Abstract
Background
The aim of this study was to compare robotic assisted and freehand facet joint puncture on a phantom model in regards to time requirements and puncture accuracy.
Material/Methods
Forty facet joints were punctured, 20 using a robotic guidance system and 20 using a freehand procedure. Side and height of the facet joints were randomized and identical for both groups. Procedural accuracy, defined as axial and sagittal deviation, as well as the number of corrections were assessed. Procedure times for each step were documented and time requirements for pre-positioning, reconstruction, planning, and total intervention were calculated.
Results
Total procedure time for robotic guidance was 259±111 seconds versus 119±77 seconds for freehand procedure (p=1.0). Procedural accuracy for robotic guidance was significantly higher with 0 corrections versus 1.3 corrections for freehand procedure (p=0.02). Needle deviation in the robotics arm was 0.35±1.1 mm in the axial and 2.15±1.2 mm in the sagittal reconstruction.
Conclusions
Robotic assisted puncture of the facet joint allowed accurate positioning of the needle with a lower number of needle readjustments. Higher procedural accuracy was marginally offset by a slightly longer intervention time.
MeSH Keywords: Pain Management; Radiology, Interventional; Robotics
Background
According to the 2010 Global Burden of Disease study, lower back pain causes more morbidity than any other conditions. It globally ranks first in the YLD (years lived with disability) ranking and is responsible for 83.0 million DALYs (disability-adjusted life years). In the upcoming years, the number of people with lower back pain will likely increase because of the ageing population [1].
Facet joint injection (FJI) defined as the precise installation of local anaesthetic and/or corticosteroid into a facet joint or around its nerve supply with the aim to relieve both pain and inflammation is a common treatment option in the case of lower back pain. FJI is commonly used in clinical practice and for the patient often is considered the last hope for pain improvement [2]. Good clinical outcome is highly dependent on accurate deposition of the anaesthetic and/or corticosteroid. This may partially explain the varied outcomes reported from different FJI studies. Recently, new procedures using laser or electromagnetic tracking, have been introduced [3,4] to allow for better needle placement during FJI.
Image CT-guided, robotic assisted interventions may allow for higher FJI accuracy, therefore lower complication rates and achieving higher clinical efficacy. Robotic navigation might also reduce radiation dose exposure and procedure time, because fewer control scans need to be performed [5,6].
Hence, this phantom model study aimed to investigate the benefits of CT-guided, robotic assisted facet joint puncture compared to the conventional freehand approach.
Material and Methods
Study design
In this phantom model study, we performed 40 facet joint punctures, 20 using a robotic targeting system and 20 using a freehand procedure, controlled by stepwise CT-scans. The primary endpoint of the study was the time required from planning the CT scan to the correct intra-articular placement of the 21-gauge puncture needle. Secondary endpoints were the accuracy of the needle placement, measured as axial and sagittal deviation, and the number of required needle adjustments.
Prior to the acquisition of a lateral topogram (Somatom Sensation 16, Siemens Healthcare, Forchheim, Germany), the phantom model (Siemens Healthcare, Forchheim, Germany) was immobilized in a vacuum fixation system (iSYS Medizintechnik GmbH, Kitzbühl, Austria). Segment and side of the punctured facet joints were randomized but identical for robotic navigated approach and freehand approach. An experienced interventional radiologist (more than 200 facet joint punctures per year) carried out the procedures. Time requirements, number of corrections, and needle deviation were documented in an Excel spreadsheet.
Robotic assisted puncture
For robotic-assisted puncture the iSYS 1.3 (iSYS Medizintechnik GmbH, Kitzbuehel, Austria) robotic targeting system was used. The phantom model and vacuum fixation system were mounted on a holding platform and placed on the CT examination table. An adjustable multifunctional arm with the connected robotic device was attached to the holding platform. The mobile CT workstation, which was connected to the local network, was placed next to the examination table (Figure 1).
Figure 1.
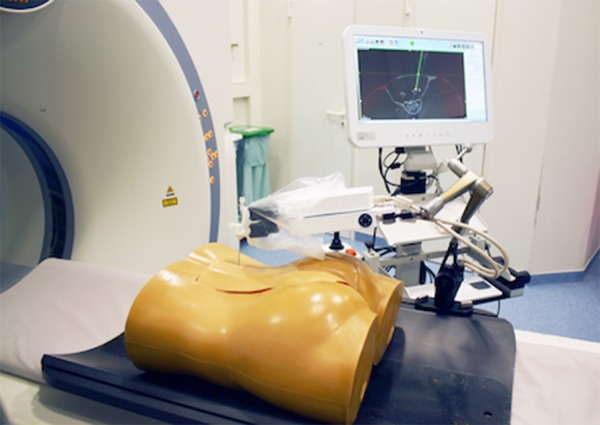
Setup of the study with the phantom device, the robotic device, and the workstation.
