Abstract
Aim
Poor growth and malnutrition are frequently reported in children with cerebral palsy in developed countries, but there is limited information from developing countries. We investigated the nutritional status of Ugandan children with cerebral palsy and described the factors associated with poor nutrition.
Methods
We examined 135 children from two to 12 years with cerebral palsy, who attended Uganda's national referral hospital. A child was considered underweight, wasted, stunted or thin if the standard deviation scores for their weight for age, weight for height, height for age and body mass index for age were ≤−2.0 using World Health Organization growth standards. Multivariable logistic regression identified the factors associated with nutritional indicators.
Results
Over half (52%) of the children were malnourished, with underweight (42%) being the most common category, followed by stunting (38%), thinness (21%) and wasting (18%). Factors that were independently associated with being malnourished were as follows: presence of cognitive impairment, with an adjusted odds ratio (aOR) of 4.5, being 5 years or older (aOR = 3.4) and feeding difficulties in the perinatal period (aOR = 3.2).
Conclusion
Malnutrition was common in Ugandan children with cerebral palsy and more likely if they were 5 years or more or had experienced neonatal complications.
Keywords: Cerebral palsy, Children, Malnutrition, Uganda, World Health Organization growth standards
Abbreviations
- aOR
adjusted odds ratio
- BMI
body mass index
- CI
confidence interval
- OR
odds ratio
- WHO
World Health Organization
Key Notes.
Poor growth and malnutrition are frequently reported in children with cerebral palsy in developed countries, but there is limited information from developing countries.
Our study examined 135 children from two to 12 years of age with cerebral palsy who attended Uganda's national referral hospital.
We found that 52% of the children were malnourished and rates were higher in those who were 5 years or more or had experienced neonatal complications.
Introduction
Cerebral palsy is a common childhood disability that affects sensory motor functions and leads to impaired motor behaviour and oral motor dysfunction. Depending on the severity of the impairments, children with cerebral palsy have feeding difficulties due to their inability to put food into their mouth and due to chewing and, or, swallowing problems. This situation makes them dependent on others, risking malnutrition, which negatively impacts on the quality of their life 1. In high‐income countries, growth and nutrition disorders are seen in one‐third of the paediatric cerebral palsy patients 2. The effects of poor nutrition on their health are devastating during early development and include the following: compromised immunity 3, cognitive problems 4, increased severity of gastro‐oesophageal reflux 5 and stunted growth 6. There are, however, limited studies from low‐income countries describing the nutritional status of children with cerebral palsy.
Challenges in the assessment of growth in children with cerebral palsy are associated with contractures, involuntary muscle spasms and limited patient cooperation due to cognitive deficits. In addition, different standards have been used resulting in diverse findings and interpretations, thus making it difficult to compare data across studies 7, 8. The development of the 2006 World Health Organization (WHO) child growth standards and the WHO Reference 2007 growth charts has provided gold standards for assessing and monitoring the growth of children and adolescents. This now makes it possible to compare data between different age groups 9. A recent study on children with cerebral palsy has confirmed that use of the WHO standard devia‐tion scores (Z‐scores) provides accurate parameters for assessing malnutrition in patients with cerebral palsy 10.
A number of factors have been associated with poor nutritional status and growth in children with cerebral palsy in high‐income countries, including self‐feeding difficulties, inadequate nutrient intake, oral motor dysfunction 11, 12, 13 and the severity of gross motor dysfunction 7. It is not clear whether these factors affect children with cerebral palsy in low‐income countries. Neither is it known whether there are specific factors associated with the different forms of malnutrition in children with cerebral palsy. In this study, we hypothesised that Ugandan children with cerebral palsy would have poorer nutritional status compared with the normal population. The aim of this study was to examine the nutritional status of children with cerebral palsy attending a specialised clinic in Uganda, as well as study factors that could potentially be associated with malnutrition.
Patients and Methods
Study setting
This study was conducted from September 2009 to August 2010 in the paediatric cerebral palsy clinic at Mulago, Uganda's national referral and teaching hospital in Kampala. The clinic has a patient turnover of about 400 children a year.
Ethical approval
The study was performed according to the Declaration of Helsinki on research on human subjects and approved by the Mulago Hospital Ethics Committee, the Makerere University School of Medicine Research and Ethics Committee and the Uganda National Council of Sciences and Technology (reference HS 628). The caregivers and, or, the children were informed about the study and gave their consent before being recruited.
Study population
Children with cerebral palsy aged two to 12 years of age who met the cerebral palsy diagnosis criteria, according to the definition by Rosenbaum et al. 14, were consecutively recruited from children visiting the cerebral palsy clinic. Initially, 151 children met the eligibility criteria, but consent was only obtained from the caregivers of 135 children. Information about the socio‐demographic and clinical characteristics of the study population is presented in Table 1, and more detailed information has previously been reported on the same cohort 15.
Table 1.
