Abstract
Rationale, aims and objectives
Clinical trial data suggest that patients who have received bisphosphonates continue to benefit from them after discontinuation. However, data from real‐world clinical practice are inconclusive. We assessed the impact of persistence and discontinuation on health resource utilization (HRU) and fracture rate in women who were prescribed oral bisphosphonates.
Method
The study used data from the UK Clinical Practice Research Datalink. Women aged 50 years or older with a first prescription of oral bisphosphonate therapy between January 2000 and December 2007 were included. Multivariate modelling compared rate ratios for fracture and HRU between patients who had discontinued medication (shorter persistence group) and patients who took their medication for longer (longer persistence group). The interactions of elapsed time (measured as 6‐month intervals) with HRU and with fracture rate for all patients within paired groups were also assessed.
Results
Overall, 36 320 patients were included. Pairwise comparisons showed that HRU and fracture rates were lower in longer persistence groups than in shorter persistence groups. Analysis by 6‐month interval showed that, across all patients in persistence group pairs, HRU significantly increased for each additional 6 months elapsed; trends towards increased risk of fracture were also seen.
Conclusion
In contrast to results from clinical trials, in this patient population the protective effect of oral bisphosphonates after discontinuation was not sufficient to reduce HRU and fracture rates to the levels that would be seen if patients had continued on therapy. Reducing the rate of treatment discontinuation may decrease the burden that osteoporosis places on both patients and health care systems.
Keywords: bisphosphonate, Clinical Practice Research Datalink, fracture, health resource utilization, medication adherence, osteoporosis
Introduction
Osteoporosis is a skeletal disorder characterized by low bone mass and microarchitectural deterioration of bone tissue [1]. Prevalence of osteoporosis increases with age [2] and is therefore predicted to increase as the average age of the population continues to rise [3, 4]. Osteoporosis increases an individual's risk of fracture, and approximately one‐half of women and one‐fifth of men aged 50 years can expect to experience a fracture during their remaining lifetime [5]. Osteoporotic fractures are not only painful and debilitating for patients; they also place a significant burden on health care systems. In the year 2000, the total direct costs arising from osteoporotic fractures in Europe were estimated to be £21 billion [6]. These costs are expected to reach £51 billion by 2050 [6].
There is substantial evidence that pharmacological intervention can reduce the risk of fracture in patients with osteoporosis. The oral bisphosphonate alendronate is the most frequently used drug for the prevention of osteoporotic fractures [7]. If alendronate is not well tolerated or effective, other agents are used, such as risedronate and ibandronate, intravenous bisphosphonates, selective oestrogen receptor modulators and strontium ranelate [7]. The most recent addition to the available treatment options for preventing osteoporotic fractures is denosumab, a receptor activator of nuclear factor kappa‐B ligand (RANKL) inhibitor that was approved in 2010 for use in patients with osteoporosis [8]. Randomized controlled trials have shown that, compared with placebo, these therapies can significantly reduce the risk of new vertebral fractures over 3 years in postmenopausal women with osteoporosis [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19]. Some of these agents can also reduce the risk of hip and other non‐vertebral fractures, depending on the patient population [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19].
Bisphosphonates function by inhibiting the activity of osteoclasts (cells responsible for bone resorption). In addition to acting directly on these cells, bisphosphonates are incorporated into the bone matrix [20]. For some types of bisphosphonate therapy, their retention in the bone may result in a sustained reduction in fracture risk even after discontinuation. Clinical trial data appear to support the theory that patients who have previously been treated with some bisphosphonates may continue to benefit from them after discontinuation. In one placebo‐controlled study, patients were found to have a reduced fracture risk when receiving alendronate for 5 years; they were subsequently randomized to either placebo or alendronate for a further 5 years. There was no increase in the risk of non‐vertebral fractures in the placebo group compared with the alendronate group during the second phase of the study [21]. Similarly, in a follow‐up of the Vertebral Efficacy With Risedronate Therapy – North America trial, the reduction in fracture risk in patients taking risedronate compared with those taking placebo was maintained for at least 1 year after treatment discontinuation [22]. However, these data are from controlled clinical trials in which patients underwent selection and careful monitoring, and therefore, persistence and compliance with medication may have been better than in routine clinical practice.
A study of patient records from the UK Clinical Practice Research Datalink (CPRD; previously known as the General Practice Research Database) found that fracture rate was reduced in adults who had recently been prescribed 6–12 months of treatment with alendronate or risedronate compared with those who had initiated treatment but had been non‐persistent for at least 6 months. Furthermore, there was no residual protection against fracture after discontinuation of either drug [23]. A study of a US health care claims database found that fracture rate increased after discontinuation of bisphosphonates in women who had been compliant [medication possession ratio (MPR) of 66% or more] for 2 years, whereas in women who had a MPR of 80% or more or who had persisted for at least 3 years, fracture rate did not increase significantly [24]. These studies suggest that the benefits observed in clinical trials following discontinuation may not translate to real‐life outcomes of osteoporosis therapy and discontinuation. However, data are inconclusive and further work is needed to investigate the effect of persistence and discontinuation on fracture rate. There is also a need to investigate how persistence is associated with health resource utilization (HRU). It is currently unclear whether more‐persistent patients have a higher HRU rate than less‐persistent patients as a result of visiting their doctors more frequently or whether better management of their disease would reduce their need for HRU. We analysed data from the UK CPRD to assess the impact of persistence on HRU and fracture rates in women treated with oral bisphosphonates.
