Abstract
We previously reported a series of de novo engineered cationic antibiotic peptides (eCAPs) consisting exclusively of arginine and tryptophan (WR) that display potent activity against diverse multidrug-resistant (MDR) bacterial strains. In this study, we sought to examine the influence of arginine compared to lysine on antibacterial properties by direct comparison of the WR peptides (8–18 residues) with a parallel series of engineered peptides containing only lysine and tryptophan. WR and WK series were compared for antibacterial activity by bacterial killing and growth inhibition assays and for mechanism of peptide–bacteria interactions by surface plasmon resonance and flow cytometry. Mammalian cytotoxicity was also assessed by flow cytometry, haemolytic and tetrazolium-based assays. The shortest arginine-containing peptides (8 and 10 mers) displayed a statistically significant increase in activity compared to the analogous lysine-containing peptides. The WR and WK peptides achieved maximum antibacterial activity at the 12-mer peptide (WK12 or WR12). Further examination of antibacterial mechanisms of the optimally active 12-mer peptides using surface plasmon resonance and flow cytometry demonstrates stronger interactions with Pseudomonas aeruginosa, greater membrane permeabilizing activity, and lower inhibitory effects of divalent cations on activity and membrane permeabilization properties of WR12 compared to WK12 (P < 0.05). Importantly, WK12 and WR12 displayed similar negligible haemolytic and cytotoxic effects at peptide concentrations up to ten times the MIC or 20 times the minimum bactericidal concentration. Thus, arginine, compared to lysine, can indeed yield enhanced antibacterial activity to minimize the required length to achieve functional antimicrobial peptides.
Keywords: Lysine, Arginine, engineered cationic antimicrobial peptides, antimicrobial peptides, multidrug-resistant antibiotics, antimicrobial agents
Introduction
The prevalence of infections associated with multidrug-resistant (MDR) bacteria underlines the need for new classes of antibiotics with novel antimicrobial mec hanisms (Boucher et al., 2009; Shi et al., 2009; Ho et al., 2012; Magiorakos et al., 2012; Bow, 2013; Agodi et al., 2014). Over the last three decades antimicrobial peptides (AMPs) have been intensely investigated as potential antibiotics against MDR bacteria (Bucki et al., 2004; Lipsky et al., 2008; Kulkarni et al., 2014). AMPs are ubiquitous effector peptides with amphipathic (usually cationic) structures representing the first line of defence against infectious pathogens (Hancock, 2001; Hancock et al., 2006). As an essential component of innate immunity, AMPs provide host epithelial surfaces and neutrophils with effective microbial killing mechanisms that are rapid and independent of the reactive oxygen species (Dashper et al., 2005) antimicrobial pathway (Hancock et al., 2006; Peschel & Sahl, 2006; Hell et al., 2010). While several bactericidal mechanisms have been identified among diverse structural classes, cationic AMPs typically recognize their microbial target by electrostatic interactions between their highly electropositive hydrophilic peptide motif and the electronegative lipids [e.g. lipid A in Gram-negative bacteria and lipoteichoic acid (Ozcan et al., 2006) in Gram-positive bacteria] on bacterial surfaces (Brogden, 2005; Mihajlovic & Lazaridis, 2010; Zhang et al., 2013). Although AMP amphiphilic sequences are highly diverse, the charge density of the cationic motif is predominantly provided by lysine or histidine residues (Park et al., 2003; Shanmugam et al., 2005; Mason et al., 2007; Georgescu et al., 2010; Khatami et al., 2014; Deslouches et al., 2015).
Despite the apparent potential of natural AMPs as therapeutic agents, their development for clinical application to treatment of infectious diseases has to date been unsuccessful, perhaps reflecting a natural adaptation to stringent specificities in terms of target pathogen and environment (Ding et al., 2003). In the last two decades, our laboratory has focused on rationally engineering AMPs to overcome intrinsic limitations of natural AMPs (Tencza et al., 1999; Phadke et al., 2002; 2003; 2005; Deslouches et al., 2005; 2013; Skinner et al., 2010 Steckbeck et al., 2014). Hence, we previously reported the rational design of an optimized engineered cationic antimicrobial peptide (eCAP) series consisting exclusively of arginine and tryptophan residues (WR series) that display broad activity against a diverse spectrum of Gram-positive and Gram-negative bacteria, including extensively drug-resistant (XDR) strains (Deslouches et al., 2013). Our preference for arginine over lysine for incorporation into eCAPs was based on various studies indicating stronger membrane interaction and perturbation properties of Arg- compared to Lys-enriched peptide sequences (Tencza et al., 1995; Mitchell et al., 2000; Phadke et al., 2002; Kalia et al., 2003; Phadke et al., 2003; Phadke et al., 2005; Su et al., 2009). However, a survey of natural AMP sequences reveals a predominance of lysine content in diverse AMP sequences, with minimal incorporation of arginine (Tossi et al., 2000; Wang & Wang, 2004; Wang et al., 2009; Seshadri Sundararajan et al., 2012). This apparent discrepancy led us to compare directly the antimicrobial activity of a parallel series of eCAPs containing only tryptophan and either arginine or lysine as the cationic component. Thus, we report here a systematic comparison of the functional properties of parallel series of eCAPs ranging in length from eight to 18 residues and composed only of tryptophan and either arginine (WR series) or lysine (WK series) arranged to form optimized amphipathic helices.
Methods
Strains.
The two bacterial strains used in this study were the laboratory strain of Pseudomonas aeruginosa (PAO1) and a clinical isolate of methicillin-resistant Staphylococcus aureus (MRSA) USA300, both provided by Dr Yohei Doi of the University of Pittsburgh and utilized in previous published studies (Deslouches et al., 2013).
Peptide synthesis.
The engineered peptides (WR and WK eCAPs series) were synthesized using standard Fmoc (9-fluorenylmethoxy carbonyl) synthesis protocols as previously described (Tencza et al., 1999; Phadke et al., 2002).0. Synthetic peptides were characterized and purified by reversed-phase HPLC on Vydac C18 or C4 columns (The Separations Group), and the identity of each peptide was confirmed by MS (Electrospray Quatro II triple quadrupole mass spectrometer; Micromass). Peptide concentrations were determined by using a quantitative ninhydrin assay as previously described (Phadke et al., 2002).
Bacterial growth inhibition and killing assays.
Antibacterial activity was examined by a standard growth inhibition assay endorsed by the Clinical and Laboratory Standards Institute (CLSI) with minor modifications as follows (Pfaller et al., 2005; Sader et al., 2007). Bacteria were incubated with each of the indicated peptides in standard Muller-Hinton broth (MHB; Sigma-Aldrich) for 18 h, at which time A600 values were measured to examine growth inhibition using a BioTek microplate reader (BioTek Instruments) (Deslouches et al., 2013). MICs were defined as the peptide concentrations reducing growth by 90 % or greater. Peptide concentrations of up to 32 µM were evaluated for antibacterial activity in the presence or absence of divalent cations Ca2+ and Mg2+; higher concentrations were not assayed to avoid solubility limitations. To examine directly peptide bactericidal activity, the test bacteria were treated for 1 h with peptides in PBS at 37°C, and residual colony forming units per ml (c.f.u. mL−1) were determined by broth dilution assay.
Comparison of WR12 and WK12 eCAP interactions with bacteria.
We compared the optimally active 12-mer peptides WR12 and WK12 for antimicrobial mechanisms by examining eCAP–bacteria interactions using surface plasmon resonance (Kuster et al., 2015a) and membrane permeabilization using flow cytometry.
Affinity for bacteria by SPR.