Demographic and clinical characteristics of the children with cerebral palsy and their caregivers
| Characteristic | Number n = 135 | Normal nutritional state n = 63 (46.7%) | Malnourisheda n = 72 (53.3%) |
|---|---|---|---|
| Age of child (%) | |||
| ≤5 years | 92 | 46 (50.0) | 46 (50.0) |
| >5 years | 43 | 17 (39.5) | 26 (60.5) |
| Sex of child (%) | |||
| Male | 72 | 34 (47.2) | 38 (52.8) |
| Female | 63 | 29 (46.0) | 34 (54.0) |
| Age of mother at child's birth (%) | |||
| ≤30 years | 107 | 52 (48.6) | 55 (51.4) |
| >30 years | 28 | 11 (39.3) | 17 (60.7) |
| Caregiver's marital status (%) | |||
| Married | 92 | 44 (47.8) | 48 (52.2) |
| Single/widowed/other | 43 | 19 (44.2) | 24 (55.8) |
| Caregiver's educational status (%) | |||
| Has completed primary school | 95 | 48 (50.5) | 47 (49.5) |
| Has not completed primary school | 40 | 15 (37.5) | 25 (62.5) |
| Gestational age at birth (%) | |||
| Full term | 117 | 53 (45.3) | 64 (54.7) |
| Preterm | 18 | 10 (55.6) | 8 (44.4) |
| Place of birth (%) | |||
| Health facility | 127 | 58 (45.7) | 69 (54.3) |
| Home | 8 | 5 (62.5) | 3 (37.5) |
| Admitted to hospital after birth (%) | |||
| Yes | 53 | 23 (43.4) | 30 (56.6) |
| No | 82 | 40 (48.8) | 42 (51.2) |
| Type of cerebral palsy (%) | |||
| Bilateral spastic | 62 | 23 (37.1) | 39 (62.9) |
| Unilateral spastic | 32 | 22 (68.8) | 10 (31.3) |
| Dyskinetic | 17 | 6 (35.3) | 11 (64.7) |
| Ataxic | 13 | 8 (61.5) | 5 (38.5) |
| Unclassifiable | 11 | 4 (36.4) | 7 (63.6) |
| Severity of gross motor function impairment (%) | |||
| Mild | 34 | 23 (67.6) | 11 (32.4) |
| Moderate | 59 | 25 (42.4) | 34 (57.6) |
| Severe | 42 | 15 (35.7) | 27 (64.3) |
| Presence of anaemiab (%) | |||
| Yes | 38 | 13 (34.2) | 25 (65.8) |
| No | 76 | 39 (51.3) | 37 (48.7) |
| Presence of cognitive impairment | |||
| Yes | 102 | 42 (41.2) | 60 (58.8) |
| No | 33 | 21 (63.6) | 12 (36.4) |
| Difficulty feeding in perinatal period (%) | |||
| Yes | 69 | 28 (40.6) | 41 (59.4) |
| No | 66 | 35 (53.0) | 31 (47.0) |
| Feeding ability (%) | |||
| Feeds self | 69 | 39 (56.5) | 30 (43.5) |
| Has to be fed | 66 | 24 (36.4) | 42 (63.6) |
Children with extreme values are included in this category.
Haemoglobin level determined in 114 children.
Study design
Assessments were carried out in three steps on a daily basis by the first author (AK‐M), who is trained in neurology, along with a medical doctor and a physiotherapist trained for the project. Step one involved screening based on questions one and five, on motor disability, from the Ten Question Screen 16. Secondly, the children who screened positive were further assessed by the physiotherapist using the decision tree for identifying CP from the Surveillance of Cerebral Palsy in Europe 17. Thirdly, the first author confirmed the cerebral palsy diagnosis and classified it according to the clinical subtype: bilateral spastic, unilateral spastic, dyskinetic or ataxic 17. No child was under medication known to affect growth or on a special diet for treatment 15.
Data collection
There were four stages to the data collection:
Structured interviews. Information from the caregiver was obtained using a structured interview questionnaire described by Kakooza‐Mwesige et al. 15. The interview included factors that could potentially be associated with malnutrition based on a previous study that explored predictors of poor growth in children in Uganda 18.
Physical examination. We assessed gross and fine motor impairment, classifying the severity of impairment into three grades – mild, moderate and severe – based on the child's ability to sit, to grasp and on self‐initiated walking and fine motor skills 15. We also checked for pedal oedema.
Anthropometric measurements. The children's weight in kilograms and height in centimetres were measured using WHO growth standards 9. Weight was measured using a SECA 813 digital scale (seca Vogel &Halke GmbH &Co., Hamburg, Germany) whose readings were recorded to the nearest 0.1 kg. If a child was unable to stand, the weight was calculated as the difference between the weight of the caregiver holding the child and the weight of the caregiver alone. Height and length were measured using a stadiometer and recorded in centimetres (cm) to the nearest 0.1 cm. Children who were at least 85 cm and able to stand flat‐footed and straight had their height measured while standing, while those who were under this height or unable to stand had their length measured while lying down. A correction factor was made to convert the measurement into height in children ≥85 cm who were measured while lying down. The length of children with contractures was measured in segments using a flexible tape measure. The mid‐upper arm circumference in children aged two to 5 years was measured using a tape measure and recorded to the nearest 0.1 cm. In patients with unilateral cerebral palsy, the unaffected side was used. The head circumference was measured by passing the tape measure over the occipital and frontal bones. Measurements were made twice and the average value recorded to the nearest 0.1 cm. Daily validation of the instruments and measurements was carried out and random checks were performed.
Laboratory investigations. Under aseptic conditions, 5 mL of venous blood was drawn. The complete blood count and haemoglobin levels were performed using a Z series Coulter counter machine (Beckman Coulter Inc, Brea, CA, USA).
Data handling and analysis
Definition of malnutrition using Z‐scores
Anthropometric indicators were constructed according to WHO growth standards based on weight, height/length, age and sex 9 and were converted into Z‐scores using the WHO Anthro 19 and AnthroPlus 20 to calculate the nutritional status. Children with a Z‐score of −2.0 or lower in any of the nutritional indicators were defined as having malnutrition 9 (Table S1). Children with extreme Z‐scores were excluded from the calculations. The weight‐for‐age Z‐score was only calculated for children up to 10 years of age and the weight‐for‐height Z‐score for those up to 5 years of age.
Pearson's chi‐square was used to determine any association between the factors considered and the dependent variables of stunting, underweight, wasting and thinness. Factors such as age and type of cerebral palsy were categorised, and the odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were computed. To determine the factors independently associated with the nutritional indicators, a multivariable analysis was performed using logistic regression methods. Factors that had a p‐value of ≤0.2 21 at univariate analysis, and factors that were assumed a priori to be associated with poor nutritional status 18, were entered into a logistic regression model using the backward stepwise (Wald) method. Missing data and categories with less than five values in each cell were excluded from the analysis. Model performance was assessed using the Hosmer–Lemeshow test.
