possibility, then they will need to be referred for flow rate +/− cystoscopy).
- Alpha blocker + PDE5‐I if LUTS + ED
- Antimuscarinic (usually tolterodine) to be used first after surgery if urgency UI.
- Antimuscarinic +PDE5‐I if postsurgery LUTS + ED (or mirabegron if adverse effects with antimuscarinics).
We recommend reviewing every 3 months with each treatment; however, patients should be able to see the healthcare provider sooner if they experience adverse events. NICE UI guidance has suggested a review either face to face or at least telephone at 4 weeks after initiating AM therapy. Therefore, a 4‐week telephone review can precede face to face 3 month review.
The treatments should be continued for as long as needed by the patient.
Other treatments
Cranberry juice
A study published in 2003 showed statistically insignificant effects of cranberry juice vs. apple juice on urinary symptoms in patients undergoing RT 101. A more recent placebo‐controlled study by Cowan et al. also demonstrated that cranberry juice did not affect urinary symptoms in patients undergoing RT, although the study was limited by the sample size and duration 102. Another study of 370 patients demonstrated that cranberry extracts significantly reduced the incidence of LUTS, including nocturia, in patients when given during RT 100.
A Cochrane review of susceptible population (including cancer patients) on cranberry juice for UTIs demonstrated that compared with placebo, water or no treatment, cranberry products did not significantly reduce the occurrence of symptomatic UTI in cancer patients. The review further stated that cranberry juice cannot be recommended for the prevention of UTIs 121.
In conclusion, although cranberry juice may have some impact on improving symptoms of LUTS, e.g. nocturia, there is no evidence for it in preventing UTIs or LUTS after cancer treatment. It is much more important to ensure patients avoid caffeinated drinks, which can aggravate storage symptoms.
Cranberry juice recommendation
Evidence grading: IIA–1B (Table 3 )
There is no significant evidence regarding the benefits of cranberry juice for LUTS and hence it cannot be recommended.
Vitamins
A study by a Japanese group in patients taking mecobalamin (vitamin B12) during and after RP demonstrated no significant effect of mecobalamin on the recovery of urinary or sexual function. However, an early non‐significant recovery effect on urinary function was suggested 103.
Vitamin supplement recommendation
Evidence grading IIA (Table 3 )
There is no evidence currently that vitamin supplements improve LUTS symptoms and as such, cannot be recommended for the management of LUTS.
Intravescical sodium hyaluronate
Sodium hyaluronate has been safely administered with success for the treatment of chemical and radiation cystitis, resulting in improvements in urinary symptoms and bladder pain (over 6–8 weeks) 104.
In clinical practice, this is very rarely used except for severe bladder pain after RT.
Intravescical sodium hyaluronate recommendation
Evidence grading 4 (Table 3 )
Very rarely used except for severe bladder pain after RT.
Alternative treatments
In a study of 37 patients, Tanaka et al. showed that Eviprostat, a herbal phytotherapeutic agent, given to patients pre‐ and post‐BT, significantly improved recovery of their urinary symptoms scores, urinary function and urinary obstruction 105.
Some men report Saw Palmetto a useful herbal alternative to an alpha blocker. However, a placebo‐controlled study of 369 men has demonstrated no differences in reduction of LUTs between Saw Palmetto and placebo 122.
Alternative treatment recommendation
Evidence grading IIA (Table 3 )
There is a lack of high quality data regarding use of alternative treatment in men after pelvic cancer therapy.
Containment devices
Fader et al. compared the performance of three continence management devices and absorbent pads used by men with intractable urinary leakage following prostate cancer surgery 106. Male devices included penile compression devices (clamp), sheath drainage systems (sheath), and body‐worn urinals (BWU). The pads were significantly more highly rated vs. sheaths, clamps and BWUs by all men overall. However, the rating of the other devices varied depending on individual needs. For example, although BWUs were rated worse than the sheath overall, the sheath was rated highest for extended period use. Generally ~50% of men stated that they used a combination of these depending on their requirements. The authors concluded that male containment devices can help men with UI and most men prefer to use a combination of devices and pads in order to meet their lifestyle needs 106.
NICE guidelines recommend offering men with storage LUTS (particularly UI) temporary containment products (e.g. pads or collecting devices) to achieve social continence until a diagnosis and management plan have been discussed 8. The ICS 2013 guidelines state that containment products play an essential role towards enhancing quality of life of individuals with incontinence 9.
A recent trial comparing the performance of three continence management devices (sheath drainage system, BWU, penile clamp) and absorbent pads used by 56 men > 1 year after treatment for prostate cancer found that the sheath was useful for extended use, especially when pad changing is difficult; the BWU was rated worse than the sheath and was mainly used for similar activities but by men who could not use a sheath (e.g. retracted penis); and the clamp was useful for short vigorous activities like swimming and exercise. It was also the most secure, least likely to leak and most discreet device but almost all men described it as uncomfortable or painful 123. The pads were useful for everyday activities, best for night‐time use, most easy to use, comfortable when dry but most likely to leak and most uncomfortable when wet. The authors concluded that pads and devices have different strengths which make them particularly suited to certain patients 123.