Surface plasmon resonance (SPR) experiments were performed on a CM4 biosensor chip using a Biacore 3000 Instrument (GE Healthcare). The WK12, WR12 and control HIV fusion inhibitor peptide T20 were synthesized by the University of Pittsburgh Peptide Synthesis Core. WK12 and WR12 peptides were solubilized in HBS-EP buffer (GE Healthcare, catalog # BR-1001-88), diluted to a concentration of 10 µM, pH 7.0, then injected onto independent CM4 surfaces at 5 μl min–1 and immobilized using standard amine coupling procedures to equivalent response units (RU). The HIV T20 peptide (Qiu et al., 2012) was diluted to 1 µM, pH 3.0, and immobilized similarly to the reference flow cell as a control for non-specific binding of analyte to a peptide surface. A sample from an overnight culture of PAO1 was pelleted, washed with PBS twice and diluted to 2×107c.f.u. ml −1 in HBS-EP. PAO1 analytes were diluted to 2×10–2, 2×10–5, 2×10–8 and 2×10–11 flowed over peptide surfaces with 1× NSB (GE Healthcare) at 25 °C, in duplicate, at a flow rate of 10 μl min–1. Association was measured for 180 s, followed by 180 s of dissociation in HBS-EP running buffer. The chip surface was regenerated with a 30 s pulse of 0.5 % SDS after each run. In addition to reference flow cell subtraction, HBS-EP buffer-only cycles were used to allow double referencing for all analyses. Binding curves were fit using a 1:1 Langmuir binding model of dissociation and the BIAevaluation software v. 4.1.1 (GE Healthcare), which was used to generate kinetic data.
Bacterial cell permeabilization by flow cytometry.
Flow cytometry was used to quantify the ability of eCAPs to permeabilize bacterial membranes resulting in propidium iodide (PI) incorporation into bacterial cells. P. aeruginosa and MRSA bacterial cells (108ml −1 in PBS) were exposed to PI (impermeable to intact cell membranes) after 45 min of treatment with 1 µM peptides in the presence or absence of 2 mM Ca2+ and Mg2+ in saline. PI incorporation into bacterial cells was quantified using a BD LSRII flow cytometer for detection of PI at 617 nm according to the manufacturer’s instructions (BD Biosciences).
Mammalian cytotoxicity assays.
Cytotoxicity was examined using three assays: haemolysis, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and flow cytometry.
Haemolytic assay.
To examine the influence of lysine and arginine substitutions on peptide haemolytic properties, human erythrocytes were treated in parallel with the respective WK or WR peptides for 1 h in PBS, and haemoglobin release was determined by spectroscopic analysis, as previously described (Deslouches et al., 2013).
MTT assay.
MTT assay was performed as previously described (Phadke et al., 2005; Deslouches et al., 2013) Briefly, freshly isolated peripheral blood mononuclear cells (PBMCs) were allowed to grow and differentiate into macrophages in the presence of granulocyte macrophage-colony stimulating factor (GM-CSF) for 5 days. Attached macrophages were exposed to peptides for 1 h and then treated with MTT (Life Technologies). After 4 h of incubation in the presence of MTT at 37 °C, formazan crystals were dissolved with acidified isopropanol (0.1 M isopropanol/HCl/10 % Triton-X-100), and A550 was measured to determine percentage viability or cytotoxicity.
Flow cytometry.
We used flow cytometry as a direct measure of mammalian cytotoxicity to complement the haemolytic and MTT assays. To assess mammalian cytotoxicity, freshly isolated human PBMCs were exposed to peptides for 1 h at 37 °C and immediately washed with PBS using a round-bottom 96-well plate. Fixable blue live/dead stain from Life Technologies was added to each sample according to the manufacturer’s instructions. The cells were washed with PBS and then fixed with 4 % formaldehyde (Thermo Scientific). After washing twice with PBS, the samples were stored at 4 °C overnight prior to analysis by flow cytometry using the BD LSR II cytometer. Peptide-treated samples were compared with untreated control for reactivity with the blue dye and data were analyzed using flowjo software (Perfetto et al., 2006).
Statistical analysis
Statistical analysis, when applicable, was determined by multiple t-tests comparison using GraphPad Prism 6 software.
Results
The goal of this study was to investigate the influence of cationic amino acid composition on antibacterial activity and cytotoxicity of engineered AMPs of increasing length differing only in use of lysine (WK series) or arginine (WR series) paired with tryptophan. Toward this goal, we designed and produced a series of eCAPs in which the arginine residues in the WR peptides were replaced with lysine to create a corresponding WK peptide series (Table 1). Comparative computational analyses reveal that the parallel WR and WK peptides differ only in cationic amino acid content; analogous WR and WK peptides are identical in terms of length, overall charge and calculated amphipathic potential, allowing a direct comparison of the functional properties imparted by the lysine and arginine, respectively.
Table 1. Primary sequences, molecular masses and hydrophobic moments of eCAPs in order of increasing length .
Previously designed (WR series) cationic peptides were modified to WK peptides via global Arg-to-Lys substitutions; hydrophobic moments were examined using the online program Heliquest (http://heliquest.ipmc.cnrs.fr/).
| Peptide sequence | Name | Charge | Molecular mass (Da) | Hydrophobic moment (μH) |
|---|---|---|---|---|
| WWRRWWRR | WR8 | +4 | 1390 | 0.93 |
| WWKKWWKK | WK8 | +4 | 1280 | 0.93 |
| WRWWRRWWRR | WR10 | +5 | 1730 | 0.94 |
| WKWWKKWWKK | WK10 | +5 | 1590 | 0.94 |
| RWWRWWRRWWRR | WR12 | +6 | 2070 | 0.99 |
| KWWKWWKKWWKK | WK12 | +6 | 1900 | 0.99 |
| WRRWWRWWRRWWRR | WR14 | +7 | 2410 | 1.03 |
| WKKWWKWWKKWWKK | WK14 | +7 | 2220 | 1.03 |
| RWWRRWWRWWRRWWRR | WR16 | +8 | 2760 | 1.02 |
| KWWKKWWKWWKKWWKK | WK16 | +8 | 2530 | 1.02 |
| WRRWWRRWWRWWRRWWRR | WR18 | +9 | 3100 | 1.04 |
| WKKWWKKWWKWWKKWWKK | WK18 | +9 | 2850 | 1.04 |
Comparative effects of arginine and lysine on antibacterial properties
Antibacterial activity.
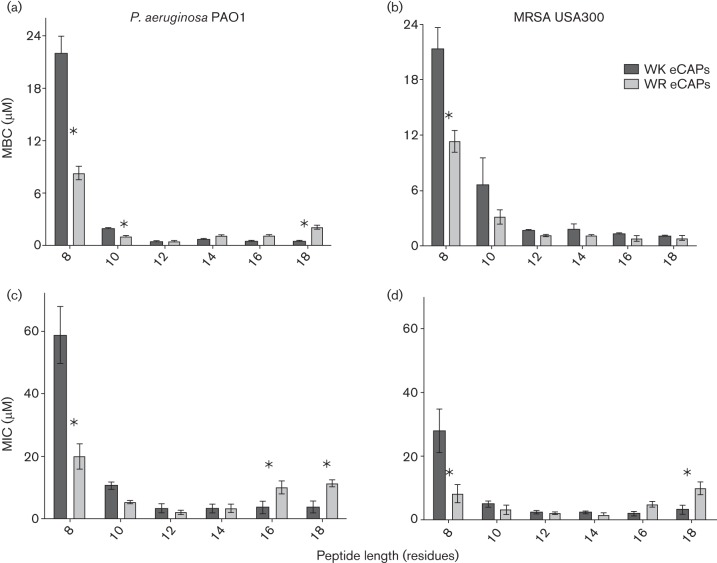
We first compared WR and WK peptides for antibacterial activity against two reference organisms, a Gram-negative (P. aeruginosa PAO1) and a Gram-positive (MRSA USA300) bacterium. For this functional comparison, each peptide series was assayed in parallel to determine both the minimum bactericidal concentration (MBC) and the MIC to assess specific activity in different environments or growth conditions (Fig. 1). The results of these assays revealed that, for each peptide series, activity increased with length and reached a maximum at 12 residues against both P. aeruginosa and MRSA targets. However, the data also generally indicate a higher level of bacterial killing (P < 0.05) in the shorter peptides (10-mer, P. aeruginosa; 8-mer, both P. aeruginosa and MRSA) containing arginine compared with lysine peptides of the same length. For example, when tested against P. aeruginosa (Fig. 1a, c), the shorter WR peptides WR8 (MBC, 8 µM and MIC, 20 µM) and WR10 (MBC, 1 µM and MIC, 5 µM) displayed higher activity (P < 0.05) compared with the analogous WK peptides WK8 (MBC, >10 µM and MIC, >32 µM) and WK10 (MBC, 2 µM and MIC, 10 µM). As peptide length increases, the WK and WR peptides demonstrated similar maximal activity at 12 residues (WK12: MBC, 0.6 µM and MIC, 2 µM; WR12: MBC, 0.4 µM and MIC, 1 µM) and 14 residues (WK14: MBC, 0.8 µM and MIC, 2 µM; WR14: MBC, 1.2 µM and MIC, 2 µM) in length against P. aeruginosa. Interestingly, while further increases in peptide length failed to significantly decrease MBC or MIC values relative to those observed for the 12-mer peptide, the WK16 (MIC: 4 µM against P. aeruginosa) and WK18 (MIC: 4 µM against P. aeruginosa and MRSA) tend to display a modest but significantly higher level of activity (P < 0.05) compared with their WR peptide analogues WR16 (MIC: 8 µM against P. aeruginosa) and WR18 (MIC: 8 µM against both P. aeruginosa and MRSA) (Fig. 1c, d).