Correlations between the different degrees of malnutrition were assessed using Pearson's correlation coefficient. Data analysis was performed using IBM SPSS Statistics software version 22.0 (IBM Corporation, Chicago, IL, USA).
Results
Nutritional status
More than half (52%, 95% CI 44–46%) of the children with cerebral palsy were malnourished, as they had a Z‐score of below 2.0 in at least one of the indicators (Fig. S1). Underweight was the most common form of malnutrition, recorded in 53 of 127 children (42%, 95% CI 33–51%), followed by stunting in 48 of 128 (38%, 95% CI 30–46%). Notably, 4% of the children were overweight. None had pedal oedema.
In Table 1, we present demographic and clinical characteristics of the total sample of 135 children with cerebral palsy and of the children in the malnourished group and the group without any signs of malnutrition. A comparison between the two groups shows an over‐representation (>60%) in the malnourished group of children ≥5 years of age, of children with bilateral spastic, dyskinetic or unclassifiable types of cerebral palsy and of children with severe gross motor function impairment and anaemia. Children whose mothers were ≥30 years of age at delivery, and whose caregiver had not completed primary school, were also more common in the malnourished group.
The distributions of the children's nutritional indicator‐based Z‐scores in relation to the WHO growth charts 9 are presented in Fig. 1. Results showed a negatively skewed distribution for most of the indicators, with the exception of mid‐upper arm circumference.
Figure 1.
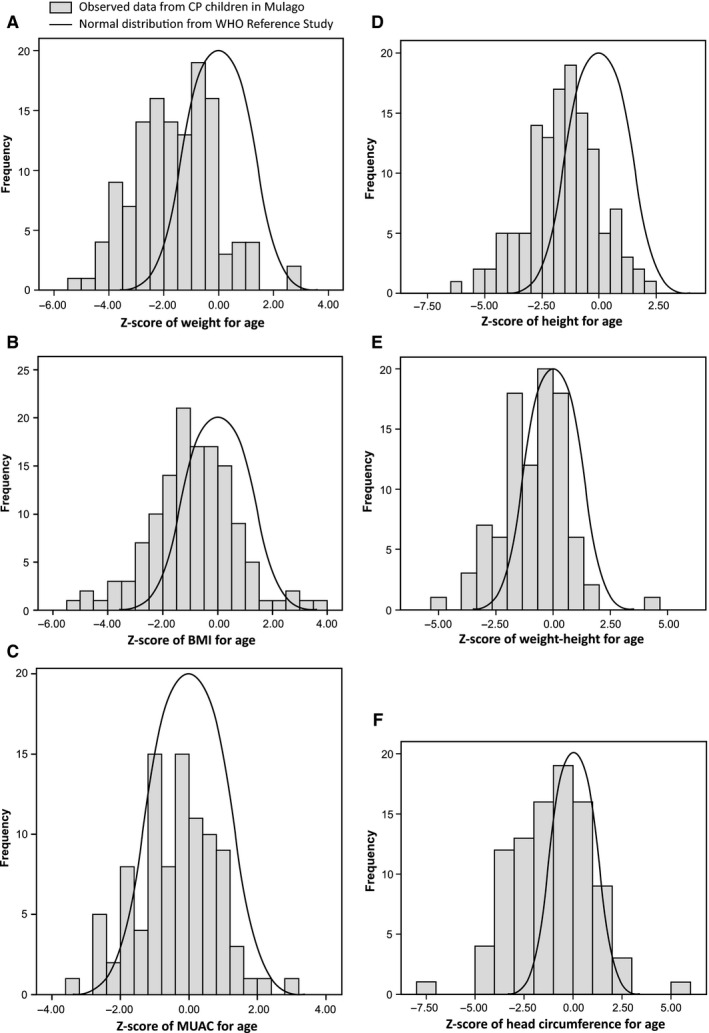
Distribution of Z‐scores in the included children with cerebral palsy (CP), compared with World Health Organization (WHO) growth standards reference Z‐scores: (A) weight‐for‐age Z‐scores, (B) BMI‐for‐age Z‐scores, (C) mid‐upper arm circumference for age Z‐scores, (D) height‐for‐age Z‐scores, (E) weight‐for‐height Z‐scores and (F) head circumference Z‐scores. The four children >10 year olds were not included in the weight‐for‐age Z‐scores calculation, and we only included 94 children up to 5 years old in calculating the mid‐upper arm circumference for age Z‐scores, weight‐for‐height Z‐score and head circumference Z‐scores. BMI = Body Mass Index, MUAC = Mid upper arm circumference.
Nine children had Z‐scores either above or below the default flag limits for individual indicators, according to WHO growth standards 9. These extreme values were deleted in the subsequent analyses as they indicated a probable measurement error. Thus, seven height‐for‐age Z‐scores, four weight‐for‐age Z‐scores and body mass index‐for‐age Z‐scores were omitted. In addition, four children were more than 10 years old, and therefore, their weight‐for‐age Z‐score was not calculated. Overall, all indicators were far below the United Nations' threshold levels and the mean scores varied between −1.57 and −0.38 (Table 2).
Table 2.
Anthropometric characteristics of the sample
| Characteristic | Number of subjects | Mean | SD | Median |
|---|---|---|---|---|
| Weight‐for‐age Z‐scorea | 127 | −1.57 | 1.48 | −1.53 |
| Height‐for‐age Z‐scoreb | 128 | −1.57 | 1.57 | −1.50 |
| BMI‐for‐age Z‐scorec | 131 | −0.92 | 1.56 | −0.94 |
| Weight‐for‐height Z‐scored | 94 | −0.84 | 1.41 | −0.68 |
| Mid‐upper arm circumference Z‐scored | 94 | −0.38 | 1.17 | −0.33 |
| Head circumference Z‐scored | 94 | −1.08 | 2.00 | −0.92 |
Mean, standard deviation and medians of Z‐scores of respective indicator. See Table S1 for definition of anthropometric indicators.