In clinical practice, pads are used first line and over time most will not need the pads or reduce to one per day for an occasional stress leak or psychological comfort (patients with T3 disease and/or over 70 years of age use pads for longer time). Sheaths are very difficult to use for some as they do not generally stay on though correctly fitted sheaths can be very helpful. Clamps can also be really helpful to some patients though good dexterity is required for use of clamps and they should be used intermittently. In addition, clamps need to be sized appropriately. To conclude, use of pads and devices depends on circumstances and lifestyle needs of patients.
In clinical practice, urinary retention occurs in 2–8% of men after BT and is predictable depending on the prostate size and presence of significant LUTS pre‐implantation. Intermittent self‐catheterisation is very useful for patients who develop retention after BT, vs. an indwelling catheter.
Generally, products available in the community for patients are inadequate for their needs as these are too big or bulky.
Containment devices recommendation
Evidence grading IIA (Table 3 ) (Recommendation here are based on consensus opinion as well as evidence‐base)
Containment devices recommendations depends on lifestyle needs of patients.
Cost‐effectiveness
The extensive use of pads together with the risk of urinary infections present an economic cost not always taken into account as the male patients generally pay for the pads themselves 18.
The NICE 2010 guidelines (not specific to cancer patients) indicate 8:
Alpha blockers are cost‐effective for men with moderate to severe symptoms.
Combination treatment is not considered cost‐effective although when alpha blockers alone are not working, adding an anticholinergic could be justified. Anticholinergic medications can impair any pre‐existing mild cognitive impediment and should be used with caution.
The cost‐effectiveness of containment products is uncertain and that the utility of these will vary among patients. Providing a choice of products appears to be the most practical way to offer cost‐effective management of LUTS patients.
Duration of treatment and referral
On average, the PFMT lasted for 6 weeks–12 months; oral treatments lasted for ≥ 12 months; and other interventions such as cranberry juice or herbal remedies lasted for ≥ 1 month. The shorter time for the later interventions may be because they are generally not prescribed by physicians.
On average, any treatment required ≥ 3 months needs to demonstrate symptom improvement, but ideally, these should be taken for as long as needed and depend on patient preference and response. In case of failure of conservative management, botulinum toxin injections for refractory OAB or surgical options, such as male slings and artificial urinary sphincter for SUI post‐prostatectomy, or indwelling urinary catheters are available. However, further research is needed on optimal treatment duration and when best to refer.
However, long‐term medical management leads to certain adverse events typical for the class of medications used. Therefore, compliance with medical therapy becomes an issue for patients. Our literature analysis demonstrates the higher rate of discontinuations with longer term treatments. Generally, a relatively high proportion of patients drop out of long‐term trials because they are unwilling to tolerate the side effects associated with the treatment 124. However, these studies are not specific to men after cancer treatment. Clinical experience suggests that compliance is related to the efficacy of drugs and whether the most bothersome symptoms of LUTS are being addressed by the said treatment. Managing expectations and providing coping strategies is important in order to improve compliance as well as preparing the patients for symptoms of LUTS after treatment.
Clinicians should also ask the patients about other over the counter or prescribed medications they are on, as some may cause urinary problems. These include antihistamines, decongestants, diuretics, opiates and tricyclic antidepressants.
Recommendation for duration of treatment
If symptoms do not improve in at least 3 months of each intervention (or a combination of these) described here, referral may be warranted to specialist urology centres.
Referral
Referral should be considered if:
Symptoms of LUTS persist after ≥ 3 months of conservative treatment or drug treatment.
Moderate to high (>8) IPSS that fails to improve in spite of interventions.
Any significant impact on QoL.
Frequency persists at > 8 times per day.
If any malignancy suspected or recurrence.
Algorithm
Based on the above recommendation, Figure 1 outlines the treatment algorithm for LUTS after treatment for pelvic cancers. A summary of recommendations from the review is highlighted in Table 5.
Figure 1.
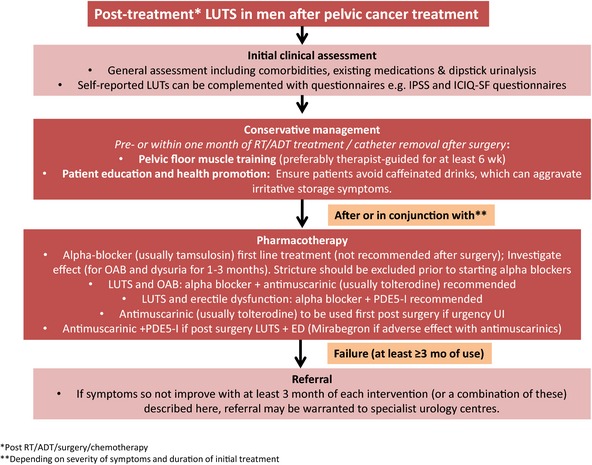
Treatment algorithm for LUTS post‐treatment for pelvic cancers
Table 5.
Summary of recommendations for LUTS post treatment for pelvic cancers
| Summary of recommendations |
|---|
Assessment
|
PFMT recommendation
|
Oral treatment recommendation
|
Other options (also included in existing guidelines)
|
Duration
|
|
Referral
Referral should be considered if:
|
Conclusions
There is a general lack of an evidence‐base for managing male LUTS after pelvic cancer treatment. More evidence is available for LUTs management of men with prostate cancer than other pelvic cancers. Patients presenting with LUTS usually have overlapping symptoms, complicating assessment and management. Large high quality studies are needed to investigate the comparative effectiveness of the various treatment options. Guideline recommendations for treatment give general recommendation and are not specific to male cancers and their specific requirements. Additionally, there are no recommendations concerning the optimal timing for the initiation or duration of the non‐invasive management of male LUTS as a result of cancer treatment.