Fig. 1.
Comparative antibacterial activity of the WR and WK peptides. Bacteria were treated with twofold serially diluted peptides at 37 °C either for 1 h in PBS (a and b) or for 18 h in MHB (c and d). MBCs or MICs for P. aeruginosa (PAO1) (a and c) or MRSA (b and d) were determined as the concentrations corresponding to a 3-log reduction in bacterial survival (MBC) or at least 90 % reduction in bacterial growth (MIC). Data plotted are mean values determined from three independent experimental trials performed in triplicate, and statistical significance is indicated by an asterisk (P < 0.05). MBCs and MICs exceeding maximum test concentrations (WK8, a–c) are shown only for comparison and clarity.
Antibacterial activities against MRSA were generally comparable to activities observed against P. aeruginosa, with maximum bacterial killing or growth inhibition achieved at the 12-residue peptide in both the WR and the WK series. Again, the data demonstrate a significantly higher level of bacterial killing (P < 0.05) by the shorter (8–10 residues) WR peptides than by the analogous WK peptides, indicating an enhancement of activity by arginine in short peptide sequences. However, this difference in activity against MRSA is not observed in the longer WR and WK peptides containing 12–18 residues (MBCs, ≤ 1.5 µM) (Fig. 1b) when tested in PBS.
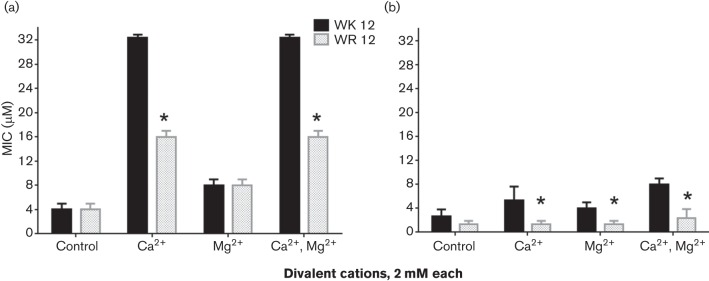
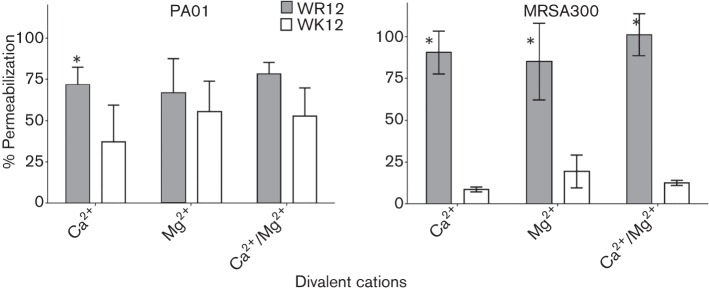
Next, we focused on the shortest peptides with optimal activity (WK12 and WR12) to further discern the differential contributions of arginine and lysine to the antibacterial mechanism and cytotoxic properties. We compared the 12-mer peptides using two XDR (Deslouches et al., 2015) clinical isolates of Gram-negative P. aeruginosa and Acinetobacter baumannii that are also resistant to the membrane-active cationic antibiotic colistin. In three independent trials, WK12 and WR12 displayed similar activity against P. aeruginosa (MIC: WK12 2.7 µM; WR12 3.3 µM) and A. baumannii (MIC: WK12 3.3 µM; WR12 2.7 µM), consistent with previous data (colistin MIC > 32 µM for both isolates). Because divalent cations usually inhibit the activity of natural AMPs (Zhang et al., 2005), we compared WK12 and WR12 for the tendency to resist the inhibitory effects of divalent cations Ca2+ and Mg2+, using growth inhibition assays. While inhibition of the activity of WK12 (Ca2+, 8-fold; Mg2+, 2-fold; both cations, 8-fold) and WR12 (Ca2+, 4-fold; Mg2+, 2-fold; both cations, 4-fold) was observed against P. aeruginosa, susceptibility of WK12 to these cations was significantly greater (P < 0.05) compared with that of WR12 (Fig. 2a). The differences in susceptibility were even significantly wider (P < 0.05) against MRSA, as WR12 was almost completely refractory to the presence of Ca2+ or Mg2+ against MRSA compared with a 4-fold (P < 0.05) inhibition of WK12 (Fig. 2b).
Fig. 2.
Arginine enhances eCAP resistance to divalent cations compared with lysine. Activity against P. aeruginosa (a) and MRSA (b) was examined in the presence or absence of divalent cations (2 mM Ca2+ or Mg2+) by growth inhibition assay, and the 12-mer peptides were compared for MIC; an asterisk indicates statistically significant difference (P < 0.05) between WR12 and WK12 for three independent experimental trials performed in triplicate.
Peptide interactions with bacterial membranes
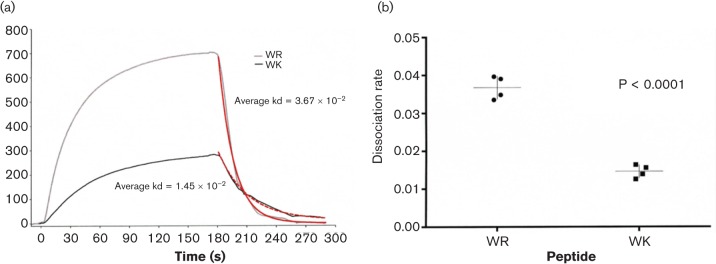
A common antimicrobial mechanism of helical AMPs is the ability to recognize their bacterial targets by electrostatic interactions and to permeabilize the cell membrane (Jelokhani-Niaraki et al., 1998; Jean-François et al., 2008; Park et al., 2008). Based on this understanding, we sought to compare the shortest eCAPs with optimal activity, WK12 and WR12, for interactions with live P. aeruginosa using SPR spectroscopy and flow cytometry (Lequin et al., 2006). SPR determination of binding affinity for WK12 and WR12 with live bacteria was carried out by passing live P. aeruginosa over chips coated with the respective peptides, as detailed in Materials and Methods. Binding analyses between peptide ligands and live bacteria analytes demonstrated over multiple dilutions two distinct binding curves for WR12 and WK12 (Fig. 3a). In general, WR12 bound whole bacteria at a faster rate than WK12, repeatedly at multiple dilutions of bacterial suspension and likewise had a significantly higher overall response (P = 0.047, paired t-test). Interestingly, bacterial binding to WR12 had a significantly faster off or dissociation rate as compared with WK12 (Fig. 3b) perhaps due to more rapid bacterial lysis by the WR12 peptide. Thus, these data indicate distinct differences in peptide–bacterial membrane interactions depending on the choice of cationic amino acid contained in the antimicrobial peptide.