Methods
Data source
This study analysed data from the UK CPRD. The CPRD is a database of computerized medical records collected by general practitioners (GPs) in the UK [25]. The records include information on patient demographics, prescriptions issued, clinical events, specialist referrals, hospital admissions and key outcomes. At the time of the study, the CPRD contained data for about 3 million living patients from over 400 practices in the UK [26]. The CPRD has been validated in numerous studies across a number of disease areas [27, 28].
Study population
The study included women aged 50 years or older with postmenopausal osteoporosis and a first prescription of oral bisphosphonate therapy (alendronate, risedronate, ibandronate or etidronate; this was the index event) between January 2000 and December 2007. Patients had to have a minimum of 12 months of computerized data before the index event and at least 6 months of data afterwards. Patients were excluded if, at the time of the index event, they had previously been prescribed any therapy for osteoporosis (excluding vitamin D and calcium supplements) or if they had a history of cancer at any time before the start of the study. All the data collected for this study were pseudoanonymized, and the study was approved by the CPRD Independent Scientific Advisory Committee.
Study design
In this retrospective cohort study, the outcomes of interest included HRU and fracture incidence. HRU measures comprised the combined number of inpatient hospitalizations (excluding accident and emergency department visits and referrals to specialist outpatient clinics) and initial referrals to any specialists. In this analysis, fractures included all bone fractures apart from those that were clearly not related to osteoporosis, such as cranial fracture or fracture likely to be due to trauma. The independent variable of interest was persistence versus discontinuation of oral bisphosphonate therapy, with duration of patients' persistence being classified as <12 months, 12–<24 months, 24–<36 months or ≥36 months. Persistence was defined as the duration of use of oral bisphosphonates. Patients with a gap of more than 3 months after the end of the coverage period of their last prescription were considered as having discontinued their medication. The coverage period was estimated using the expected time between each prescription, which was determined using the dose and preparations information listed in the British National Formulary [29]. The MPR was also calculated; this measure of treatment compliance was defined as the proportion of the time between treatment initiation and discontinuation for which the patient had access to medication, assuming that the medication was used in accordance with the approved prescribing information.
Covariates assessed included age, history of fracture, previous HRU, co‐medications and co‐morbidities. Data for these covariates were taken from patient records for the 12‐month period before the index date.
Data on hospitalizations, referrals and fractures were collected until patients left the study, re‐started treatment after discontinuation, or until the study end date of December 2008 was reached.
Analyses
Descriptive statistics were used to characterize patients by duration of persistence. Multivariate modelling analyses (generalized linear mixed modelling) compared HRU and fracture incidence between those who stayed on medication and those who discontinued. Pairwise analyses compared patients with persistence durations of <12 months, 12–<24 months, 24–<36 months and ≥36 months. The follow‐up period for outcome events began after the end of the persistence period of the less‐persistent group in each comparison (Fig. 1). Therefore, rate ratios compared patients who had discontinued medication (shorter persistence group) with those who took their medication for longer (longer persistence group). For example, for the comparison of patients who were persistent for <12 months versus 24–<36 months, events were counted over the period of month 12 to month 36 following treatment initiation. All the patients with persistence of <12 months would have discontinued medication by month 12, whereas all the patients with persistence of 24–<36 months would still be on medication. The differing lengths of follow‐up in the various persistence groups were accounted for by offsetting the length of follow‐up in the model.
Figure 1.
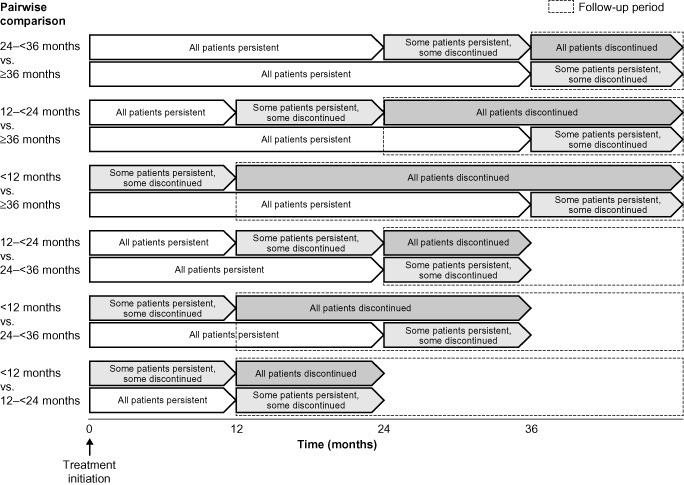
Persistence groups of patients receiving oral bisphosphonates, used for pairwise comparisons and data collection periods.