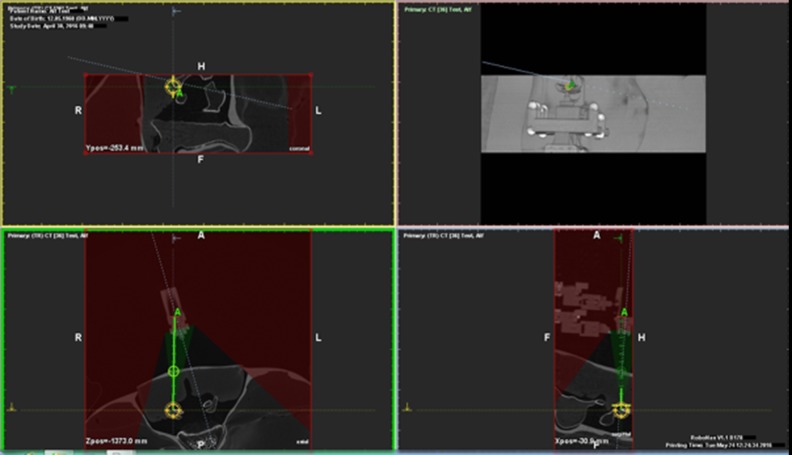
In order to manually pre-position the robotic targeting system at the approximate position of the percutaneous entry point, a lateral topogram was acquired, which was used to define the height of the facet joint in the z-axis. The examination table was moved to the defined height and, using the gantry laser, the robot was manually locked into position at the presumable percutaneous entry point. After positioning of the device, a CT scan was acquired and sent to the workstation, where an automatic registration of the robotic device and its 13 radio-opaque markers was performed by the navigation software (RoboNav, MedCom Gesellschaft für medizinische Bildverarbeitung GmbH, Darmstadt, Germany). The robotic device has a range of motion of ±2 cm in both axes and of −32° in every direction. The navigation software displays all reachable target and entry points, determined by the registered position of the robotic device (Figure 2). Target and percutaneous entry points were defined by the physician. Afterwards, the needle guidance of the robot was moved into position. The needle was then manually placed through the needle guidance, whereby the depth of the needle was calculated after defining the target and entry point. After injection, another CT scan was performed and sent to the workstation. Axial and sagittal deviation between planned and conducted punctures were noted.
Figure 2.
Planning screen of the workstation. Reachable target and entry points are automatically calculated based on the registration of the robotic device and can be set within the green conic field.
Free hand puncture
According to the navigated puncture, a lateral topogram was acquired to define the height of the facet joint. A grid was placed on the presumed percutaneous entry point and a CT scan was acquired. The percutaneous entry point and the needle trajectory were planned by the physician at the CT workstation. The examination table was moved to the defined position. The gantry laser marked the height of the facet joint and the needle was placed freehand. A 3-slice biopsy scan was performed to control the position of the needle. If not placed intra-articular, the position of the needle was corrected and another biopsy scan was performed stepwise until the facet joint was reached.
Time requirements
During robotic assisted and freehand needle placement procedures, the times were documented (Figure 3). The following time intervals were calculated for the robotic assisted procedure: time required for pre-positioning of the robotic device, time required for reconstruction, time required for transfer to the workstation (measured from the CT scan to the start of the planning), and time required for planning needle trajectory at the robotic workstation. Total intervention time (measured from the CT scan until the final needle position was reached) was calculated for robotic assisted and freehand procedure.
Figure 3.