Fig. 3.
eCAP binding differences to live P. aeruginosa. WK12 and WR12 peptide binding to PAO1 was detailed using SPR. (a) A representative curve for each peptide is displayed. Sensogram plot provides binding over time, where association is visualized as an increasing response, during which PAO1 was flowed over immobilized peptides including the negative control peptide T20, an HIV fusion inhibitor. Dissociation is visualized as a decreasing response, during which PAO1 was replaced with buffer. Red lines indicate dissociation rates determined using BIAevaluation software. (b) WR12 demonstrated a significantly faster dissociation rate as compared with WK, determined with via unpaired t-test (P < 0.0001) in the GraphPad software package.
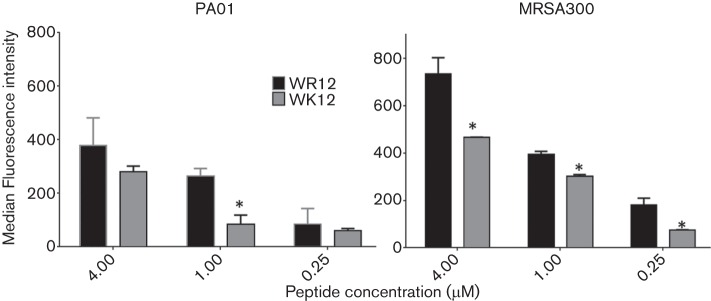
Based on the differential binding of the 12-mer peptides to P. aeruginosa, we next used flow cytometry to compare membrane permeabilization properties against both P. aeruginosa and MRSA by assaying PI incorporation in bacterial suspensions in PBS. The data demonstrate that, compared with WK12, WR12 has a significantly greater concentration-dependent permeabilizing effect (by up to 60 %) against P. aeruginosa (P < 0.05, 1 µM) and MRSA (P < 0.05) (Fig. 4).
Fig. 4.
Arginine enhances bacterial membrane permeabilization compared with lysine. P. aeruginosa (left) and MRSA (right) were treated with 0.25–4.0 µM WK12 and WR12 in PBS for 30 min, and then fluorescence intensity due to PI incorporation was quantified by flow cytometry as a measure of membrane permeabilization; an asterisk indicates a statistically significant difference between WR12 and WK12 for three independent experimental trials performed in triplicate.
Next we compared arginine and lysine for the ability of the 12-mer peptides to resist the inhibitory effects of divalent cations (Ca2+ and Mg2+) using a series of flow cytometry assays to quantify membrane permeabilization by PI incorporation of WR12- and WK12-treated bacteria. As shown in Fig. 5, the data are normalized for the effects of 2 mM Ca2+, Mg2+, or both Ca2+ and Mg2+, against P. aeruginosa or MRSA using PI incorporation in saline alone (100 % permeabilization). WK12 demonstrates a significant (P < 0.05) reduction (up to 60 % for PAO1 and up to 90 % for MRSA) in membrane permeabilization in the presence of divalent cations compared with moderate (P. aeruginosa) or negligible inhibition (MRSA) of WR12, consistent with the growth inhibition assay data shown in Fig. 2.
Fig. 5.
Membrane permeabilization: arginine confers eCAP resistance to divalent cations compared with lysine. P. aeruginosa (left) and MRSA (right) were treated with 1 µM WK12 and WR12 in PBS and in the presence or absence of divalent cations (2mM each); the data are normalized with respect to eCAP-specific permeabilization in saline alone; an asterisk indicates a statistically significant difference (P < 0.05) between WR12 and WK12 for three independent experimental trials performed in triplicate.
Comparative effects of arginine and lysine on haemolytic and cytotoxic properties
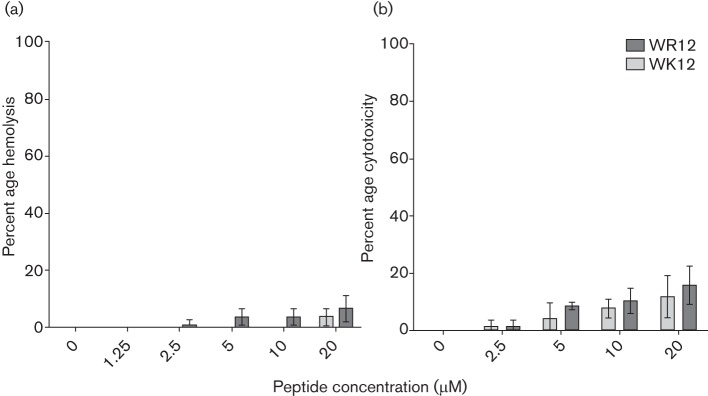
As WR12 and WK12 were the shortest peptides to display optimal activity in respective series, we continued to focus on these two peptides for examination of haemolytic activity and cytotoxicity. As an initial measure of the influence of lysine and arginine on peptide cytotoxic properties, we used a standard red blood cell (RBC) lysis assay (Deslouches et al., 2013) in which freshly isolated human erythrocytes in PBS were exposed to the WK12 and WR12 peptides for 1 h, and the release of haemoglobin associated with each peptide was measured. The data summarized in Fig. 6(a) demonstrate that both WK12 and WR12 displayed less than 10 % haemolytic activity at peptide concentrations up to 20 µM, which is approximately 10 times the MIC or >20 times the MBC (Fig. 1) against P. aeruginosa or S. aureus. There was no significant difference (P < 0.05) in the haemolytic activity of WR12 and WK12 at each peptide concentration.
Fig. 6.
Host toxicity by haemolytic and tetrazolium-based assays. Human RBCs were treated for 1 h with WK12 or WR12 in PBS, and percentage haemolysis was determined by spectrophotometric analysis of released haemoglobin using a standard curve of RBC lysis (a). To determine the cytotoxicity against nucleated cells, human monocyte-derived macrophages were treated with peptides for 1 h and cytotoxicity was determined by MTT assay (b). Data plotted are mean values (in triplicate) from three independent experimental trials, and statistical analysis by multiple t-tests showed no significant differences between WR12 and WK12 for each test concentration (P > 0.2).
We next examined potential cytotoxic properties of the WR12 and WK12 peptides by treatment of nucleated cells, primary human monocyte-derived macrophages (Hadnagy et al., 1993; Varney et al., 2002). As revealed by the tetrazolium-based (MTT) assay (Fig. 6b), WK12 and WR12 demonstrated no detectable cytotoxicity at the peptide concentrations similar to the respective MBC or MIC values of 1–2 µM. At the maximum peptide concentration of 20 µM (approximately 10 times the MIC and 20 times the MBC), WR12 displayed a mean macrophage toxicity of about 16 % compared with 13 % for WK12.
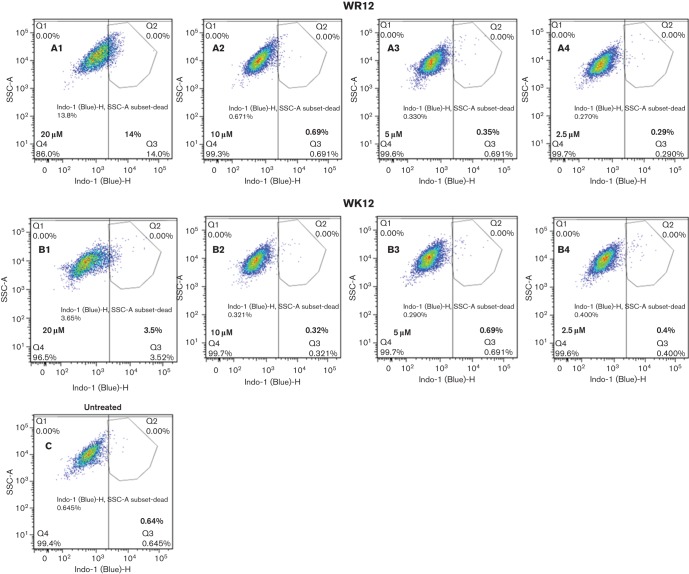
As a complementary measure of peptide cytotoxicity, we also determined the effects of exposure of freshly isolated PBMCs to WK12 and WR12 using flow cytometry to quantify dead and live cells by differential incorporation of a fluorescent amine-reactive dye (Perfetto et al., 2006). As summarized in Fig. 7, the level of viable PBMCs (around 99 %) after treatment with either WR12 (A2 to A4) or WK12 (B2 to B4) at 2.5–10 µM was comparable to PBMC controls (C) that were not exposed to peptide treatment, thus indicating a similar lack of apparent cytotoxicity for the WR12 and WK12 peptides at concentrations of or below 10 µM. At a peptide concentration of 20 µM, treatment of PBMCs resulted in cell viability values of about 86 % after exposure to WR12 and 96 % after exposure to WK12. These data indicate similar cytotoxic properties of WK12 and WR12 at peptide concentrations up to 10 times the MBC and more than five times the MIC values. However, WK12 may display lower cytotoxicity to PBMCs at higher peptide concentrations, perhaps due to the greater cell-penetrating properties of arginine-rich peptides compared with lysine-rich peptides.