In this review, we have attempted to provide a comprehensive review of the evidence together with consensus opinion from clinical practice in order to develop recommendations for this patient group. It must be noted, however, that most of the studies presented here were performed in patients with UI rather than LUTS. For many men after cancer treatment LUTS is one of several symptoms and comorbidities and as such requires a holistic approach for its assessment and subsequent management. In addition, LUTS can cause great distress and functional limitations for men.
Clear assessment of the aetiology along with information on techniques to help men cope is essential in managing symptoms. The interventions and algorithm recommended here can be applied in clinical practice to improve management of LUTS in men with pelvic cancers although further testing of recommended management strategies is warranted.
Author contributions
All authors took part in the survey for clinicians, reviewed and edited the publication, which was produced by Sara Faithfull with editorial assistance from Dr Sabah Al‐Lawati at Right Angle.
Conflict of interests
None of the authors have received any financial compensation for writing this publication.
Acknowledgements
Funding for the development of this clinical guidance was provided by Macmillan Cancer Support. The authors would also like to acknowledge Right Angle Communications for editorial assistance.
Disclosure
None.
References
- 1. Cancer Research UK website. http://www.cancerresearchuk.org/cancer-info/cancerstats/incidence/uk-cancer-incidence-statistics (accessed January 2015).
- 2. International Continence Society Factsheet. http://www.ics.org/Documents/DocumentsDownload.aspx?DocumentID=148 (accessed January 2015).
- 3. Andreyev HJ, Muls AC, Norton C et al. The practical management of gastrointestinal symptoms of pelvic radiation disease. Frontline Gastroenterol 2015; 6(1): 53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Payne H, Adamson A, Bahl A et al. Chemical‐ and radiation‐induced haemorrhagic cystitis: current treatments and challenges. BJU Int 2013; 112(7): 885–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirby MG, White ID, Butcher J et al. Development of UK recommendations on treatment for post‐surgical erectile dysfunction. Int J Clin Pract 2014; 68(5): 590–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White ID, Wilson J, Aslet P et al. Development of UK guidance on the management of erectile dysfunction resulting from radical radiotherapy and androgen deprivation therapy for prostate cancer. Int J Clin Pract 2015; 69(1): 106–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsieh CI, Lung AL, Chang LI, Sampselle CM, Lin CC, Liao YM. Prevalence, associated factors, and relationship to quality of life of lower urinary tract symptoms: a cross‐sectional, questionnaire survey of cancer patients. Int J Clin Pract 2013; 67(6): 566–75. [DOI] [PubMed] [Google Scholar]
- 8. National Clinical Guideline Centre . CG97 Lower urinary tract symptoms, 2010.
- 9. Abrams P, Cardozo L, Fall M et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub‐committee of the International Continence Society. Neurourol Urodyn 2002; 21(2): 167–78. [DOI] [PubMed] [Google Scholar]
- 10. Rees J, Bultitude M, Challacombe B. The management of lower urinary tract symptoms in men. BMJ 2014; 348: g3861. [DOI] [PubMed] [Google Scholar]
- 11. Agarwal A, Eryuzlu LN, Cartwright R et al. What is the most bothersome lower urinary tract symptom? Individual‐ and population‐level perspectives for both men and women. Eur Urol 2014; 65(6): 1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McVary K. Lower urinary tract symptoms and sexual dysfunction: epidemiology and pathophysiology. BJU Int 2006; 97(Suppl. 2): 23–8. [DOI] [PubMed] [Google Scholar]
- 13. Rosen R, Altwein J, Boyle P et al. Lower urinary tract symptoms and male sexual dysfunction: the Multinational Survey of the Aging Male (MSAM‐7). Eur Urol 2003; 44: 637–49. [DOI] [PubMed] [Google Scholar]
- 14. Kirby M, Chapple C, Jackson G et al. Erectile dysfunction and lower urinary tract symptoms: a consensus on the importance of co‐diagnosis. Int J Clin Pract 2013; 67(7): 606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oelke M, Bachmann A, Descazeaud A et al.; European Association of Urology . EAU guidelines on the treatment and follow‐up of non‐neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2013; 64(1): 118–40. [DOI] [PubMed] [Google Scholar]
- 16. Overgard M, Angelsen A, Lydersen S, Mørkved S. Does physiotherapist‐guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? a randomised controlled trial Eur Urol 2008; 54(2): 438–48. [DOI] [PubMed] [Google Scholar]
- 17. Khoder WY, Trottmann M, Stuber A, Stief CG, Becker AJ. Early incontinence after radical prostatectomy: A community based retrospective analysis in 911 men and implications for preoperative counseling. Urol Oncol 2013; 31(7): 1006–11. [DOI] [PubMed] [Google Scholar]