Only patients with an MPR of 80% or more were included in the multivariate analyses, to assess the effect of medication persistence on outcomes more accurately. Analyses were adjusted for age, co‐medications (glucocorticoids, immunosuppressants, anticonvulsants, vitamin C, vitamin D and/or calcium supplements and proton pump inhibitors) and co‐morbidities (irritable bowel syndrome, diabetes, ischaemic heart disease, congestive heart disease, chronic obstructive pulmonary disease, hyperthyroidism, chronic liver disease, metabolic disorders, renal disorders, systemic lupus erythematosus and arthritis).
We also investigated the interaction between each outcome (HRU rate and fracture rate) and time by considering the change in rate of HRU and fracture for each 6‐month period. The modelling analyses did not adjust for an inflated zero distribution, but allowed for over‐ and under‐dispersion.
Results
Patient characteristics
Baseline characteristics are presented in Table 1. A total of 36 320 patients were included, with a median age of 74 years. Overall, in the 12 months before the index date, 15.5% of patients had experienced a fracture and 6.4% had experienced a fracture of the wrist, hip or vertebra. The lifetime recorded incidence of fracture at baseline was 35.7% overall, with a lifetime incidence of 12.3% at the wrist, hip or vertebra. Vitamin D and/or calcium supplements were the most commonly prescribed co‐medications in the 12 months before the index date (65.0% of patients). Glucocorticoids and proton pump inhibitors were also frequently prescribed (29.8% and 25.4% of patients, respectively; Table 1).
Table 1.
Baseline characteristics and medical history among all patients and by length of persistence period
| All patients (n = 36 320) | Length of persistence period | ||||
|---|---|---|---|---|---|
| <12 months (n = 9477) | 12–<24 months (n = 7331) | 24–<36 months (n = 5832) | ≥36 months (n = 13 680) | ||
| Median age at index date, years (min., max.) | 74 (50, 105) | 74 (50, 105) | 75 (50, 105) | 75 (50, 102) | 73 (50, 99) |
| Median number of GP events in the 12 months before the index date (min., max.)a | 22 (1, 344) | 22 (1, 282) | 23 (1, 344) | 23 (1, 128) | 20 (1, 122) |
| Median number of hospital visits in the 12 months before the index date (min., max.)b | 0 (0, 155) | 0 (0, 19) | 0 (0, 12) | 0 (0, 9) | 0 (0, 155) |
| Fractures in the 12 months before the index date, n (%) | |||||
| Wrist | 249 (0.7) | 58 (0.6) | 59 (0.8) | 41 (0.7) | 91 (0.7) |
| Hip | 1418 (3.9) | 339 (3.6) | 358 (4.9) | 323 (5.5) | 461 (3.4) |
| Vertebral | 641 (1.8) | 152 (1.6) | 142 (1.9) | 104 (1.8) | 243 (1.8) |
| All | 5642 (15.5) | 1286 (13.6) | 1247 (17.0) | 1090 (18.7) | 2019 (14.8) |
| Fractures at any time in the patients' history, n (%) | |||||
| Wrist | 934 (2.6) | 243 (2.6) | 198 (2.7) | 141 (2.4) | 352 (2.6) |
| Hip | 2622 (7.2) | 657 (6.9) | 619 (8.4) | 508 (8.7) | 838 (6.1) |
| Vertebral | 909 (2.5) | 221 (2.3) | 191 (2.6) | 154 (2.6) | 343 (2.5) |
| All | 12 957 (35.7) | 3186 (33.6) | 2712 (37.0) | 2214 (38.0) | 4845 (35.4) |
| Concomitant medication in the 12 months before the index date, n (%) | |||||
| Glucocorticoids | 10 811 (29.8) | 3274 (34.5) | 2330 (31.8) | 1720 (29.5) | 3487 (25.5) |
| Immunosuppressants | 2228 (6.1) | 630 (6.6) | 471 (6.4) | 345 (5.9) | 782 (5.7) |
| Anticonvulsants | 1379 (3.8) | 372 (3.9) | 300 (4.1) | 222 (3.8) | 485 (3.5) |
| Vitamin C | 134 (0.4) | 28 (0.3) | 41 (0.6) | 23 (0.4) | 42 (0.3) |
| Vitamin D and/or calcium supplements | 23 593 (65.0) | 6344 (66.9) | 4991 (68.1) | 3904 (66.9) | 8354 (61.1) |
| Proton pump inhibitors | 9238 (25.4) | 2574 (27.2) | 2116 (28.9) | 1612 (27.6) | 2936 (21.5) |
| HRTc | 331 (0.9) | 220 (2.3) | 65 (0.9) | 31 (0.5) | 15 (0.1) |
| Co‐morbidities in the 12 months before the index date, n (%) | |||||
| Irritable bowel syndrome | 384 (1.1) | 105 (1.1) | 72 (1.0) | 54 (0.9) | 153 (1.1) |
| Diabetes | 2143 (5.9) | 589 (6.2) | 466 (6.4) | 397 (6.8) | 691 (5.1) |
| Ischaemic heart disease | 2085 (5.7) | 589 (6.2) | 456 (6.2) | 344 (5.9) | 696 (5.1) |
| Congestive heart disease | 523 (1.4) | 174 (1.8) | 131 (1.8) | 83 (1.4) | 135 (1.0) |
| COPD | 1549 (4.3) | 542 (5.7) | 351 (4.8) | 238 (4.1) | 418 (3.1) |
| Hyperthyroidism | 155 (0.4) | 51 (0.5) | 30 (0.4) | 23 (0.4) | 51 (0.4) |
| Chronic liver disease | 104 (0.3) | 25 (0.3) | 24 (0.3) | 18 (0.3) | 37 (0.3) |
| Metabolic disorders | 1495 (4.1) | 386 (4.1) | 333 (4.5) | 247 (4.2) | 529 (3.9) |
| Renal disorders | 113 (0.3) | 40 (0.4) | 46 (0.6) | 12 (0.2) | 15 (0.1) |
| Systemic lupus erythematosus | 51 (0.1) | 17 (0.2) | 12 (0.2) | 5 (0.1) | 17 (0.1) |
| Arthritis | 1084 (3.0) | 293 (3.1) | 227 (3.1) | 179 (3.1) | 385 (2.8) |
Includes GP consultations, opening of patient records, repeat prescriptions and viewing electronic pathology reports.