Fig. 7.
Cytotoxic effects of WK and WR peptides determined by flow cytometry examination of live/dead cell staining: WR12, panels A1 to A4; WK12, panels B1 to B4; untreated cells, panel C; data are representative of two independent experimental trials.
Discussion
The aim of the current study was to test directly the functional properties of lysine and arginine as determinants of the antibacterial and cytotoxic properties of a defined series of peptides ranging from eight to 18 residues in length and containing only tryptophan in addition to the respective cationic amino acid. These comparative studies were based on the previously published and well-characterized WR eCAPs that contain only arginine and tryptophan with optimal helicity achieved at 12 and 14 residues (Deslouches et al., 2013), thus allowing a straightforward substitution of lysine for arginine to produce a parallel WK series that were identical in terms of length and overall charge properties. In addition, computational modelling of the WK and WR peptides revealed identical calculated hydrophobic moment values for the respective peptide pairs, an important consideration as the amphipathic helical structure of engineered peptides has been shown to be a major determinant of antibacterial properties. Thus, WK and WR peptide series provided a unique opportunity to isolate and define the respective contributions of the two cationic amino acids to antibacterial and cytotoxic properties to inform future eCAP design.
The results of the current studies reveal that despite the very similar physiochemical properties of lysine and arginine, their contribution to antimicrobial peptide functional properties can differ. Specifically, the comparison of bacterial killing activity against the reference Gram-negative P. aeruginosa and the Gram-positive MRSA under different environmental conditions (MBC versus MIC/dynamic growth) revealed a higher level of bacterial killing by 8- and 10-mer WR peptides compared with the analogous WK peptides. This difference in bacterial killing suggests a significant difference in the functional interaction between the WR and WK peptides with the same target bacterial membrane.
Both the WR and the WK peptides achieved maximal bacterial killing at the 12-mer peptide (Kuster et al., 2015a) with no significant decrease in MBC or MIC values observed with longer peptides up to 18 residues in length. Furthermore, the 12-mer peptides retained activity against two colistin-resistant and XDR clinical isolates of P. aeruginosa and A. baumannii. Interestingly, in a study of LL37 peptide, Wang (2008) identified the minimum length peptide KR-12 that achieved antibacterial activity comparable to that of the full-length LL-37, achieving an MIC of 40 µM against Echerichia coli K12. The identification in separate studies of 12-mer peptides as a minimal length to achieve maximal antibacterial activity may reflect the fact that the 12-mer achieves the levels of positive charge and amphipathicity to optimize membrane interactions and the minimal peptide length to completely span the bacterial membrane.
We have previously reported the identification of a minimum peptide length that achieves maximum bacterial killing (Deslouches et al., 2013). In the case of peptides composed exclusively of arginine and valine or arginine, valine, and tryptophan, the optimally functional minimum length peptide was 24 residues long (Deslouches et al., 2005). In contrast, engineered cationic peptides containing only arginine and tryptophan achieved maximum bactericidal activity at 12-residue length with confirmation of helicity by circular dichroism (Deslouches et al., 2013), suggesting that a minimum of three helical turns is required for optimal interaction with bacteria and bactericidal activity. Importantly, based on the helicity of other Lys-rich helical peptides (e.g. LL37, magainin; Park et al., 2003; Lai et al., 2011) Lys substitution is predicted to retain the helical content of the peptides. In these series of eCAPs, increasing peptide length with the same amino acid content failed to increase antimicrobial activity. Thus, while peptide length, charge and minimum helical turns are determinants of antibacterial activity, amino acid content may be a predominant parameter in peptide design.
The current data, summarized in Fig. 1, also suggest that the longer WK peptides (14–18 residues) may display a significantly lower MIC or MBC compared with the analogous WR peptide, especially in the MIC assay environment. However, a possible explanation for this apparent differential may in fact be the observed lower solubility of long WR peptides compared with WK peptides in complex solutions. As shown in our previous circular dichroism studies, WR16 and WR18 have a tendency to aggregate, possibly due to intermolecular interactions between the stable positively charged guanidinium group of Arg and pi electrons of the Trp indole ring (Schibli et al., 2002; Deslouches et al., 2013). These longer peptides (WR16 and WR18) tend to precipitate more substantially than shorter WR eCAPs in complex media, even at relatively low micromolar concentrations. In this report, the environmental effect is evident in the observed differences in activity of the 16–18 residue peptides against MRSA in MHB and PBS (Fig. 1b, d). While the current studies focus mainly on functional differences based on Lys or Arg, we are systematically investigating the mechanistic and structural basis for these differences through extensive biophysical studies (e.g. circular dichroism and X-ray diffraction) of eCAP-membrane interactions using WK/WR peptides and other eCAP series.
The optimal activity observed at 12 residues in length for both the WR and the WK series necessitated further consideration of WR12 and WK12 for comparative antimicrobial mechanisms and cytotoxic properties. Despite similar activity of WR12 and WK12 initially observed in the bacterial killing and growth inhibition assays, physiologically relevant changes in assay conditions (presence of divalent cations) revealed significant differences in activity between these two peptides, indicating that arginine enhanced activity compared with lysine. Such differences in resistance to divalent cations warranted a more extensive exploration of bacterial interactions with these two peptides.
The comparative studies of antimicrobial mechanisms of WK12 and WR12 further highlight the role of arginine in optimization of antimicrobial properties compared with lysine. Using SPR assays, we demonstrated interesting differences in the interactions of the optimally active 12-mer peptides of the WK and WR series with intact bacteria. SPR data indicated that WR12 bound P. aeruginosa to higher levels than WK12. While definitive rates were not achievable due to the nature of the whole-cell bacteria binding kinetics, the binding curve repeatedly indicated a faster binding of WR12 to bacteria in addition to greater amounts. The observed significantly faster dissociation rate of the WR12 peptide and bacteria, when evaluated in light of the flow cytometry permeabilization data, could be attributed to more rapid bacterial membrane lysis by the WR12 peptide.