- 18. Marchiori D, Bertaccini A, Manferrari F, Ferri C, Martorana G. Pelvic floor rehabilitation for continence recovery after radical prostatectomy: role of a personal training re‐educational program. Anticancer Res 2010; 30(2): 553–6. [PubMed] [Google Scholar]
- 19. Mariotti G, Sciarra A, Gentilucci A et al. Early recovery of urinary continence after radical prostatectomy using early pelvic floor electrical stimulation and biofeedback associated treatment. J Urol 2009; 181(4): 1788–93. [DOI] [PubMed] [Google Scholar]
- 20. Gandaglia G, Albersen M, Gallina A et al. Post‐operative PDE‐5 inhibitors use improves recovery of urinary continence following open bilateral nerve‐sparing radical prostatectomy. Eur Urol Suppl 2012; 11(1): E284–E84. [Google Scholar]
- 21. Viswanathan AN, Yorke ED, Marks LB, Eifel PJ, Shipley WU. Radiation dose–volume effects of the urinary bladder. Int J Rad Oncol Bio Phys 2010; 76: S116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burgio KL, Goode PS, Urban DA et al. Preoperative biofeedback assisted behavioral training to decrease postprostatectomy incontinence: a randomized, controlled trial. J Urol 2006; 175: 196–201. [DOI] [PubMed] [Google Scholar]
- 23. Nilsson A, Schumacher M, Johansson E et al. Age at surgery, educational level and long term urinary incontinence after radical prostatectomy. BJU Int 2011; 108: 1572–7. [DOI] [PubMed] [Google Scholar]
- 24. Burgio KL, Newman DK, Rosenberg MT, Sampselle C. Impact of behaviour and lifestyle on bladder health. Int J Clin Pract 2013; 67(6): 495–504. [DOI] [PubMed] [Google Scholar]
- 25. Thomas R, Holm M, Williams M et al. Lifestyle factors correlate with the risk of late pelvic symptoms after prostate radiotherapy. Clin Oncol 2013; 25: 246–51. [DOI] [PubMed] [Google Scholar]
- 26. Reynolds WS, Shikanov SA, Katz MH, Zagaja GP, Shalhav AL, Zorn KC. Analysis of continence rates following robot‐assisted radical prostatectomy: strict leak‐free and pad‐free continence. Urology 2010; 75(2): 431–6. [DOI] [PubMed] [Google Scholar]
- 27. Wagg A, Verdejo C, Molander U. Review of cognitive impairment with antimuscarinic agents in elderly patients with overactive bladder. Int J Clin Pract 2010; 64(9): 1279–86. [DOI] [PubMed] [Google Scholar]
- 28. Rosenberg MT, Staskin DR, Kaplan SA, MacDiarmid SA, Newman DK, Ohl DA. A practical guide to the evaluation and treatment of male lower urinary tract symptoms in the primary care setting. Int J Clin Pract 2007; 61(9): 1535–46. [DOI] [PubMed] [Google Scholar]
- 29. White AJ, Reeve BB, Chen RC, Stover AM, Irwin DE. Urinary incontinence and health‐related quality of life among older Americans with and without cancer: a cross‐sectional study. BMC Cancer 2013; 13: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Resnick M, Guzzo T, Cowan J, Knight SJ, Carroll PR, Penson DF. Factors associated with satisfaction with prostate cancer care: results from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE). BJU Int 2013; 111(2): 213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park SW, Kim TN, Nam JK et al. Recovery of overall exercise ability, quality of life, and continence after 12‐week combined exercise intervention in elderly patients who underwent radical prostatectomy: a randomized controlled study. Urology 2012; 80(2): 299–30. [DOI] [PubMed] [Google Scholar]
- 32. Marinkovic SP, Rovner ES, Moldwin RM, Stanton SL, Gillen LM, Marinkovic CM. The management of overactive bladder syndrome. BMJ 2012; 344: e2365. [DOI] [PubMed] [Google Scholar]
- 33. Sacco E, Prayer‐Galetti T, Pinto F et al. Urinary incontinence after radical prostatectomy: incidence by definition, risk factors and temporal trend in a large series with a long‐term follow‐up. BJU Int 2006; 97(6): 1234–41. [DOI] [PubMed] [Google Scholar]
- 34. Tsui JF, Shah MB, Weinberger JM et al. Pad count is a poor measure of the severity of urinary incontinence. J Urol 2013; 190(5): 1787–90. [DOI] [PubMed] [Google Scholar]
- 35. Koelbl H, Mostwin J, Boiteux JP et al. Pathophysiology In: Abrams P, Cardozo L, Khoury S, Wein A, eds. Incontinence. 2nd International Consultation on Incontinence, 2 edn. UK: Plymbridge Distributors Ltd, Plymbridge, 2002: 203–41. [Google Scholar]
- 36. Ficazzola MA, Nitti VW. The aetiology of post‐radical prostatectomy incontinence and correlation of symptoms with urodynamic findings. J Urol 1998; 160(4): 1317–20. [PubMed] [Google Scholar]
- 37. Song C, Lee J, Hong J, Choo MS, Kim CS, Ahn H. Urodynamic interpretation of changing baldder function and voiding pattern after radical prostatectomy: a long term follow‐up. BJUI 2010; 106: 681–6. [DOI] [PubMed] [Google Scholar]
- 38. Thüroff JW, Abrams P, Andersson KE et al. EAU guidelines on urinary incontinence. Eur Urol 2011; 59(3): 387–400. [DOI] [PubMed] [Google Scholar]
- 39. Bauer RM, Gozzi C, Hübner W et al. Contemporary management of postprostatectomy incontinence. Eur Urol 2011; 59(6): 985–96. [DOI] [PubMed] [Google Scholar]