Includes inpatient hospitalizations; not emergency room visits or day surgeries, unless referred.
Includes HRT used at any time during the study.
GP, general practitioner; COPD, chronic obstructive pulmonary disease; HRT, hormone replacement therapy.
Among the 36 320 patients, 9477 (26.1%) discontinued oral bisphosphonates after less than 12 months on therapy, 7331 (20.2%) were persistent for 12–<24 months, 5832 (16.1%) were persistent for 24–<36 months and 13 680 (37.7%) were persistent for 36 months or longer. Most patients (85.1%) had an MPR of 80% or more; very few (3.9%) had an MPR of less than 60% (Fig. 2).
Figure 2.
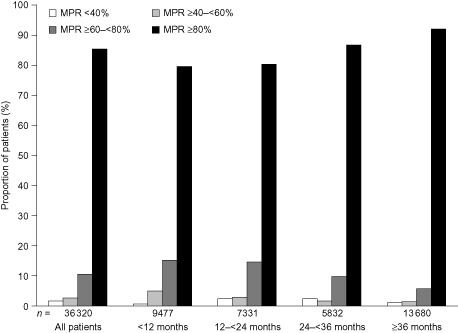
Medication possession ratio (MPR) according to length of persistence with oral bisphosphonates.
HRU
In total, the 30 912 patients with an MPR of 80% or more experienced 19 388 hospitalizations while persistent and 7170 hospitalizations during the post‐persistence period. We used multivariate modelling to compare HRU rate between those who stayed on medication and those who discontinued, according to different durations of persistence (Table 2). HRU rate after the end of the shorter persistence period was significantly higher in the group of patients who discontinued before 12 months than in those who remained on treatment for up to 24 months [hazard ratio (HR): 2.14; 95% confidence interval (CI): 1.38–3.33; P = 0.0007], for 24–<36 months (HR: 1.98; 95% CI: 1.63–2.41; P < 0.0001) and for ≥36 months (HR: 1.29; 95% CI: 1.16–1.44; P < 0.0001). HRU rate was also significantly higher after discontinuation in patients who persisted for 12–<24 months than those who persisted for 24–<36 months (HR: 5.29; 95% CI: 1.94–14.4; P = 0.0012). There were no significant differences in HRU rate between patients with ≥36 months' persistence and those with 12–<24 months' or 24–<36 months' persistence (Table 2).
Table 2.
Hazard ratios comparing outcome rates by duration of persistence in patients with an MPR ≥80%
| Pairwise comparison | HRU rate, HR (95% CI) | Fracture rate, HR (95% CI) |
|---|---|---|
| <12 months' persistence vs. 12–<24 months' persistence | 2.14 (1.38–3.33) | 1.68 (0.51–5.58) |
| P = 0.0007 | P = 0.395 | |
| <12 months' persistence vs. 24–<36 months' persistence | 1.98 (1.63–2.41) | 2.25 (1.35–3.77) |
| P < 0.0001 | P = 0.002 | |
| <12 months' persistence vs. ≥36 months' persistence | 1.29 (1.16–1.44) | 1.69 (1.28–2.24) |
| P < 0.0001 | P = 0.0002 | |
| 12–<24 months' persistence vs. 24–<36 months' persistence | 5.29 (1.94–14.4) | 0.36 (0.02–6.81) |
| P = 0.0012 | P = 0.4943 | |
| 12–<24 months' persistence vs. ≥36 months' persistence | 1.00 (0.81–1.23) | 0.96 (0.53–1.74) |
| P = 0.9936 | P = 0.8903 | |
| 24–<36 months' persistence vs. ≥36 months' persistence | 1.10 (0.70–1.75) | 4.22 (1.11–16.1) |
| P = 0.6761 | P = 0.0347 |
Multivariate modelling adjusted for age, co‐medication and co‐morbidities. For each comparison, follow‐up was from the end of the shorter persistence period.
CI, confidence interval; HR, hazard ratio; HRU, health resource utilization; MPR, medication possession ratio.