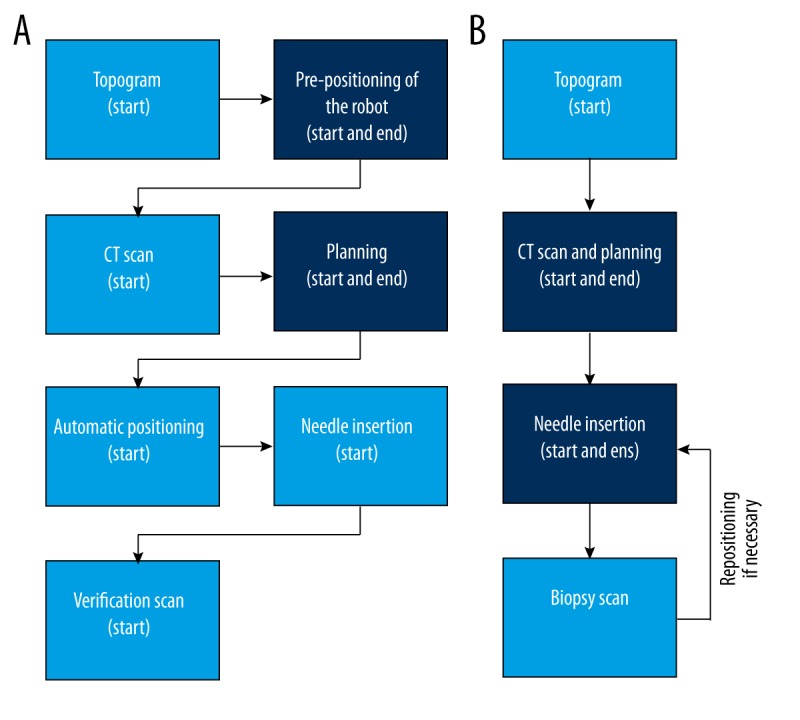
Workflow of the robotic-assisted (A) and freehand approach (B) with the documented time points
Statistical analysis
The JMP statistics software package (SAS Institute, Cary, NC, USA) was used to perform all statistical calculations. The Shapiro-Wilk test was used to assess the normality of the distribution of investigated parameters. Data are expressed as mean ± standard deviation (SD). Differences between total intervention times were compared using the paired t-test, reported with degrees of freedom in parentheses, the t statistic, and the significance level. The number of required needle corrections were compared using Pearsons’ chi-squared test, reported with degrees of freedom and sample size in parentheses, the Pearson chi-square value, and the significance level. A p-value of p≤0.05 was considered the cut-off point of statistical significance.
Results
Segment and side of the punctured facet joint are listed by their frequency in Table 1.
Table 1.
Identical segment and side of the punctured facet joint for robotic assisted and freehand technique.
| Segment | Left side | Right side |
|---|---|---|
| L1 | 2 | 2 |
| L2 | 2 | 1 |
| L3 | 0 | 3 |
| L4 | 3 | 4 |
| L5 | 2 | 1 |
The facet joint was reachable by the robotic device after initial pre-positioning in all cases (20 of 20, 100%), no position adjustments after the registration were necessary.
Table 2 shows the measured time intervals in seconds. Differences between the total intervention time were not significant (t (19)=4.64, p=1.0).Using the robotic device, the facet joint was reached within the first puncture in all cases (20 of 20, 100%; Figure 4). For the freehand approach, a maximum of four corrections was necessary (median, 1 correction), the frequencies are shown in Table 3. The number of required corrections was significantly greater in the freehand group (c2 (4, N=40)=11.61, p=0.02).
Table 2.
Measured time spans in seconds in robotic assisted and freehand approach.
| Time span | Robotic assisted (sec) | Freehand (sec) |
|---|---|---|
| Device pre-positioning | 57±20 (min. 23, max. 97) | – |
| CT reconstruction | 130±66 (min. 42, max. 314) | – |
| Trajectory planning | 70±36 (min. 29, max. 189) | – |
| Total intervention time | 259±111 (min. 118, max. 517) | 119±77 (min. 40, max. 344) |
Figure 4.
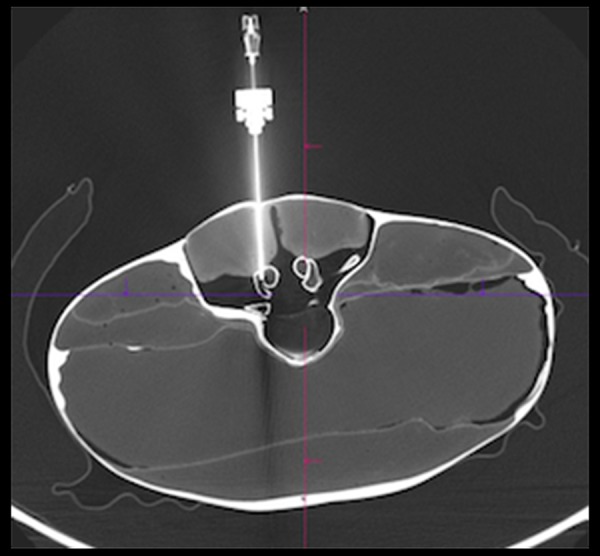
Verification scan showing correct intra-articular needle placement in the robotic-assisted group.
Table 3.
Frequency of required needle corrections resp. biopsy scans in the freehand approach.
| Number of required corrections | Frequency |
|---|---|
| 0 | 11 |
| 1 | 4 |
| 2 | 2 |
| 3 | 2 |
| 4 | 1 |
In the robotic assisted group, the mean deviation from the planned trajectory to the documented needle position was: 0.35±1.1 mm (minimum 2, maximum 3) in the axial and 2.15±1.2 mm (minimum 0, maximum 4) in the sagittal reconstruction.