- 40. Fowler FJ Jr, Barry MJ, Lu‐Yao G, Wasson JH, Bin L. Outcomes of external‐beam radiation therapy for prostate cancer: a study of Medicare beneficiaries in three surveillance, epidemiology and end results areas. J Clin Oncol 1996; 14: 2258–65. [DOI] [PubMed] [Google Scholar]
- 41. Van Cangh PJ, Richard F, Lorge F et al. Adjuvant radiation therapy does not cause urinary incontinence after radical prostatectomy: results of a prospective randomized study. J Urol 1998; 159: 164–6. [DOI] [PubMed] [Google Scholar]
- 42. Ferrer M, Suárez JF, Guedea F et al.; Multicentric Spanish Group of Clinically Localized Prostate Cancer . Health‐related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2008; 72(2): 421–32. [DOI] [PubMed] [Google Scholar]
- 43. Colombo R, Rocchini L, Suardi N et al. Neoadjuvant short‐term intensive intravesical mitomycin C regimen compared with weekly schedule for low‐grade recurrent non‐muscle‐invasive bladder cancer: preliminary results of a randomised phase 2 study. Eur Urol 2012; 62(5): 797–802. [DOI] [PubMed] [Google Scholar]
- 44. Lammers RJ, Witjes JA, Inman BA et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non‐muscle‐invasive bladder cancer: a systematic review. Eur Urol 2011; 60(1): 81–93. [DOI] [PubMed] [Google Scholar]
- 45. Addeo R, Caraglia M, Bellini S et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol 2010; 28(4): 543–8. [DOI] [PubMed] [Google Scholar]
- 46. Jevtana Summary of Product Characteristics. Sanofi
- 47. Doxorubicin Summary of Product Characteristics. https://www.medicines.org.uk/emc/ (accessed November 2011).
- 48. Paclitaxel Summary of Product Characteristics. https://www.medicines.org.uk/emc/ (accessed November 2011).
- 49. Denham JW, Wilcox C, Lamb DS et al. Rectal and urinary dysfunction in the TROG 03.04 RADAR trial for locally advanced prostate cancer. Radiother Oncol 2012; 105(2): 184–92. [DOI] [PubMed] [Google Scholar]
- 50. Stone NN, Marshall DT, Stone JJ, Cesaretti JA, Stock RG. Does neoadjuvant hormonal therapy improve urinary function when given to men with large prostates undergoing prostate brachytherapy? J Urol 2010; 183(2): 634–9. [DOI] [PubMed] [Google Scholar]
- 51. Grant JD, Litwin MS, Kwan L, Lee SP, Steinberg ML, King CR. Does hormone therapy exacerbate the adverse effects of radiotherapy in men with prostate cancer? A quality of life study. J Urol 2011; 185(5): 1674–80. [DOI] [PubMed] [Google Scholar]
- 52. Crook J, Fleshner N, Roberts C, Pond G. Long‐term urinary sequelae following 125iodine prostate brachytherapy. J Urol 2008; 179(1): 141–5. [DOI] [PubMed] [Google Scholar]
- 53. Axcrona K, Aaltomaa S, da Silva CM et al. Androgen deprivation therapy for volume reduction, lower urinary tract symptom relief and quality of life improvement in patients with prostate cancer: degarelix vs goserelin plus bicalutamide. BJU Int 2012; 110(11): 1721–8. [DOI] [PubMed] [Google Scholar]
- 54. Mason M, Maldonado Pijoan X, Steidle C et al. Neoadjuvant androgen deprivation therapy for prostate volume reduction, lower urinary tract symptom relief and quality of life improvement in men with intermediate‐ to high‐risk prostate cancer: a randomised non‐inferiority trial of degarelix versus goserelin plus bicalutamide. Clin Oncol (R Coll Radiol) 2013; 25(3): 190–6. [DOI] [PubMed] [Google Scholar]
- 55. Anderson J, Al‐Ali G, Wirth M et al. Degarelix versus goserelin (plus antiandrogen flare protection) in the relief of lower urinary tract symptoms secondary to prostate cancer: results from a phase IIIb study (NCT00831233). Urol Int 2013; 90(3): 321–8. [DOI] [PubMed] [Google Scholar]
- 56. You D, Jeong IG, Kim SW et al. Impacts of leuprolide acetate on quality of life in patients with prostate cancer: a prospective multicenter study. Scand J Urol Nephrol 2010; 44(6): 399–405. [DOI] [PubMed] [Google Scholar]
- 57. Uchida T, Tomonaga T, Kim H et al. Improved outcomes owing to high‐intensity focused ultrasound devices version‐up for the treatment of patients with localized prostate cancer. J Urol 2015; 193(1): 103–10. [DOI] [PubMed] [Google Scholar]
- 58. Mearini L, Nunzi E, Giovannozzi S, Lepri L, Lolli C, Giannantoni A. Urodynamic evaluation after high‐intensity focused ultrasound for patients with prostate cancer. Prostate Cancer 2014; 2014: 462153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baco E, Gelet A, Crouzet S et al. Hemi salvage high‐intensity focused ultrasound (HIFU) in unilateral radiorecurrent prostate cancer: a prospective two‐centre study. BJU Int 2014; 114(4): 532–40. [DOI] [PubMed] [Google Scholar]
- 60. Berge V, Baco E, Dahl AA, Karlsen SJ. Health‐related quality of life after salvage high‐intensity focused ultrasound (HIFU) treatment for locally radiorecurrent prostate cancer. Int J Urol 2011; 18(9): 646–51. [DOI] [PubMed] [Google Scholar]
- 61. Lopez‐Alcina E, Sánchez‐Ballester F, Montoliu‐Garcia A, Ramada‐Benlloch F, Juan‐Escudero J, Marques‐Vidal E. Lower urinary tract symptoms after high intensity focused ultrasounds (HIFU) treatment for localized prostate cancer. 5 year experience of first Spanish series. Abstract 449 ICS 2012.