Four children were excluded because of outlier values and four children were >10 years.
Seven children were omitted because of outlier values.
Four children were omitted because of outlier values.
Only children ≤5 years old were included.
Several children had poor nutritional status based on more than one of the nutritional indicators (Fig. 2). We found that 11 children had a combination of three indicators, such as being underweight (low weight‐for‐age Z‐score), stunting (low height‐for‐age Z‐score) and thinness (low BMI‐for‐age Z‐score), while 35 children had a combination of two of the indicators (Fig. 2A). Similarly, nine children had a combination of wasting (low weight‐for‐height Z‐score), stunting (low height‐for‐age Z‐score) and underweight (low weight‐for‐age Z‐score), with 35 having a combination of two of these indicators (Fig. 2B). In agreement with this co‐occurrence of several indicators of poor nutrition within the same child, there was a positive correlation in the whole group between the parameters of underweight (low weight‐for‐age Z‐score) and thinness (low BMI‐for‐age Z‐score), stunting (low height‐for‐age Z‐score) and wasting (low weight‐for‐height Z‐score) (r = 0.374, p < 0.001; r = 0.512, p < 0.001; and r = 0.381, p < 0.001, respectively). The relationship between wasting and stunting and between wasting and thinness was also statistically significant (r = 0.184, p < 0.033; r = 0.632, p < 0.001, respectively). No relationship was found between stunting and thinness (r = 0.078, p < 0.366).
Figure 2.
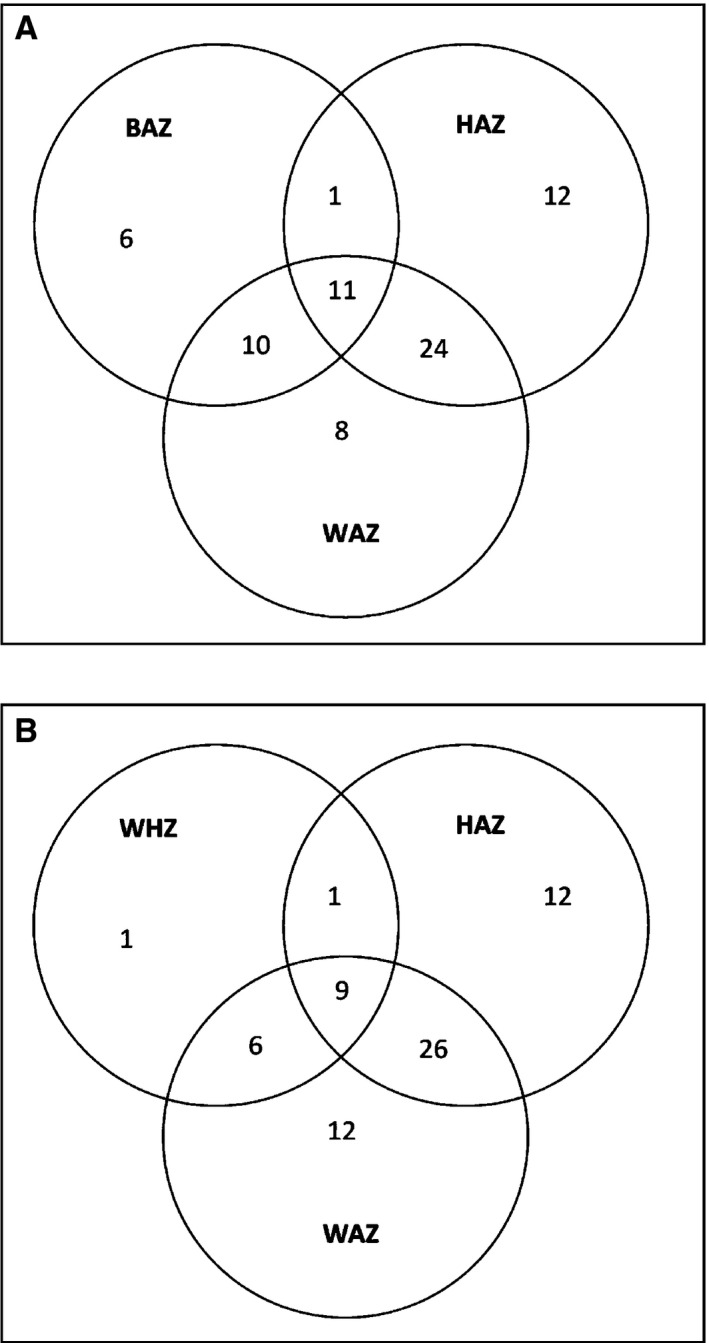
Venn diagrams to illustrate the relationship between nutritional status and various factors among the children, classified by the anthropometric indicators of (A) thinness [BMI‐for‐age Z‐score (BAZ)], stunting [height‐for‐age Z‐score (HAZ)] and underweight [weight‐for‐age Z‐score (WAZ)] in two to 12 year olds and (B) wasting [weight‐for‐height Z‐score (WHZ)], stunting [height‐for‐age Z‐score (HAZ)] and underweight [weight‐for‐age Z‐score (WAZ)] in two to 5 year olds. The overlap between the different indicators is illustrated. Regarding weight for age, the Z‐scores for four children were omitted because of outlier values. A further four children >10 years of age were not included in this calculation. Similarly, height‐for‐age Z‐score results for seven children and BMI‐for‐age Z‐score results for four children were excluded from this calculation because of outlier values. Finally, with regard to weight‐for‐height Z‐score, only 94 children ≤5 years old were included in the calculation.