Discussion
Nowadays, injections into the facet joint are performed using a freehand technique and under CT control. Hence, frequent repositioning of the needle is necessary. This causes higher radiation doses for the patients and increases their risk for related complications [7]. Correspondingly, we observed up to four needle repositions with a median of one in our study.
Robotic assisted punctures on the other hand have been associated with higher accuracy compared to the freehand technique, especially for complex needle trajectories [8]. With fewer readjustments, the required radiation dose can also be reduced [9]. Our study confirmed these results; using the robotic guidance system, the needle was placed intra-articular at the first attempt and no repositioning of the needle was required. Exact positioning of the needle intra-articular with minimal axial and sagittal deviation might increase therapeutic efficiency. Further studies with patients have to be conducted to confirm this benefit.
The intervention time was longer, although not significantly, using the robotic guidance system. On average, the procedure took 259 seconds (minimum 118 seconds, maximum 517 seconds) within the robotic assisted group, and 119 seconds (minimum 40 seconds, maximum 344 seconds) when performed freehand. This difference might be explained by the long reconstruction times, which were primarily caused by the required 1 mm CT slice thickness and the low computing power of our CT scanner. The planning itself, defining target and entry point within the reachable range of the robot, took on average only 70 seconds. In no case did the robot have to be re-pre-positioned because the target could not be reached.
Even though the procedure took longer using the robotic guidance system, it is, with approximately four minutes, still fast and a recommendable technique especially for inexperienced interventional radiologists.
The study had several limitations, being a phantom model study the high accuracy reached within the robotic assisted group might not be reached in a real world patient setting. A further limitation was that only one interventional radiologist performed all robotic assisted and freehand punctures. Especially the freehand puncture is highly depend on the experience of the interventional radiologist and might therefore vary considerably.
Conclusions
The high accuracy achieved when using the robotic targeting system can allow even an inexperienced interventional radiologist to place the needle intra-articular without repositioning. We believe this advantage outweighs the fact that the robotic intervention takes slightly longer.
Footnotes
Conflict of interest
No conflict of interest to declare.
Source of support: Departmental sources
References
- 1.Hoy D, March L, Brooks P, et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–74. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- 2.Airaksinen O, Brox JI, Cedraschi C, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(Suppl 2):S192–300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruners P, Penzkofer T, Nagel M, et al. Electromagnetic tracking for CT-guided spine interventions: phantom, ex-vivo and in-vivo results. Eur Radiol. 2009;19(4):990–94. doi: 10.1007/s00330-008-1227-z. [DOI] [PubMed] [Google Scholar]
- 4.Krombach GA, Schmitz-Rode T, Brabrand K, et al. Initial experiences with a new optical target system (SimpliCT) for CT-guided punctures. RöFo Fortschritte auf dem Gebiete der Röntgenstrahlen und der Nukl. 2000;172(6):557–60. doi: 10.1055/s-2000-3748. [DOI] [PubMed] [Google Scholar]
- 5.Cornelis F, Takaki H, Laskhmanan M, et al. Comparison of CT fluoroscopy-guided manual and CT-guided robotic positioning system for in vivo needle placements in swine liver. Cardiovasc Intervent Radiol. 2015;38(5):1252–60. doi: 10.1007/s00270-014-1016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer LP, Pregler B, Niessen C, et al. Robot-assisted microwave thermoablation of liver tumors: A single-center experience. Int J Comput Assist Radiol Surg. 2016;11(2):253–59. doi: 10.1007/s11548-015-1286-y. [DOI] [PubMed] [Google Scholar]
- 7.Mbalisike EC, Vogl TJ, Zangos S, et al. Image-guided microwave thermoablation of hepatic tumours using novel robotic guidance: An early experience. Eur Radiol. 2015;25(2):454–62. doi: 10.1007/s00330-014-3398-0. [DOI] [PubMed] [Google Scholar]
- 8.Koethe Y, Xu S, Velusamy G, et al. Accuracy and efficacy of percutaneous biopsy and ablation using robotic assistance under computed tomography guidance: A phantom study. Eur Radiol. 2014;24(3):723–30. doi: 10.1007/s00330-013-3056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato R, Katada K, Anno H, et al. Radiation dosimetry at CT fluoroscopy: Physician’s hand dose and development of needle holders. Radiology. 1996;201(2):576–78. doi: 10.1148/radiology.201.2.8888264. [DOI] [PubMed] [Google Scholar]