- 62. Miller RJ Jr, Cohen JK, Shuman B, Merlotti LA. Percutaneous, transperineal cryosurgery of the prostate as salvage therapy for post radiation recurrence of adenocarcinoma. Cancer 1996; 77: 1510–4. [DOI] [PubMed] [Google Scholar]
- 63. Shinohara K, Connolly JA, Presti JC Jr, Carroll PR. Cryosurgical treatment of localized prostate cancer (stages T1 to T4): preliminary results. J Urol 1996; 156: 115–20. [PubMed] [Google Scholar]
- 64. Pisters LL, von Eschenbach AC, Scott SM et al. The efficacy and complications of salvage cryotherapy of the prostate. J Urol 1997; 157: 921–5. [PubMed] [Google Scholar]
- 65. Cespedes RD, Pisters LL, von Eschenbach AC, McGuire EJ. Long‐term follow‐up of incontinence and obstruction after salvage cryosurgical ablation of the prostate: results in 143 patients. J Urol 1997; 157: 237–40. [PubMed] [Google Scholar]
- 66. Ghafar MA, Johnson CW, De La Taille A et al. Salvage cryotherapy using an argon based system for locally recurrent prostate cancer after radiation therapy: the Columbia experience. J Urol 2001; 166: 1333–8. [PubMed] [Google Scholar]
- 67. Han KR, Cohen JK, Miller RJ et al. Treatment of organ confined prostate cancer with third generation cryosurgery: preliminary multi‐center experience. J Urol 2003; 170: 1126–30. [DOI] [PubMed] [Google Scholar]
- 68. Malcolm JB, Fabrizio MD, Barone BB et al. Quality of life after open or robotic prostatectomy, cryoablation or brachytherapy for localized prostate cancer. J Urol 2010; 183(5): 1822–8. [DOI] [PubMed] [Google Scholar]
- 69. Ball AJ, Gambill B, Fabrizio MD et al. Prospective longitudinal comparative study of early health‐related quality‐of‐life outcomes in patients undergoing surgical treatment for localized prostate cancer: a short‐term evaluation of five approaches from a single institution. J Endourol 2006; 20(10): 723–31. [DOI] [PubMed] [Google Scholar]
- 70. Williams SB, Lei Y, Nguyen PL et al. Comparative effectiveness of cryotherapy vs brachytherapy for localised prostate cancer. BJU Int 2012; 110(2 Pt 2): E92–8. [DOI] [PubMed] [Google Scholar]
- 71. Prostate Cancer UK website. http://prostatecanceruk.org/information/living-with-prostate-cancer/urinary-problems (accessed August 2014).
- 72. Campbell SE, Glazener CM, Hunter KF, Cody JD, Moore KN. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev 2012; 13: CD001843. [DOI] [PubMed] [Google Scholar]
- 73. Centemero A, Rigatti L, Giraudo D et al. Preoperative pelvic floor muscle exercise for early continence after radical prostatectomy: a randomised controlled study. Eur Urol 2010; 57(6): 1039–43. [DOI] [PubMed] [Google Scholar]
- 74. Centemero A, Rigatti L, Giraudo D et al. Effectiveness of pre‐operative pelvic floor muscle training for post‐prostatectomy early continence recovery. Eur Urol Suppl 2009; 8(4): 334. [Google Scholar]
- 75. Dieperink KB, Johansen C, Hansen S et al. The effects of multidisciplinary rehabilitation: RePCa‐a randomised study among primary prostate cancer patients. Br J Cancer 2013; 109(12): 3005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dubbelman Y, Groen J, Wildhagen M, Rikken B, Bosch R. The recovery of urinary continence after radical retropubic prostatectomy: a randomized trial comparing the effect of physiotherapist‐guided pelvic floor muscle exercises with guidance by an instruction folder only. BJU Int 2010; 106(4): 515–22. [DOI] [PubMed] [Google Scholar]
- 77. Faithfull S, Cockle‐Hearne J, Khoo V. Self‐management after prostate cancer treatment: evaluating the feasibility of providing a cognitive and behavioural programme for lower urinary tract symptoms. BJU Int 2011; 107(5): 783–90. [DOI] [PubMed] [Google Scholar]
- 78. Filocamo MT, Li Marzi V, Del Popolo G et al. Effectiveness of early pelvic floor rehabilitation treatment for post‐prostatectomy incontinence. Eur Urol 2005; 48(5): 734–8. [DOI] [PubMed] [Google Scholar]
- 79. Geraerts I, Van Poppel H, Devoogdt N et al. Influence of preoperative and postoperative pelvic floor muscle training (PFMT) compared with postoperative PFMT on urinary incontinence after radical prostatectomy: a randomized controlled trial. Eur Urol 2013; 64(5): 766–72. [DOI] [PubMed] [Google Scholar]
- 80. Glazener C, Boachie C, Buckley B et al. Urinary incontinence in men after formal one‐to‐one pelvic‐floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): two parallel randomised controlled trials. Lancet 2011; 378(9788): 328–37. [DOI] [PubMed] [Google Scholar]
- 81. Goode PS, Burgio KL, Johnson TM et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence a randomized controlled trial. JAMA 2011; 305(2): 151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lin YH, Yang MS, Lin VCH, Yu TJ, Chiang PH. The effectiveness of pelvic floor exercise on urinary incontinence in radical prostatectomy patients. Int J Urol Nurs 2011; 5(3): 115–22. [Google Scholar]