Factors associated with poor nutritional status
The association between malnutrition and several of the demographic and clinical variables is shown in Table 3a. At unadjusted analysis, microcephaly, bilateral cerebral palsy type, and inability to feed independently were associated with malnutrition. However, at adjusted analysis, only an age of more than 5 years, cognitive impairment and a history of feeding difficulties during the first week of life (the perinatal period) were significantly associated with malnutrition (p < 0.05).
Table 3.
(a) Factors associated with malnutrition in children with cerebral palsy. (b) Factors associated with underweight in children with cerebral palsy (c) Factors associated with stunting in children with cerebral palsy (d) Factors associated with thinness in children with cerebral palsy (e) Factors associated with wasting in children with cerebral palsy
| Subcategory n (%) | Unadjusted OR (95% CI) | p‐value | Adjusted OR (95% CI) | p‐value | |
|---|---|---|---|---|---|
| (a) Characteristic (N = 126)a | |||||
| Child >5 years old | |||||
| Yes (36) | 23 (63.9) | 1.9 (0.8, 4.3) | 0.102 | 3.4 (1.2, 9.7) | 0.020 |
| No (90) | 43 (47.8) | 1.00 | 1.00 | ||
| Difficulty feeding in the perinatal period | |||||
| Yes (64) | 38 (59.4) | 1.7 (0.9, 3.6) | 0.110 | 3.2 (1.3, 7.9) | 0.008 |
| No (62) | 28 (45.2) | 1.00 | 1.00 | ||
| Presence of cognitive impairment | |||||
| Yes (93) | 54 (58.1) | 2.4 (1.0, 5.5) | 0.032 | 4.5 (1.6, 12.5) | 0.004 |
| No (33) | 12 (36.4) | 1.00 | 1.00 | ||
| Anaemia (HB >11.0 g/dL) | |||||
| Yes (35) | 24 (68.6) | 2.4 (1.0, 5.7) | 0.038 | 2.3 (0.9, 5.8) | 0.076 |
| No (72) | 34 (47.2) | 1.00 | 1.00 | ||
| (b) Characteristic (N = 127)b | |||||
| Child >5 years old | |||||
| Yes (35) | 20 (57.1) | 2.3 (1.0, 5.3) | 0.030 | 6.0 (1.9, 19.0) | 0.002 |
| No (92) | 33 (35.9) | 1.00 | 1.00 | ||
| History of infection in the perinatal period | |||||
| Yes (33) | 19 (57.6) | 2.4 (1.4, 5.4) | 0.032 | 3.6 (1.2, 10.3) | 0.017 |
| No (94) | 34 (36.2) | 1.00 | 1.00 | ||
| Presence of microcephaly | |||||
| Yes (74) | 38 (51.4) | 2.6 (1.2, 5.6) | 0.009 | 2.9 (1.1, 7.4) | 0.024 |
| No (53) | 15 (28.3) | 1.00 | 1.00 | ||
| Delay in sitting without support | |||||
| Yes (80) | 41 (51.2) | 3.0 (1.3, 6.7) | 0.005 | 2.5 (0.9, 6.9) | 0.077 |
| No (47) | 12 (25.5) | 1.00 | 1.00 | ||
| Presence of cognitive impairment | |||||
| Yes (94) | 45 (47.9) | 2.8 (1.2, 7.0) | 0.018 | 4.9 (1.5, 15.8) | 0.008 |
| No (33) | 8 (24.2) | 1.00 | 1.00 | ||
| (c) Characteristic (N = 128)c | |||||
| History of being kept in hospital after birth | |||||
| Yes (52) | 24 (46.2) | 1.8 (0.8, 3.8) | 0.094 | 2.6 (1.1, 5.9) | 0.028 |
| No (76) | 24 (31.6) | 1.00 | 1.00 | ||
| Signs of ADHD | |||||
| Yes (45) | 21 (46.7) | 1.8 (0.8, 3.8) | 0.115 | 2.3 (0.9, 5.6) | 0.058 |
| No (83) | 27 (32.5) | 1.00 | 1.00 | ||
| (d) Characteristic (N = 131)d | |||||
| Child's ability to feed self | |||||
| Unable (63) | 21 (33.3) | 4.4 (1.7, 11.1) | 0.001 | 5.2 (1.9, 14.0) | 0.001 |
| Able (68) | 7 (10.2) | 1.00 | 1.00 | ||
| Sex of the child | |||||
| Male (68) | 18 (26.5) | 1.9 (0.8, 4.5) | 0.139 | 2.5 (0.9, 6.5) | 0.053 |
| Female (63) | 10 (15.9) | 1.00 | 1.00 | ||
| Duration of breastfeeding | |||||
| ≤1 year (64) | 18 (28.1) | 2.2 (0.9, 5.2) | 0.065 | 2.3 (0.9, 5.9) | 0.070 |
| >1 year (67) | 10 (14.9) | 1.00 | 1.00 | ||
| (e) Characteristic (N = 94)e | |||||
| History of infection in the neonatal period | |||||
| Yes (21) | 6 (28.6) | 2.2 (0.7, 7.1) | 0.157 | 3.9 (1.0, 15.7) | <0.048 |
| No (73) | 11 (15.1) | 1.00 | |||
| Duration of breastfeeding | |||||
| ≤1 year (42) | 10 (23.8) | 2.0 (0.6, 5.8) | 0.195 | 2.9 (0.8, 10.1) | 0.088 |
| >1 year (52) | 7 (13.5) | 1.00 | 1.00 | ||
| Mode of communication | |||||
| Nonverbal (34) | 10 (29.4) | 3.1 (1.1, 9.3) | 0.032 | 4.1 (1.2, 14.0) | 0.025 |
| Verbal (60) | 7 (11.7) | 1.00 | |||
| Type of CP | |||||
| Bilateral CP (43) | 12 (27.9) | 3.6 (1.1, 11.1) | 0.023 | 3.6 (1.0, 12.2) | 0.038 |
| Other CP type (51) | 5 (9.8) | 1.00 | |||
Number = 126 after excluding outlier values, children ≤5 years old and those of age >10 years. The variables child's duration of breastfeeding, mode of communication and history of trauma to the head had a p‐value <0.2 and were also included in the logistic regression model but were not significant.