Fractures
Overall, the patient population with an MPR of 80% or more experienced 7483 fractures during their persistence periods and 1873 fractures during follow‐up after discontinuation. We used the same multivariate modelling method to compare fracture rate between those who stayed on medication and those who discontinued by different durations of persistence (Table 2). Fracture rate was significantly higher among patients with <12 months' persistence than among those with 24–<36 months' persistence (HR: 2.25; 95% CI: 1.35–3.77; P = 0.002) and those with ≥36 months' persistence (HR: 1.69; 95% CI: 1.28–2.24; P = 0.0002). Additionally, patients with 24–<36 months' persistence had a significantly higher fracture rate than those with ≥36 months' persistence (HR: 4.22; 95% CI: 1.11–16.1; P = 0.0347). There was a trend towards a higher fracture rate in patients with <12 months' persistence than in those with 12–<24 months' persistence, but it was not significant (Table 2).
Interaction between time and HRU and fracture rates
We also investigated the interaction between time (using 6‐month intervals) and HRU and fracture rates (Table 3). Although the biggest increase in HRU rate with each additional 6 months elapsed during the follow‐up period was seen when comparing patients who persisted for <12 months with those who persisted for 12–<24 months (HR 1.27; 95% CI: 1.15–1.41; P < 0.0001), significant interactions between each 6 months and HRU rate were observed when comparing all other persistence periods (Table 3).
Table 3.
Hazard ratios comparing each successive 6‐month period of follow‐up with rates of HRU and fracture in patients with an MPR ≥80%
| Pairwise comparison between persistence groups | HRU rate, HR* (95% CI) | Fracture rate, HR* (95% CI) |
|---|---|---|
| <12 months vs. 12–<24 months | 1.27 (1.15–1.41) | 1.08 (0.81–1.42) |
| P < 0.0001 | P = 0.610 | |
| <12 months vs. 24–<36 months | 1.16 (1.12–1.20) | 1.19 (1.09–1.30) |
| P < 0.0001 | P = 0.0002 | |
| <12 months vs. ≥36 months | 1.03 (1.02–1.03) | 1.06 (1.04–1.09) |
| P < 0.0001 | P < 0.0001 | |
| 12–<24 months vs. 24–<36 months | 1.26 (1.1–1.4) | 1.16 (0.84–1.59) |
| P = 0.0002 | P = 0.3805 | |
| 12–<24 months vs. ≥36 months | 1.03 (1.02–1.03) | 1.07 (1.04–1.09) |
| P < 0.0001 | P < 0.0001 | |
| 24–<36 months vs. ≥36 months | 1.03 (1.01–1.04) | 1.04 (1.00–1.08) |
| P = 0.0001 | P = 0.0579 |
*HR for the interaction between each 6‐month period and HRU or fracture rate.
Multivariate modelling adjusted for age, co‐medication and co‐morbidities. For each comparison, follow‐up was from the end of the shorter persistence period.
CI, confidence interval; HR, hazard ratio; HRU, health resource utilization; MPR, medication possession ratio.
We saw significant interactions between each 6‐month period elapsed during the follow‐up period and fracture rate when comparing patients with <12 months' persistence and those with 24–<36 months' persistence. Significant interactions between 6‐month periods and fracture rate were also seen when comparing patients with both <12 months' persistence and 12–<24 months' persistence with ≥36 months' persistence. A strong trend towards an interaction was seen when comparing patients with 24–<36 months' persistence and patients with ≥36 months' persistence (HR: 1.04; 95% CI: 1.00–1.08; P = 0.0579; Table 3).
Discussion
We found that patients who persisted for longer periods with oral bisphosphonates tended to have lower HRU and fewer fractures. Multivariate modelling was used to compare patients who discontinued medication with those who continued medication, according to duration of persistence. Patients with <12 months' persistence had significantly higher HRU and fracture rates than those who persisted for various longer periods. This indicates that, for patients who persisted for <12 months, oral bisphosphonates did not substantially protect against increased HRU or fracture risk after discontinuation. There was a significant benefit in terms of reducing HRU rate for patients who persisted for 12–<24 months compared with those who persisted for <12 months. In terms of reducing fracture rate, however, patients needed to persist for at least 2 years in order to gain a considerable benefit over those who persisted for <12 months.
Interpretation of data from those patients who achieved persistence of ≥12 months was more challenging. For patients with 12–<24 months' persistence, the HRU rate was five times higher from month 24 to month 36 than for those with 24–<36 months' persistence, although the 95% CI was quite wide for this comparison. Evidence for the benefit of persisting beyond 36 months in terms of HRU, however, was limited. This could reflect the limited follow‐up data available after 36 months on the study or could suggest that this patient population had more severe disease, rather than a lack of benefit from persisting for longer periods. Among patients with persistence of ≥12 months, we saw a significant difference in fracture rate only when comparing patients with 24–<36 months' persistence with patients with ≥36 months' persistence.
Analysis by each 6‐month interval showed that, irrespective of duration of persistence, HRU rate significantly increased for each additional 6 months of the follow‐up period, during which time the number of patients off‐therapy would have increased. An increased fracture rate for each additional 6 months of follow‐up was seen at all durations of persistence, and reached significance in three of the six comparisons. These data are consistent with our other analyses and suggest that HRU and fracture rates increase with time since discontinuation. Another contributing factor to the increased rates of HRU and fracture could be the increasing age of patients over the study period.