- 83. Nilssen SR, Morkved S, Overgard M, Lydersen S, Angelsen A. Does physiotherapist‐guided pelvic floor muscle training increase the quality of life in patients after radical prostatectomy? A randomized clinical study. Scand J Urol Nephrol 2012; 46(6): 397–404. [DOI] [PubMed] [Google Scholar]
- 84. Patel MI, Yao JN, Hirschhorn AD, Mungovan SF. Preoperative pelvic floor physiotherapy improves continence after radical retropubic prostatectomy. Int J Urol 2013; 20(10): 986–92. [DOI] [PubMed] [Google Scholar]
- 85. Ribeiro LH, Prota C, Gomes CM et al. Long‐term effect of early postoperative pelvic floor biofeedback on continence in men undergoing radical prostatectomy: a prospective, randomized, controlled trial. J Urol 2010; 184(3): 1034–9. [DOI] [PubMed] [Google Scholar]
- 86. Serda BC, Vesa J, del Valle A, Monreal P. Urinary incontinence and prostate cancer: a progressive rehabilitation program design. Actas Urol Esp 2010; 34(6): 522–3. [PubMed] [Google Scholar]
- 87. Tienforti D, Sacco E, Marangi F et al. Efficacy of an assisted low‐intensity programme of perioperative pelvic floor muscle training in improving the recovery of continence after radical prostatectomy: a randomized controlled trial. BJU Int 2012; 110(7): 1004–10. [DOI] [PubMed] [Google Scholar]
- 88. Van Kampen M, De Weerdt W, Van Poppel H, De Ridder D, Feys H, Baert L. Effect of pelvic‐floor re‐education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. The Lancet 2000; 355: 98–102. [DOI] [PubMed] [Google Scholar]
- 89. Wille S, Sobottka A, Heidenreich A, Hofmann R. Pelvic floor exercises, electrical stimulation and biofeedback after radical prostatectomy: results of a prospective randomized trial. J Urol 2003; 170(2 Pt 1): 490–3. [DOI] [PubMed] [Google Scholar]
- 90. Zahariou A, Kyriakides A. S40 Intensive pelvic floor muscle training after radical prostatectomy improves continence outcomes. Eur Urol Suppl 2009; 8(8): 620. [Google Scholar]
- 91. Cornu J‐N, Merlet B, Ciofu C et al. Duloxetine for mild to moderate postprostatectomy incontinence: preliminary results of a randomised placebo‐controlled trial. Eur Urol 2011; 59(1): 148–54. [DOI] [PubMed] [Google Scholar]
- 92. Filocamo MT, Li Marzi V, Del Popolo G et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. Eur Urol 2007; 51(6): 1559–64. [DOI] [PubMed] [Google Scholar]
- 93. Neff D, Guise A, Guralnick ML et al. Duloxetine for the treatment of post‐prostatectomy stress urinary incontinence. Can Urol Assoc J 2013; 7(5–6): E260–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jang J‐H, Kang S‐B, Lee S‐M et al. Randomized controlled trial of tamsulosin for prevention of acute voiding difficulty after rectal cancer surgery. World J Surg 2012; 36(11): 2730–7. [DOI] [PubMed] [Google Scholar]
- 95. Oyama N, Aoki Y, Ito H et al. Alpha 1‐adrenoceptor blocker may improve not only voiding but also storage lower urinary tract symptoms caused by (125) I brachytherapy for prostate cancer. World J Urol 2010; 28(5): 609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shimizu N, Minami T, Sugimoto K et al. Efficacy of silodosin in patients undergoing brachytherapy: a randomized trial involving a pressure flow study. World J Urol 2014; 32(6): 1423–32. [DOI] [PubMed] [Google Scholar]
- 97. Tsumura H, Satoh T, Ishiyama H et al. Comparison of prophylactic naftopidil, tamsulosin, and silodosin for 125I brachytherapy‐induced lower urinary tract symptoms in patients with prostate cancer: randomized controlled trial. Int J Radiat Oncol Biol Phys 2011; 81(4): e385–92. [DOI] [PubMed] [Google Scholar]
- 98. Zhang Z, Cao Z, Xu C et al. Solifenacin is able to improve the irritative symptoms after transurethral resection of bladder tumors. Urology 2014; 84(1): 117–21. [DOI] [PubMed] [Google Scholar]
- 99. Gacci M, Ierardi A, Rose AD et al. Vardenafil can improve continence recovery after bilateral nerve sparing prostatectomy: results of a randomized, double blind, placebo‐controlled pilot study. J Sex Med 2010; 7(1 Pt 1): 234–43. [DOI] [PubMed] [Google Scholar]
- 100. Bonetta A, Di Pierro F. Enteric‐coated, highly standardized cranberry extract reduces risk of UTIs and urinary symptoms during radiotherapy for prostate carcinoma. Cancer Manag Res 2012; 4: 281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Campbell G, Pickles T, D'Yachkova Y. A randomised trial of cranberry versus apple juice in the management of urinary symptoms during external beam radiation therapy for prostate cancer. Clin Oncol 2003; 15(6): 322–8. [DOI] [PubMed] [Google Scholar]