The differences between these two peptides (WK12 and WR12) are further underlined by the effects of divalent cations on bacterial membrane permeabilization. Divalent cations play an important role in the stability of the Gram-negative bacterial membrane (Clifton et al., 2015) and influence the antibacterial properties of AMPs (Sugiarto & Yu, 2007). These cations also interact with proteoglycan and wall teichoic/lipoteichoic acids of Gram-positive bacteria (Swoboda et al., 2010; Thomas & Rice, 2014), a process targeted by the anionic antibiotic daptomycin (Straus & Hancock, 2006). Conversely, the activity of cationic AMPs tends to be inhibited by divalent cations (Dashper et al., 2005; Zhang et al., 2005; Sugiarto & Yu, 2007), and the strength of that inhibition may vary with the test peptide and type of (Gram-positive or Gram-negative) organisms as supported by the data. Because of the presence of Ca2+ and Mg2+ in biological matrices (e.g. blood), resistance to these cations is an important consideration in eCAP design. While the activity of both WR12 and WK12 at 2 mM Ca2+ and Mg2+ was moderately inhibited against P. aeruginosa, WK12 was more significantly affected. Similarly, the ability of the lysine analogue to permeabilize MRSA cell membrane was substantially inhibited (MRSA) in the presence of these cations compared with WR12, indicating that arginine significantly enhanced membrane permeabilization properties and resists the effects of divalent cations compared with lysine (P < 0.05). Based on previous comparative studies of our eCAPs LBU2 and WLBU2, resistance to cations has been attributed mainly to tryptophan content (Deslouches et al., 2005; Phadke et al., 2005). However, the current results, combined with previous data, indicate that both arginine and tryptophan play an important role in eCAP optimization to overcome certain limitations of natural AMPs. Interestingly, the high frequency of lysine in natural AMPs (e.g. LL37) compared to arginine is consistent with the observed inhibition of activity in certain environments (e.g. pH, divalent cations) (Pezzulo et al., 2012) which can be overcome by design optimization (Deslouches et al., 2005; Deslouches et al., 2013). An interesting question is why these two 12-mer peptides display similar MBCs in PBS but differential binding and membrane permeabilizing properties under the same conditions. A plausible explanation could be that the lysine-rich peptide may also kill bacteria via a secondary mechanism, as shown for other antimicrobial peptides (Hale & Hancock, 2007).
To overcome the intrinsic limitations of natural AMPs that have to date limited clinical applications, we previously reported novel engineered cationic antimicrobial peptides composed of only arginine and tryptophan that display highly effective bacterial killing and low mammalian cell toxicity under diverse environmental conditions and at minimal peptide lengths. These arginine-rich engineered peptides produce negligible cytoxicity at concentrations lower than 20 µM. In the current study, we compared WK12 and WR12 for the influence of lysine or arginine on eCAP cytotoxic properties by three different methods: standard haemolytic assays, MTT assays and viability assays by flow cytometry. The haemolytic assay reflects direct eCAP effects on mammalian RBC membrane, the MTT assay evaluates mainly eCAP influence on metabolic activity (mitochondrial function) and flow cytometry is a direct measure of cell viability (live/dead cells) (Perfetto et al., 2006). All three assays consistently revealed minor or negligible haemolytic or cytotoxic effects of both peptides at test concentrations lower than 20 µM. The data show a modest difference (3–10 %) in haemolytic or cytotoxic effects between WR12 and WK12 at 20 µM, a concentration that is approximately 20 and 40 times the MBCs for WR12 or 15 and 30 times MBCs for WK12 for MRSA and P. aeruginosa, respectively. Hence, WK12 and WR12 demonstrate comparable in vitro selectivity, which is consistent with previously published reports (Deslouches et al., 2013).
Taken together, the current data further highlight the importance of optimizing the amino acid content, overall charge and total length as determinants of bacterial killing by eCAPs. Previous studies from our lab demonstrated the marked enhancement of bacterial killing properties by the substitution of tryptophan for valine in the peptide sequence(Deslouches et al., 2005). Here we demonstrate an enhancement of bacterial killing properties by short arginine-rich peptides compared with analogous lysine-rich peptides toward achieving minimal peptide length with maximal bacterial killing and lower inhibitory effects by divalent cations. Thus, these studies continue to elucidate useful guidelines that can inform the continued engineering of eCAPs to target specific bacteria in diverse environments.
Acknowledgements
We thank Kazi Islam and the staff of the University of Pittsburgh Peptide Synthesis facility for peptide production and Tyler Hall for expert technical assistance. This project, executed as part of the routine work of the authors for the University of Pittsburgh, was supported in part by the NIH grant 5P30 DK072506 awarded to the Cystic Fibrosis Research Center of the University of Pittsburgh and by funds from the Center for Vaccine Research of the University of Pittsburgh. All authors contributing to this study receive ongoing financial support from the University of Pittsburgh as staff or faculty members of the Department of Microbiology and Molecular Genetics and Center for Vaccine Research.
Authors of this publication (R.C.M., J.D.S., J.K.C.) hold stock in Peptilogics. However, the Kuster et al., 2015b research findings included in this publication may not necessarily be related to the interests of Peptilogics.
Abbreviations:
- AMP
antimicrobial peptide
- eCAP
engineered cationic antimicrobial peptide
- MDR
multidrug-resistant
- RBC
red blood cell
- PI
propidium iodide
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- XDR
extensively drug-resistant
- MRSA
methicillin-resistant Staphylococcus aureus
- PBMC
peripheral blood mononuclear cell
- MBC
minimum bactericidal concentration
- SPR
surface plasmon resonance
References
- Agodi A., Voulgari E., Barchitta M., Quattrocchi A., Bellocchi P., Poulou A., Santangelo C., Castiglione G., Giaquinta L, Santangelo G.(2014). Spread of a carbapenem- and colistin-resistant Acinetobacter baumannii ST2 clonal strain causing outbreaks in two Sicilian hospitals. J Hosp Infect 86 260–266. 10.1016/j.jhin.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Boucher H. W., Talbot G. H., Bradley J. S., Edwards J. E., Gilbert D., Rice L. B., Scheld M., Spellberg B., Bartlett J.(2009). Bad bugs, no drugs: No ESKAPE! an update from the infectious diseases society of america. Clin Infect Dis 48 1–12. 10.1086/595011 [DOI] [PubMed] [Google Scholar]
- Bow E. J.(2013). There should be no ESKAPE for febrile neutropenic cancer patients: The dearth of effective antibacterial drugs threatens anticancer efficacy. J Antimicrob Chemother 68 492–495. 10.1093/jac/dks512 [DOI] [PubMed] [Google Scholar]
- Brogden K. A.(2005). Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3 238–250. 10.1038/nrmicro1098 [DOI] [PubMed] [Google Scholar]