Number 127 after excluding outlier values and children of age >10 years. The following variables child's ability to feed self, duration of breastfeeding, presence of anaemia, type of residence, mode of communication, history of infection in the neonatal period and history of assistance to breathe at birth had a p‐value <0.2 and were also included in the logistic regression model, but were not significant.
Number = 128 after excluding all other outlier values. Child's type of cerebral palsy (CP), presence of cognitive impairment, history of difficult delivery and history of assistance to breathe at birth had a p‐value <0.2 and were also included in the logistic regression model, but were not significant.
Number = 131 after excluding all other outlier values. The variables child's type of cerebral palsy (CP), mode of communication, presence of cognitive impairment/visual impairment/attention‐deficit hyperactivity disorder (ADHD)/epilepsy, age at solid food introduction, history of deep jaundice in the neonatal period, history of seizures in the neonatal period and history of infection in the neonatal period had a p‐value <0.2 and were also included in the logistic regression model, but were not significant.
Number = 94 after including only those ≤5 years old. The variables child's history of difficulty in feeding in the perinatal period, difficulty breathing in the neonatal period and presence of visual impairment had a p‐value <0.2 and were also included in the logistic regression model, but were not significant. n represents the number for each nutritional indicator; a p‐value <0.05 indicates statistical significance.
CP, cerebral palsy; ADHD, attention‐deficit hyperactivity disorder; CI, confidence interval; OR, odds ratio.
Factors associated with underweight are shown in Table 3b. Being under the age of five or having a cognitive impairment was also associated with being underweight after the adjusted analysis (p > 0.05). These two factors were, respectively, six and five times more likely in children with underweight compared with children without underweight. There were also significant associations with children having microcephaly and a history of infection during the first week of life (p < 0.05).
Factors associated with stunting are shown in Table 3c. The only significant association was found when a child had been admitted to hospital during the postnatal period (p = 0.028).
Factors associated with thinness are given in Table 3d. Children were most likely to be thin if they required assistance to be fed (p = 0.001). Table 3e shows that children were more likely to be wasted if they were nonverbal (p = 0.025), had a history of infection in the neonatal period (p < 0.048) and, or, had bilateral cerebral palsy (p = 0.038).
The regression models fitted the data as all had nonsignificant Hosmer–Lemeshow chi‐square (p > 0.05). The p‐values for the models of malnutrition, underweight, stunting, thinness and wasting were p = 0.594, p = 0.681, p = 0.473, p = 0.502 and p = 0.652, respectively (data not shown).
Discussion
More than half of the children with cerebral palsy (52%) who visited the specialist clinic at Mulago Hospital during the 1‐year study period were malnourished. This was a higher prevalence than indicated in previous reports on clinical samples from both high‐income countries 22 and other low‐income countries 23. Stunting, indicated by low height for age, occurred in 38% of the children and was a marker of chronic malnourishment and subsequent growth retardation. Despite stunting being multifactorial in its aetiology, it reflects inadequate nutrition in relation to the body's needs over a long period. It is often accompanied by recurrent and chronic illness. Compared with the prevalence of stunting in the normal Ugandan child population of children under the age of five, which is 33% 24, our findings only showed a 5% increase. One assumption was that caregivers master the nutritional situation in a longer perspective despite cerebral palsy being a chronic illness with known feeding difficulties. Alternatively, our figures may not reflect the frequency in the general cerebral palsy population, but, rather, indicate a tendency for caregivers to seek health care when there is an acute illness.
By contrast, we found that wasting (18%) was less common than stunting in the cerebral palsy group but was still almost four times more prevalent than the national average of 5% 24. Wasting, measured by weight for height, reflects a recent episode of weight loss, often due to an acute stress factor, including illness. This finding supports the fact that children with cerebral palsy seek health care more often as result of an acute illness or emergency situation than due to prolonged chronic undernourishment. Weight for height is a commonly accepted measure of global acute malnutrition and is used in emergency situations as it indicates rapid weight loss. The mean weight‐for‐height Z‐score of −0.84, however, was slightly above the critical level of <−1.00 set by the United Nations for acute child malnutrition 25. The increased prevalence of wasting could possibly be due to the children being at the margin of poor feeding. In our study, three indicators were used to reflect wasting, namely weight for height (18%), BMI (21%) and mid‐upper arm circumference for age (9%). The lower frequency of mid‐upper arm circumference for age suggests that it is a less sensitive anthropometric measure in these children, as has been noted elsewhere 26 and that the triceps skin‐fold thickness should rather be used, as has been recommended 3. Irrespective of the parameter used, children with wasting have an increased risk of dying and require appropriate medical and nutritional therapy, as defined by the WHO 27.
Underweight, measured by weight for age, was the most common nutritional problem. It can be caused by both chronic and acute malnutrition. This means that a child can be underweight as a result of being stunted, wasted or thin or as a result of a combination of any of these indicators of malnutrition, as depicted in the Venn diagrams in Fig. 2. In our population, 42% of the children with cerebral palsy were underweight, which is three times higher than the normal Ugandan population 24, further confirming the poor nutrition of these cerebral palsy children in relation to local standards. This figure is comparable to a similar study of Greek children with cerebral palsy, using the same WHO growth standards, which found that 38.1% were undernourished based on weight for age 10. However, these two studies differ from a study of a sample of Egyptian children with cerebral palsy that reports a three times higher frequency 28. This discrepancy could stem from the different populations of cerebral palsy children, but also from the use of other growth reference standards for children with cerebral palsy 11.