- 102. Cowan CC, Hutchison C, Cole T et al. A randomised double‐blind placebo‐controlled trial to determine the effect of cranberry juice on decreasing the incidence of urinary symptoms and urinary tract infections in patients undergoing radiotherapy for cancer of the bladder or cervix. Clin Oncol 2012; 24(2): e31–8. [DOI] [PubMed] [Google Scholar]
- 103. Matsushita M, Nakagawa H, Namiki S et al. Effects of urinary function and erectile function on the use of mecobalamin after nerve sparing radical prostatectomy. Nihon Hinyōkika Gakkai Zasshi 2009; 100(1): 7–11. [DOI] [PubMed] [Google Scholar]
- 104. Sommariva ML, Sandri SD, Ceriani V. Efficacy of sodium hyaluronate in the management of chemical and radiation cystitis. Minerva Urologica E Nefrologica. Ital J Urol Nephrol 2010; 62(2): 145–50. [PubMed] [Google Scholar]
- 105. Tanaka T, Morimoto K, Nishikawa N et al. Suppressive effects of eviprostat, a phytotherapeutic agent, on lower urinary tract symptoms in prostate cancer patients treated with brachytherapy. LUTS 2012; 4(1): 25–8. [DOI] [PubMed] [Google Scholar]
- 106. Fader M, Macaulay M, Broadbridge J, Cottenden A, Birch B, Moore KN. A trial of devices for the management of urinary incontinence following treatment for prostate cancer. Neurourol Urodyn 2013; 32(6): 891–2. [Google Scholar]
- 107. Coyne KS, Sexton CC, Kopp Z et al. Assessing patients' descriptions of lower urinary tract symptoms (LUTS) and perspectives on treatment outcomes: results of qualitative research. Int J Clin Pract 2010; 64(9): 1260–78. [DOI] [PubMed] [Google Scholar]
- 108. Newman DK. Pelvic floor muscle rehabilitation using biofeedback. Urol Nurs 2014; 34(4): 193–202. [PubMed] [Google Scholar]
- 109. Wyman JF, Burgio KL, Newman DK. Practical aspects of lifestyle modifications and behavioural interventions in the treatment of overactive bladder and urgency urinary incontinence. Int J Clin Pract 2009; 63(8): 1177–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Song C, Doo CK, Hong JH, Choo MS, Kim CS, Ahn H. Relationship between the integrity of the pelvic floor muscles and early recovery of continence after radical prostatectomy. J Urol 2007; 178(1): 208–11. [DOI] [PubMed] [Google Scholar]
- 111. Moore K, Bradley C, Burgio B et al. Adult conservative treatment In: Abrams P, Cardozo L, Khoury S, Wein A, eds. Incontinence: proceedings from the 5th International Consultation on Incontinence. Plymouth, UK: Health Publications, 2013: 1101–228. [Google Scholar]
- 112. National Kidney and Urologic Diseases Information Clearinghouse (NKUDIC) website. http://kidney.niddk.nih.gov/KUDiseases/pubs/uimen/index.aspx (accessed July 2014)
- 113. Merck Books . www.merckbooks.com/mmha/pdf/table2_excerpt.pdf (accessed January 2015).
- 114. Tsakiris P, Oelke M, Michel M. Drug‐induced urinary incontinence. Drugs Aging 2008; 25(7): 541–9. [DOI] [PubMed] [Google Scholar]
- 115. British National Formulary website. http://www.evidence.nhs.uk/formulary/bnf/current/1-gastro-intestinal-system/12-antispasmodics-and-other-drugs-altering-gut-motility/antimuscarinics (accessed January 2015).
- 116. Wagg A, Compion G, Fahey A, Siddiqui E. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int 2012; 110: 1767–74. [DOI] [PubMed] [Google Scholar]
- 117. NICE TA290 . Mirabegron for treating symptoms of overactive bladder. https://www.nice.org.uk/guidance/ta290/chapter/1-guidance (accessed November 2014).
- 118. Lucas MG, Bosch RJ, Burkhard FC et al.; European Association of Urology . EAU guidelines on assessment and nonsurgical management of urinary incontinence. Eur Urol 2012; 62(6): 1130–42. [DOI] [PubMed] [Google Scholar]
- 119. Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo‐controlled clinical trial. Eur Urol 2012; 61: 917–25. [DOI] [PubMed] [Google Scholar]
- 120. Salem BA, Karam EG. Duloxetine and suicide attempts: a possible relation. Clin Pract Epidemiol Ment Health 2008; 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2012; 10: CD001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Barry MJ, Meleth S, Lee JY et al.; Complementary and Alternative Medicine for Urological Symptoms (CAMUS) Study Group . Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA 2011; 306(12): 1344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Macaulay M, Broadbridge J, Gage H et al. A trial of devices for urinary incontinence following treatment for prostate cancer. BJU Int 2014. Dec 11 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 124. Roehrborn CG. Current medical therapies for men with lower urinary tract symptoms and benign prostatic hyperplasia: achievements and limitations. Rev Urol 2008; 10(1): 14–25. [PMC free article] [PubMed] [Google Scholar]