- Bucki R., Pastore J. J., Randhawa P., Vegners R., Weiner D. J., Janmey P. A.(2004). Antibacterial activities of rhodamine b-conjugated gelsolin-derived peptides compared to those of the antimicrobial peptides cathelicidin LL37, magainin II, and melittin. Antimicrob Agents Chemother 48 1526–1533. 10.1128/AAC.48.5.1526-1533.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton L. A., Skoda M. W., Le Brun A. P., Ciesielski F., Kuzmenko I., Holt S. A., Lakey J. H.(2015). Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir 31 404–412. 10.1021/la504407v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashper S. G., O'Brien-Simpson N. M., Cross K. J., Paolini R. A., Hoffmann B., Catmull D. V., Malkoski M., Reynolds E. C.(2005). Divalent metal cations increase the activity of the antimicrobial peptide kappacin. Antimicrob Agents Chemother (Bethesda) 49 2322–2328. 10.1128/AAC.49.6.2322-2328.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslouches B., Phadke S. M., Lazarevic V., Cascio M., Islam K., Montelaro R. C., Mietzner T. A.(2005). De novo generation of cationic antimicrobial peptides: Influence of length and tryptophan substitution on antimicrobial activity. Antimicrob Agents Chemother 49 316–322. 10.1128/AAC.49.1.316-322.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslouches B., Steckbeck J. D., Craigo J. K., Doi Y., Burns J. L., Montelaro R. C.(2015). Engineered cationic antimicrobial peptides to overcome multidrug resistance by ESKAPE pathogens. Antimicrob Agents Chemother (Bethesda) 59 1329–1333. 10.1128/AAC.03937-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslouches B., Steckbeck J. D., Craigo J. K., Doi Y., Mietzner T. A., Montelaro R. C.(2013). Rational design of engineered cationic antimicrobial peptides consisting exclusively of arginine and tryptophan, and their activity against multidrug-resistant pathogens. Antimicrob Agents Chemother (Bethesda) 57 2511–2521. 10.1128/AAC.02218-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Yang L., Weiss T. M., Waring A. J., Lehrer R. I., Huang H. W.(2003). Interaction of antimicrobial peptides with lipopolysaccharides. Biochemistry 42 12251–12259. 10.1021/bi035130+ [DOI] [PubMed] [Google Scholar]
- Georgescu J., Munhoz V. H., Bechinger B.(2010). NMR structures of the histidine-rich peptide LAH4 in micellar environments: Membrane insertion, ph-dependent mode of antimicrobial action, and DNA transfection. Biophys J 99 2507–2515. 10.1016/j.bpj.2010.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadnagy W., Seemayer N. H., Happel A., Kiell A.(1993). Human monocyte-derived macrophage cultures: An alternative test system for the detection of pulmonary toxicity induced by inhaled particulate pollutants. Toxicol in Vitro 7 365–371. 10.1016/0887-2333(93)90029-5 [DOI] [PubMed] [Google Scholar]
- Hale J. D., Hancock R. E.(2007). Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti Infect Ther 5 951–959. 10.1586/14787210.5.6.951 [DOI] [PubMed] [Google Scholar]
- Hancock R. E.(2001). Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect Dis 1 156–164. 10.1016/S1473-3099(01)00092-5 [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Brown K. L., Mookherjee N.(2006). Host defence peptides from invertebrates--emerging antimicrobial strategies. Immunobiology 211 315–322. 10.1016/j.imbio.2005.10.017 [DOI] [PubMed] [Google Scholar]
- Hell E., Giske C. G., Nelson A., Römling U., Marchini G.(2010). Human cathelicidin peptide LL37 inhibits both attachment capability and biofilm formation of staphylococcus epidermidis. Lett Appl Microbiol 50 211–215. 10.1111/j.1472-765X.2009.02778.x [DOI] [PubMed] [Google Scholar]
- Ho J. Y., Cira N. J., Crooks J. A., Baeza J., Weibel D. B.(2012). Rapid identification of ESKAPE bacterial strains using an autonomous microfluidic device. PLoS One 7, . 10.1371/journal.pone.0041245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-François F., Elezgaray J., Berson P., Vacher P., Dufourc E. J.(2008). Pore formation induced by an antimicrobial peptide: Electrostatic effects. Biophys J 95 5748–5756. 10.1529/biophysj.108.136655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelokhani-Niaraki M., Nakashima K., Kodama H., Kondo M.(1998). Interaction and orientation of an alpha-aminoisobutyric acid- and tryptophan-containing short helical peptide pore-former in phospholipid vesicles, as revealed by fluorescence spectroscopy. J Biochem 123 790–797. 10.1093/oxfordjournals.jbchem.a022006 [DOI] [PubMed] [Google Scholar]
- Kalia V., Sarkar S., Gupta P., Montelaro R. C.(2003). Rational site-directed mutations of the LLP-1 and LLP-2 lentivirus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation. J Virol 77 3634–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatami M. H., Bromberek M., Saika-Voivod I., Booth V.(2014). Molecular dynamics simulations of histidine-containing cod antimicrobial peptide paralogs in self-assembled bilayers. Biochimica Et Biophysica Acta (BBA) - Biomembranes 1838 2778–2787. 10.1016/j.bbamem.2014.07.013 [DOI] [PubMed] [Google Scholar]
- Kulkarni M. M., Karafova A., Kamysz W., McGwire B. S.(2014). Design of protease-resistant pexiganan enhances antileishmanial activity. Parasitol Res 113 1971–1976. 10.1007/s00436-014-3847-3 [DOI] [PubMed] [Google Scholar]
- Kuster D. J., Liu C., Fang Z., Ponder J. W., Marshall G. R.(2015a). High-resolution crystal structures of protein helices reconciled with three-centered hydrogen bonds and multipole electrostatics. PLoS One 10, 10.1371/journal.pone.0123146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster D. W., Govindan S., Springer T. I., Martin J. L., Finley N. L., Sadayappan S.(2015b). A hypertrophic cardiomyopathy-associated MYBPC3 mutation common in populations of South Asian descent causes contractile dysfunction. J Biol Chem 290 5855–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y., Adhikarakunnathu S., Bhardwaj K., Ranjith-Kumar C. T., Wen Y., Jordan J. L., Wu L. H., Dragnea B., San Mateo L., Kao C. C.(2011). LL37 and cationic peptides enhance TLR3 signaling by viral double-stranded RNAs. PLoS One 6, . 10.1371/journal.pone.0026632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb H. M., Wiseman L. R.(1998). Pexiganan acetate. Drugs 56 1047–1052. 10.2165/00003495-199856060-00011 [DOI] [PubMed] [Google Scholar]
- Lequin O., Ladram A., Chabbert L., Bruston F., Convert O., Vanhoye D., Chassaing G., Nicolas P, Amiche M..(2006). Dermaseptin S9, an alpha-helical antimicrobial peptide with a hydrophobic core and cationic termini. Biochemistry 45 468–480. 10.1021/bi051711i [DOI] [PubMed] [Google Scholar]
- Lipsky B. A., Holroyd K. J., Zasloff M.(2008). Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: A randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis 47 1537–1545. 10.1086/593185 [DOI] [PubMed] [Google Scholar]
- Magiorakos A. P., Srinivasan A., Carey R. B., Carmeli Y., Falagas M. E., Giske C. G., Harbarth S., Hindler J. F., Kahlmeter G, other authers. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18 268–281. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- Mason A. J., Bertani P., Moulay G., Marquette A., Perrone B., Drake A. F., Kichler A., Bechinger B.(2007). Membrane interaction of chrysophsin-1, a histidine-rich antimicrobial peptide from red sea bream. Biochemistry 46 15175–15187. 10.1021/bi701344m [DOI] [PubMed] [Google Scholar]
- Mihajlovic M., Lazaridis T, Lazaridis. (2010). Antimicrobial peptides in toroidal and cylindrical pores. Biochim Biophys Acta 1798 1485–1493. 10.1016/j.bbamem.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. J., Kim D. T., Steinman L., Fathman C. G., Rothbard J. B, Steinman. (2000). Polyarginine enters cells more efficiently than other polycationic homopolymers. J Pept Res 56 318–325. 10.1034/j.1399-3011.2000.00723.x [DOI] [PubMed] [Google Scholar]
- Ozcan A. V., Demir M., Onem G., Goksin I., Baltalarli A., Topkara V. K., Kaleli I.(2006). Topical versus systemic vancomycin for deep sternal wound infection caused by methicillin-resistant staphylococcus aureus in a rodent experimental model. Tex Heart Inst J 33 107–110. [PMC free article] [PubMed] [Google Scholar]
- Park S. C., Kim M. H., Hossain M. A., Shin S. Y., Kim Y., Stella L., Wade J. D., Park Y., Hahm K. S..(2008). Amphipathic alpha-helical peptide, HP (2-20), and its analogues derived from helicobacter pylori: Pore formation mechanism in various lipid compositions. Biochim Biophys Acta 1778 229–241. 10.1016/j.bbamem.2007.09.020 [DOI] [PubMed] [Google Scholar]
- Park Y., Lee D. G., Jang S. H., Woo E. R., Jeong H. G., Choi C. H., Hahm K. S.(2003). A leu-lys-rich antimicrobial peptide: Activity and mechanism. Biochim Biophys Acta 1645 172–182. 10.1016/S1570-9639(02)00541-1 [DOI] [PubMed] [Google Scholar]