Factors associated with poor nutritional status
Circumstances surrounding the neonatal period and factors reflecting the severity of the cerebral palsy condition were significantly associated with the nutritional condition. Admission to hospital directly after birth, a history of infections or feeding problems in the neonatal period or cognitive impairment were all associated with malnutrition. These correlations suggest that the nutrition of these children was compromised from early on. In particular, there was a correlation between admission to hospital and stunting. This confirms that the current priority actions, which are aimed at the first 1000 most critical days of life, are appropriate when it comes to prevention. It would be worthwhile to closely monitor and support children with such a history in an attempt to prevent malnutrition. The results also suggest that it is crucial to keep an eye on children with bilateral cerebral palsy, microcephaly or cognitive impairment, given that there is a three to four times higher likelihood that they will be malnourished. These conditions are consequences of severe brain damage early in life, and they are often accompanied by comorbidities such as epilepsy, visual impairment, feeding difficulties and speech and language difficulties. These findings highlight the negative impact that neurological impairment has on the nutritional status of children with cerebral palsy 13.
Being unable to eat without help from a caregiver was significantly associated with thinness in our population. This finding confirms that feeding problems are an issue for children with cerebral palsy. It has been reported that feeding regulated by the caregiver may lead to underfeeding due to the risk of the caregiver overestimating both the time spent feeding the child and the child's caloric intake 29. Several previous studies have shown that poor oral motor functioning affects a child's ability to consume calories and nutrients, and this consequently leads to malnutrition 11.
Children under 5 years of age were six times more likely to be underweight than children over this age. This corresponds with the findings by Stevenson et al. 30 who noted that the linear growth Z‐score worsened with age in children with cerebral palsy, independently of their nutritional status, implying that there may be other factors that influence growth. In our group, there seemed to be a declining number of children with increasing age, which could be attributed to decreased survival. An interesting observation from our analysis is that, despite the overlap (Fig. 2) and correlation among the different nutritional indicators, the only factors that were associated with more than two indicators (underweight and malnutrition) were being over the age of five and the presence of a cognitive impairment. Other factors were only correlated to one nutritional indicator. The reason for the differences between the factors in each model (nutritional indicator) is not known, but this finding suggests that there is an interaction of factors that contribute to the development of each of these nutritional indicators in children with cerebral palsy.
Study limitations
This was a clinical study of children with cerebral palsy who attended a tertiary referral hospital in Uganda, which means that our findings are subject to selection bias and probably deviate from the general population.
Other limitations are related to the method of data collection. We were not able to conduct serial anthropometric measurements of these children, which would have provided a better indication of the nutritional status. The segmental length measurements in the children with contractures may have yielded imprecise results. A substantial part of the history involved asking the caregiver to recall events that had happened some years before, and this provided potential bias associated with the caregivers' recall.
Clinical considerations
Malnutrition was common among children with cerebral palsy attending a specialised clinic in a tertiary hospital in sub‐Saharan Africa. Underweight and stunting were the most prevalent conditions, but wasting was also three times more common than the general population. Our findings are of specific clinical relevance because they highlighted the concerns of poor growth and nutrition in children with cerebral palsy from a low‐income country using the latest WHO growth standards. Complications in the perinatal period were associated with malnutrition, highlighting the importance of providing comprehensive emergency obstetric and neonatal care. Older children over 5 years of age, children with severe cerebral palsy and those with intellectual disability were at greater risk of malnutrition. To optimise function and quality of life for children with cerebral palsy and allow early intervention and prevention of malnutrition, close monitoring to enable early detection should be implemented as a first step, in particular for children with these risk factors. In addition, nutritional rehabilitation programmes need to be developed and adapted for children with cerebral palsy in this specific environment to be evaluated for efficacy.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This investigation was carried out with support from the following: the Belgian Government through the Belgian Technical Cooperation, Uganda Country Office, the Karolinska Institute, Sweden, the Swedish International Development Agency, the African Doctoral Dissertation Research Fellowship from the African Population and Health Research Centre in partnership with the International Development Research Centre, the Ford Foundation and the Foundation FrimurarnaBarnhuset, Swedish Research Council. The funding agencies had no role in the data collection or analysis, the interpretation of results or the decision to submit this research for publication.
Supporting information
Figure S1. Distribution (%) of children with cerebral palsy (CP) by nutrition Z‐scores for those who were malnourished (underweight, stunted, thin or wasted), based on weight for age (WFA) (underweight), height for age (HFA) (stunted), body mass index (BMI) for age (thinness) and weight for length/height (WFH) (wasting).
Table S1. Definition of anthropometric indicators. Z‐scores of −2.0 or lower were used as threshold values.
Acknowledgements
We would like to thank the children and caregivers who participated in this study and the clinical staff from Mulago Hospital and Makerere University College of Health Sciences. Special thanks go to Richard Kasiita for conducting the screening assessments, William Magala and Nancy Egwayu for professional support and Magnus Backheden for statistical help.