One limitation of the study is that all HRU data were collected, regardless of the reason for the resource utilization. Reduced HRU rate in more persistent patients could therefore also be due to greater persistence with other medications that they had been prescribed. In addition, patients with osteoporosis tend to be elderly and are likely to have a number of co‐morbidities and co‐medications that could mask any beneficial effects that bisphosphonates have on HRU because of osteoporosis, although we did adjust for common co‐morbidities and co‐medications.
Another potential limitation of the study is that the CPRD does not record whether prescriptions were dispensed or whether patients took their prescribed medications as recommended. Furthermore, although the CPRD is generally considered to be a high‐quality source of information, prescriptions given in secondary care are often not recorded. It is possible, therefore, that some patients may have been excluded despite having been prescribed oral bisphosphonates or indeed intravenous bisphosphonates, which are typically prescribed and administered in the secondary care setting. However, we would expect the majority of prescriptions for oral bisphosphonates to originate from primary care. Similarly, owing to the fact that zoledronic acid and intravenous ibandronate were approved late in the study period (2005 and 2004, respectively), contribution from such cases is likely to be minimal. Additional limiting factors are that GPs may not have full records of each inpatient hospitalization or fracture, resulting in incomplete records in the CPRD, and that the rate of social care utilization was not examined; therefore, this study may underestimate the resource burden imposed by fractures in the elderly.
Excluding fractures likely to be trauma‐associated and focusing on those of the wrist, hip and vertebra might also lead to an underestimation of fracture incidence [30, 31]; however, fractures of the wrist, hip and vertebra are generally considered more likely to be because of osteoporosis [32, 33], and similar methods for defining osteoporotic fractures have been used in other studies [34, 35].
It should be noted that the MPR was high in this study population. This could be a consequence of the MPR being assessed over the persistence period rather than the entire follow‐up period. To mitigate this, persistence was defined using a wide time frame (i.e. 90 days), but there was a small number of patients with a relatively low MPR. Finally, the wide CIs observed for the HRs for HRU rate suggest that a larger number of events would provide greater precision in assessing the effect of persistence on HRU rate.
The patient population may be atypical in that there was a high level of glucocorticoid use (29.8%) compared with that in the general population: data from another study using the CPRD suggest that glucocorticoids have been prescribed to 2.5% of 70–79‐year‐olds and just 0.9% of the overall population [36]. This could limit the potential for these results to provide a generalized overview of HRU and fracture rates at a population level. However, the high level of glucocorticoid use could be explained in part by a tendency of doctors to be more aware of bone health in patients taking glucocorticoids. Therefore, patients on glucocorticoids are more likely to be identified as being at risk of fragility fracture and subsequently becoming eligible for preventive therapy with bisphosphonates. The overall history of fracture in our study (35.7%) was also greater than that seen in a previous CPRD study of patients prescribed bisphosphonates, which reported that 27.4% of women had a history of fracture [23]. However, this is probably because our study included all fractures over the patients' lifetimes. In addition, our study included only women aged 50 years or older, whereas the previous study included women and men aged 18 years or older [23].
In contrast to the overall history of fracture, the rate of fractures in the 12 months before the index event that were likely to be due to osteoporosis (wrist, hip and vertebral fractures) was low in our study. This could reflect under‐diagnosis and under‐recording in UK clinical practice [37, 38], but also suggests that previous osteoporotic fractures were rarely the trigger for initiation of osteoporosis therapy and that the patients included in this analysis may be a low‐risk group. The high prevalence of low‐risk patients among those receiving osteoporosis treatments in the UK may mask the anti‐fracture benefits of treatment. The frequent use of bisphosphonates for primary rather than secondary fracture prevention has also been reported in another recent study, in which only women at the highest risk of fracture benefited from bisphosphonate treatment [39]. Better targeting of therapy to high‐risk patients would allow us to assess the efficacy of these agents better and to improve the cost‐effectiveness of osteoporosis treatment.
In conclusion, patients persisting with oral bisphosphonates for less than 2 years are unlikely to benefit from substantial protection against fracture after discontinuation. Differences in HRU and fracture rates were seen even when comparing patients with 24–<36 months' persistence and ≥36 months' persistence, suggesting that, even after more than 2 years on treatment, the protective effect after discontinuation is not always sufficient to reduce HRU and fracture rates to the levels that would be seen if patients continued on therapy. The greatest clinical effectiveness was seen in patients with 2, 3 or more years of persistence with treatment at a high MPR. UK guidelines recommend that treatment review for oral bisphosphonates should be performed every 5 years [40], which implies that prescribing clinicians should aim for patients to be persistent over this period. The data from this study do not facilitate a direct analysis of the validity of this recommended 5‐year course. They do, however, indicate the importance of persistence and they reveal distinct real‐world trends in increased HRU and fracture rates after discontinuation for all durations of treatment. Awareness of these trends can help prescribing doctors to make informed treatment decisions. Alternative treatments with better persistence profiles may reduce the rates of HRU and fractures caused by treatment discontinuation and help to alleviate the burden osteoporosis places both on patients and on health care systems.