- Perfetto S. P., Chattopadhyay P. K., Lamoreaux L., Nguyen R., Ambrozak D., Koup R. A., Roederer M.(2006). Amine reactive dyes: An effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods 313 199–208. 10.1016/j.jim.2006.04.007 [DOI] [PubMed] [Google Scholar]
- Peschel A., Sahl H. G.(2006). The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4 529–536. 10.1038/nrmicro1441 [DOI] [PubMed] [Google Scholar]
- Pezzulo A. A., Tang X. X., Hoegger M. J., Abou Alaiwa M. H., Ramachandran S., Moninger T. O., Karp P. H., Wohlford-Lenane C. L., Haagsman H. P., other authors(2012). Reduced airway surface ph impairs bacterial killing in the porcine cystic fibrosis lung. Nat New Biol 487 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Boyken L., Hollis R. J., Messer S. A., Tendolkar S., Diekema D. J.(2005). In vitro susceptibilities of clinical isolates of candida species, cryptococcus neoformans, and aspergillus species to itraconazole: Global survey of 9,359 isolates tested by clinical and laboratory standards institute broth microdilution methods. J Clin Microbiol 43 3807–3810. 10.1128/JCM.43.8.3807-3810.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke S. M., Deslouches B., Hileman S. E., Montelaro R. C., Wiesenfeld H. C., Mietzner T. A.(2005). Antimicrobial peptides in mucosal secretions: The importance of local secretions in mitigating infection. J Nutr 135 1289–1293. [DOI] [PubMed] [Google Scholar]
- Phadke S. M., Islam K., Deslouches B., Kapoor S. A., Beer Stolz D., Watkins S. C., Montelaro R. C., Pilewski J. M., Mietzner T. A.(2003). Selective toxicity of engineered lentivirus lytic peptides in a CF airway cell model. Peptides 24 1099–1107. 10.1016/j.peptides.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Phadke S. M., Lazarevic V., Bahr C. C., Islam K., Stolz D. B., Watkins S., Tencza S. B., Vogel H. J., Montelaro R. C., Mietzner T. A.(2002). Lentivirus lytic peptide 1 perturbs both outer and inner membranes of serratia marcescens. Antimicrob Agents Chemother 46 2041–2045. 10.1128/AAC.46.6.2041-2045.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S., Yi H., Hu J., Cao Z., Wu Y., Li W.(2012). The binding mode of fusion inhibitor T20 onto HIV-1 gp41 and relevant t20-resistant mechanisms explored by computational study. Curr HIV Res 10 182–194. 10.2174/157016212799937191 [DOI] [PubMed] [Google Scholar]
- Sader H. S., Ferraro M. J., Reller L. B., Schreckenberger P. C., Swenson J. M., Jones R. N.(2007). Reevaluation of clinical and laboratory standards institute disk diffusion breakpoints for tetracyclines for testing enterobacteriaceae. J Clin Microbiol 45 1640–1643. 10.1128/JCM.00143-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibli D. J., Epand R. F., Vogel H. J., Epand R. M.(2002). Tryptophan-rich antimicrobial peptides: Comparative properties and membrane interactions. Biochem Cell Biol 80 667–677. 10.1139/o02-147 [DOI] [PubMed] [Google Scholar]
- Sekimata M., Homma Y, Wang Z., Wang G..(2004). Sequence-specific transcriptional repression by an mbd2-interacting zinc finger protein MIZF. Nucleic Acids Res 32 590–592. (Database issue), D. 10.1093/nar/gkh249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri Sundararajan V., Gabere M. N., Pretorius A., Adam S., Christoffels A., Lehväslaiho M., Archer J. A., Bajic V. B.(2012). DAMPD: A manually curated antimicrobial peptide database. Nucleic Acids Res 40 D1108–D1112. 10.1093/nar/gkr1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam G., Polavarapu P. L., Gopinath D., Jayakumar R.(2005). The structure of antimicrobial pexiganan peptide in solution probed by fourier transform infrared absorption, vibrational circular dichroism, and electronic circular dichroism spectroscopy. Biopolymers 80 636–642. 10.1002/bip.20132 [DOI] [PubMed] [Google Scholar]
- Shi S. H., Kong H. S., Xu J., Zhang W. J., Jia C. K., Wang W. L., Shen Y., Zhang M., Zheng S. S.(2009). Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transpl Infect Dis 11 405–412. 10.1111/j.1399-3062.2009.00421.x [DOI] [PubMed] [Google Scholar]
- Skinner M. C., Kiselev A. O., Isaacs C. E., Mietzner T. A., Montelaro R. C., Lampe M. F.(2010). Evaluation of WLBU2 peptide and 3-o-octyl-sn-glycerol lipid as active ingredients for a topical microbicide formulation targeting chlamydia trachomatis. Antimicrob Agents Chemother (Bethesda) 54 627–636. 10.1128/AAC.00635-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckbeck J. D., Deslouches B., Montelaro R. C.(2014). Antimicrobial peptides: New drugs for bad bugs? Expert Opin Biol Ther 14 11–14. 10.1517/14712598.2013.844227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. K., Hancock R. E. W.(2006). Mode of action of the new antibiotic for gram-positive pathogens daptomycin: Comparison with cationic antimicrobial peptides and lipopeptides. Biochimica Et Biophysica Acta (BBA) - Biomembranes 1758 1215–1223. 10.1016/j.bbamem.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Su Y., Doherty T., Waring A. J., Ruchala P., Hong M.(2009). Roles of arginine and lysine residues in the translocation of a cell-penetrating peptide from (13)C, (31)P, and (19)F solid-state NMR. Biochemistry 48 4587–4595. 10.1021/bi900080d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiarto H., Yu P. L.(2007). Effects of cations on antimicrobial activity of ostricacins-1 and 2 on E. coli O157:H7 and S. aureus 1056MRSA. Curr Microbiol 55 36–41. 10.1007/s00284-006-0554-z [DOI] [PubMed] [Google Scholar]
- Swoboda J. G., Campbell J., Meredith T. C., Walker S, Campbell. (2010). Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem 11 35–45. 10.1002/cbic.200900557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tencza S. B., Creighton D. J., Yuan T., Vogel H. J., Montelaro R. C., Mietzner T. A.(1999). Lentivirus-derived antimicrobial peptides: Increased potency by sequence engineering and dimerization. J Antimicrob Chemother 44 33–41. 10.1093/jac/44.1.33 [DOI] [PubMed] [Google Scholar]
- Tencza S. B., Douglass J. P., Creighton D. J., Montelaro R. C., Mietzner T. A.(1997). Novel antimicrobial peptides derived from human immunodeficiency virus type 1 and other lentivirus transmembrane proteins. Antimicrob Agents Chemother 41 2394–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tencza S. B., Miller M. A., Islam K., Mietzner T. A., Montelaro R. C.(1995). Effect of amino acid substitutions on calmodulin binding and cytolytic properties of the LLP-1 peptide segment of human immunodeficiency virus type 1 transmembrane protein. J Virol 69 5199–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. J., Rice C. V.(2014). Revised model of calcium and magnesium binding to the bacterial cell wall. Biometals 27 1361–1370. 10.1007/s10534-014-9797-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossi A., Sandri L., Giangaspero A.(2000). Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55 4–30. [DOI] [PubMed] [Google Scholar]
- Varney M. L., Olsen K. J., Mosley R. L., Bucana C. D., Talmadge J. E., Singh R. K.(2002). Monocyte/macrophage recruitment, activation and differentiation modulate interleukin-8 production: A paracrine role of tumor-associated macrophages in tumor angiogenesis. In Vivo 16 471– 4710. [PubMed] [Google Scholar]
- Wang G.(2008). Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem 283 32637–32643. 10.1074/jbc.M805533200 [DOI] [PubMed] [Google Scholar]
- Wang G., Li X., Wang Z, Li X., Wang Z..(2009). APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res 37 D933–D937. (Database issue), D 10.1093/nar/gkn823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Parente J., Harris S. M., Woods D. E., Hancock R. E. W., Falla T. J.(2005). Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob Agents Chemother (Bethesda) 49 2921–2927. 10.1128/AAC.49.7.2921-2927.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Shi W., Tang S., Li J., Yin S., Gao X., Wang L., Zou L., Zhao J., other authors(2013). The influence of cathelicidin LL37 in human anti-neutrophils cytoplasmic antibody (anca)-associated vasculitis. Arthritis Res & Ther 15 R161. 10.1186/ar4344 [DOI] [PMC free article] [PubMed] [Google Scholar]