References
- 1. Samson‐Fang L, Fung E, Stallings VA, Conaway M, Worley G, Rosenbaum P, et al. Relationship of nutritional status to health and societal participation in children with cerebral palsy. J Pediatr 2002; 141: 637–43. [DOI] [PubMed] [Google Scholar]
- 2. Kuperminc MN, Stevenson RD. Growth and nutrition disorders in children with cerebral palsy. Dev Disabil Res Rev 2008; 14: 137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chandra RK, Kumari S. Effects of nutrition on the immune system. Nutrition 1994; 10: 207–10. [PubMed] [Google Scholar]
- 4. Corbett S, Drewett R. To what extent is failure to thrive in infancy associated with poorer cognitive development? A review and meta analysis. J Child Psychol Psychiatry 2004; 45: 641–54. [DOI] [PubMed] [Google Scholar]
- 5. Lewis D, Khoshoo V, Pencharz PB, Golladay ES. Impact of nutritional rehabilitation on gastroesophageal reflux in neurologically impaired children. J Pediatr Surg 1994; 29: 167–9 discussion 9–70. [DOI] [PubMed] [Google Scholar]
- 6. Stevenson RD, Conaway M, Chumlea WC, Rosenbaum P, Fung EB, Henderson RC, et al. Growth and health in children with moderate‐to‐severe cerebral palsy. Pediatrics 2006; 118: 1010–8. [DOI] [PubMed] [Google Scholar]
- 7. Day SM, Strauss DJ, Vachon PJ, Rosenbloom L, Shavelle RM, Wu YW. Growth patterns in a population of children and adolescents with cerebral palsy. Dev Med Child Neurol 2007; 49: 167–71. [DOI] [PubMed] [Google Scholar]
- 8. Krick J, Murphy‐Miller P, Zeger S, Wright E. Pattern of growth in children with cerebral palsy. J Am Diet Assoc 1996; 96: 680–5. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organisation . Child Growth Standards based on length/height, weight and age. Acta Paediatr 2006; Suppl.450: 76–85. [DOI] [PubMed] [Google Scholar]
- 10. Karagiozoglou‐Lampoudi T, Daskalou E, Vargiami E, Zafeiriou D. Identification of feeding risk factors for impaired nutrition status in paediatric patients with cerebral palsy. Acta Paediatr 2012; 101: 649–54. [DOI] [PubMed] [Google Scholar]
- 11. Fung EB, Samson‐Fang L, Stallings VA, Conaway M, Liptak G, Henderson RC, et al. , et al. Feeding dysfunction is associated with poor growth and health status in children with cerebral palsy. J Am Diet Assoc 2002; 102: 361–73. [DOI] [PubMed] [Google Scholar]
- 12. Rogers B. Feeding method and health outcomes of children with cerebral palsy. J Pediatr 2004; 145(Suppl. 2): 28–32. [DOI] [PubMed] [Google Scholar]
- 13. Marchand V, Motil KJ. Nutrition support for neurologically impaired children: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2006; 43: 123–35. [DOI] [PubMed] [Google Scholar]
- 14. Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of CP April 2006. Dev Med Child Neurol 2007; 109(Suppl.): 8–14. [PubMed] [Google Scholar]
- 15. Kakooza‐Mwesige A, Forssberg H, Eliasson AC, Tumwine JK. Cerebral palsy in children in Kampala, Uganda: clinical subtypes, motor function and co‐morbidities. BMC Res Notes 2015; 8: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Durkin M. The epidemiology of developmental disabilities in low‐income countries. Ment Retard Dev Disabil Res Rev 2002; 8: 206–11. [DOI] [PubMed] [Google Scholar]
- 17. SCPE . Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol 2000; 42:816–24. [DOI] [PubMed] [Google Scholar]
- 18. Wamani H, Nordrehaug AA, Peterson S, Tumwine JK, Tylleskar T. Predictors of poor anthropometric status among children under 2 years of age in rural Uganda. Public Health Nutr 2006; 9: 320–6. [DOI] [PubMed] [Google Scholar]
- 19. WHO Anthro for personal computers, version 3.2.2 , 2011. Software for assessing growth and development of the world's children. Geneva, Switzerland: WHO, 2010. [Google Scholar]
- 20. WHO AnthroPlus for personal computers Manual . Software for assessing growth of the world's children and adolescents. Geneva, Switzerland: WHO, 2009. [Google Scholar]
- 21. Collett D. Modelling binary data. London, England: Chapman and Hall, 1991: 23–37. [Google Scholar]
- 22. Troughton KE, Hill AE. Relation between objectively measured feeding competence and nutrition in children with cerebral palsy. Dev Med Child Neurol 2001; 43: 187–90. [PubMed] [Google Scholar]
- 23. Singhi P, Saini AG. Changes in the clinical spectrum of cerebral palsy over two decades in North India – an analysis of 1212 cases. J Trop Pediatr 2013; 59: 434–40. [DOI] [PubMed] [Google Scholar]
- 24. Uganda Bureau of Statistics (UBOS) and ICF International Inc . Uganda demographic and health survey. 2011. Kampala, Uganda: UBOS, editor. Maryland, Calverton, USA ICF International Inc; 2012. [Google Scholar]
- 25. IFRC, UNHCR, WFP, WHO . The management of nutrition in major emergencies. Geneva, Switzerland: WHO, 2000. [Google Scholar]
- 26. Burden ST, Stoppard E, Shaffer J, Makin A, Todd C. Can we use mid upper arm anthropometry to detect malnutrition in medical inpatients? A validation study. J Hum Nutr Diet 2005; 18: 287–94. [DOI] [PubMed] [Google Scholar]
- 27. WHO/WFP/UNSCN/UNICEF . Community‐based Management of Severe Acute Malnutrition: a Joint Statement by the World Health Organisation, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children's Fund, 2006.
- 28. Tomoum HY, Badawy NB, Hassan NE, Alian KM. Anthropometry and body composition analysis in children with cerebral palsy. Clin Nutr 2010; 29: 477–81. [DOI] [PubMed] [Google Scholar]
- 29. Stallings VA, Zemel BS, Davies JC, Cronk CE, Charney EB. Energy expenditure of children and adolescents with severe disabilities: a cerebral palsy model. Am J Clin Nutr 1996; 64: 627–34. [DOI] [PubMed] [Google Scholar]
- 30. Stevenson RDHR, Cater LV, Blackman JA. Clinical correlates of linear growth in children with cerebral palsy. Dev Med Child Neurol 1994; 36: 135–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution (%) of children with cerebral palsy (CP) by nutrition Z‐scores for those who were malnourished (underweight, stunted, thin or wasted), based on weight for age (WFA) (underweight), height for age (HFA) (stunted), body mass index (BMI) for age (thinness) and weight for length/height (WFH) (wasting).
Table S1. Definition of anthropometric indicators. Z‐scores of −2.0 or lower were used as threshold values.