Conflict of interest
S. Ferguson, M. Feudjo Tepie, A. Taylor, C. Critchlow, L. Spangler and M. Iqbal are employees of Amgen and hold stock. A. Roddam is an employee of GSK and holds stock in Amgen Inc. and GSK. J. Bayly has acted as a consultant and participated in speakers' bureaus for Amgen.
Acknowledgements
The authors thank Kim Allcott PhD from Oxford PharmaGenesis, UK who provided medical writing support. Funding for this support was provided by Amgen (Europe) GmbH. This study was funded by Amgen Inc.
Ferguson, S. , Feudjo Tepie, M. , Taylor, A. , Roddam, A. , Critchlow, C. , Iqbal, M. , Spangler, L. , and Bayly, J. (2016) The impact of persistence with bisphosphonates on health resource utilization and fracture risk in the UK: a study of patient records from the UK Clinical Practice Research Datalink. Journal of Evaluation in Clinical Practice, 22: 31–39. doi: 10.1111/jep.12422.
References
- 1. Szulc, P. & Bouxsein, M. L. (2011) Vertebral fracture initiative: PART I osteoporosis and related fractures: an under‐diagnosed and under‐treated public health issue. Available at: http://www.iofbonehealth.org/what‐we‐do/training‐and‐education/educational‐slide‐kits/vertebral‐fracture‐teaching‐program (last accessed 5 August 2015).
- 2. Dennison, E. , Mohamed, M. A. & Cooper, C. (2006) Epidemiology of osteoporosis. Rheumatic Diseases Clinics of North America, 32 (4), 617–629. [DOI] [PubMed] [Google Scholar]
- 3. Cummings, S. R. & Melton, L. J. (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet, 359 (9319), 1761–1767. [DOI] [PubMed] [Google Scholar]
- 4. Hernlund, E. , Svedbom, A. , Ivergård, M. , et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. Archives of Osteoporosis, 8 (1–2), 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Staa, T. P. , Dennison, E. M. , Leufkens, H. G. & Cooper, C. (2001) Epidemiology of fractures in England and Wales. Bone, 29 (6), 517–522. [DOI] [PubMed] [Google Scholar]
- 6. Kanis, J. A. & Johnell, O. (2005) Requirements for DXA for the management of osteoporosis in Europe. Osteoporosis International, 16 (3), 229–238. [DOI] [PubMed] [Google Scholar]
- 7. National Institute for Health and Clinical Excellence (2011) Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women (amended). Available at: http://www.nice.org.uk/guidance/ta161 (last accessed 5 August 2015).
- 8. European Medicines Agency (2010) Prolia: summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/001120/WC500093526.pdf (last accessed 5 August 2015).
- 9. Black, D. M. , Cummings, S. R. , Karpf, D. B. , et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet, 348 (9041), 1535–1541. [DOI] [PubMed] [Google Scholar]
- 10. Black, D. M. , Delmas, P. D. , Eastell, R. , et al (2007) Once‐yearly zoledronic acid for treatment of postmenopausal osteoporosis. The New England Journal of Medicine, 356 (18), 1809–1822. [DOI] [PubMed] [Google Scholar]
- 11. Chesnut, I. C. , Skag, A. , Christiansen, C. , et al (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. Journal of Bone and Mineral Research, 19 (8), 1241–1249. [DOI] [PubMed] [Google Scholar]
- 12. Cummings, S. R. , Black, D. M. , Thompson, D. E. , et al (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA: The Journal of the American Medical Association, 280 (24), 2077–2082. [DOI] [PubMed] [Google Scholar]
- 13. Eisman, J. A. , Civitelli, R. , Adami, S. , et al (2008) Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2‐year results from the DIVA study. The Journal of Rheumatology, 35 (3), 488–497. [PubMed] [Google Scholar]
- 14. Harris, S. T. , Watts, N. B. , Genant, H. K. , et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA: The Journal of the American Medical Association, 282 (14), 1344–1352. [DOI] [PubMed] [Google Scholar]
- 15. Liberman, U. A. , Weiss, S. R. , Broll, J. , et al (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. The New England Journal of Medicine, 333 (22), 1437–1443. [DOI] [PubMed] [Google Scholar]
- 16. Lyles, K. W. , Colon‐Emeric, C. S. , Magaziner, J. S. , et al (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. The New England Journal of Medicine, 357 (18), 1799–1809. [Multicenter Study, Randomized Controlled Trial, Research Support, Non‐U.S. Gov't]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McClung, M. R. , Geusens, P. , Miller, P. D. , et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. The New England Journal of Medicine, 344 (5), 333–340. [DOI] [PubMed] [Google Scholar]
- 18. Reginster, J. , Minne, H. W. , Sorensen, O. H. , et al (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporosis International, 11 (1), 83–91. [DOI] [PubMed] [Google Scholar]
- 19. Sambrook, P. , Cranney, A. & Adachi, J. D. (2010) Risk reduction of non‐vertebral fractures with intravenous ibandronate: post‐hoc analysis from DIVA. Current Medical Research and Opinion, 26 (3), 599–604. [DOI] [PubMed] [Google Scholar]
- 20. Nancollas, G. H. , Tang, R. , Phipps, R. J. , et al (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone, 38 (5), 617–627. [DOI] [PubMed] [Google Scholar]
- 21. Black, D. M. , Schwartz, A. V. , Ensrud, K. E. , et al (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long‐term Extension (FLEX): a randomized trial. JAMA: The Journal of the American Medical Association, 296 (24), 2927–2938. [DOI] [PubMed] [Google Scholar]
- 22. Watts, N. B. , Chines, A. , Olszynski, W. P. , et al (2008) Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporosis International, 19 (3), 365–372. [DOI] [PubMed] [Google Scholar]
- 23. Gallagher, A. M. , Rietbrock, S. , Olson, M. & van Staa, T. P. (2008) Fracture outcomes related to persistence and compliance with oral bisphosphonates. Journal of Bone and Mineral Research, 23 (10), 1569–1575. [DOI] [PubMed] [Google Scholar]
- 24. Curtis, J. R. , Westfall, A. O. , Cheng, H. , Delzell, E. & Saag, K. G. (2008) Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporosis International, 19 (11), 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walley, T. & Mantgani, A. (1997) The UK general practice research database. Lancet, 350 (9084), 1097–1099. [DOI] [PubMed] [Google Scholar]
- 26. MHRA (2008) Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency (MHRA) database research. 2nd annual report April 2007–March 2008. Available at: http://webarchive.nationalarchives.gov.uk/20140506174544/http://www.mhra.gov.uk/home/groups/pl‐a/documents/committeedocument/con028614.pdf (last accessed 5 August 2015).
- 27. Herrett, E. , Thomas, S. L. , Schoonen, W. M. , Smeeth, L. & Hall, A. J. (2010) Validation and validity of diagnoses in the General Practice Research Database: a systematic review. British Journal of Clinical Pharmacology, 69 (1), 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan, N. F. , Harrison, S. E. & Rose, P. W. (2010) Validity of diagnostic coding within the General Practice Research Database: a systematic review. The British Journal of General Practice, 60 (572), e128–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. BNF Publications (2008) British National Formulary. BNF Publications, UK: BNF.org. Available at: http://www.bnf.org/bnf/index.htm (last accessed 5 August 2015). [Google Scholar]
- 30. Court‐Brown, C. M. & Caesar, B. (2006) Epidemiology of adult fractures: a review. Injury, 37 (8), 691–697. [DOI] [PubMed] [Google Scholar]
- 31. Mackey, D. C. , Lui, L. Y. , Cawthon, P. M. , et al (2007) High‐trauma fractures and low bone mineral density in older women and men. JAMA: The Journal of the American Medical Association, 298 (20), 2381–2388. [DOI] [PubMed] [Google Scholar]
- 32. Melton, L. J. III , Thamer, M. , Ray, N. F. , et al (1997) Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. Journal of Bone and Mineral Research, 12 (1), 16–23. [DOI] [PubMed] [Google Scholar]
- 33. Warriner, A. H. , Patkar, N. M. , Curtis, J. R. , et al (2011) Which fractures are most attributable to osteoporosis? Journal of Clinical Epidemiology, 64 (1), 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morin, S. N. , Lix, L. M. & Leslie, W. D. (2014) The importance of previous fracture site on osteoporosis diagnosis and incident fractures in women. Journal of Bone and Mineral Research, 29 (7), 1675–1680. [DOI] [PubMed] [Google Scholar]
- 35. Islam, S. , Liu, Q. , Chines, A. & Helzner, E. (2009) Trend in incidence of osteoporosis‐related fractures among 40‐ to 69‐year‐old women: analysis of a large insurance claims database, 2000–2005. Menopause (New York, N.Y.), 16 (1), 77–83. [DOI] [PubMed] [Google Scholar]
- 36. van Staa, T. P. , Leufkens, H. G. , Abenhaim, L. , Begaud, B. , Zhang, B. & Cooper, C. (2000) Use of oral corticosteroids in the United Kingdom. QJM: Monthly Journal of the Association of Physicians, 93 (2), 105–111. [DOI] [PubMed] [Google Scholar]
- 37. Clark, E. M. , Gould, V. , Morrison, L. , Ades, A. E. , Dieppe, P. & Tobias, J. H. (2012) Randomized controlled trial of a primary care‐based screening program to identify older women with prevalent osteoporotic vertebral fractures: Cohort for Skeletal Health in Bristol and Avon (COSHIBA). Journal of Bone and Mineral Research, 27 (3), 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Delmas, P. D. , van de Langerijt, L. , Watts, N. B. , et al (2005) Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. Journal of Bone and Mineral Research, 20 (4), 557–563. [DOI] [PubMed] [Google Scholar]
- 39. Leslie, W. D. , Lix, L. M. , Johansson, H. , et al (2012) Does osteoporosis therapy invalidate FRAX for fracture prediction? Journal of Bone and Mineral Research, 27 (6), 1243–1251. [DOI] [PubMed] [Google Scholar]
- 40. National Osteoporosis Guideline Group (2014) Osteoporosis: Clinical Guideline for Prevention and Treatment. University of Sheffield. November 2014. [Google Scholar]


